Abstract
Background:
Structural and functional abnormalities have been found in language-related brain regions in patients with schizophrenia. We previously reported findings pointing to differences in word processing between people with schizophrenia and individuals who are at high-risk for schizophrenia using a voxel-based (whole brain) fMRI approach. We now extend this finding to specifically examine functional activity in three language related cortical regions using a larger cohort of individuals.
Method:
A visual lexical discrimination task was performed by 36 controls, 21 subjects at high genetic-risk for schizophrenia, and 20 patients with schizophrenia during blood oxygenation level dependent (BOLD) fMRI scanning. Activation in bilateral inferior frontal gyri (Brodmann's area 44-45), bilateral inferior parietal lobe (Brodmann's area 39-40), and bilateral superior temporal gyri (Brodmann's area 22) was investigated. For all subjects, two-tailed Pearson correlations were calculated between the computed laterality index and a series of cognitive test scores determining language functioning.
Results:
Regional activation in Brodmann's area 44-45 was left lateralized in normal controls, while high-risk subjects and patients with schizophrenia or schizoaffective disorder showed more bilateral activation. No significant differences among the three diagnostic groups in the other two regions of interest (Brodmann's area 22 or areas 39-40) were found. Furthermore, the apparent reasons for loss of leftward language lateralization differed between groups. In high-risk subjects, the loss of lateralization was based on reduced left hemisphere activation, while in the patient group, it was due to increased right side activation. Language ability related cognitive scores were positively correlations with the laterality indices obtained from Brodmann's areas 44-45 in the high-risk group, and with the laterality indices from Brodmann's areas 22 and 44-45 in the patient group.
Conclusions:
This study reinforces previous language related imaging studies in high-risk subjects and patients with schizophrenia suggesting that reduced functional lateralization in language related frontal cortex may be a vulnerability marker for schizophrenia. Future studies will determine whether it is predictive of who develops illness.
Keywords: fMRI; schizophrenia; High risk, genetic; Language lateralization; ROI based study
Introduction
Although first-degree relatives of individuals with schizophrenia have an almost 10-fold increased risk of developing schizophrenia (Gottesman & Shields, 1972; Gottesman, 1991), the underlying genetic mechanism for the pathogenesis of the disorder is not well understood. By studying individuals who are at high-risk for developing schizophrenia, one can determine biological factors that may be important for distinguishing people who are likely to later develop the illness. Subjects at high genetic risk for schizophrenia can be defined as individuals between the ages of 13 and 30, who have never been diagnosed with schizophrenia, but have a first-degree relative with the illness (Johnstone et al, 2000; Li et al, 2007). These individuals share on average 50% of genes with their diagnosed relatives and thus have a heightened risk of developing the disorder.
Several studies indicate that patients with schizophrenia may have anomalies of language functioning (reviewed in Delisi, 2001). Structural and functional magnetic resonance imaging (MRI/fMRI) made it possible to investigate the anatomical and neural basis of disturbed language processing in schizophrenia in vivo. Anatomical abnormalities in patients with schizophrenia in regions associated with language processing in the frontal, temporal and parietal lobes have been found in previous structural MRI studies (reviewed in Shenton, 2001). Volume reductions in the inferior parietal lobule, supramarginal gyrus and left angular gyrus, which are parts of the neuroanatomical circuitry for language comprehension, have been reported (Buchanan et al, 2004; Nierenberg et al, 2005). Several functional MRI studies have revealed abnormal language related brain activation patterns in patients with schizophrenia (Table 1). Studies employing either language listening tasks or verbal fluency tasks have reported that patients with schizophrenia exhibited reduced left hemisphere dominant activation or reversed language dominance (Curtis et al, 1998; Kiehl & Liddle, 2001; Kircher et al, 2001; Menon et al, 2001; Ngan et al, 2003; Sommer et al, 2001, 2003; Woodruff et al, 1997). While the findings varied across studies, overall, they suggest that structural and functional abnormalities exist in the brain regions associated with language processing in individuals with schizophrenia. If comparable anomalies are found in high-risk individuals before they experience the symptoms of the illness, these changes might then be considered as possible vulnerability markers for the illness.
Table 1.
Functional MRI findings in language related brain areas in schizophrenia.
SZ=schizophrenia, NC=Normal Controls, m=males, f=females.
| Reference | Sample size (n) |
Mean age (yrs) |
Sex (m/f) |
Task design | Major findings |
|---|---|---|---|---|---|
| Kircher et al, 2001 | SZ 6, NC 6 |
SZ 34.3, NC 34.0 |
All m | Speech generation task. Seven Rorschach cards were presented on the screen during scanning and subjects were asked to describe them. Event- related design. |
Severity of thought disorder was correlated positively with activity in the cerebellar vermis, right body of caudate, right precentral gyrus; and correlated negatively with left superior temporal gyrus, and the posterior part of middle temporal gyrus. |
| Kiehl & Liddle, 2001 | SZ 11, NC 11 |
SZ 26.6, NC 27.0 |
6m/5f in SZ, 6m/5f in NC |
Auditory oddball task. Event-related design. |
Patients showed reduced activation in the bilateral anterior superior temporal gyri, left supramarginal gyrus, right superior and inferior parietal lobule, anterior and posterior cingulated, thalamus, and right lateral frontal cortex |
| Menon et al, 2001 | SZ 11, NC 13 |
SZ 44.6 NC 42.5 |
All m | 2-back auditory working memory (WM) task. Block design. |
Patients showed decreased lateralization of activation and significant WM related activation deficits in bilateral dorsolateral prefrontal cortices, frontal operculum, inferior parietal , and superior parietal cortex. |
| Ngan et al, 2003 | SZ 14, NC 29 |
SZ 35.1, NC 29.3 |
12m/2f in SZ, 21m/8f in NC |
Auditory oddball task. Event-related design. |
Patients showed greater differential activation between speech and nonspeech in right temporal cortex, left superior frontal cortex, and the left temporal-parietal junction. The magnitude of the difference in the left temporal parietal junction was significantly correlated with severity of disorganized thinking. |
| Woodruff et al, 1997 | SZ trait- positive 8, trait- negative 7, NC 8 |
SZ trait- positive 36, trait- negative 34.6, NC 35.3 |
All m | Auditory perception of speech task. Block design. |
Reduced in the left superior temporal gyrus but increased in the right middle temporal gyrus in the combined schizophrenic groups relative to the healthy comparison group. Comparison of the trait-positive and trait-negative patients revealed no clear difference in the power of temporal cortical activation. |
| Curtis et al, 1998 | SZ 5, NC 5 |
SZ 29.6, NC 31.6 |
All m | Verbal fluency task. Block design. |
Schizophrenic subjects showed significantly reduced power of response in the left dorsal prefrontal cortex, the inferior frontal gyrus, and the insula but significantly increased power of response in the medial parietal cortex. |
| Sommer et al, 2001 | SZ 12, NC 12 |
SZ 27.0, NC 28.0 |
All m | Verb generation and semantic decision reverse read task. Block design. |
Reduced leftward language-laterality in SZ group was related to increased action in the right hemisphere. |
| Sommer er al, 2003 | SZ 12, NC 12 |
SZ 33.6 NC 32.0 |
All f | The same task as in Sommer et al, 2001 |
Reduced leftward language-laterality in SZ group was related to increased action in the right hemisphere. No gender difference was found compared with the findings in Sommer et al, 2001. |
The anatomical pathways for language processing were first documented by Broca and Wernicke in the 19th Century (Broca, 1861; Wernicke, 1874). The development of functional imaging tools has redefined models of language processing so that they now include modulation by cognitive processes, semantic orientation and input pathway relevance which none-the-less remain rooted in the original anatomical structures (Price, 2000). In this model, the left superior temporal gyri, incorporating the primary auditory cortex and Wernicke's area, process heard words, while the posterior fusiform gyri and lingual gyri within the visual cortex process written words. Wernicke's area and more posterior and inferior temporal regions, such as the midfusiform and temporo-parietal regions including the angular gyrus are involved in the semantic processing of words, while inferior lateral frontal regions, incorporating Broca's area and the motor cortices, provide motor and speech output responses. The planning of language articulation is proposed to take place in the frontal operculum and anterior insula. Figure 1 summarizes the relevant areas of interest. In this study, we focused attention on the functional activation in three specific cortical regions of interest (ROIs), which were selected from the anatomical pathways for language processing suggested above, in subjects who are at genetic high risk for developing schizophrenia, and diagnosed patients with schizophrenia. The chosen regions were bilateral inferior frontal gyri—Brodmann's area 44-45, the bilateral inferior parietal lobe—Brodmann's area 39-40, and bilateral superior temporal gyri—Brodmann's area 22. In a recent initial study (Li et al, 2007) we reported differences in language processing between controls (n=15) and both high risk (n=15) and schizophrenic (n=15) cohorts using voxel-wise (whole brain) analysis of fMRI data acquired during a word discrimination task. The present study is an extension of that study using the same fMRI language paradigm in a larger cohort and now examining activation in specific language regions of interest. In addition the relationships between language activation and verbal cognition, and heritability as well, were assessed outside the scanner.
Figure 1.
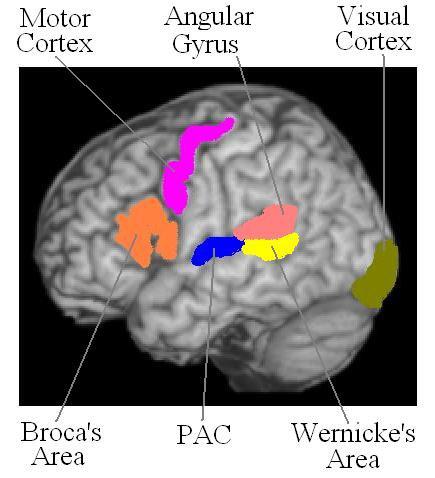
The major anatomical pathways for language processing
Materials and methods
Subjects
A total of 77 subjects were included in this study (36 normal controls, 21 subjects at high genetic risk for schizophrenia and 20 patients with schizophrenia (N=9) or schizoaffective disorder (N=11). Six patients were siblings of the unaffected individuals who comprised the high risk group. Although none of the genetically at high-risk subjects had a history of psychosis, 6 were diagnosed with DSM-IV axis II schizotypal personality disorder and four had a history of at least one episode of major depression (3 of which were in the schizotypal group). Data from 15 subjects in each group were analyzed in the preliminary voxel-based analysis study (Li et al, 2007). A total of 45 of the current 77 subjects overlap with the previous study. Individuals were considered at high genetic risk if they were within the peak age of risk for schizophrenia (defined as ages 13-30) and they either originated from families in which at least one first-degree relative (parent or sibling) had a diagnosis of schizophrenia or schizoaffective disorder or they had a very high prevalence of the illness within multiple generations of their families. Individuals were considered at low risk for schizophrenia (and thus controls) if they had no personal or family history of any psychotic disorder, psychiatric hospitalization or suicide in a first or second-degree relative, and provided that upon evaluation they were found to have no evidence of a psychotic illness (schizophrenia, bipolar disorder or psychosis not otherwise specified). Low-risk controls were age and sex matched as close as possible to the individuals at high-risk for illness (Table 2). The normal controls and high-risk subjects were never treated with medication for psychotic symptoms, whereas the schizophrenia patients were on a combination of conventional and atypical medications.
Table 2.
Subject characteristics and cognitive test scores analyzed by one-way ANOVA
| Normal controls (n = 36) |
High-risk subjects (n = 21) |
Patients (n = 20) |
F | d.f. | P | |
|---|---|---|---|---|---|---|
| Age (range) |
22.9±5.4 (16-35) |
20.1±4.3 (13-30) |
37.7±10.3 (20-55) |
46.8 | 2, 74 | 0.000 |
| Male/Female | 17/19 | 7/14 | 13/7 | Chi-sq.=9.7 | 2, 74 | 0.083 |
| L/R Handed | 2/34 | 3/18 | 2/18 | -- | -- | -- |
| Education(years) | 14.2±2.21 | 12.6±2.75 | 14.5±2.20 | 0.815 | 2, 74 | 0.606 |
| Racial composition | 21 Ca, 15 Nc | 15 Ca, 6 Nc | 17 Ca, 3 Nc | Chi-sq.=3.1 | 2, 74 | 0.09 |
| WAIS-VCI | 107.9±17.5 | 105.9±12.8 | 108.6±15.0 | 0.168 | 2, 74 | 0.846 |
| PPVT-III | 102.9±13.6 | 101.5±12.9 | 102.5±16.6 | 0.7 | 2, 74 | 0.932 |
| WRAT | 103.7±11.2 | 106.3±8.5 | 106.6±11.9 | 0.596 | 2, 74 | 0.553 |
Ca: Caucasian
Nc: Non-Caucasian
The high-risk cohort was recruited by placing advertisements in newspapers and newsletters distributed by multiple chapters of The National Alliance for The Mentally Ill (NAMI). In addition, families who previously participated in a genetic study of schizophrenia (Delisi et al, 2002) were contacted for eligibility for the current study. Controls were solicited from the community by public advertisement. All subjects underwent an interview using the DIGS (Diagnostic Interview for Genetic Studies; Nurnberger et al, 1994) and diagnoses made based on the interview, information from family members and medical records as appropriate.
This study received Institutional Review Board Approval for human subjects' research at the Nathan S. Kline Institute for Psychiatric Research, a New York State Institution, and at New York University School of Medicine. All subjects gave written informed consent for their participation after being carefully explained the nature of the study and its procedures.
Experimental task
A Visual Word Discrimination task was used in this study. The experimental task has been described in detail by Li et al, (2007). A practice session with instructions was given to all subjects prior to the fMRI scanning.
Briefly, “A” blocks were Lexical Decision Blocks. In each “A” block, English language words (all concrete nouns) were presented on a screen in a random fashion, interspersed with pseudowords matched to the real words for number of letters. Subjects were instructed to use their right hand to press the left button when a word appeared and to press the right button when the letters displayed were not considered to be a real word. “B” blocks were Non-Linguistic Control Blocks. They consisted of two kinds of non-linguistic symbols, denoting “left” and “right”. This task was included to control for motor response and activity in the visual cortex. The subjects were instructed to press the left button in response to the symbol denoting “left” and the right button in the response to the symbol denoting “right”. “C” rest blocks contained a blank screen. During the rest block a blank screen was presented and subjects were instructed to keep their eyes open, remain relaxed and motionless.
The visual word discrimination task was repeated four times. Each block comprised 10 stimuli, each presented for 1000 ms with an interstimulus interval of 3000 ms. Each sequence consisted of 8 s of initial fixation, followed by 11 stimulation blocks of 30 s each. The stimuli were presented in random order within blocks. The order of blocks within sequences and the order of trials within blocks differed across the three tasks, but were identical for all subjects.
Only the first three task sequences for each subject were used in the final analysis. The fourth sequence exhibited substantial head motion in many subjects and little activation in all groups, and was therefore excluded in subsequent analysis. No other data sets were excluded.
Cognitive tasks related with language abilities
The overall cognitive ability and lexical discrimination ability of each subject were tested by general measures of IQ and a series of language related cognitive tests (Table 2).
For each subject, the age-appropriate Wechesler IQ was tested with select subtests (WAIS-III for adult (Wechsler, 1997) and WISC-IV for children (Wechsler, 2004). The Verbal Comprehension Index (WAIS-VCI) was used as the primary measure of verbal capacity.
The Wide Range Achievement Test – Reading (WRAT-Reading) was also performed. Subjects were asked to decode a list of progressively harder words. This test is used as a measure of reading ability (Wilkinson, 1993).
The Peabody Picture Vocabulary Test (PPVT-III) was performed by each subject. The PPVT is a test of receptive language where the subject is shown panels of four pictures and asked to point to the one that best describes a word spoken by the examiner (Dunn & Dunn, 1997).
MRI Scans
Imaging was performed on a 1.5T MRI system (Siemens Vision systems, Erlangen Germany). For each experiment 169 functional volumes sensitive to blood oxygen level dependent (BOLD) contrast were acquired with a T2-weighted gradient-echo EPI sequence [TR=2s, TE=50ms, flip angle=85°, Matrix=64×64, FOV=224, pixel size=3.5×3.5, time = 3×5min 38s]. Each volume was comprised of 22 axial slices of 5mm slice thickness with no gap. The first four volumes acquired during the initial fixation period were discarded.
fMRI analyses
The functional images were first corrected for acquisition time differences and head motion (scans with head movement < 1.5 mm were considered acceptable), then spatially normalized to a standard template (Montreal Neurological Institute), resampled to 3×3×3 mm and smoothed by a 8 mm full width half maximum Gaussian filter to improve signal-to-noise ratio. All these preprocesses were undertaken using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/).
Regions of interest (ROIs) were identified in which brain activation was determined. Regions were defined on the T1-weighted standard template (Montreal Neurological Institute) by using the WFU-PickAtlas software (Maldjian et al., 2003, 2004), which provides a method for generating ROI masks based on the Talairach Daemon database (http://www.fmri.wfubmc.edu). The selected regions were the bilateral inferior frontal gyri (Brodmann's area 44-45), bilateral inferior parietal lobe – angular and supramarginal gyri (mainly Brodmann's areas 39-40), and bilateral superior temporal gyri (mainly Brodmann's area 22).
For each subject, the average time series was calculated for each ROI by taking the mean value of the BOLD signal intensities from all voxels within that ROI. One-way Analysis of Variance (ANOVA) was applied to test the hypothesis that the mean of the time series under each of the three task conditions were equal by setting the average time series of the ROI as the dependent variable and the three task conditions as the independent factors. Three contrasts, language blocks vs. rest condition, control blocks vs. rest condition, and language blocks vs. control blocks, were set prior to the analysis. A four seconds time shift was employed to model the delayed hemodynamic response of brain activation. Scheffe Test was used for post hoc correction for multiple testing. The t value of the first contrast (language blocks vs. rest condition) in each ROI of the subjects denoted the significance of the regional activation by the language task, and was used as the variable for between-groups analysis.
The non-linguistic control condition was originally designed to locate the specific language activation by subtraction from the linguistic condition. However, in our preliminary whole brain activation study (Li et al, 2007), we found that in high-risk subjects and schizophrenia patients, regions of language processing were also involved during the non-linguistic control condition. Thus in these subjects, no significantly activated brain region was found during this subtraction paradigm. Thus in the current study, we used the rest condition instead of the control condition as the baseline condition.
Statistical analyses
The group differences in lateralization were compared by a General Linear Model, with controls, high-risk subjects and diagnosed patients as the groups, and hemisphere (left vs. right) as repeated measures, the t values representing the significant regions of activation by the language task as the dependent variables, and age and gender as covariates. Bonferroni correction was used for correcting for multiple comparisons. The language related laterality index, LI = (L−R)/(|L|+|R|), was calculated for each ROI, where L and R were the t values representing the regional activation by the language task in the left and right hemispheres. The more positive the LI, the more left lateralized the activation.
For all subjects, two-tailed Pearson correlations were determined between the regional brain activation and demographic measures as well as each of the language ability related cognitive test scores. Two-tailed Pearson correlations were also determined between the laterality indices and the cognitive test scores as well as the genetic test measures.
Heritability estimates were calculated for Brodmann's areas 22, 39-40, 44-45, using maximum-likelihood variance components analysis implemented in the Sequential Oligogenic Linkage Analysis Routines (SOLAR) version 4.0.2 software package (Almasy and Blangero 1998). The software uses the pedigree covariance matrix to take into account all family relationships (affected and unaffected family members). The variance-components model, as depicted in Equation (1), assumes that variation in the trait can be partitioned into genetic and random environmental components.
| (1) |
where Ω is the covariance matrix derived from the family pedigree, Φ is the matrix of kinship values, represents the additive genetic variance, I is the identity matrix, and is the random environmental variance (Almasy and Blangero 1998). Because this model allows for complex pedigree data beyond parent-offspring pairs (i.e. includes all family information: parent/offspring, sib/sib, sister/sister, brother/brother. Brother/sister, grand parent, avuncular, half sib, cousin), the resulting heritability estimates are more accurate than those obtained using only nuclear family members. For this study, each laterality index (LI) was entered into a separate univariate model. Heritability was estimated as the ratio of genetic variance to total phenotypic variance. Sex and age were entered into each trait model as covariates but their effects were not significant.
Results
Table 2 presents a summary of the subject test results and general demographics from each cohort. The mean age of high-risk subjects did not differ significantly from that of the normal controls (P=0.325), while the mean age of diagnosed patients with schizophrenia was relatively older than the other two groups. There were no significant between-group differences in sex, handedness and years of education completed. No significant between-group differences were found in the performance scores of any of the language related cognitive tests.
As indicated in Table 3, there were no significant group-by-hemisphere interactions in the Brodmann's area 22 or Brodmann's areas 39-40, whereas Brodmann's area 44-45 had significant group-by-hemisphere interactions (P = 0.04). More specifically, the regional mean activation was much greater in left hemisphere in both Brodmann's areas 22 and 39-40 among the groups. However, for the regional mean activation in Brodmann's area 44-45, normal controls were more left lateralized, whereas the high-risk subjects and patients with schizophrenia exhibited significantly greater bilateral activations.
Table 3.
Repeated-measures ANOVA analysis for group-by-hemisphere ROI study
| Mean activation |
F | d.f. | P | |||
|---|---|---|---|---|---|---|
| Normal controls (n = 36) |
High-risk subjects (n = 21) |
Schizophrenic patients (n = 20) |
||||
| B22 | 0.438 | 2 | 0.647 | |||
| Left | 0.225 | 1.249 | 0.816 | |||
| Right | −0.2 | 0.648 | 0.018 | |||
| B39-40 | 0.512 | 2 | 0.603 | |||
| Left | 2.37 | 2.638 | 2.925 | |||
| Right | 1.445 | 1.256 | 1.265 | |||
| B44-45 | 4.327 | 2 | 0.04 | |||
| Left | 3.065 | 2.542 | 3.493 | |||
| Right | 1.908 | 2.028 | 3.018 | |||
Significance level is P<0.05, confidence interval are 95%;
B22 for Brodmann's area 22;
B39-40 for Brodmann's areas 39-40;
B44-45 for Brodmann's areas 44-45
The group-by-hemisphere mean activation analysis is insensitive to the individual subject activation laterality, which we are primarily concerned with. Therefore, in order to clarify the group-by-hemisphere laterality interaction, we analyzed the language related laterality index (LI) for the three bilateral ROIs of all groups (Table 4 and Figure 3). A One-way ANOVA revealed significant difference (P = 0.02) in Brodmann's areas 44-45 among the three groups, whereas no significant differences were found in the other two ROIs. Post hoc t tests in Brodmann's area 44-45 showed that the high-risk subjects and patients had significantly smaller LIs than the normal controls, but no significant differences between the high-risk subjects and the patients with schizophrenia.
Table 4.
One way ANOVA and post hoc t tests for Language Lateralization (LI) analysis
| Normal controls (n = 36) |
High-risk subjects (n = 21) |
Schizophrenic patients (n = 20) |
F | d.f. | P | |
|---|---|---|---|---|---|---|
| B22 | −0.027 | 0.0837 | 0.0623 | 0.893 | 2, 74 | 0.414 |
| B39-40 | 0.1246 | 0.1992 | 0.0296 | 1.689 | 2, 74 | 0.192 |
| B44-45 | 0.1802 | −0.1252 | −0.1022 | 6.905 | 2, 74 | 0.02 |
| Post hoc t test on the LI of B44-45 | ||||||
| Controls vs. High-risk | 55 | 0.002 | ||||
| High-risk vs. Patients | 39 | 0.841 | ||||
| Patients vs. Controls | 54 | 0.004 | ||||
Significance level is P<0.05, confidence interval are 95%;
B22 for Brodmann's area 22;
B39-40 for Brodmann's areas 39-40;
B44-45 for Brodmann's areas 44-45
Figure 3.
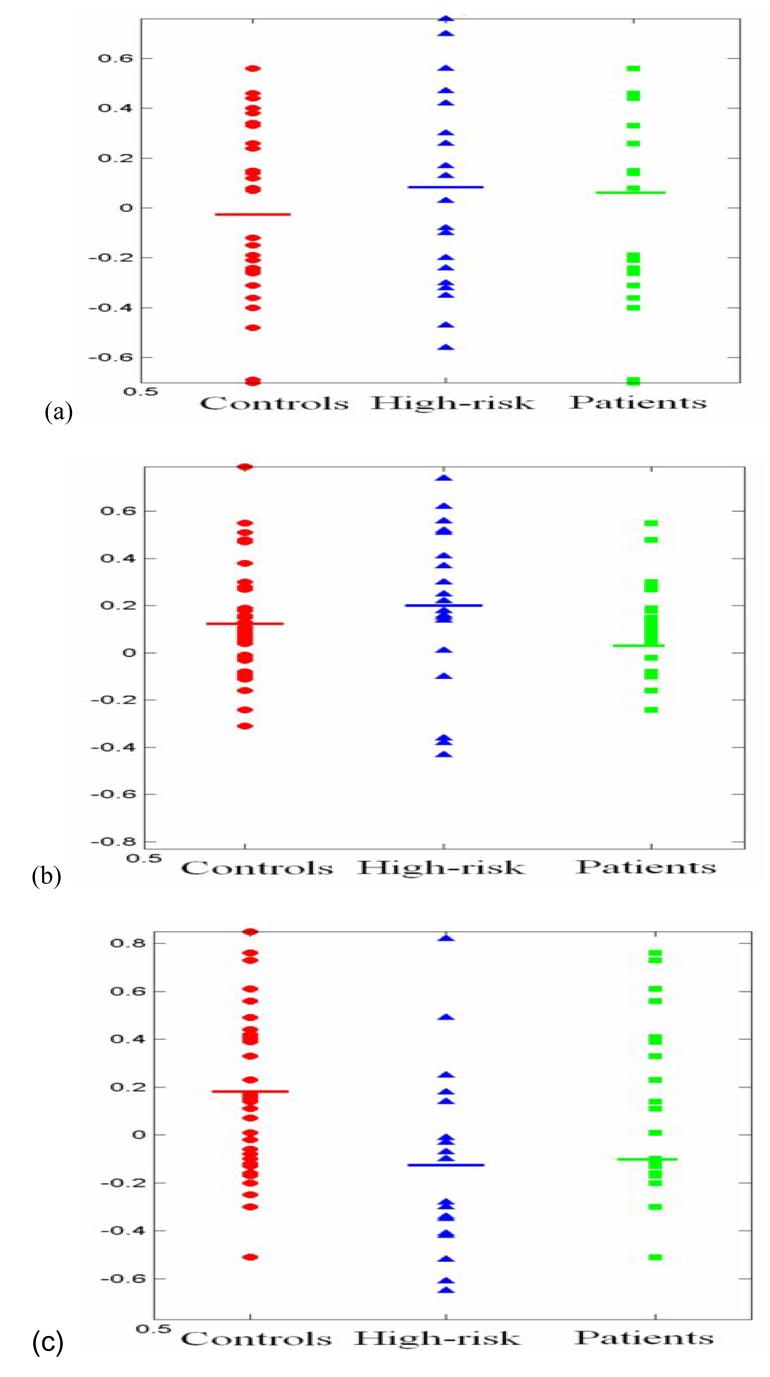
Language Lateralization (LI) of normal controls, high-risk subjects for schizophrenia and schizophrenia patients; (a) superior temporal gyri (Brodmann's 22 area), (b) inferior parietal lobe (Brodmann's areas 39-40), (c) inferior frontal gyri (Brodmann's areas 44-45)
No significant correlations were found between the brain activation and any demographic measures (age, sex, handedness, education, race) for any ROI in any group. The results of the correlation analyses between brain activation and individual cognitive scores are presented in Table 5. Significant negative correlations between PPVT test scores and brain activation in the right hemispherical Brodmann's areas 44-45 were present in the high-risk group (R=−0.483, P=0.026). Thus higher activation was correlated with lower functioning on this test. In the patient group, significant negative correlations between brain activation and WRAT test scores were observed in both sides Brodmann's areas 44-45 (R=−0.635, P=0.003 for left side and R=−0.477, P=0.039 for right side), and in left side Brodmann's areas 39-40 (R=−0.598, P=0.007).Thus, similarly, the higher the activity, the lower the performance in these areas. In addition, Table 6 depicted the results of correlations between the language related cognitive scores and laterality indices, from which we found significant positive correlation in high-risk group in Brodmann's areas 44-45 (LI v.s. PPVT-III: P=0.031), and patient group in Brodmann's areas 44-45 (LI v.s WRAT: P=0.032).
Table 5.
Correlations between brain activity and cognitive test scores
| WAIS-VCI | PPVT | WRAT | |||||
|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | ||
| Normal controls |
B22_l | 0.158 | 0.358 | 0.04 | 0.819 | 0.16 | 0.352 |
| B22_r | −0.108 | 0.529 | 0.019 | 0.911 | 0.022 | 0.897 | |
| B39-40_l | 0.013 | 0.938 | 0.019 | 0.913 | 0.099 | 0.567 | |
| B39-40_r | 0.064 | 0.712 | 0.075 | 0.664 | 0.231 | 0.175 | |
| B44-45_l | 0.227 | 0.182 | 0.093 | 0.591 | 0.273 | 0.107 | |
| B44-45_r | 0.112 | 0.517 | −0.007 | 0.969 | 0.07 | 0.686 | |
| High-risk subjects |
B22_l | −0.068 | 0.771 | −0.206 | 0.37 | 0.066 | 0.778 |
| B22_r | 0.191 | 0.407 | 0.181 | 0.434 | −0.11 | 0.635 | |
| B39-40_l | −0.213 | 0.354 | −0.328 | 0.147 | 0.097 | 0.676 | |
| B39-40_r | −0.024 | 0.919 | −0.282 | 0.215 | 0.283 | 0.213 | |
| B44-45_l | −0.272 | 0.233 | −0.019 | 0.935 | 0.1 | 0.667 | |
| B44-45_r | −0.075 | 0.748 | −0.483 | 0.026 | −0.12 | 0.603 | |
| Schizophrenia patients |
B22_l | −0.135 | 0.581 | 0.00 | 0.999 | −0.304 | 0.206 |
| B22_r | −0.024 | 0.923 | 0.166 | 0.498 | 0.032 | 0.896 | |
| B39-40_l | −0.395 | 0.094 | −0.262 | 0.279 | −0.598 | 0.007 | |
| B39-40_r | −0.099 | 0.686 | −0.109 | 0.658 | −0.221 | 0.363 | |
| B44-45_l | −0.304 | 0.206 | −0.263 | 0.277 | −0.635 | 0.003 | |
| B44-45_r | −0.046 | 0.852 | −0.103 | 0.675 | −0.477 | 0.039 | |
B22 for Brodmann's area 22;
B39-40 for Brodmann's areas 39-40;
B44-45 for Brodmann's areas 44-45;
_l for left side activation;
_r for right side activation;
P<0.05 for significant correlation;
R for correlation coefficient.
Table 6.
Correlations between brain activity lateralization (LI) and cognitive test scores (P)
| Normal controls | High-risk subjects | Schizophrenia patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B22 | B39-40 | B44-45 | B22 | B39-40 | B44-45 | B22 | B39-40 | B44-45 | |
| WAIS-VCI | 0.202 | 0.391 | 0.52 | 0.325 | 0.408 | 0.067 | 0.675 | 0.79 | 0.089 |
| WRAT | 0.308 | 0.075 | 0.939 | 0.349 | 0.693 | 0.055 | 0.99 | 0.091 | 0.032 |
| PPVT-III | 0.208 | 0.723 | 0.314 | 0.659 | 0.942 | 0.031 | 0.053 | 0.315 | 0.068 |
B22 for Brodmann's area 22;
B39-40 for Brodmann's areas 39-40;
B44-45 for Brodmann's areas 44-45
Estimates of heritability for Brodmann's areas 22 and 39-40 were h2 =0.00 indicating no heritable genetic variation, whereas the estimate of heritability for Brodmann's areas 44-45 was h2 =0.24 indicating low heritable genetic variation. These tend to suggest that the three traits are potentially be under epigenetic or environmental control.
Discussion
The current study was performed to extend and clarify our previous preliminary report whole brain voxel-based fMRI analysis on a smaller cohort of individuals (Li et al, 2007). In the current study fMRI activation in specific regions of interest based on our previous exploratory analysis and based on our knowledge of language relevant structures was quantified and lateralization of activations in these structures evaluated. Significantly decreased lateralization in Brodmann's areas 44-45 of the frontal lobe was found in the patients and high-risk subjects as compared to normal controls. Specifically (Table 3), the decreased lateralization in high-risk subjects mainly resulted from decreased activation in the left Brodmann's 44-45 ROI. In contrast, activation was increased bilaterally in the individuals with schizophrenia and the decreased lateralization was mainly caused by increased activation on the right. In addition, the verbal cognition as tested by the WRAT test was significantly correlated with the brain activations in Brodmann's areas 44-45 in the patient group (Table 5).
Most previous fMRI based ROI studies calculated the number of activated voxels in the ROI with a threshold applied to the activation – active or not. The current study calculated the actual amount of f fMRI signal intensity in each voxel within each total ROI. This value can clearly better reflect the level of the neuronal activity than a binary representation (active or not by a threshold). Nevertheless, the relative increase of activity in the right hemisphere found in the current study in schizophrenia is consistent with finding of previous fMRI studies (Crow, 2000; Curtis et al, 1998; Menon et al, 2001; Ngan et al, 2003; Sommer et al, 2001, 2003; Woodruff et al, 1997), and adds to the increasing literature showing altered patterns of language activations and thus changes in the normal lateralization in schizophrenia. These functional MRI studies suggest that functional abnormalities in the brain regions associated with language processing in patients with schizophrenia may be related to the underlying pathological basis for schizophrenia, as previously suggested (Crow, 1998; DeLisi, 2001; Morey et al, 2005).
Few fMRI studies have previously been reported in individuals at high-risk for schizophrenia and particularly using language processing tasks. Whyte et al, (2006) reported increased fMRI activation in the right inferior frontal gyrus and cerebellum in high-risk subjects compared to controls during a word classification recognition task, although they did not study people who were already diagnosed with schizophrenia. In contrast, our previous fMRI study showed more bilateral activation in high-risk individuals, as well as those with schizophrenia than controls (Li et al, 2007) using the same task as in the current study. The preliminary study also showed that control subjects' discrimination of words from pseudowords significantly activated left side Brodmann's 44 more strongly than when non-linguistic symbols were discriminated. However, high-risk subjects and their siblings with schizophrenia activated this region similarly for both language and non-language tasks. Unlike the Whyte et al (2006) study, our current analyses now shows that high-risk subjects have less leftward activation than controls, while those with schizophrenia have increased rightward activation. It is possible that as well as using a different language task, the Whye et al. study may have sampled subjects who already exhibited some psychotic symptoms and thus were more similar to the current subjects with schizophrenia. It is also unclear why the current study found decreased activation in the left hemisphere in the high-risk cohort and increased activation in the right hemisphere in the subjects with schizophrenia as a basis for the reduced lateralization. One can only speculate that the evolution of chronic schizophrenia from an early high-risk state to chronic illness is an active and changing process and thus one would not expect individuals at high risk to have brain function equivalent to that of chronic patients. Only future longitudinal studies can clarify this process.
Some other recent findings in high-risk for schizophrenia subjects using different imaging modalities seem to converge on frontal lobe abnormalities. A DTI study in the same high-risk sample reported white matter deficit in the left frontal lobe (DeLisi et al, 2006). A gyrification index study reported by another research group suggested reduced cortical folding in the left frontal lobe of individuals at high risk for developing schizophrenia (Jou et al, 2005). Pantelis et al (2003) found that high-risk subjects, who did later develop schizophrenia, had less gray matter in the right inferior frontal cortex (Brodmann's areas 44-45) as compared to the high-risk subjects who did not develop the disorder. We also note that although the Laterality Index for the patients with schizophrenia-spectrum disorder and high-risk subject are similarly reduced, the activations in both hemispheres of the diagnosed patients were greater than in the normal controls. It is unclear what caused the increased activation in the schizophrenia group, although it may be associated with the use of anti-psychotic medications. Some previous studies suggest that anti-psychotic medications may contribute to the altered brain activity in schizophrenia patients (Stephan et al, 2001). However, others suggest that treatment with anti-psychotic medication may normalize brain function (Davis et al, 2005). Although speculative, the finding of lack of language lateralization in high-risk cohort and their ill siblings may be genetic in origin, as Crow originally suggested in 1989. He proposed (based on Annett's inherited “right-shift” factor, Annett, 1992; 1999) that the normal cerebral lateralization process was disrupted during development by genetic control of the growth of the right-sided language-related cortex, rather than through enhancement of these areas in the left hemisphere (Annett, 1992). It was hypothesized that the right shift factor may play a role in mechanisms of promoting proneness for schizophrenia (Annett, 1999; Crow, 2000).
The present high-risk for schizophrenia study found significant correlations between brain activation and the language related cognitive test scores in the high-risk and patient groups. According to this finding, we hypothesize that the reduced brain lateralization in language related frontal cortex may be associated with language processing impairments in schizophrenia, and the language related cognitive symptoms appear prior to diagnosis.
Our sample included both male and female subjects. A meta-analysis reported no significant difference in language lateralization between men and women (Sommer et al, 2004). In addition sex was one of the covariates in the activation analysis in our study. The patient group included patients with schizophrenia and schizoaffective disorder. These individuals all had a chronic psychotic condition and were at one time diagnosed with schizophrenia. The distinction between schizoaffective and schizophrenia is known to be highly unreliable (Nurnberger et al., 1994) and in addition schizoaffective disorder is known to be highly genetically related to schizophrenia. That is, in family studies both schizophrenia and schizoaffective diagnoses appear within the same families. We thus feel justified in using these families with both diagnoses as a unified group of subjects.
In summary, the current study attempted to regionally determine whether a language paradigm in fMRI may be a useful candidate method to develop as a possible screening measure for early detection of schizophrenia. There were some limitations. First, the patients were taking anti-psychotic medications, possibly affecting neural activation. Second, the sample size was still relatively small. Future work will need a much larger cohort of high-risk individuals, followed longitudinally, to determine who eventually develops schizophrenia and whether the prior language study would have been predictive of illness.
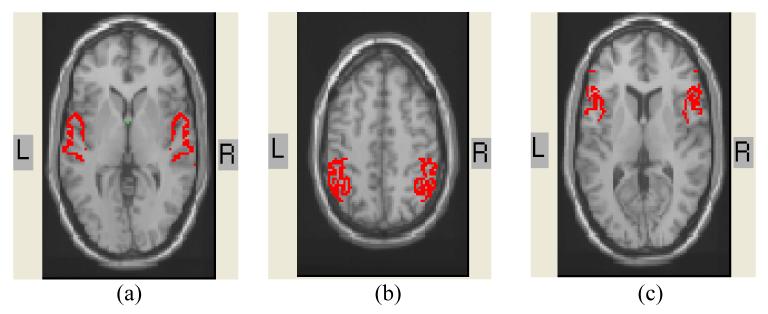
Figure 2.
Average Talairach coordinates for (a) bilateral superior temporal gyri (Brodmann's area 22); (b) bilateral inferior parietal lobe (Brodmann's areas 39-40); (c) bilateral inferior frontal gyri (Brodmann's areas 44-45:)
Acknowledgments
This project was partially supported by a grant from NIMH, R21 MH071720.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. Parallels between asymmetries of planum temporale and hand skill. Neuropsycholigia. 1992;30:951–962. doi: 10.1016/0028-3932(92)90048-q. [DOI] [PubMed] [Google Scholar]
- Annett M. The theory of an agnostic right shift gene in schizophrenia and autism. Schizophrenia Research. 1999;39:177–183. doi: 10.1016/s0920-9964(99)00072-9. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siège de la faculté du language articulé; suivies d'une observation d'aphemie. Bulletin de la Société Anatomique de Paris. 1861;6:330–357. [Google Scholar]
- Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, Barta PE, Pearlson GD. Morphometric assessment of the hetermodel association cortex in schizophrenia. Am J Psychiatry. 2004;161:322–331. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- Crow T, Ball J, Bloom S, Brown R, Bruton C, Colter N, Frith CEC, Owens D, Roberts G. Schizophrenia as an anomaly of development of cerebral asymmetry. Arch. Gen. Psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Nuclear schizophrenic symptoms as a window on the relationship between thought and speech. Br J Psychiatry. 1998;173:303–309. doi: 10.1192/bjp.173.4.303. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Invited commentary on: functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. The genetics of asymmetry and psychosis. Br J Psychiatry. 2000;176:61–63. doi: 10.1192/bjp.176.1.61. [DOI] [PubMed] [Google Scholar]
- Curtis VA, Bullmore ET, Brammer MJ, et al. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry. 1998;155:1056–1063. doi: 10.1176/ajp.155.8.1056. [DOI] [PubMed] [Google Scholar]
- Davis CE, Jeste DV, Eyler LT. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr Res. 2005;78:45–60. doi: 10.1016/j.schres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Speech Disorder in Schizophrenia: Review of the literature and new study of the relation to Uniquely Human Capacity for language. Schizophrenia Bulletin. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sherrington R, Shaw S, Nanthakumar B, Shields G, Smith AB, Wellman N, Larach NW, Loftus J, Razi K, Stewart J, Vita A, De Hurt M, Crow TJ, Sherrington R. A genome-wide scan of 382 affected sibling-pairs with schizophrenia suggests linkage to chromosomes 2cen and 10p. Am J of Psychiatry. 2002;159:803–812. doi: 10.1176/appi.ajp.159.5.803. [DOI] [PubMed] [Google Scholar]
- Delisi LE, Szulc KU, Bertisch H, Majcher M, Brown K, Bappal A, Branch CA, Ardekani BA. Early detection of schizophrenia by diffusion weighted imaging. Psychiatry Research. 2006 doi: 10.1016/j.pscychresns.2006.04.010. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3rd edition American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Gottesman II, Shields J. Schizophrenia and Genetics; a Twin Study Vantage Point. Academic Press Inc; New York: 1972. [Google Scholar]
- Gottesman II. Schizophrenia Genesis: The Origins of Madness. W.H. Freeman and Company; New York: 1991. [Google Scholar]
- Johnstone EC, Abukmeil SS, Byrne M, Clafferty R, Grant E, Hodges A, et al. Edinburgh high risk study – findings after four years: demographic, attainment and psychopathological issues. Schizophrenia Research. 2000;46:1–15. doi: 10.1016/s0920-9964(99)00225-x. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Hardan AY, Keshavan MS. Reduced cortical folding in individuals at high risk for schizophrenia: a pilot study. Schizophrenia Research. 2005;75:309–313. doi: 10.1016/j.schres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophrenia Research. 2001;48:159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Liddle PF, Brammer MJ, Williams SC, Murray RM, McGuire PK. Neural correlates of formal thought disorder in schizophrenia: preliminary findings from a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:769–774. doi: 10.1001/archpsyc.58.8.769. [DOI] [PubMed] [Google Scholar]
- Leudar I, Thomas P, Johnston M. Self-repair in dialogues of schizophrenics: Effects of hallucinations and negative symptoms. Brain and Language. 1992;43:487–511. doi: 10.1016/0093-934x(92)90114-t. [DOI] [PubMed] [Google Scholar]
- Li X, Branch C, Bertisch H, Brown K, Szule K, Ardekani B, Delisi LE. An fMRI study of language processing in people at high genetic risk for schizophrenia. Schizophrenia Research. 2007;91:62–72. doi: 10.1016/j.schres.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft R. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of f MRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Menon V, Anagnoson RT, Mathalon DH, Glover GH, Pfefferbaum A. Functional neuroanatomy of auditory working memory in schizophrenia:relation to positive and negative symptoms. Neuroimage. 2001;13:433–446. doi: 10.1006/nimg.2000.0699. [DOI] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan ET, Vouloumanos A, Cairo TA, Laurens KR, Bates AT, Anderson CM, et al. Abnormal processing of speech during oddball target detectionin schizophrenia. Neuroimage. 2003;20:889–897. doi: 10.1016/S1053-8119(03)00385-9. [DOI] [PubMed] [Google Scholar]
- Nierenberg J, Salisbury DF, Levitt JJ, David EA, McCarley RW, Shenton ME. Reduced left angular gyrus volume in first-episode schizophrenia. Am J Psychiatry. 2005;162:1539–1542. doi: 10.1176/appi.ajp.162.8.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies, rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. The Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J. Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Frumin M, McCarley RW, Maier S, Wesin CF, Fischer IA, Dickey CC, Kikinis R. MR morphometric findings in schizophrenia. In: Dougherty DRS, Rosenbaum J, editors. Psychiatric neuroimaging strategies: research and clinical application. American Psychiatric Association; Washington DC: 2001. [Google Scholar]
- Sommer IE, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001;52:57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, Kahn RS. Language lateralization in female patients with schizophrenia: an fMRI study. Schizophr Res. 2003;60:183–190. doi: 10.1016/s0920-9964(02)00300-6. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn R. Do woman really have more bilateral language representation than men? A meta-analysis of function imaging studies. Brain. 2004;127:1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Magnotta VA, White T, Arndt S, Flaum M, O'Leary DS, et al. Effects of olanzapine on cerebellar functional connectivity in schizophrenia measured by fMRI during a simple motor task. Psychol Med. 2001;2001:1065–1078. doi: 10.1017/s0033291701004330. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition The Psychological Corporation; San Antonio, TX: 2004. [Google Scholar]
- Wernicke C. Der aphasiche symptomenkomplex. Cohen and Weigert; Breslau, Poland: 1874. [Google Scholar]
- Whyte MC, Whalley HC, Simonotto E, Flett S, Shillcock R, Marshall I, Goddard NH, Johnstone EC, Lawrie SM. Event-related fMRI of word classification and successful word recognition in subjects at genetically enhanced risk of schizophrenia. Psychol Med. 2006;36:142–1439. doi: 10.1017/S0033291706008178. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The wide range achievement test. 3rd edition Wide Range, Inc; Wilmington, DE: 1993. [Google Scholar]
- Woodruff PW, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SC, et al. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry. 1997;15:1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]