Abstract
Scleritis and peripheral ulcerative keratitis (PUK) can present as isolated conditions or as part of a systemic inflammatory or infectious disorder. Both are serious ocular conditions that can result in vision loss and therefore require early diagnosis and treatment. Nearly two-thirds of patients with non-infectious scleritis require systemic glucocorticoid therapy, and one fourth need a glucocorticoid-sparing agent, as well. Essentially all patients with non-infectious PUK require systemic glucocorticoids. Detailed clinical history, thorough physical examination, and thoughtful laboratory evaluations are all important in the exclusion of underlying disorders and extraocular involvement.
Keywords: Scleritis, peripheral ulcerative keratitis, glucocorticoids, cyclophosphamide, vasculitis, rheumatoid arthritis
Scleritis and peripheral ulcerative keratitis (PUK) are two ocular disorders that require urgent attention for the purpose of diagnosis, treatment, and detection of underlying systemic inflammatory diseases.
Part 1: Scleritis
Anatomy of the Sclera
The sclera, which serves as the protective outer layer of the eye (Figure 1), is composed of connective tissue: collagen, elastin, and proteoglycans. The sclera starts at the limbus, where it is continuous with the cornea, and ends at the optic canal, where it is continuous with the dura. The six extraocular muscles insert into the sclera. Scleral tissue receives its sensory innervation from the short posterior and long ciliary nerves [1]. The sclera, an avascular structure, receives its nourishment from adjacent vascularized tissues: the choroidal plexus beneath and the episcleral plexus above. The episclera has two vascular plexi, one superficial whose vessels are arranged radially, and another deep whose vessels adhere to the sclera.
Figure 1.

Anatomy of the eye
Epidemiology of Scleritis
Scleritis can occur in any age group but usually presents between ages 30 and 50 [2,3]. Women are affected approximately twice more often than men, and there is no racial or geographic predilection [2,3]. There is no known HLA association [4]. The prevalence of scleritis in the general population is estimated to be 6 cases per 100,000 peopl, but has been described in between 0.2% and 6.3% of patients with RA and up to 7% of those with Wegener’s granulomatosis [5-7].
Pathogenesis of scleritis
Studies on the pathogenesis of scleritis are limited. However, the data available support an important if not predominant role for T cells in the inflammatory process [8]. Inflammatory cells, mostly T cells and macrophages, are found on biopsy specimens [8,9]. Evidence of vasculitis with neutrophil invasion and necrosis of the vessel wall was observed in one histopathologic study but not in a second one [8,10]. Antibody deposition has also been described in one study [8]. One group that divided scleritis into categories by morphology found that zonal necrotizing granulomas were more common in patients with associated systemic autoimmune conditions and that nonzonal diffuse scleral inflammation was more common in patients without an associated systemic condition [11].
Clinical History in Scleritis
The chief complaint of patients with scleritis is pain that responds poorly to analgesics. The pain is described as dull, aching, or boring and may be severe and constant. Because the extraocular muscles insert into the sclera, the pain typically worsens with eye movement. The pain often awakens patients from sleep and is typically worse in early morning. The pain can spread to the periorbital region, brow, forehead, temple, ear, or jaw, and may be disproportionate to the clinical findings.
Up to 20% of patients with scleritis have little or no pain [12]. The absence of pain is observed in three settings: 1) milder disease (e.g., diffuse anterior or nodular scleritis, as opposed to the necrotizing type [see below]; 2) patients already on immunosuppressive medications at the time their symptoms begin; and, 3) scleromalacia perforans, a rare complication of advanced RA. Other complaints may include tearing, photophobia, and decreased vision. Patients with anterior scleritis usually notice eye redness. Patients with posterior scleritis may complain of ptosis and swelling of the periorbital tissue.
Clinical Findings in Scleritis
Patients with anterior scleritis present with a red eye. However, in some cases, the redness is located underneath the lid and may be easily overlooked without a focused eye examination. The redness has a violaceous hue that is best seen in natural sunlight. Slit lamp examination reveals scleral edema and dilatation of both the superficial and deep episcleral vessels. The eye in patients with scleritis is tender to palpation. Ocular erythema persists after the application of topical phenylephrine. Approximately 25% of patients have bilateral disease at presentation, but scleritis ultimately becomes bilateral in about 50% of patients [3,12,13].
Classification of Scleritis
The Watson and Hayreh classification of scleritis [12] divides the disorder into anterior and posterior types based upon the anatomic distribution of disease. Anterior scleritis is further subdivided into diffuse, nodular, necrotizing with inflammation, and necrotizing without inflammation (scleromalacia perforans). Although these forms of scleritis correspond roughly to different degrees of severity, it is unusual for a case of scleritis to evolve from one type to another, e.g., from diffuse anterior scleritis to necrotizing scleritis.
Diffuse Anterior Scleritis
Diffuse anterior disease, the most common clinical presentation of scleritis, occurs in approximately 60% of patients [3]. Although the onset of disease is insidious, ocular inflammation may involve a substantial proportion or all of the anterior sclera at presentation (Figure 2). The normal radial pattern of the episcleral vessels is lost and one may see beading and tortuosity of the vessels. Signs of corneal inflammation such as peripheral corneal infiltrates or mild corneal thinning may be present [13]. Frank corneal ulceration is not typical as it is more common in necrotizing scleral disease (described below). Progression to nodular or necrotizing scleritis has been observed, but is uncommon [3, 14]. Resolution of the ocular inflammation in diffuse anterior scleritis may leave a bluish gray hue caused by rearrangement of the scleral collagen fibers (Figure 3). This does not represent scleral thinning, which is an uncommon sequela of anterior scleritis. Systemic disorders have been described in up to 45% of patients with diffuse anterior scleritis, most commonly RA [13,15].
Figure 2.
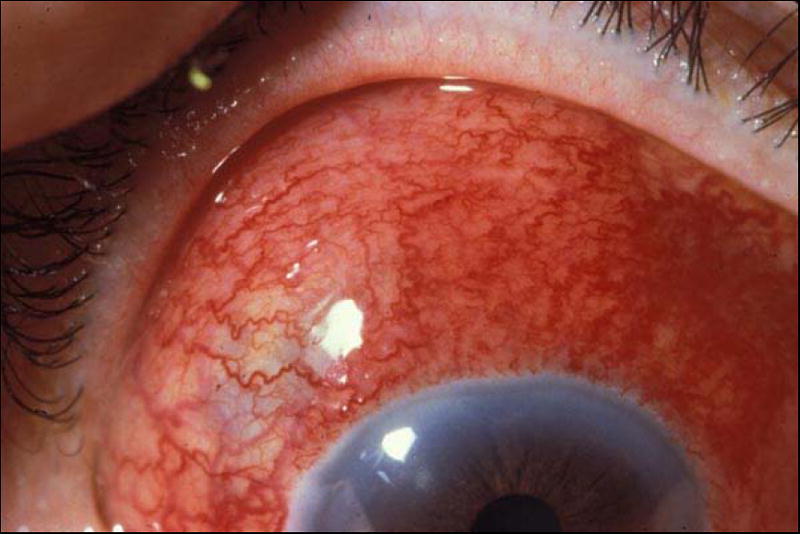
Diffuse anterior scleritis. In addition to the bright red episcleral vessels, there is a deep violaceous hue to the sclera that indicates scleral inflammation.
Figure 3.
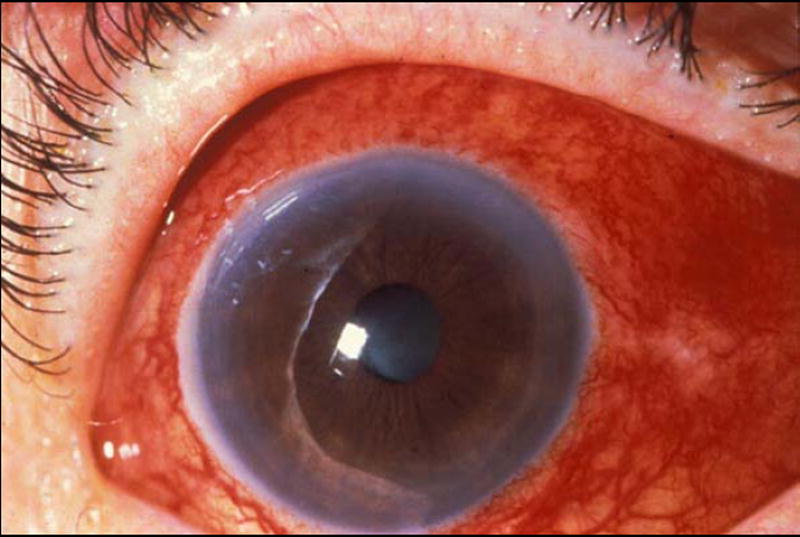

Active (left) and resolved (right) diffuse anterior scleritis. Resolution of diffuse anterior scleritis in a 50 year-old man with Wegener’s granulomatosis after treatment with prednisone and cyclophosphamide. Resolution of the scleral inflammation has left a bluish gray hue that represents rearrangement of the collagen fibers in the sclera.
Nodular Anterior Scleritis
Nodular scleritis is the second most common clinical presentation of anterior scleritis (Figure 4), accounting for approximately 20% of scleritis cases [3]. Patients with nodular scleritis present with a firm, immobile, and tender nodule that typically is found close to the limbus. Similar to diffuse anterior scleritis, progression to other forms of scleritis is uncommon [3, 14]. A systemic disease is diagnosed in 40-50% of patients [13].
Figure 4.

Nodular anterior scleritis. Nodular anterior scleritis in a 44 year-old man with hepatitis C. Along with areas of diffuse scleral inflammation, a discrete, raised, scleral nodule is seen near the limbus at the 5 o’clock position.
Necrotizing Anterior Scleritis with Inflammation
Necrotizing scleritis is the most serious clinical presentation of anterior scleritis. This condition has an older age of onset compared to the other types of scleritis and a higher proportion of patients (50-80%) have an underlying systemic disease [14]. The two diseases most often associated with necrotizing anterior scleritis are Wegener’s granulomatosis and RA [13,16]. Necrotizing scleritis typically requires therapy with glucocorticoids and/or immunosuppressive drugs to control the disease [3]. Patients present with typical signs of anterior scleritis combined with areas of white sclera surrounded by edema and congestion (Figure 5). The areas of white sclera represent capillary closure of the episcleral vasculature, with infarction and necrosis of the underlying sclera. Involvement of adjacent ocular structures, including the cornea, ciliary body, and trabecular meshwork is observed with secondary corneal ulceration, uveitis, or increased intraocular pressure [3]. After resolution of scleritis with appropriate treatment, there is thinning of the sclera with translucency, and the choroid often is seen through the sclera (Figure 6). Despite such thinning, rupture of the globe is rare.
Figure 5.

Necrotizing anterior scleritis. Necrotizing anterior scleritis in a 49 year-old woman with hepatitis C associated cryoglobulinemia. Along with nasal episcleral and scleral inflammation, there is an area of scleral whitening that represents an area of necrosis.
Figure 6.

Resolved necrotizing anterior scleritis. Resolution of idiopathic necrotizing scleritis in a 36 year-old women after treatment with prednisone and mycophenolate mofetil. The sclera is thin and translucent in the area of previous necrotizing inflammation. The choroidal hue can be appreciated through this area.
Scleromalacia Perforans (Necrotizing Anterior Scleritis Without Inflammation)
Scleromalacia perforans, also known as necrotizing anterior scleritis without inflammation, is a rare but severe form of scleritis that tends to involve both eyes and presents without redness, pain, or edema. Often there is thinning and atrophy of the episclera and loss of the episcleral vasculature (Figure 7). Localized areas of yellow-white infarcted tissue are seen. Such areas are demarcated clearly from surrounding tissue. Thinning may become so pronounced that the choroid is covered by conjunctivae alone. Given the lack of typical symptoms, patients often present with discolored sclera or blurred vision secondary to astigmatism due to scleral thinning. The classic patient with scleromalacia perforans is an elderly woman with longstanding RA [13].
Figure 7.

Necrotizing anterior scleritis without inflammation (scleromalacia perforans). The patient presented with scleral necrosis and minimal pain. The bluish scleral hue is created by the transmission of choroidal pigment through the thin sclera.
Posterior Scleritis
Posterior scleritis is defined as involvement of the sclera by inflammation posterior to the insertion of the medial and lateral rectus muscles. This form of scleritis is difficult to diagnose because of the secluded nature of its ocular inflammation and its non-specific clinical features [13]. Patients may present with deep-seated headaches, pain upon movements of the eye, proptosis, ptosis, double vision, and reduced visual acuity. Ocular examination may be normal or may disclose localized or generalized exudative retinal detachment (21% in one study of 99 patients with posterior scleritis), optic nerve edema (18%), subretinal mass lesion (13%), choroidal effusion (4%), uveitis (2%), and vasculitis (2%) [2]. Posterior scleritis may also be accompanied by macular edema, retinal striae, and choroidal folds.
Patients with posterior scleritis are less likely to have an underlying systemic disease compared to those with anterior scleritis [3, 12]. Diagnosis can be made based on the typical B-scan ultrasonography appearance of thickened sclera with fluid in Tenon’s space (Figure 8). An ultrasonographic finding referred to as the T-sign is created by hypoechogenic fluid that forms an interface between the optic nerve and the sclera.
Figure 8.

B-scan ultrasonography of posterior scleritis. Idiopathic posterior scleritis in a 51 year-old woman. The figure demonstrates thickened sclera with hypoechogenic fluid in the space behind the sclera.
Differential Diagnosis of Scleritis
A variety of other etiologies of red eyes must be considered in the differential diagnosis of scleritis. These are shown in Table 1. Episcleritis refers to inflammation of the superficial vessels within the episclera. Ocular erythema in episcleritis is limited usually to only a sector of the eye and is not associated with pain, vision changes, or discharge. Examination reveals episcleral injection, which is more likely to be bright red than deeply violaceous in color.
Table 1.
Differential diagnosis of scleritis.
|
The vessels involved in episcleritis are more superficial than those in scleritis, have a radial pattern, and can be moved with a cotton tip applicator. Furthermore, palpation of the area does not result in pain. Phenylephrine blanches the episcleral vessels in episcleritis but will not do so in scleritis. The condition tends to be self-limited but intermittently recurrent. Episcleritis is less likely to be associated with a systemic autoimmune condition than scleritis [17]. Ocular complications are also less likely in episcleritis, with vision loss occurring in none of the 37 patients in one study and only two of 94 (not related directly to episcleritis) in another [3,17]. Treatment is not necessary unless the redness is bothersome to the patient. Therapy may include topical glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs).
Conjunctivitis can be caused by a variety of processes including bacterial or viral infections, allergies, and prolonged contact lens use. Symptoms include tearing, discharge, and a foreign body sensation. Examination reveals a follicular or papillary reaction of the palpebral conjunctivae. Treatment of bacterial conjunctivitis consists of antibiotic eyedrops. Treatment of viral conjunctivitis is supportive, with cool compresses and artificial tears. Allergic conjunctivitis can also be treated with cool compresses as well as artifical tears and a topical anti-histamine or mast cell inhibitor eyedrops.
Blepharitis refers to inflammation of the eyelid margins. Blepharitis symptoms include burning, tearing, and crusting of the eyelashes. Symptoms are typically worse in the morning. Ocular examination reveals crusting around the eyelashes, oil inspissation, and telangiectasias of the eyelid margin. Treatment for blepharitis involves warm compresses followed by gentle massage to clean the eyelashes and oil gland openings. Oral antibiotics from the tetracycline class are used in more severe cases.
Keratoconjunctivitis sicca (KCS) can also present with red eyes. Symptoms include foreign body sensation and burning, typically worse at the end of the day. Although it is paradoxical, the eyes in KCS may also tear. Examination reveals a decreased tear lake and roughness of the corneal surface. Treatment of KCS includes tear replacement therapy, plugs in the cannuliculi, and topical cyclosporine.
Anterior uveitis refers to inflammation of the iris and ciliary body. Symptoms include pain, sensitivity to light, and blurred vision. The redness is more pronounced over the perilimbal (circumcorneal) region, which overlies the inflamed ciliary body. The pupil can be miotic and poorly reactive to light. Examination reveals cells and flare in the anterior chamber. The first-line therapy for anterior uveitis consists of topical glucocorticoid eyedrops and a mydriatic agent. (Dilating the pupils is essential to the prevention of synechiae between the iris and lens). More severe cases are treated with oral glucocorticoids or immunosuppressive drug therapy.
Systemic Conditions Associated With Scleritis
Fifty percent of patients with scleritis are diagnosed with an associated systemic condition [3, 18]. Disorders associated with scleritis can be divided three categories: inflammatory, infectious, and other (Table 2). Autoimmune conditions are found in approximately 40% of patients and infections in approximately 7% [3, 18]. The most commonly associated rheumatic diseases are RA (18-33%); systemic vasculitis (7-19%), of which Wegener’s granulomatosis is the most common; systemic lupus erythematosus (SLE)(4-7%); inflammatory bowel disease (4-7%), and relapsing polychondritis (3%) [3, 17]. Less commonly associated conditions include sarcoidosis [19, 20], cryoglobulinemia [21], and hypocomplementemic urticarial vasculitis [22]. The most commonly associated infection is herpes zoster [18].
Table 2.
Systemic Diseases Associated With Scleritis [1].
| Autoimmune diseases | Infections | Other |
|---|---|---|
| Rheumatoid arthritis* | Viruses (Varicella zoster virus, herpes simplex virus, hepatitis C) | Surgically induced |
| Inflammatory bowel disease | Bacteria | Drug induced |
| Relapsing polychondritis | Mycobacteria | Pamidronate |
| Systemic lupus erythematosus | Spirochetes (Treponema pallidum, Borrelia burgdorferi) | Fluvirin |
| Spondyloarthropathies | Fungi | Trauma |
| Sarcoidosis | Ameoba | |
| Vasculitides | Parasite | |
| Wegener’s granulomatosis* | ||
| Polyarteritis nodosa | ||
| Cryoglobulinemia | ||
| Cogan’s Syndrome | ||
| Hypocomplementemic urticarial vasculitis | ||
| Temporal arteritis | ||
| Takayasu arteritis |
Most commonly associated conditions
In patients with scleritis and an associated systemic condition, the underlying disease is already known in about 80% of patients at the time scleritis presents. In one study, approximately 15% of patients were diagnosed with a new systemic condition as a result of the initial evaluation, and an additional 8% developed a systemic disease during follow-up [18]. A systemic vasculitis was more likely to be discovered by the initial diagnostic evaluation than were other rheumatic diseases [18]. Among patients with no systemic diagnosis after initial evaluation, a new rheumatic disease was diagnosed at a rate of 4% per person-year [18]. Among patients with apparent scleritis who have a known underlying inflammatory condition, an important consideration is the exclusion of an infectious complication of immunosuppression.
Clinical, Radiologic, and Laboratory Evaluation
Evaluation should begin with careful history, review of systems, and physical examination. Specific questions should be directed toward symptoms of systemic conditions commonly associated with scleritis including RA, Wegener’s granulomatosis, and inflammatory bowel disease. Infectious etiologies also should be considered and a history of trauma or surgical insult should be sought.
Routine testing typically includes a complete blood count and metabolic panel and a urinalysis with microscopy (Table 3). In addition, serologic testing for antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA), rheumatoid factor, and antibodies to cyclic citrullinated peptides (anti-CCP antibodies) should also be performed. Additional serologic assays designed to exclude inflammatory conditions (e.g., for complement levels or other types of autoantibodies) may be useful if dictated by the presence of specific clinical features or by the results of initial serologic testing.
Table 3.
Clinical, Radiologic, and Laboratory Evaluation for Patients with Scleritis.
| Standard | Directed based on history and physical examination |
|---|---|
| Complete blood count | Tuberculin skin test |
| Complete metabolic panel | Sacroiliac joint x-rays |
| Urinalysis with microscopic analysis | Sinus imaging |
| Antineutrophil cytoplasmic antibody assay
Antinuclear antibody assay Rheumatoid factor Anti-cyclic citrullinated peptide antibodies |
Viral hepatitis panel |
| Chest radiograph | IgE levels |
| Rapid plasma reagin | Gastrointestinal evaluation |
| Fluorescent treponemal antibody absorption (FTA/Abs) assay | Cultures for bacteria, virus, fungi |
| Lyme antibody | Scleral biopsy |
Spirochetal infections should be excluded with a rapid plasma reagin (RPR) test, a fluorescent treponemal antibody (FTA-ABS) assay, and serological testing for Lyme disease. Chest imaging should be performed with either a radiograph or computed tomographic (CT) scan. The latter is preferred if suspicion for Wegener’s granulomatosis is strong. Other potential directed tests may include a tuberculin skin test, sacroiliac joint radiographs (for spondyloarthropathy), a CT scan of the sinuses, serologies for viral hepatitis panel (hepatitis B and C). When an infectious form of scleritis is suspected, cultures and/or a scleral biopsy may be needed to secure the diagnosis.
If posterior scleritis is suspected, ultrasonography is useful in evaluating the posterior globe for scleral thickening and fluid in Tenon’s capsule (Figure 8). When fluid in Tenon’s capsule is seen in the plane of the optic nerve, the finding is called the “T-sign”. CT scan of the orbits also can be of use in posterior scleritis, showing thickening of the sclera or orbital inflammation.
Complications of Scleritis
Scleritis can be associated with significant eye morbidity [13]. Visual loss can result from a number of complications including uveitis, cataracts, corneal melts (see PUK, below), glaucoma, and posterior segment disease. Specific complications of posterior scleritis can include optic disk edema, cystoid macular edema (swelling of the macula), exudative retinal detachment, and choroidal folds (wrinkles in the choroid – the layer of the eye interposed between the retina and sclera). Other less common complications include scleral thinning and globe rupture with minor trauma.
In a retrospective study of 172 patients, anterior uveitis was found in 42% of patients with scleritis, cataracts were detected in 17%, PUK in 14%, glaucoma in 13%, and posterior segment disease in 6% [17]. In that same study, 37% of patients experienced a decrease in vision due to scleritis, defined as a loss of two or more lines of vision during follow up or a visual acuity of 20/80 or worse at presentation. Vision loss was more commonly seen in patient with necrotizing scleritis (82%) [17]. Another retrospective study of 290 patients found that vision loss (defined as a permanent drop in Snellen acuity of two or more lines) occurred in 9% of patients with diffuse anterior scleritis, 26% of patients with nodular scleritis, 74% of those with necrotizing disease, and 84% of those with posterior scleritis [14]. Vision loss (loss of 2 or more Snellen lines from initial best corrected acuity) is much more likely in patients with posterior scleritis (31% [2]) or an underlying systemic disorder (27% [3]).
Treatment of Scleritis
In a retrospective study of 97 patients with scleritis, 30% required nonsteroidal anti-inflammatory drugs (NSAIDs) alone, 31% required oral glucocorticoids, and 26% required immunosuppressive drugs in addition to glucocorticoids to control their disease [3]. Treatments varied with the specific type of scleritis. Nodular anterior scleritis responded more often to NSAIDs (57%), whereas necrotizing scleritis often required systemic immunosuppressive drugs (70%). Posterior scleritis was more often treated with oral glucocorticoids (83%) and occasionally immunosuppressive drugs (17%) [3].
A step-ladder approach typically is utilized in the treatment of scleritis. The first line therapy in treating non-necrotizing scleritis that is not caused by an infection is an NSAID. Two drugs NSAIDs have been shown to be effective: flurbiprofen 100 mg three times daily and indomethacin 25-50 mg three times daily [3, 13]. If one NSAID does not relieve the pain and inflammation, another may be tried.
Systemic glucocorticoids are used in three general settings: when NSAIDs prove ineffective; in cases of necrotizing anterior scleritis; and in cases of posterior scleritis. The usual starting dose is 1 mg/kg/day (maximum of 60 mg/daily) followed by a tapering schedule based on clinical response. Table 4 describes the typical tapering schedule used in our clinic. In patients whose disease appears particularly aggressive, pulse methylprednisolone can be administered intravenously at a dose of one gram/day for three days, following by the initiation of prednisone 60 mg/day. The role of depot glucocorticoid treatment is controversial, given the potential risk for scleral perforation with this therapy [24-26].
Table 4.
Typical prednisone taper.
| Dose | Duration |
|---|---|
| 1 mg/kg (maximum 60 mg/day) | 1 month |
| 40-60 mg/day | Taper by 10 mg/week |
| 20-40 mg/day | Taper by 5 mg/week |
| 10-20 mg/day | Taper by 2.5 mg/week |
| 0-10 mg/day | Taper by 1-2.5 mg/week |
Immunosuppressive drug therapy is instituted under several clinical circumstances: for necrotizing scleritis, which usually requires cyclophosphamide plus glucocorticoids from the outset of treatment; when other types of scleritis are not controlled by one month of high dose glucocorticoids; when more than 10 mg/day of prednisone is required to maintain disease control; and when glucocorticoid-related adverse effects become an issue [3, 27].
A variety of glucocorticoid sparing agents have been used in the treatment of scleritis (Table 5). Cyclophosphamide (up to 2 mg/kg/day) is the drug of choice for patients with necrotizing scleritis and in patients with scleritis associated with a systemic vasculitis such as Wegener’s granulomatosis [3]. The justification for use of an alkylating agent in such cases is the high risk of progressive ocular damage, extraocular vasculitic lesions, and for death [28]. For patients with non-necrotizing scleritis who require a glucocorticoid-sparing agent, first line treatment typically consists of methotrexate (up to 25 mg/week), azathioprine (up to 200 mg/day), or mycophenolate mofetil (1 gram twice daily). In a retrospective study that examined the clinical outcomes of 50 patients treated with these agents, 46% achieved disease quiescence and were able to taper their prednisone to ≤ 10 mg/day of prednisone [Galor et al, unpublished data]. Depending on severity of disease, treatment typically is continued for one to two years after control of inflammation.
Table 5.
Treatments for non-infectious scleritis.
| Antimetabolites | T cell inhibitors | Alkylating agents | Biologics |
|---|---|---|---|
| Methotrexate*
(start 15 mg weekly) (max 25 mg weekly) |
Cyclosporine**
(start 2 mg/kg twice daily) (max 2.5 mg/kg twice daily) |
Cyclophosphamide†
(start up to 2 mg/kg daily) (max 200 mg/daily) |
Infliximab**
(start 5 mg/kg every 6 weeks) (max 10mg/kg every 4 weeks) |
| Azathioprine
(start 2 mg/kg daily) (max 200 mg daily) |
Tacrolimus
(start 1 mg twice daily) (increase dose until therapeutic) |
Chlorambucil
(start 0.1 mg/kg daily) (max 12 mg/daily) |
Rituximab
(optimal dose uncertain) |
| Mycophenolate mofetil*
(start 1 gram twice daily) (max 1.5 gram twice daily) |
First line treatment in cases where inability to control disease on desired prednisone dose. Generally start for active disease on ≥10 mg prednisone/day
First line agent in cases of systemic vasculitis
Second line agents added if maximal tolerated dose of antimetabolite alone does not allow for control of disease on desired prednisone dose.
Possible second-line agents for scleritis include calcineurin inhibitors (cyclosporine or tacrolimus), infliximab, or rituximab [29-33]. However, none of these agents has been studied rigorously to date. In some cases of necrotizing anterior scleritis or scleromalacia perforans, surgical therapy is required to address extensive scleral thinning and avoid globe rupture. Scleral grafting surgery may be performed with donor sclera, periostium, or fascia lata. Simultaneous efforts to control the underlying inflammation with medical therapy are essential when surgery is required.
The treatment of scleritis with immunosuppressive medications is fraught with the potential for treatment-related morbidity and mortality. Patients on high-dose glucocorticoids and other immunosuppressive agents require careful monitoring, with frequent clinic visits and bloodwork. Patients on cyclophosphamide should have a complete blood count checked not less often than every two weeks. Prophylaxis against Pneumocystis carinii (jiroveci) pneumonia (PCP) is also critical, particularly when the combination of glucocorticoids and another immunosuppressive agent is used. One common approach to PCP prophylaxis is to use trimethoprim-sulfamethoxazole (one single-strength tablet daily). Evaluation for and prophylaxis against glucocorticoid-induced bone loss must also be considered.
Part 2: Peripheral ulcerative keratitis
Peripheral ulcerative keratitis (PUK) is a form of ocular inflammation that involves the outer portions of the cornea and may be associated with systemic conditions such as RA, Wegener’s granulomatosis, and other systemic conditions. The most severe complication of PUK, known as corneal melt syndrome, is the progression of marginal corneal thinning to perforation of the cornea. Corneal melt syndromes can lead to the abrupt and permanent loss of vision in the involved eye.
Anatomy of the cornea
The cornea is a transparent, avascular structure that allows light into the eye and serves as the eye’s main focusing instrument (Figure 8). The peripheral cornea, the site of disease in PUK, is distinct from the central cornea both in its anatomic and physiologic characteristics [34]. The peripheral cornea is near the capillary bed and partly derives its nutrients from the capillary arcades [35]. Proximity to this vascular and lymphatic arcade may explain the peripheral location of PUK, as inflammatory cells and mediators can gain access to this part of the cornea more readily.
Epidemiology of PUK
PUK is less common that scleritis, with an incidence of 3 cases per million per year in one study from England [36]. Women and men appear to be affected equally [37, 38].
Pathogenesis of PUK
The pathogenesis of PUK has not been elucidated completely, but both T cell and antibody mediated pathways have been implicated in the disease process [39]. Abnormal T cell responses have been found in several studies on PUK [39-41]. It is hypothesized that T cells lead to antibody production and the formation of immune complexes that deposit in the peripheral cornea [42]. The complement pathway is activated with recruitment of inflammatory cells to the cornea. Collagenases and other proteases are secreted by neutrophils and macrophages, which leads to destruction of the peripheral corneal stroma [39]. Histopathologic examinations of cornea and conjunctiva from patients with PUK reveal a multitude of inflammatory cells including plasma cells, neutrophils, mast cells, and eosinophils [39].
Clinical History in PUK
Patients with PUK present with ocular pain and redness. Other symptoms may include tearing, photophobia, and decreased vision due to astigmatism or corneal opacity.
Clinical Findings in PUK
PUK typically presents as a crescent-shaped corneal ulcer found within 2 mm of the limbus (Figure 9). The epithelium is absent overlying the ulcer, and the underlying stroma is thinned. There is a variable amount of cellular infiltrate within the stroma, and inflammation involving the contiguous conjunctiva and sclera. In a review of 47 PUK patients, scleritis was present simultaneously in 36% [37]. Conversely, in a retrospective study of patients with scleritis, 14% of patients also had PUK. PUK is observed most often in patients with necrotizing scleritis and is bilateral in up to 40% of cases [17,37,38].
Figure 9.

Peripheral ulcerative keratitis. Idiopathic peripheral ulcerative keratitis in a 43 year-old man. A crescent-shaped corneal ulcer with minimal discharge is seen at the nasal limbus.
A Mooren’s ulcer is a subtype of PUK typified by extreme eye pain, no associated scleral involvement or systemic findings, and an overhanging corneal edge.
Differential Diagnosis of PUK
The differential diagnosis of PUK includes other conditions that affect the peripheral cornea and lead to peripheral corneal thinning or scarring. These include non-inflammatory conditions (e.g., Terrien’s marginal degeneration, pellucid marginal degeneration, and senile furrows) as well as others associated with inflammation or infection (e.g., staphylococcal marginal keratitis, phlyctenulosis, vernal keratoconjunctivitis). Other local insults may also lead to peripheral corneal pathology: poorly-fitting contact lenses, corneal exposure, misdirected lashes (trichiasis), and complications of ocular surgical procedures. The presence of keratoconjunctivitis sicca (KCS; see above) and meibomian gland dysfunction must be evaluated in every patient with corneal thinning, as these factors may cause or contribute to peripheral corneal abnormalities.
Systemic Conditions Associated With PUK
As in scleritis, PUK may be associated with systemic conditions and may be an early manifestation of an underlying vasculitis [36,43]. One retrospective study from a tertiary care referral center reported an underlying systemic disease was found 25 of 47 patients [37]. Although most systemic conditions were known prior at the time of diagnosis, approximately one quarter of the predisposing conditions were undiagnosed, reinforcing the importance of a careful medical history, comprehensive review of systems, and appropriate laboratory testing [37]. RA, found in 34% of patients in one study and 42% in another, was the most common associated disease [37, 38]. Other systemic conditions associated with PUK include the ANCA-associated vasculitides (Wegener’s granulomatosis, the Churg-Strauss syndrome, and microscopic polyangiitis), polyarteritis nodosa, relapsing polychondritis, and systemic lupus erythematosus [37,38].
In RA, corneal melt usually occurs in a patient with longstanding disease, the same type of patient prone to the development of rheumatoid vasculitis: nodular, erosive, rheumatoid factor-positive. In one study, there was a mean of 19.6 years between diagnosis of RA and PUK [36]. In Wegener’s granulomatosis and other forms of systemic vasculitis, corneal melt can occur earlier in the disease course. Regardless of the underlying condition with which it is associated, however, corneal melting can occur swiftly once inflammation begins within the cornea. Visual loss can ensue within days.
Ocular and systemic infections may also cause or be associated with PUK. Microbial pathogens implicated in the etiology of PUK include bacteria (Staphylococcus and Streptococcus species), spirochetes (Treponema pallidum), Mycobacteria (tuberculosis), viruses (hepatitis C, herpes simplex virus, varicella zoster virus), acanthameoba, and fungi [39].
Laboratory Evaluation in PUK
The clinical, radiological, and laboratory evaluation in PUK is identical to that of scleritis (see above) (Table 3).
Complications of PUK
PUK may be associated with significant eye and systemic morbidity. In one retrospective review of 24 patients with scleritis associated PUK, all 24 patients had impending corneal perforation, 16 (67%) had an associated anterior uveitis, and 20 (83%) had decreased vision, defined as a decrease in visual acuity of 2 or more Snellen lines at the end of follow up or visual acuity of 20/80 or worse at presentation [38]. In another retrospective review of 47 patients with PUK, 34% had impending or frank corneal perforations (defined as peripheral corneal thinning of 75-100%), 47% required a tectonic graft procedure, 9% had an associated anterior uveitis, and 43% had visual acuity of 20/400 or worse at presentation [37]. In addition to the high risk of ocular damage from PUK, eye inflammation of this nature is a harbinger of active inflammatory disease in other organ systems with major potential for morbidity and mortality [28].
Treatment of PUK
The treatment of PUK is determined by the severity of findings within the cornea and the extent of extra-ocular disease. Systemic glucocorticoids are the cornerstone of therapy for non-infectious PUK. Glucocorticoids are started at a dose of 1 mg/kg/day (maximum of 60 mg daily), followed by a tapering schedule based on clinical response. As in scleritis, patients in whom loss of vision is imminent should be treated with pulse methylprednisolone, 1 gram/day for three days. Topical glucocorticoids are not an appropriate therapy for PUK.
Alkylating agents may be used in conjunction with glucocorticoids in cases of PUK with imminent danger of corneal perforation and in cases associated with a systemic vasculitis. Because of the high likelihood of ocular morbidity resulting from ineffective treatment of PUK, the usual approach is to employ both cyclophosphamide (up to 2 mg/kg/day) in addition to high-dose glucocorticoids from the outset of therapy. As with scleritis, steroid-sparing agents are also added when an initial course of high-dose glucocorticoids does not control the disease, when disease control cannot be maintained with less than 10 mg/day of prednisone, and when significant concern related to glucocorticoid-related adverse effects exists [27]. Methotrexate, azathioprine, mycophenolate mofetil, and the biologic agents are all employed in a fashion similar to their use in scleritis (Table 5).
The treatment of PUK with immunosuppressive medications is fraught with the potential for treatment-related morbidity and mortality. Patients on high-dose glucocorticoids and other immunosuppressive agents require careful monitoring, with frequent clinic visits and bloodwork. Patients on cyclophosphamide should have a complete blood count checked not less often than every two weeks. Prophylaxis against Pneumocystis carinii (jiroveci) pneumonia (PCP) is also critical, particularly when the combination of glucocorticoids and another immunosuppressive agent is used. One common approach to PCP prophylaxis is to use trimethoprim-sulfamethoxazole (one single-strength tablet daily). Evaluation for and prophylaxis against glucocorticoid-induced bone loss must also be considered.
Surgical resection of conjunctival tissue adjacent to PUK has been promoted as a means of decreasing access of inflammatory cells and factors to the peripheral cornea [37, 44]. This treatment is controversial, as it is not uniformly agreed that resection alters the course of disease. Surgical management of PUK is used in cases of impending perforation to preserve globe integrity. Several surgical options exist depending on the size of perforation. These include the use of a tissue adhesive bandage contact lens, a lamellar graft, and tectonic corneal grafting.
A study of 34 patients with RA-associated PUK and/or necrotizing scleritis found decreased mortality and ocular morbidity in patients treated with immunosuppressive medication [28]. A lower mortality was seen (6% versus 53%) in patients managed with cyclophosphamide (12 patients) or antimetabolite agents (5 patients) compared to those managed with oral glucocorticoids and NSAIDS alone. Progression of ocular disease occurred less often (0% versus 76%) in the former group, as well. Furthermore, no patient treated with immunosuppressive agents developed extraocular vasculitis while on medication. Another retrospective review of 12 patients with RA-associated PUK found that 68% of patients had stable or improved visual acuities after systemic treatment with cyclophosphamide or methotrexate and surgical therapy (required in 63% of eyes) [45].
A retrospective study of 38 patients with PUK who underwent adjacent conjunctival biopsy found vasculitis in 20 biopsies. Eighteen of the 20 patients were treated with chemotherapy; seventeen had no progression of ulceration. The two patients not treated with chemotherapy stabilized with surgical management. Of 18 patients without vasculitis on biopsy, 11 were treated with chemotherapy. All remained stable or improved with combination surgery and chemotherapy. Of the remaining 7 patients, 6 remained stable after conjunctival resection alone [37].
Acknowledgments
Grant Support: Supported by a grant EY-13707 (Dr. Thorne) from the National Eye Institute, Bethesda, Maryland, and by unrestricted funds form Research to Prevent Blindness (Dr. Galor).
Footnotes
Proprietary Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okhravi N, Odufuwa B, McCluskey P, Lightman S. Scleritis. Surv Ophthalmol. 2005;50(4):351–63. doi: 10.1016/j.survophthal.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999;106(12):2380–6. doi: 10.1016/S0161-6420(99)90543-2. [DOI] [PubMed] [Google Scholar]
- 3.Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130(4):469–76. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 4.Joysey VC, Roger JH, Ashworth F, et al. Parallel studies of HLA antigens in patients with rheumatic heart disease and scleritis: comparisons with three control populations. J Rheumatol Suppl. 1977;3:84–8. [PubMed] [Google Scholar]
- 5.Smith JR, Mackensen F, Rosenbaum JT. Therapy insight: scleritis and its relationship to systemic autoimmune disease. Nat Clin Pract Rheumatol. 2007;3(4):219–26. doi: 10.1038/ncprheum0454. [DOI] [PubMed] [Google Scholar]
- 6.Haynes BF, Fishman ML, Fauci AS, Wolff SM. The ocular manifestations of Wegener’s granulomatosis. Fifteen years experience and review of the literature. Am J Med. 1977;63(1):131–41. doi: 10.1016/0002-9343(77)90125-5. [DOI] [PubMed] [Google Scholar]
- 7.Bullen CL, Liesegang TJ, McDonald TJ, DeRemee RA. Ocular complications of Wegener’s granulomatosis. Ophthalmology. 1983;90(3):279–90. doi: 10.1016/s0161-6420(83)34574-7. [DOI] [PubMed] [Google Scholar]
- 8.Fong LP, Sainz de la Maza M, Rice BA, Kupferman AE, Foster CS. Immunopathology of scleritis. Ophthalmology. 1991;98(4):472–9. doi: 10.1016/s0161-6420(91)32280-2. [DOI] [PubMed] [Google Scholar]
- 9.Bernauer W, Buchi ER, Daicker B. Immunopathological findings in posterior scleritis. Int Ophthalmol. 1994;18(4):229–31. doi: 10.1007/BF00951803. [DOI] [PubMed] [Google Scholar]
- 10.Bernauer W, Watson PG, Daicker B, Lightman S. Cells perpetuating the inflammatory response in scleritis. Br J Ophthalmol. 1994;78(5):381–5. doi: 10.1136/bjo.78.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riono WP, Hidayat AA, Rao NA. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology. 1999;106(7):1328–33. doi: 10.1016/S0161-6420(99)00719-8. [DOI] [PubMed] [Google Scholar]
- 12.Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60(3):163–91. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwam B. Scleritis. In: Krachmer J, Mannis M, Holland E, editors. Cornea and External Disease: Clinical Diagnosis and Management. 1. II. Saint Louis: Mosby; 1997. pp. 1479–1491. [Google Scholar]
- 14.Tuft SJ, Watson PG. Progression of scleral disease. Ophthalmology. 1991;98(4):467–71. doi: 10.1016/s0161-6420(91)32269-3. [DOI] [PubMed] [Google Scholar]
- 15.Hakin KN, Watson PG. Systemic associations of scleritis. Int Ophthalmol Clin. 1991;31(3):111–29. doi: 10.1097/00004397-199103130-00010. [DOI] [PubMed] [Google Scholar]
- 16.Foster C. The sclera. New York: Springer-Verlag; 1994. [Google Scholar]
- 17.Sainz de la Maza M, Jabbur NS, Foster CS. Severity of scleritis and episcleritis. Ophthalmology. 1994;101(2):389–96. doi: 10.1016/s0161-6420(94)31325-x. [DOI] [PubMed] [Google Scholar]
- 18.Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004;111(3):501–6. doi: 10.1016/j.ophtha.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Dursun D, Akova YA, Bilezikci B. Scleritis associated with sarcoidosis. Ocul Immunol Inflamm. 2004;12(2):143–8. doi: 10.1080/09273940490895353. [DOI] [PubMed] [Google Scholar]
- 20.Qazi FA, Thorne JE, Jabs DA. Scleral nodule associated with sarcoidosis. Am J Ophthalmol. 2003;136(4):752–4. doi: 10.1016/s0002-9394(03)00454-9. [DOI] [PubMed] [Google Scholar]
- 21.Kedhar SR, Belair ML, Jun AS, Sulkowski M, Thorne JE. Scleritis and peripheral ulcerative keratitis with hepatitis C virus-related cryoglobulinemia. Arch Ophthalmol. 2007;125(6):852–3. doi: 10.1001/archopht.125.6.852. [DOI] [PubMed] [Google Scholar]
- 22.Thorne JE, Hernandez MI, Rencic A, Nousari HC. Severe scleritis and urticarial lesions. Am J Ophthalmol. 2002;134(6):932–4. doi: 10.1016/s0002-9394(02)01814-7. [DOI] [PubMed] [Google Scholar]
- 23.Galor A, Thorne JE, Jabs DA. Rheumatic disease and scleritis. Ophthalmology. 2007;114(6):1232. doi: 10.1016/j.ophtha.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Albini TA, Zamir E, Read RW, Smith RE, See RF, Rao NA. Evaluation of subconjunctival triamcinolone for nonnecrotizing anterior scleritis. Ophthalmology. 2005;112(10):1814–20. doi: 10.1016/j.ophtha.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Zamir E, Read RW, Smith RE, Wang RC, Rao NA. A prospective evaluation of subconjunctival injection of triamcinolone acetonide for resistant anterior scleritis. Ophthalmology. 2002;109(4):798–805. doi: 10.1016/s0161-6420(01)01018-1. discussion 805-7. [DOI] [PubMed] [Google Scholar]
- 26.Tu EY, Culbertson WW, Pflugfelder SC, Huang A, Chodosh JC. Therapy of nonnecrotizing anterior scleritis with subconjunctival corticosteroid injection. Ophthalmology. 1995;102(5):718–24. doi: 10.1016/s0161-6420(95)30963-3. [DOI] [PubMed] [Google Scholar]
- 27.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis. Effects of systemic immunosuppression. Ophthalmology. 1984;91(10):1253–63. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- 29.Murphy CC, Ayliffe WH, Booth A, Makanjuola D, Andrews PA, Jayne D. Tumor necrosis factor alpha blockade with infliximab for refractory uveitis and scleritis. Ophthalmology. 2004;111(2):352–6. doi: 10.1016/S0161-6420(03)00721-8. [DOI] [PubMed] [Google Scholar]
- 30.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113(12):2317–23. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi-Simab K, Lamprecht P, Nolle B, Ai M, Gross WL. Successful treatment of refractory anterior scleritis in primary Sjogren’s syndrome with rituximab. Ann Rheum Dis. 2005;64(7):1087–8. doi: 10.1136/ard.2004.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minami R, Miyamura T, Watanabe H, Takahama S, Yamamoto M, Suematsu E. Successful treatment of a patient with refractory Wegener’s granulomatosis by rituximab. Nihon Rinsho Meneki Gakkai Kaishi. 2007;30(2):133–8. doi: 10.2177/jsci.30.133. [DOI] [PubMed] [Google Scholar]
- 33.Cheung CM, Murray PI, Savage CO. Successful treatment of Wegener’s granulomatosis associated scleritis with rituximab. Br J Ophthalmol. 2005;89(11):1542. doi: 10.1136/bjo.2005.075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abelson M, Ousler G, Plummer A. An In-depth Look at ‘Our Two Corneas’. Review of Ophthalmology 2005. 2005 Aug 1;12 [Google Scholar]
- 35.Freeman RD. Oxygen consumption by the component layers of the cornea. J Physiol. 1972;225(1):15–32. doi: 10.1113/jphysiol.1972.sp009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKibbin M, Isaacs JD, Morrell AJ. Incidence of corneal melting in association with systemic disease in the Yorkshire Region, 1995-7. Br J Ophthalmol. 1999;83(8):941–3. doi: 10.1136/bjo.83.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tauber J, Sainz de la Maza M, Hoang-Xuan T, Foster CS. An analysis of therapeutic decision making regarding immunosuppressive chemotherapy for peripheral ulcerative keratitis. Cornea. 1990;9(1):66–73. [PubMed] [Google Scholar]
- 38.Sainz de la Maza M, Foster CS, Jabbur NS, Baltatzis S. Ocular characteristics and disease associations in scleritis-associated peripheral keratopathy. Arch Ophthalmol. 2002;120(1):15–9. doi: 10.1001/archopht.120.1.15. [DOI] [PubMed] [Google Scholar]
- 39.Dana M, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19(5):625–43. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Foster C, Kenyon K, Greiner J. The immunopathology of Mooren’s ulcer. Am J Ophthalmol. 1979;88:149–59. doi: 10.1016/0002-9394(79)90459-8. [DOI] [PubMed] [Google Scholar]
- 41.Mondino B, Brown S, Rabin B. Cellular immunity in Mooren’s ulcer. Am J Ophthalmol. 1978;85:788–91. doi: 10.1016/s0002-9394(14)78106-1. [DOI] [PubMed] [Google Scholar]
- 42.Mondino B. Inflammatory diseases of the peripheral cornea. Ophthalmology. 1988;95:463–72. doi: 10.1016/s0161-6420(88)33164-7. [DOI] [PubMed] [Google Scholar]
- 43.Ladas JG, Mondino BJ. Systemic disorders associated with peripheral corneal ulceration. Curr Opin Ophthalmol. 2000;11(6):468–71. doi: 10.1097/00055735-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Messmer EM, Foster CS. Vasculitic peripheral ulcerative keratitis. Surv Ophthalmol. 1999;43(5):379–96. doi: 10.1016/s0039-6257(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 45.Messmer EM, Foster CS. Destructive corneal and scleral disease associated with rheumatoid arthritis. Medical and surgical management. Cornea. 1995;14(4):408–17. doi: 10.1097/00003226-199507000-00010. [DOI] [PubMed] [Google Scholar]


