Abstract
The beneficial actions of nonsteroid anti-inflammatory drugs (NSAID) can be associated with inhibition of cyclo-oxygenase (COX)-2 whereas their harmful side effects are associated with inhibition of COX-1. Here we report data from two related assay systems, the human whole blood assay and a modified human whole blood assay (using human A549 cells as a source of COX-2). This assay we refer to as the William Harvey Modified Assay. Our aim was to make meaningful comparisons of both classical NSAIDs and newer COX-2-selective compounds. These comparisons of the actions of >40 NSAIDs and novel COX-2-selective agents, including celecoxib, rofecoxib and diisopropyl fluorophosphate, demonstrate a distribution of compound selectivities toward COX-1 that aligns with the risk of serious gastrointestinal complications. In conclusion, this full in vitro analysis of COX-1/2 selectivities in human tissues clearly supports the theory that inhibition of COX-1 underlies the gastrointestinal toxicity of NSAIDs in man.
Nonsteroid anti-inflammatory drugs (NSAIDs) are among the most widely prescribed drugs worldwide, being the drugs of first choice in the treatment of rheumatic disorders and other degenerative inflammatory joint diseases. Inhibition of cyclo-oxygenase (COX), and therefore prostaglandin production, is the common mechanism of action of the NSAIDs (1). As is now well appreciated, COX exists as two isoforms. In general terms, cyclo-oxygenase-1 (COX-1) is constitutive and present in, for example, the endothelium, stomach and kidney whereas cyclo-oxygenase-2 (COX-2) is induced by proinflammatory cytokines and endotoxin in cells in vitro and at inflammatory sites in vivo (see ref. 2). This led some of us to the previous proposition that the side effects of NSAIDs correlate with their ability to inhibit COX-1 whereas the therapeutic, anti-inflammatory effects of these agents are attributable to their ability to inhibit COX-2 (3). A number of subsequent analyses have been published demonstrating the potencies against COX-1 and COX-2 of a large number of NSAIDs and novel COX-2-selective inhibitors (see ref. 2). Although these analyses have used a wide range of assay systems, from isolated purified enzymes to intact cells, the assay most widely accepted is the human whole blood assay (4–7). This assay has the advantage of using readily available human cells and taking into account the binding of NSAIDs to human plasma proteins. However, thus far, there are no single studies published that compare the relative abilities of all members of the NSAID family to inhibit COX-1 versus COX-2 on a common and appropriate assay system. Without such information, it is not possible to determine the predictive nature of such assays for the use of NSAIDs in the patient population. Here we present data derived from both the human whole blood assay (WBA) and a human modified whole blood assay (WHMA) for >40 NSAIDs and COX-2-selective inhibitors. These data support the concept that inhibition of COX-1 is responsible for the serious gastrointestinal (GI) complications induced by NSAIDs in humans (8).
METHODS
Cell Culture.
Human airway epithelial cells, A549 cells (European Collection of Animal Cell Cultures, ref. no. 86012804) were cultured in 96-well plates with DMEM supplemented with 10% fetal calf serum and l-glutamine (4 mM). To induce the expression of COX-2, A549 cells were exposed to interleukin-1β (10 ng⋅ml−1) for 24 h (9).
Human Whole Blood Assay (WBA).
Blood was collected by venupuncture into heparin (19 units/ml) and then was aliquoted in 100-μl volumes into the individual wells of 96-well plates. For COX-1 assays, blood then was treated with test agents or vehicle (usually 0.1% vol/vol dimethyl sulfoxide) followed 60 min later by calcium ionophore, A23187 (50 μM). After 30 min, the plates were centrifuged (1,500 × g, 4°C, 5 min), and the plasma was removed and immediately frozen. For WBA COX-2 assays, blood was treated with aspirin (12 μg/ml) to inactivate COX-1, and then 6 h later with lipopolysaccharide (10 μg/ml) plus test agents or vehicle. Incubation then was continued for a further 18 h, after which time the plates were spun, and the plasma was removed and frozen. Concentrations of thromboxane (Tx) B2 (as a measure of TxA2 formation and so COX activity) in samples from both protocols then were determined by radioimmunoassay. Data is reported as being from COX-1 and WBA-COX-2 protocols.
William Harvey Human Modified Whole Blood Assay (WHMA).
For assay of COX-1, experiments were conducted as above, and all COX-1 data were pooled. For assay of COX-2, the medium was removed from A549 cells, which had been exposed to interleukin-1β for the preceding 24 h, and human blood (100 μl) added together with test agents or vehicle. Sixty minutes later, A23187 (50 μM) was added, followed 30 min later by diclofenac (1 mM) to inhibit (>98%) the formation of prostanoids. The plates then were centrifuged, and plasma was removed (as above). Concentrations of prostaglandin E2 (PGE2) in samples then were determined by radioimmunoassay as a measure of the activity of COX-2 in the A549 cells. Data is reported as being from the WHMA-COX-2 protocol.
Materials.
Radiolabeled [3H]TxB2 and [3H]PGE2 were obtained from Amersham. Celecoxib, L-745,337, SC58125, and rofecoxib were synthesized by Boehringer Ingelheim; 6-methoxy-2-napthylacetic acid (6MNA) was a gift from SmithKline Beecham; diisopropyl fluorophosphate was a gift from Merck-Frosst Labs (Pointe Claire, PQ, Canada); tomoxiprole was a gift from NicOx S.A. (Nice, France); ketorolac, meclofenamate, niflumic acid, NS398, and valeryl salicylate were obtained from SPI Bio (Massy Cedex, France); and sulindac sulfide was purchased from Affiniti (Exeter, U.K). All other compounds and reagents were obtained from Sigma.
Calculations.
For each blood sample, the “control” formation of TxB2 or PGE2 was assessed as the mean of six determinations. For each experiment, the effects of the compounds were calculated and represented as percent of control by using the mean control value. Concentration response curves were fitted, and IC50 and IC80 values were derived, by using prism (GraphPad, San Diego). COX-1/WBA-COX-2 (WBA) and COX-1/WHMA-COX-2 (WHMA) selectivities were determined as the ratios of the IC50 and IC80 values.
RESULTS
Prostanoid Production.
In the presence of drug vehicle, the productions of prostanoids in the assay systems were: COX-1, 32.3 ± 1.9 ng⋅ml−1 TxB2; WBA-COX-2, 12 ± 0.6 ng⋅ml−1 TxB2; and WHMA-COX-2, 41.8 ± 1.9 ng⋅ml−1 PGE2 (n = 24–31). In blood treated with aspirin and then incubated for 18 h in the absence of lipopolysaccharide, there was no detectable formation of TxB2 or PGE2.
Inhibitor Potencies.
The agents tested readily divided into four groups in terms of their potencies as inhibitors of COX-1 and COX-2 (Table 1; Figs. 1–4). The first group consists of compounds that can produce full inhibition of both COX-1 and COX-2 with relatively poor selectivity. This group contained most of the currently used NSAIDs, including, for instance, diclofenac, ibuprofen, naproxen, piroxicam, and sulindac (Fig. 1) as well as 6MNA, the active metabolite of nabumetone. Aspirin could not be assessed in the WBA-COX-2 assay because of its instability in whole blood but was active in the WHMA-COX-2 assay. Taken together with the COX-1 assay, our data demonstrated a selectivity of aspirin of ≈4-fold toward COX-1. The second group contained compounds such as etodolac, meloxicam, and nimesulide, all of which show a preferential selectivity toward COX-2 (>5-fold in the WHMA/COX-1 determination) (Fig. 1). It must not be overlooked, however, that these compounds all have the potential to produce full inhibition of COX-1. Of interest, our data also indicate that celecoxib should be included in this second group (Fig. 1). The third group contained compounds that inhibit COX-2 with only a very weak activity against COX-1 and included the experimental compounds diisopropyl fluorophosphate, L-745,337, NS398, and SC58125 together with rofecoxib, all of which were designed as COX-2-selective agents (Fig. 2). The fourth group contained compounds that appeared to be only weak inhibitors of COX-1 and COX-2, such as many of the salicylates. As expected, this fourth group also included nabumetone, which, unlike its metabolite 6MNA, only produced weak inhibition of both COX isoforms.
Table 1.
Potencies of all compounds tested as inhibitors of prostanoid formation determined in the COX-1 assay, WBA-COX-2, and WHMA-COX-2
| Compound | COX-1
|
WBA-COX-2
|
WHMA-COX-2
|
IC50 ratios
|
IC80 ratios
|
Ranking at IC80 ratios
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50, μM | IC80, μM | IC50, μM | IC80, μM | IC50, μM | IC80, μM | WBA COX-1 | WHMA COX-1 | WBA COX-1 | WHMA COX-1 | WBA COX-1 | WHMA COX-1 | |
| 6MNA | 42 | 130 | 146 | 580 | n.d. | n.d. | 3.5 | n.d. | 4.5 | n.d. | 27 | n.d. |
| Aspirin | 1.7 | 8.0 | >100 | >100 | 7.5 | 30 | >100 | 4.4 | >100 | 3.8 | 34 | 23 |
| Carprofen | 0.087 | 19 | 4.3 | 75 | n.d. | n.d. | 50 | n.d. | 3.9 | n.d. | 25 | n.d. |
| Diclofenac | 0.075 | 1.0 | 0.038 | 0.27 | 0.020 | 0.23 | 0.5 | 0.3 | 0.27 | 0.23 | 10 | 9 |
| Fenoprofen | 3.4 | 23 | 41 | 100 | 5.9 | 24 | 12 | 1.7 | 4.3 | 1.0 | 26 | 18 |
| Flufenamate | 3.0 | 80 | 9.3 | 79 | n.d. | n.d. | 3.1 | n.d. | 1.0 | n.d. | 13 | n.d. |
| Flubiprofen | 0.075 | 1.0 | 5.5 | 24 | 0.77 | 51 | 73 | 10 | 24 | 51 | 31 | 27 |
| Ibuprofen | 7.6 | 58 | 7.2 | 67 | 20 | 150 | 0.9 | 2.6 | 1.2 | 2.6 | 14 | 20 |
| Indomethacin | 0.013 | 0.46 | 1.0 | 5.0 | 0.13 | 2.0 | 80 | 10 | 11 | 4.3 | 29 | 24 |
| Ketoprofen | 0.047 | 1.0 | 2.9 | 22 | 0.24 | 6.0 | 61 | 5.1 | 22 | 6.0 | 31 | 25 |
| Ketorolac | 0.00019 | 0.0034 | 0.086 | 4.0 | 0.075 | 1.0 | 453 | 395 | 1176 | 294 | 33 | 28 |
| Meclofenamate | 0.22 | 3.0 | 0.7 | 8.0 | 0.2 | 1.0 | 3.2 | 0.91 | 2.7 | 0.3 | 22 | 11 |
| Mefenamic acid | 25 | >100 | 2.9 | >100 | 1.3 | >100 | 0.11 | 0.049 | - | - | - | - |
| Naproxen | 9.3 | 110 | 28 | 260 | 35 | 330 | 3.0 | 3.8 | 2.4 | 3.0 | 18 | 22 |
| Niflumic acid | 25 | 77 | 5.4 | 35 | 11 | 74 | 0.22 | 0.43 | 0.45 | 1.0 | 12 | 16 |
| Piroxicam | 2.4 | 15 | 7.9 | 31 | 0.17 | 7.0 | 3.3 | 0.1 | 2.1 | 0.47 | 17 | 13 |
| Sulindac sulphide | 1.9 | 38 | 55 | 100 | 1.21 | 11 | 29 | 0.64 | 2.6 | 0.29 | 20 | 10 |
| Suprofen | 1.1 | 3.0 | 8.7 | 56 | 8.3 | 100 | 7.7 | 7.3 | 19 | 33 | 30 | 26 |
| Tenidap | 0.081 | 5.0 | 2.9 | 13 | n.d. | n.d. | 35.2 | n.d. | 2.6 | n.d. | 21 | n.d. |
| Tolmetin | 0.35 | 5.0 | 0.82 | 43 | 1.3 | 13 | 2.3 | 3.8 | 8.6 | 2.6 | 28 | 21 |
| Tomoxiprol | 7.6 | 35 | 20 | 84 | 0.32 | 13 | 2.7 | 0.042 | 2.4 | 0.37 | 19 | 12 |
| Zomepirac | 0.43 | 2.0 | 0.81 | 6.0 | 0.096 | 2.0 | 1.9 | 0.22 | 3.0 | 1.0 | 23 | 17 |
| Celexocib | 1.2 | 28 | 0.83 | 6.0 | 0.34 | 3.0 | 0.7 | 0.3 | 0.21 | 0.11 | 8 | 7 |
| Etodolac | 12 | 69 | 2.2 | 8.0 | 0.94 | 3.0 | 0.2 | 0.1 | 0.12 | 0.043 | 6 | 5 |
| Meloxicam | 5.7 | 22 | 2.1 | 7 | 0.23 | 2.0 | 0.37 | 0.040 | 0.32 | 0.091 | 11 | 6 |
| Nimesulide | 10 | 41 | 1.9 | 7.0 | 0.39 | 7.0 | 0.19 | 0.038 | 0.17 | 0.17 | 7 | 8 |
| Diisopropyl fluorophosphate | >100 | >100 | 0.76 | 4.0 | 0.17 | 5.0 | <0.01 | <0.01 | <0.01 | <0.01 | 1= | 1= |
| L745,337 | >100 | >100 | 8.6 | 41 | 1.3 | 17 | <0.01 | <0.01 | <0.01 | <0.01 | 1= | 1= |
| NS398 | 6.9 | 65 | 0.35 | 1.0 | 0.042 | 1.0 | 0.051 | 0.0061 | 0.015 | 0.015 | 5 | 4 |
| Rofecoxib | 63 | >100 | 0.84 | 6.0 | 0.31 | 5.0 | 0.013 | 0.0049 | <0.05 | <0.05 | 4 | 3 |
| SC58125 | >100 | >100 | 2.0 | 10 | n.d. | n.d. | >0.01 | n.d. | <0.01 | n.d. | 1= | n.d. |
| 5-Aminosalicylic acid | 410 | >1000 | 61 | >1000 | n.d. | n.d. | 0.15 | n.d. | - | n..d. | - | n.d. |
| Ampyrone | 55 | 270 | 203 | 1000 | 85 | 670 | 3.7 | 1.5 | 3.7 | 2.5 | 24 | 19 |
| Diflunisal | 113 | 530 | 8.2 | 140 | 134 | 400 | 0.1 | 1.2 | 0.26 | 0.75 | 9 | 14 |
| Nabumetone | 460 | >1000 | >1000 | >1000 | 290 | >1000 | - | - | - | - | - | - |
| Paracetamol | >100 | >100 | 49 | >100 | 64 | >100 | - | - | - | - | - | - |
| Resveratrol | 30 | >100 | 39 | >100 | n.d. | n.d. | 1.3 | n.d. | - | - | - | n.d. |
| Salicin | >100 | >100 | >100 | >100 | n.d. | n.d. | - | n.d. | - | - | - | n.d. |
| Salicylaldehyde | >100 | >100 | >100 | >100 | n.d. | n.d. | - | n.d. | - | - | - | n.d. |
| Sodium salicylate | 4956 | 49000 | 34440 | 101000 | 482 | 45000 | 6.9 | 0.10 | 2.1 | 0.92 | 16 | 15 |
| Sulfasalazine | 3242 | 6400 | 2507 | 8300 | n.d. | n.d. | 0.8 | n.d. | 1.3 | n.d. | 15 | n.d. |
| Sulindac | >100 | >100 | >100 | >100 | 58 | >100 | - | - | - | - | - | - |
| Tamoxifen | 15 | >100 | 95 | >100 | n.d. | n.d. | 6.4 | n.d. | - | - | - | n.d. |
| Ticlopidine | 52 | >100 | 47 | >100 | n.d. | n.d. | 0.9 | n.d. | - | - | - | n.d. |
| Valeryl salicylate | 42 | >100 | 2.3 | >100 | n.d. | n.d. | 0.053 | n.d. | - | n.d. | - | n.d. |
Data is presented in the following column order: alphabetical listing of agents after division into four main groups: (top) compounds that can produce full inhibition of both COX-1 and COX-2 with poor COX-2 selectivity; (second) compounds that can produce full inhibition of COX-1 and COX-2 with >5× preference towards inhibiting COX-2 (WHMA/COX-1 < 0.2); (third) compounds that appear to be only weak inhibitors of COX-1 and COX-2. Shown are potencies (micromolar IC50 and IC80 values) of compounds against COX-1, WBA-COX-2, and WHMA-COX-2. Selectivities of compounds towards COX-1 were determined as IC50 and IC80 ratios for both WBA-COX-2/COX-1 and WHMA-COX-2/COX-1. Ranking of compounds as inhibitors of COX-2 relative to COX-1 are based on ordering of IC80 ratios; higher ranking numbers are associated with increased selectivity towards COX-1. n.d., not done.
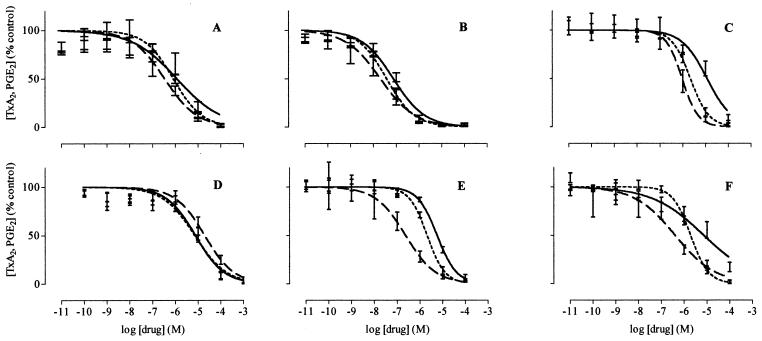
Figure 1.
The effects of celecoxib (A), diclofenac (B), etodolac (C), ibuprofen (D), meloxicam (E), and nimesulide (F) on the activity of COX-1 (solid line), WBA-COX-2 (short dashed line), and WHMA-COX-2 (long dashed line). Results are expressed as percent of control and are represented as mean ± SEM. (n = 5–8).
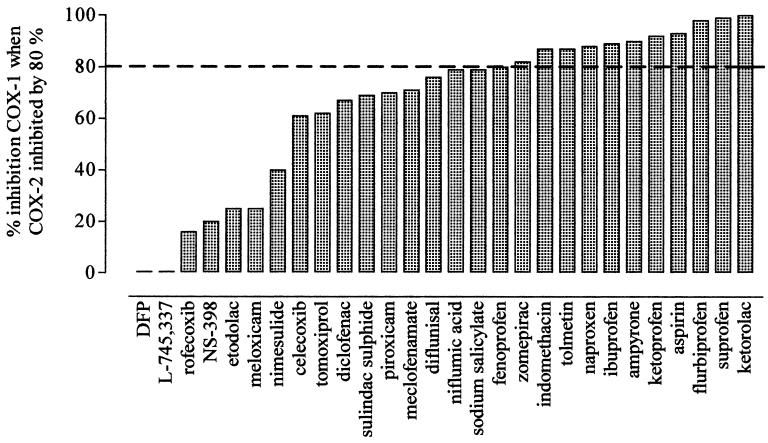
Figure 4.
Analysis of the percent inhibition of COX-1 seen when COX-2 (WHMA) is inhibited by 80%. The dotted line indicates equiactivity, i.e., an 80% inhibition of COX-1.
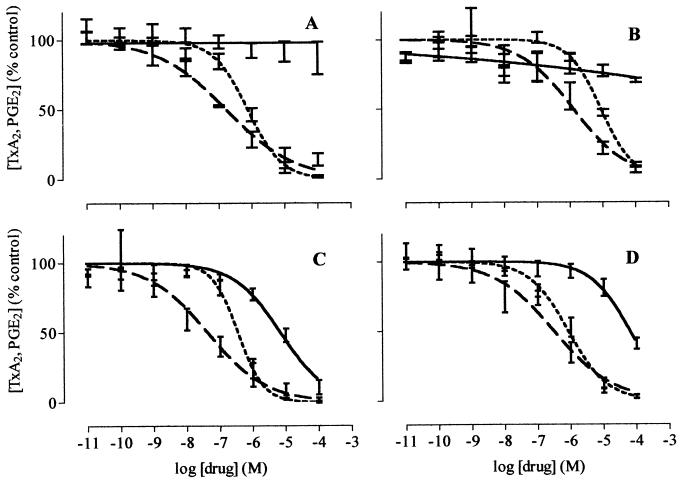
Figure 2.
The effects of diisopropyl fluorophosphate (A), L-745,337 (B), NS398 (C), and rofecoxib (D) on the activity of COX-1 (solid line), WBA-COX-2 (short dashed line), and WHMA-COX-2 (long dashed line). Results are expressed as percent of control and are represented as mean ± SEM. (n = 5–8).
DISCUSSION
Here, using simple assay systems, we have investigated the relative potencies as inhibitors of COX-1 and COX-2 of a wide range of NSAIDs as well as representatives of the newer COX-2 selective agents. In particular, however, we also included all of those agents for which good epidemiological data of the risk of serious GI complications existed (8). This was a deliberate approach because, although some of these compounds were previously tested in other human whole blood assays (e.g., refs. 4–7), they have not been tested together within a single assay system.
When comparing the potencies of NSAIDs against COX-1 and COX-2, IC50 values are often used. However, there are assumptions underlying such an approach that are not necessarily correct. In particular, as is clear from Figs. 1 and 2, the inhibitor curves are often not parallel. Thus, as the concentration of a NSAID varies, so does its relative potency. Second, NSAIDs are used therapeutically at doses that produce more than a 50% reduction in prostanoid formation. Indeed, a survey of the literature established that, for diclofenac (10), etodolac (11), indomethacin (12, 13), fenoprofen (12), flurbiprofen (14), ketoprofen (12), ketorolac (13, 15), meclofenamate (12), meloxicam (16), naproxen (17), nimesulide (18), piroxicam (19), sulindac (20), and tolmetin (12), the steady-state plasma concentrations of these drugs, as well as the peak concentrations of aspirin (12), would produce average inhibitions in our assay systems of 82 ± 5% (COX-1), 74 ± 5% (WBA-COX-2), and 89 ± 2% (WHMA-COX-2) (n = 15). Comparison of the potencies of the NSAIDs against COX-1 and COX-2 at the IC80 value, therefore, appears more appropriate. In making these comparisons, we used data both from the WBA and from the WHMA. This second assay was developed because the potencies of NSAIDs as inhibitors of prostanoid formation are influenced by the supply of arachidonic acid both in vitro (21) and in vivo (22). Clearly, in the standard human whole blood assay, there is a substantial difference between the time courses of the incubations for testing inhibition of COX-1 and COX-2 (1 h vs. 18 h) and, hence, in the rate of prostanoid formation and so in the supply of arachidonic acid. The human whole blood plus A549 cell assay provides a system in which COX-2-containing cells are exposed to NSAIDs for the same time periods and in which the same stimulus is applied at the end of this incubation period, as for the matched COX-1 assay system. Of interest, a number of the compounds tested appeared more potent in the WHMA-COX-2 than the WBA-COX-2. This could be explained by variations in either the metabolism or the plasma binding of compounds within the blood samples during the different time courses of the WBA and WHMA. Alternatively, it could be explained by different levels or sources of free arachidonic acid within the cells expressing COX-2 in the two assay systems, or even to the binding characteristics of the NSAIDs to COX-2 (23).
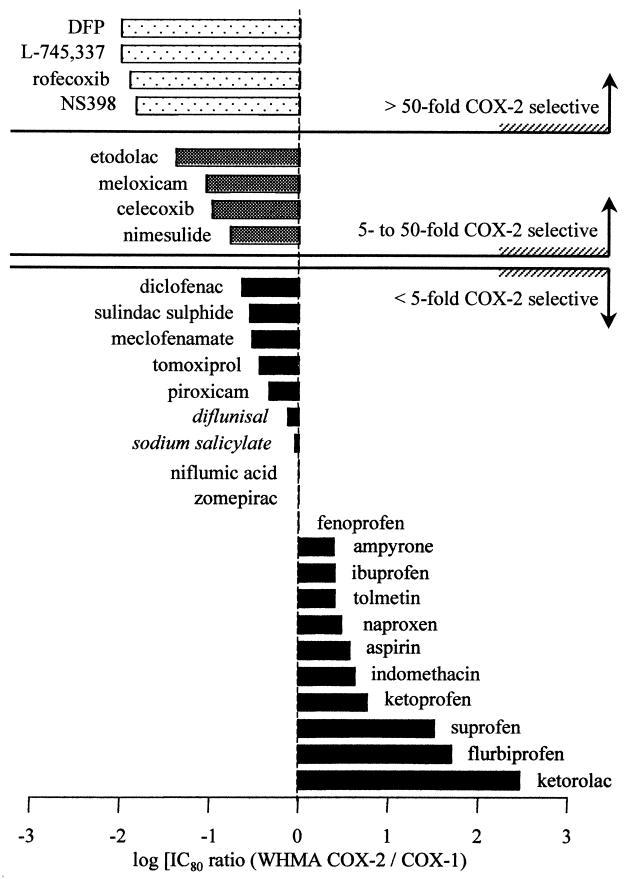
When making our comparisons from the two assay systems we found that the agents tested could be divided into four main groups: (i) compounds capable of producing full inhibition of both COX-1 and COX-2 with poor selectivity; (ii) compounds capable of producing full inhibition of COX-1 and COX-2 with preference toward COX-2; (iii) compounds that strongly inhibited COX-2 with only weak activity against COX-1; and (iv) compounds that appeared to be only weak inhibitors of COX-1 and COX-2 (Table 1; Fig. 3). It is of interest to compare these groupings of NSAIDs to epidemiological studies of NSAID-induced GI toxicity. This is an area of particular interest, for NSAIDs cause serious gastric damage leading to hospitalization in some 100,000 patients per year in the U.S. alone (24). The relationship between NSAID use and serious GI complications has, therefore, been examined in a number of studies. One of the most complete recent studies is a meta-analysis of reports between 1985 and 1994 (8) in which 11 NSAIDs (plus azapropazone) were ordered for their association with serious complications. The order of the NSAIDs, from least to most damaging, was 1-ibuprofen, 2-diclofenac, 3-diflunisal, 4-fenoprofen, 5-aspirin, 6-sulindac, 7-naproxen, 8-indomethacin, 9-piroxicam, 10-ketoprofen, and 11-tolmetin, with azapropazone last. (We have not included azapropazone in any of our subsequent analyses). Group 1 (see Table 1) contained all of the NSAIDs included in this analysis. This is consistent with the idea that NSAIDs produce serious GI complications by significantly inhibiting the activity of COX. Further comparison of the COX-1 selectivities of these compounds (Fig. 3) demonstrates that compounds associated with the greatest GI toxicity have the greatest COX-1 selectivity. These include tolmetin, indomethacin, ketoprofen (8), and, in particular, ketorolac. It is notable that we found ketorolac to be the most COX-1 selective of all of the NSAIDs we tested because this compound is ≈5× more gastrotoxic than other NSAIDs (25). Clearly, this is in keeping with the idea that COX-1 inhibition underlies the serious GI complications of NSAIDs; ketorolac is an extreme outlier both in our assay system and in epidemiological reports.
Figure 3.
Determinable log [IC80 ratio (WBA-COX-2/COX-1)] for all agents assayed (see Table 1). The “0 line” indicates equipotency, i.e., an IC80 ratio of 1. Italics indicate compounds with very low potency.
Because all of the compounds contained within group 1 have the potential to produce full inhibition of both COX-1 and COX-2, their associated risk of producing GI toxicity can be strongly influenced by dose. This can be readily appreciated by reference to Fig. 4. Here, we have displayed the extent of COX-1 inhibition produced by individual NSAIDs at concentrations that cause 80% inhibition of COX-2. This analysis essentially provides the answer to the important question, If a NSAID is used at levels sufficient to inhibit COX-2 by 80%, i.e., to produce some therapeutic effect, by how much will COX-1 be inhibited? As can be seen, the classical NSAIDs produce inhibitions of ≈80% or more. This implies that, even for a drug such as diclofenac, which is >4-fold selective for COX-2 in terms of IC80 values, therapeutically relevant selectivity will be very difficult to achieve; i.e., the concentration of diclofenac necessary to produce 80% inhibition of COX-2 will produce almost 70% inhibition of COX-1. To extend this line of reasoning, it is also clear that, when relative selectivities differ by only slight amounts, other variables, such as ingested dose and plasma half-life, will have a particular influence on NSAID toxicity (26). This may well be especially true for piroxicam, which we did not find in our assays to be notably COX-1-selective despite its well established GI toxicity. Piroxicam, however, has a much longer elimination half-life (30 to 70 h) (19) than other NSAIDs, and plasma half-life has been previously correlated with GI toxicity (27).
The second grouping of NSAIDs consists of preferential COX-2 inhibitors. In Fig. 3, we have classified these as compounds with between 5- and 50-fold selectivity for COX-2 over COX-1. Possibly more importantly, Fig. 4 implies that the selectivity of these compounds could be usefully exploited. For example, the concentrations of etodolac and meloxicam sufficient to inhibit COX-2 by 80% produce only 25% inhibition of COX-1. Despite the sparse epidemiological data, controlled trials [e.g., for meloxicam (28, 29)] show that these preferential compounds have an improved GI toxicity profile. It must be remembered, however, that increasing the dosage of these agents could readily increase GI toxicity due to inhibition of COX-1 because all of the compounds in this group are capable of inhibiting this isoform of COX (Fig. 1).
It is interesting that, in our assays, celecoxib was found to be a member of the preferential group of COX-2 inhibitors. This is in contrast to data derived by using recombinant human COX-1 and COX-2 from broken insect cells. In this system, celecoxib is between 155- and 3,200-fold selective for COX-2 over COX-1 (23). This difference may be attributable to the fact that celecoxib inhibition of both COX-1 and COX-2 is initially competitive with respect to substrate and is characterized by similar affinity for COX-1 and COX-2. There is a second, slow, time-dependent binding of celecoxib to COX-2 but not COX-1 that may well produce the selectivity seen in other assay systems (23). It is currently not clear why celecoxib does not demonstrate such selectivity in either the WBA or WHMA. It is unlikely that these assay systems in some way delay the time-dependent binding of celecoxib to COX-2. For instance, in the isolated human enzyme assays, this secondary binding takes place in seconds rather than minutes (23), and the WHMA assay included a preincubation period of 60 min, and the WBA included a 24-h incubation period.
Our data also reinforce the concept that compounds within group 3 that inhibit COX-2 with only a very weak activity against COX-1 will produce few serious GI complications when used in the general population. As is clear from both the direct inhibitor curves (Fig. 2) and the derived data (Figs. 3 and 4), these compounds produce very little effect on COX-1 and should have a large therapeutic window. There are preliminary reports that rofecoxib has a low GI toxicity, but, until appropriate comparative clinical trials have been completed, no firm conclusions can be drawn (30). Furthermore, it must be remembered that studies in animals (31) suggest that when used in the presence of existing GI damage, COX-2-selective inhibitors might slow the repair process in man due to reductions in the production of protective COX-2 products (32).
Group 4 contains weak inhibitors of COX-1 and COX-2 for which reliable data with regard to inhibition of COX-1 and COX-2 could not be derived. These compounds are not, therefore, displayed in Figs. 3 and 4. Clearly, however, the weak ability of the group 4 compounds to inhibit prostanoid production explains their general lack of, or very low, GI toxicity. Sodium salicylate, for example, only caused inhibition of prostanoid formation at concentrations far in excess of those achieved in vivo (13) and in accordance with its relatively low GI toxicity (33). As expected, this fourth group also contained nabumetone whereas its active metabolite, 6MNA (34), was a member of the first group. This classification is in accordance with the results of Patrigiani et al. (4) who found that oral dosing of nabumetone at 1 g per day for 7 days reduced COX-1 activity in the WBA by 70%. The plasma concentration of drug achieved with such dosing (34) would correlate with the activity of 6MNA but not nabumetone, which we report here. As a cautionary remark to other investigators, we would like to note that we also tested six additional samples of “6MNA” supplied from commercial sources. These all were found to be essentially inactive, with potencies in the various assay systems similar to that of nabumetone. Possibly such variations in supply may explain some of the confusion regarding the activity of and selectivity of nabumetone and 6MNA. We found nabumetone to be essentially inactive and 6MNA to be active with a selectivity at the IC80 values of 4.5-fold toward COX-1 (WBA).
In conclusion, we have conducted a full and careful in vitro analysis of COX-1/2 selectivities for a large range of NSAIDs and COX-2-selective compounds. The distribution of potencies of these agents as inhibitors of COX-1 relative to COX-2 supports our earlier premise (3) that inhibition of COX-1 underlies the gastrointestinal toxicity of NSAIDs.
Acknowledgments
T.D.W. holds a British Heart Foundation Lectureship (BS/95003), and J.A.M. is a Wellcome Career Development fellow. This work was supported by a grant from Boehringer Ingelheim.
ABBREVIATIONS
- NSAID
nonsteroidal anti-inflammatory drug
- COX
cyclo-oxygenase
- WBA
whole blood assay
- WHMA
William Harvey human modified whole blood assay
- Tx
thromboxane
- PGE2
prostaglandin E2
- 6MNA
6-methoxy-2-napthylacetic acid
- GI
gastrointestinal
References
- 1.Vane J R. Nat New Biol. 1971;231:232–239. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Vane J R, Bakhle Y S, Botting R M. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell J A, Akarasereenont P, Thiemermann C, Flower R J, Vane J R. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrignani P, Panara M R, Greco A, Fusco O, Natoli C, Iacobelli S, Cipollone F, Ganci A, Créminon, Maclouf J, et al. J Pharmacol Exp Ther. 1994;271:1705–1712. [PubMed] [Google Scholar]
- 5.Brideau C, Kargman S, Liu S, Dallob A L, Ehrich E W, Rodger I W, Chan C-C. Inflamm Res. 1996;45:68–74. doi: 10.1007/BF02265118. [DOI] [PubMed] [Google Scholar]
- 6.Young J M, Panah S, Satchawatcharaphong C, Cheung P S. Inflamm Res. 1996;45:246–253. doi: 10.1007/BF02259611. [DOI] [PubMed] [Google Scholar]
- 7.Patrigiani P, Panara M R, Sciulli M G, Santini G, Renda G, Patrono C. J Physiol Pharmacol. 1997;48:623–631. [PubMed] [Google Scholar]
- 8.Henry D H, Lim L L-Y, Garcia Rodriguez L A, Perez Gutthann S, Carson J L, Griffin M, Savage R, Logan R, Moride Y, Hawkey C, et al. Br Med J. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell J A, Belvisi M G, Akarasereenont P, Robbins R A, Kwon O J, Croxtall J, Barnes P J, Vane J R. Br J Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies N M, Anderson K E. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Brocks D R, Jamali F. Clin Pharmacokinet. 1994;26:259–274. doi: 10.2165/00003088-199426040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Rainsford K D, Velo G P. New Developments in Antirheumatic Therapy. Dordrecht, The Netherlands: Kluwer; 1989. [Google Scholar]
- 13.Insell P A. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: Health Professions Div.; 1996. pp. 617–657. [Google Scholar]
- 14.Davies N M. Clin Pharmacokinet. 1995;28:100–114. doi: 10.2165/00003088-199528020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Buckley M M-T, Brogden R N. Drugs. 1990;39:86–109. doi: 10.2165/00003495-199039010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Noble S, Balfour J A. Drugs. 1996;51:424–430. doi: 10.2165/00003495-199651030-00007. [DOI] [PubMed] [Google Scholar]
- 17.Davies N M, Anderson K E. Clin Pharmacokinet. 1997;32:268–293. doi: 10.2165/00003088-199732040-00002. [DOI] [PubMed] [Google Scholar]
- 18.Davis R, Brogden R N. Drugs. 1994;48:431–454. doi: 10.2165/00003495-199448030-00009. [DOI] [PubMed] [Google Scholar]
- 19.Olkkola K T, Brunetto A V, Mattila M J. Clin Pharmacokinet. 1994;26:107–120. doi: 10.2165/00003088-199426020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Davies N M, Watson M S. Clin Pharmacokinet. 1997;32:437–459. doi: 10.2165/00003088-199732060-00002. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell J A, Saunders M, Barnes P J, Newton R, Belvisi M G. Mol Pharmacol. 1997;51:907–912. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton L C, Mitchell J A, Tomlinson A M, Warner T D. FASEB J. 1999;13:245–251. doi: 10.1096/fasebj.13.2.245. [DOI] [PubMed] [Google Scholar]
- 23.Gierse J K, Koboldt C M, Walker M C, Seibert K, Isakson P C. Biochem J. 1999;339:607–614. [PMC free article] [PubMed] [Google Scholar]
- 24.Fries J F. J Clin Rheumatol. 1998;4:S11–S16. doi: 10.1097/00124743-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Rodriguez L A, Cattaruzzi C, Grazia Troncon M, Agostinis L. Arch Intern Med (Moscow) 1998;158:33–39. doi: 10.1001/archinte.158.1.33. [DOI] [PubMed] [Google Scholar]
- 26.McGettigan D, Henry D, Page J. In: Clinical Significance and Potential of Selective COX- 2 Inhibitors. Vane J R, Botting R, editors. London: William Harvey Press; 1998. pp. 617–657. [Google Scholar]
- 27.Henry D, Dobson A, Turner C. Gastroenterology. 1993;105:1078–1088. doi: 10.1016/0016-5085(93)90952-9. [DOI] [PubMed] [Google Scholar]
- 28.Hawkey C, Kahan A, Steinbruck K, Alegre C, Baumelou E, Begaud B, Dequeker J, Isomaki H, Littlejohn G, Mau J, et al. Br J Rheumatol. 1998;37:937–945. doi: 10.1093/rheumatology/37.9.937. [DOI] [PubMed] [Google Scholar]
- 29.Dequeker J, Hawkey C, Kahan A, Steinbruck K, Alegre C, Baumelou E, Begaud B, Isomaki H, Littlejohn G, Mau J, et al. Br J Rheumatol. 1998;37:946–951. doi: 10.1093/rheumatology/37.9.946. [DOI] [PubMed] [Google Scholar]
- 30.Hawkey C J. Lancet. 1999;353:307–314. doi: 10.1016/s0140-6736(98)12154-2. [DOI] [PubMed] [Google Scholar]
- 31.Reuter B K, Asfaha S, Buret A, Sharkey K A, Wallace J L. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCartney, S. A., Mitchell, J. A., Vojnovic, I., Farthing, M. J. G. & Warner, T. D. (1998) Aliment. Pharmacol. Ther., in press. [DOI] [PubMed]
- 33.Whittle B J R, Higgs G A, Eakins K E, Moncada S, Vane J R. Nature (London) 1980;284:271–273. doi: 10.1038/284271a0. [DOI] [PubMed] [Google Scholar]
- 34.Davies N M. Clin Pharmacokinet. 1997;33:403–416. doi: 10.2165/00003088-199733060-00001. [DOI] [PubMed] [Google Scholar]