Abstract
Hydrogels were the first biomaterials developed for human use. The state-of-the-art and potential for the future are discussed. Recently, new designs have produced mechanically strong synthetic hydrogels. Protein based hydrogels and hybrid hydrogels containing protein domains present a novel advance; such biomaterials may self-assemble from block or graft copolymers containing biorecognition domains. One of the domains, the coiled-coil, ubiquitously found in nature, has been used as an example to demonstrate the developments in the design of smart hydrogels. The application potential of synthetic, protein-based, DNA-based, and hybrid hydrogels bodes well for the future of this class of biomaterials.
Keywords: hydrogels, smart biomaterials, self-assembly, hybrid biomaterials, bionanotechnology
1. Introduction
Hydrogels are water-swollen polymeric materials that maintain a distinct three-dimensional structure. They were the first biomaterials designed for use in the human body [1,2]. Traditional methods of biomaterials synthesis include crosslinking copolymerization, crosslinking of reactive polymer precursors, and crosslinking via polymer-polymer reaction. These methods of hydrogel synthesis were limited in the control of their detailed structure. Due to side reactions the networks contain cycles, unreacted pendant groups, and entanglements. Other inadequacies of traditional hydrogels have been poor mechanical properties and slow or delayed response times to external stimuli [2].
Novel approaches in hydrogel design have revitalized this field of biomaterials research. New ideas on the design of hydrogels with substantially enhanced mechanical properties [3–6], superporous [7] and comb-type grafted hydrogels [8] with fast response times, self-assembling hydrogels from hybrid graft copolymers with property-controlling protein domains [9,10], and from genetically engineered triblock copolymers [11,12] are just a few examples of hydrogel biomaterials with a smart future.
What are the limitations for further developments of the basic science and in the applications of hydrogels? Let’s discuss individual groups of hydrogels and point out the possibilities for their development into intelligent materials with applications in therapeutics, sensors, microfluidic systems, nanoreactors, and interactive surfaces.
2. Synthetic (traditional) hydrogels
Traditional synthetic methods have produced numerous hydrogel materials with excellent properties, e.g. hydrogel implants [13] and soft contact lenses [14]. Previously, these synthetic pathways did not permit an exact control of chain length, sequence, and three-dimensional structure. Developments in controlled radical polymerization, such as atom transfer radical polymerization (ATRP), reversible addition-fragmentation transfer (RAFT) polymerization, and nitroxide-mediated polymerization [15] have provided the potential to produce macromolecules with a narrow molecular weight distribution. New catalysts (transition metal complexes) and novel experimental approaches (polymerization under vacuum or at low temperature) afford control of the polymerization of α-amino acid N-carboxyanhydrides and permit the production of well-defined synthetic polypeptides [16].
2.1 Hydrogels with excellent mechanical properties
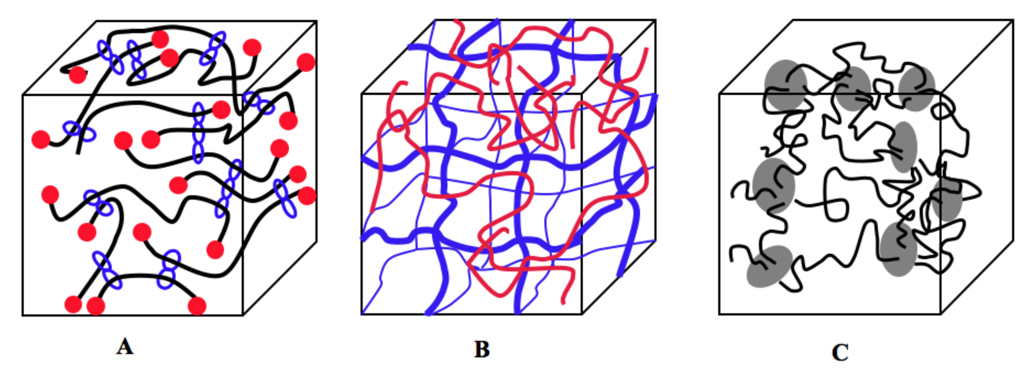
Recently, there have been several important developments, which have broadened the applicability of hydrogel materials. Three main approaches, namely introduction of sliding crosslinking agents [3], double network hydrogels [4], and nanocomposite (clay filled) hydrogels [5] have markedly improved the mechanical properties of hydrogels (Figure 1).
Figure 1.
Synthetic hydrogels with excellent mechanical properties. (A) Topological sliding hydrogel. α-Cyclodextrin moieties threaded on PEG chains, end-capped with a bulky group, are crosslinked by trichlorotriazine producing double ring crosslinks freely movable along the PRG chains (adapted from ref. [3]); (B) Double network hydrogels composed from two hydrophilic networks, one highly crosslinked, the other loosely crosslinked [4]; and (C) Nanocomposite (clay filled) hydrogels synthesized by radical polymerization of N-isopropylacrylamide in the presence of uniformly dispersed clay sheets (adapted from ref. [5]).
A new concept of chain crosslinking - sliding crosslinking agents - was introduced by Okamura and Ito [3]. By chemically crosslinking two cyclodextrin molecules, each threaded on a different PEG chain (end-capped with a bulky group, such as adamantan), a sliding double ring crosslinking agent was produced. This resulted in outstanding mechanical properties – a high degree of swelling in water and a high stretching ratio without fracture. The sliding crosslinks in these so called topological gels apparently equalized the tension along the polymer chains (“pulley effect”) [3].
Double networks (DN) are a subset of interpenetrating networks (IPNs) formed by two hydrophilic networks, one highly crosslinked, the other loosely crosslinked [4]. For example, a DN composed of two mechanically weak hydrophilic networks, poly(2-acrylamido-2-methylpropanesulfonic acid) and polyacrylamide, provides a hydrogel with outstanding mechanical properties. Hydrogels containing about 90% water possessed an elastic modulus of 0.3 MPa and fracture stress of ~10 MPa, demonstrating both hardness and toughness. This was explained by effective relaxation of locally applied stress and dissipation of crack energy through combination of the different structures and densities of the two networks [4].
Nanocomposite (clay filled) hydrogels are organic-inorganic hybrids. They are based on N-isopropylacrylamide (NIPAAm) with hectorite, [Mg5.34Li0.66Si8O20(OH)4]Na0.66, as multifunctional crosslinker [5]. Exfoliated clay platelets are uniformly dispersed in an aqueous medium containing the NIPAAm monomer; polyNIPAAm chains are grafted on the clay surface by one or two ends. Here, up to 1500% elongation-at-break values were obtained. It is interesting to note that mechanically robust hydrogels could only be prepared by radical polymerization of NIPAAm in the presence of clay. Mixing of polyNIPAAm with clay did not produce homogeneous hydrogels with robust mechanical properties. These hydrogels possessed extremely high surface hydrophobicities, as demonstrated by contact angle measurements. The main contributing factor was likely the alignment of N-isopropyl groups at the gel-air interface [6].
3. Stimuli-sensitive hydrogels
Some hydrogels undergo continuous or discontinuous changes in swelling that are mediated by external stimuli such as changes in pH, temperature, ionic strength, solvent type, electric and magnetic fields, light, and the presence of chelating species. The majority of stimuli responsive hydrogels were created using conventional (traditional) methods of synthesis of a relatively small number of synthetic polymers, especially (meth)acrylate derivatives and their copolymers. In 1968, Dušek and Paterson [17] theoretically predicted that changes in external conditions might result in abrupt changes of the hydrogel’s degree of swelling (phase transition). Indeed, ten years later, Tanaka et al. [18] and others [19] have verified the theory by experimental observations.
Lately, numerous polypeptide based responsive hydrogels have been designed, synthesized, and evaluated. These include hydrogels formed from block copolypeptides [20], recombinant segments of natural structural proteins like elastin [21] and silk [22], tandemly repeated silk-like and elastin-like peptide blocks [23], and recombinant triblock copolymers of a random polypeptide sequence flanked by two coiled-coil blocks [11,12].
In another promising approach, peptide and/or protein segments have been used to introduce degradability [24], temperature-induced phase transition [25], and sensitivity to the presence of biologically active molecules [26] into the hydrogel structure. Water-soluble synthetic polymers have been crosslinked with biological molecules, such as oligopeptides [24], oligodeoxyribonucleotides [27], stereospecific D,L-lactic acid oligomers [28], through antigen-antibody binding [26], or by intact native proteins [29].
Hydrogels containing functional proteins as a part of their structure have tremendous potential applications. Recently, calmodulin (CaM) was selected as the biological element in stimuli-responsive CaM-phenothiazine hydrogels [30]. Both the modified CaM and polymerizable phenothiazine were immobilized within the polymer network. In the presence of Ca2+, the immobilized phenothiazine derivative was bound to CaM, forming physical crosslinks within the hydrogel. Upon the removal of Ca2+, the immobilized phenothiazine derivative was released from the CaM binding site, and the CaM protein underwent a conformational change from a more constrictive conformation to its native conformation, which caused the hydrogel to swell. The potential to use this hydrogel as a valve in a microfluidic device was demonstrated [30].
Crosslinking of elastic protein, resilin, via dityrosine bonds produced hydrogels with a resiliency exceeding that of unfilled polybutadiene. In addition, the hydrogel displayed negligible hysteresis upon compression [31].
4. Self-assembled hydrogels
Hydrogels may self-assemble from block and graft copolymers driven by hydrophobic interactions, e.g. of A blocks in ABA triblock copolymers containing a hydrophilic B block capped with (short) hydrophobic A blocks [32,33]. Designing hydrogel-forming copolymers, using recognition motifs found in nature, such as coiled-coils, enhances the potential for the formation of precisely defined three-dimensional structures. The coiled-coil, a supercoil formed by two or more strands of α-helices [34], will be used as an example to demonstrate the potential for the design of well-organized hydrogel structures. The primary sequence of a typical coiled-coil is composed of 7-residue repeats, designated as heptads. The amino acid residues in a heptad are conventionally denoted as “a, b, c, d, e, f, g”. Hydrophobic residues at positions “a” and “d” form an inter-helical hydrophobic core, providing a stabilizing interface between the helices. Charged residues at positions “e” and “g” form electrostatic interactions, which contribute to coiled-coil stability and mediate specific association among helices (Figure 2). The versatility of this motif, especially the possibility to manipulate its stability and specificity by modifying the primary structure, bodes well for the successful design of a new class of hydrogel biomaterials.
Figure 2.
Coiled-coil protein domains. (A) Cartoon depicting the interaction of two heptads. The hydrophobic core is formed by the interactions of amino acid residues in positions a and d. (B) Helical wheel representation of two-stranded, antiparallel α-helical coiled-coils formed by the dimerization of CCE and CCK. The view is shown looking down the superhelical axis from the N-terminus of CCE and from the C-terminus of the CCK. CC denotes the coiled-coil peptides. E and K denote peptides in which most of the e and g positions are occupied by either glutamic acid or lysine, respectively. (C) Schematic illustration of the parallel or antiparallel orientation of two coiled-coil strands. (D) Primary structure of CCE and CCK. The sequences are written in the one-letter amino acid code. Positions a and d of the heptad repeats are underlined and form the hydrophobic core of the coiled-coil. N and C denote the amino and carboxy ends of the α-helix, respectively. Cartoons are from ocw.mit.edu/NR/rdonlyres/Biology.
4.1 Self-assembly from genetically engineered block copolymers
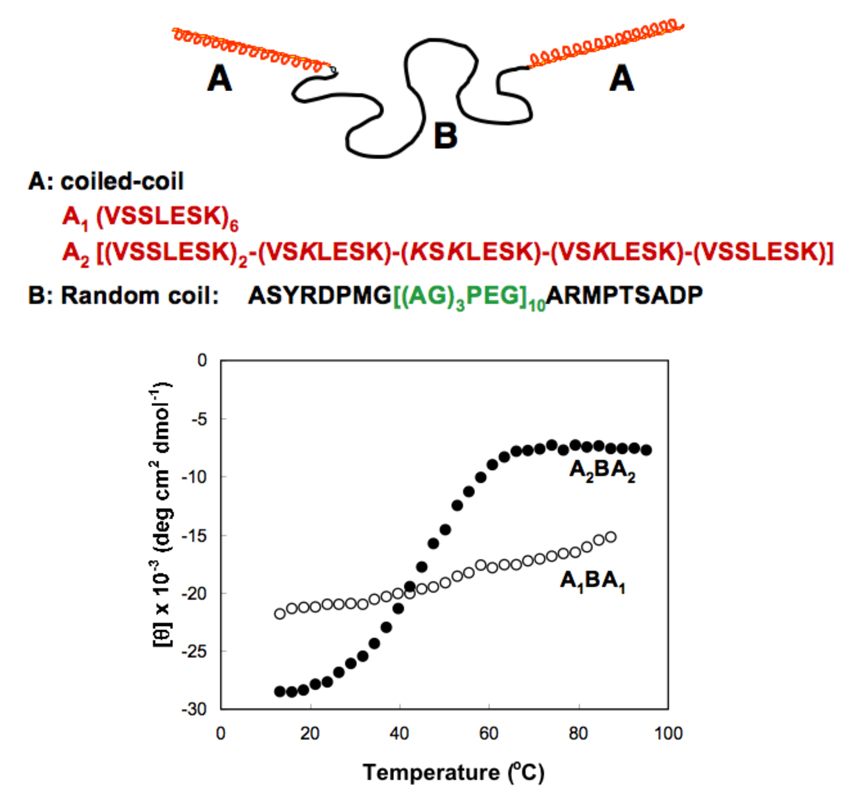
ABA block copolymers, where the block A is a coiled-coil forming peptide and block B a random coil, self-assemble into hydrogels [11,12,35,36]. The self-assembly occurs as a balance between the oligomerization of the helical ends and swelling of the central water-soluble polyelectrolyte segment. Consequently, temperature and/or pH-responsiveness may be achieved by manipulating the amino acid sequence of the coiled-coil domains. Minor modifications in their structure have a strong impact on the stimuli-sensitivity of self-assembled hydrogels. For example, the thermal stability of the coiled-coil containing proteins can be manipulated in a predictable way by substituting amino acids in the coiled-coil domain (Figure 3). An important observation was that these structures were reversible after denaturation [12]. Changes in environmental conditions (temperature, pH, removal of denaturing agent by dialysis) result in refolding of the a-helical structure. Experimental data clearly indicate that manipulation of the structure of the coiled-coil sequence in triblock copolymers, composed of two coiled-coil blocks flanking a random coil, permits rational design of reversible, stimuli-sensitive hydrogels.
Figure 3.
Temperature dependence of the secondary structures of the protein polymers. CD signal (ellipticity) at 222 nm as a function of temperature (modified from ref. [12]). In A2, K replaced V in the a position of the 4th heptad, and three additional K residues replaced S in the c positions of the 3rd, 4th, and 5th heptads (indicated by bold italics).
4.1 Hybrid systems
Hybrid hydrogels are usually referred to as hydrogel systems that possess components from at least two distinct classes of molecules, for example, synthetic polymers and biological macromolecules, interconnected either covalently or non-covalently [37]. Conjugation of peptide domains and synthetic polymers may lead to novel materials with properties superior to those of individual components. Compared to synthetic polymers, proteins and protein modules have well-defined and homogeneous structures, consistent mechanical properties, and cooperative folding/unfolding transitions. The peptide domain may impose a level of control over the structure formation at the nanometer level; the synthetic part may contribute to the biocompatibility of the hybrid material [38]. The synergistic combination of two types of structures may produce new materials that possess unprecedented levels of structural organization and novel properties [38]. In principle, the responsiveness of hydrogels may be directly related to the defined structure of the protein crosslinks. By optimizing the amino acid sequence, responsive hybrid hydrogels tailor-made for a specific application may be designed.
4.2.1 Crosslinking of polymer precursors with genetically engineered protein domains
A new strategy of hybrid hydrogel synthesis entails the non-covalent attachment of genetically engineered coiled-coil protein motifs to hydrophilic synthetic HPMA copolymer backbone. The physical crosslinking was established by self-assembly of the coiled-coil domains. A temperature-induced hydrogel collapse was observed that corresponded to the structural transition of the coiled-coil domains from an elongated helix to an unfolded state. Hydrogels formed by crosslinking of HPMA copolymer precursors with coiled-coil modules underwent dramatic volume transitions (de-swelling up to 10-fold) at the melting temperature of the coiled-coil modules [9]. This is a new temperature response mechanism for hydrogels that can be tuned over a wide temperature range by assembling gels with coiled-coils that have different melting temperatures [25]. These results seemed to indicate that the properties of a well-defined coiled-coil protein motif can be imposed onto a hybrid hydrogel containing synthetic polymer-based primary chains. Given the immense potential of tailoring material properties with genetically engineered proteins this strategy adds a new dimension to the field of “smart” hydrogel-based biomaterials [39].
4.2.2 Hydrogels self-assembled from graft copolymers via formation of coiled-coil antiparallel heterodimers
Recently, self-assembly of graft copolymers into hybrid hydrogels was demonstrated [10,40]. A novel hybrid hydrogel system based on HPMA copolymers consisted of a hydrophilic polymer backbone and a pair of oppositely charged peptide grafts [10]. Two distinct pentaheptad peptides (CCE and CCK) were designed to create a dimerization motif and serve as physical crosslinkers. Consequently, the graft copolymers, CCE-P (P is the HPMA copolymer backbone) and CCK-P, self-assembled into hybrid hydrogels in situ; the process was modulated by the formation of antiparallel heterodimeric coiled-coils (Figure 4). This approach possesses the advantage of decreased steric hindrance of the polymer backbone due to the “in-register” alignment of the peptide grafts. Equimolar mixtures of the graft copolymers, CCE-P/CCK-P, have been observed to self-assemble into hydrogels in PBS (phosphate buffer) solution at neutral pH at concentrations as low as 0.1 wt.%. The formation of these hybrid hydrogels was reversible [10].
Figure 4.
(A) Self-assembly of HPMA graft copolymers, CCK-P and CCE-P, containing oppositely charged peptide grafts (P is the HPMA copolymer backbone; see Figure 2 for structure of CCE and CCK). Aqueous solutions of CCE-P or CCK-P did not form gels. In contrast, gel-like materials were formed from equimolar mixtures of CCE-P/CCK-P at low concentrations. (B) pH dependence of the secondary structure of CCE, CCK, and equimolar mixture CCE/CCK expressed as the content of α-helix. (C) Microrgeology of 1% w/v solutions of CCE-P, CCK-P, and equimolar mixture of CCE-P/CCK-P. Mean square displacement of tracking particles as a function of time. (D) CD spectra of the equimolar mixture CCK-P/CCE-P before (full tilted squares) and after (empty triangles) denaturation by guanidine hydrochloride at 25 °C at a 20 µM peptide concentration and after removal of guanidine hydrochloride by dialysis (empty squares). Adapted from [10].
4.2.3 Phenomena to be studied
There is a critical need to study the major factors involved in self-assembly of hybrid hydrogels and to establish structural and physicochemical criteria for the formation of reproducible, reversible three-dimensional hydrogel networks with precisely defined structures. An important aspect to be evaluated is the kinetics of self-assembly [41]. Dynamic light scattering experiments in the pre-gelation period are a good start. Other factors to be studied are the homogeneity of crosslink distribution within the hydrogel structure, “in-register” alignment of oppositely charged peptide grafts, the impact of the presence of (therapeutic proteins) during self-assembly on the characteristics of the hydrogels, and viscoelastic properties of the networks. Microrheology, NMR, CD, FRET, and other physicochemical techniques may provide the preliminary insight into the details of hydrogel structure and into structure – property relationships.
Also, the problems related to mixing of two components (CCK-P and CCE-P) are important. If graft copolymers contain coiled-coil forming sequences with a high degree of biorecognition (high binding constant), then the mixing may not be random. The formation of the first heterodimer, before the thorough mixing of the graft copolymers, may decrease the probability that additional grafts on the same macromolecule will form heterodimers. To evaluate this phenomenon, the approach previously used for genetically engineered block copolymers could be adopted. Dissolving block copolymers using conditions when the α-helical sequences are denatured, followed by the removal of the denaturing agent (guanidium chloride) by dialysis, revealed that hydrogels self-assembled (due to refolding of the α-helices) at lower polymer concentrations when compared to dissolving the polymers in water or PBS [42].
4.3 Hydrogels whose self-assembly was mediated by DNA recognition
DNA sequences have been frequently used as biorecognition motifs in the design of new biomaterials. Just a few examples follow to illustrate this rapidly developing field. Biorecognition of complementary oligonucleotides grafted on a hydrophilic polymer backbone mediated self-assembly of graft copolymers into hydrogels [27]. In another design, hydrogels were synthesized by copolymerizing acrylamide with dimethacryloylated 5′,3′- diaminoalkyl-modified (single strand) ssDNAs as crosslinking agents [43]. DNA motifs were used to assembly nanoparticles into macroscopic materials [44,45], and dendrimers into hydrogels [46]. An interesting approach is the use of an enzyme-catalyzed process to mediate hydrogel formation. Branched DNA molecules containing complementary sticky ends with palindromic sequences were hybridized, followed by ligation catalyzed with T4 DNA ligase [47]. DNA may impose changes in the supramolecular structure of hybrid copolymers. A diblock copolymer, ssDNA-b-polypropyleneoxide, forms spherical micelles in aqueous solutions. Using molecular recognition mediated by long DNA templates, these micelles can be transformed into amphiphilic rods [48].
5. Applications of hydrogels
Potential applications of all the types of hydrogels discussed above include: tissue engineering, synthetic extracellular matrix, implantable devices, biosensors, separation systems (valves to control permeability across porous membranes, or materials for affinity separations based on the specific recognition of monomeric strands), materials controlling the activity of enzymes, phospholipid bilayer destabilizing agents, materials controlling reversible cell attachment, nanoreactors with precisely placed reactive groups in three-dimensional space, smart microfluidics with responsive hydrogels, and energy-conversion systems [49–56].
The fact that the self-assembly of block and graft copolymers into hydrogels takes place in aqueous environments at physiological conditions provides opportunities to develop delivery systems for molecules which are sensitive to, or incompatible with, organic solvents. The goal is to develop in-situ self-assembling hydrogels suitable for the delivery of a wide range of biologically active compounds.
6. Conclusions and future prospects
In conclusion, the direction of where smart hydrogels will go in the future should be addressed. Yes, all scientific evidence seems to indicate that basic and translational research in hydrogels has a bright future. Numerous new designs, e.g. involving protein domains containing non-canonical amino acids [57], successful attempts to control the morphology of self-assembling peptide fibers [58], artificial glycoproteins for controlling cell responses [59], hydrogels as the building material for microchemotaxis devices [60], enhanced use of DNA recognition motifs [61,62], and improved synthetic methods [63] demonstrate the versatility of the hybrid hydrogel approach. An outstanding example of the potential of stimuli-sensitive hydrogels in the development of bionanotechnology products is the design of optical systems that do not require mechanical components. Jiang’s laboratory developed a tunable liquid lens that permits autonomous focusing. The design was based on a temperature-sensitive hydrogel integrated into a microfluidic system [64].
Obviously, there will be set-backs on the way forward [65], but the scientific and translational potential of hydrogel biomaterials makes me confident in predicting a smart future.
Acknowledgments
The hydrogel research in author‘s laboratory was supported in part by NIH grants EB00251 and EB005288. I thank my coworkers for their contributions as reflected in the references, Dr. Jiyuan Yang for valuable discussions and help with the artwork, and Jon Callahan for carefully revising the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wichterle O, Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 2.Kopeček J, Yang J. Hydrogels as smart materials. Polym Int. 2007 [Google Scholar]
- 3.Okumura Y, Ito K. The polyrotaxane gels: a topological gel by figure-of-eight cross-links. Adv Mat. 2001;13:485–487. [Google Scholar]
- 4.Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mat. 2003;15:1155–1158. [Google Scholar]
- 5.Haraguchi K, Takehisa T. Nanocomposite hydrogels: a unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv Mat. 2002;14:1120–1124. [Google Scholar]
- 6.Haraguchi K, Li HJ, Okumura N. Hydrogels with hydrophobic surfaces: Abnormally high contact angles for water on PNIPA nanocomposite hydrogels. Macromolecules. 2007;40:2299–2302. [Google Scholar]
- 7.Chen J, Park H, Park K. Synthesis of superporous hydrogels: hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Comb-type grafted hydrogels with rapid de-swelling response to temperature changes. Nature. 1995;374:240–242. [Google Scholar]
- 9.Wang C, Stewart RJ, Kopeček J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature. 1999;397:417–420. doi: 10.1038/17092. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Xu C, Wang C, Kopeček J. Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules. 2006;7:1187–1195. doi: 10.1021/bm051002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Breedveld V, Kopeček J. Reversible hydrogels from self-assembling genetically engineered block copolymers. Biomacromolecules. 2005;6:1739–1749. doi: 10.1021/bm050017f. [DOI] [PubMed] [Google Scholar]
- 13.Voldřich Z, Tománek Z, Vacík J, Kopeček J. Long-term experience with the poly(glycol monomethacrylate) gel in plastic operations of the nose. J Biomed Mater Res. 1975;9:675–685. doi: 10.1002/jbm.820090612. [DOI] [PubMed] [Google Scholar]
- 14.Nicolson PC, Vogh J. Soft contact lens polymers: an evolution. Biomaterials. 2001;22:3273–3283. doi: 10.1016/s0142-9612(01)00165-x. [DOI] [PubMed] [Google Scholar]
- 15.Matyjaszewski K, Davis TP, editors. Handbook of radical polymerization. New York: John Wiley; 2002. [Google Scholar]
- 16.Deming TJ. Polypeptide and polypeptide hybrid copolymer synthesis via NCA polymerization. Adv Polym Sci. 2006;202:1–18. [Google Scholar]
- 17.Dušek K, Patterson D. Transition in swollen polymer networks induced by intramolecular condensation. J Polym Sci Part A-2. 1968;6:1209–1216. [Google Scholar]
- 18.Tanaka T. Collapse of gels and the critical endpoint. Phys Rev Lett. 1978;40:820–823. [Google Scholar]
- 19.Hrouz J, Ilavský M, Ulbrich K, Kopeček J. The photoelastic behaviour of dry and swollen networks of poly(N,N-diethylacrylamide) and of its copolymers with N-tert.butylacrylamide. Europ Polym J. 1981;17:361–366. [Google Scholar]
- 20.Nowak AP, Breedveld V, Pakstis L, Ozbas B, Pine DJ, Pochan D, Deming TJ. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417:424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 21.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J Phys Chem B. 1997;101:11007–11028. [Google Scholar]
- 22.Prince JT, McGrath KP, DiGirolamo CM, Kaplan DL. Construction, cloning, and expression of synthetic genes encoding spider dragline silk. Biochemistry. 1991;281:10879–10885. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- 23.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv Drug Deliv Rev. 2002;54:1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 24.Ulbrich K, Strohalm J, Kopeček J. Polymers containing enzymatically degradable bonds. 6. Hydrophilic gels cleavable by chymotrypsin. Biomaterials. 1982;3:150–154. doi: 10.1016/0142-9612(82)90004-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Kopeček J, Stewart RJ. Hybrid hydrogels crosslinked by genetically engineered coiled-coil block proteins. Biomacromolecules. 2001;2:912–920. doi: 10.1021/bm0155322. [DOI] [PubMed] [Google Scholar]
- 26.Miyata T, Asami T, Uragami T. A reversibly antigen-responsive hydrogel. Nature. 1999;399:766–769. doi: 10.1038/21619. [DOI] [PubMed] [Google Scholar]
- 27.Nagahara S, Matsuda T. Hydrogel formation via hybridization of oligonucleotides derivatized in water-soluble vinyl polymers. Polymer Gels Networks. 1996;4:111–127. [Google Scholar]
- 28.De Jong SJ, De Smedt SC, Wahls MWC, Demeester J, Kettenes-van den Bosch JJ, Hennink WE. Novel self-assembled hydrogels by stereocomplex formation in aqueous solution of enantiomeric lactic acid oligomers grafted to dextran. Macromolecules. 2000;33:3680–3686. [Google Scholar]
- 29.Tada D, Tanabe T, Tachibana A, Yamauchi K. Drug release from hydrogel containing albumin as crosslinker. J Biosci Bioeng. 2005;100:551–555. doi: 10.1263/jbb.100.551. [DOI] [PubMed] [Google Scholar]
- 30.Ehrick JD, Deo SK, Browning TW, Bachas LG, Madou MJ, Daunert S. Genetically engineered protein in hydrogels tailors stimuli-responsive characteristic. Nature Mater. 2005;4:298–302. doi: 10.1038/nmat1352. [DOI] [PubMed] [Google Scholar]
- 31.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 32.Tae G, Kornfeld JA, Hubbell JA. Sustained release of human growth hormone from in situ forming hydrogels using self-assembly of fluoroalkyl-ended poly(ethylene glycol) Biomaterials. 2005;26:5259–5266. doi: 10.1016/j.biomaterials.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 33.Serero Y, Aznar R, Porte G, Berret JF, Calvet D, Collet A, Viguier M. Associating polymers: From ,,flowers“ to transient networks. Phys Rev Lett. 1998;81:5584–5587. [Google Scholar]
- 34.Mason JM, Arndt KM. Coiled coil domains: Stability, specificity, and biological implications. ChemBioChem. 2004;5:170–176. doi: 10.1002/cbic.200300781. [DOI] [PubMed] [Google Scholar]
- 35.Shen W, Zhang K, Kornfeld JA, Tirrell DA. Tunig the erosion rate of artificial protein hydrogels through control of network topology. Nature Mater. 2006;5:153–158. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 36.Shen W, Kornfeld JA, Tirrell DA. Structure and mechanical properties of artificial protein hydrogels assembled through aggregation of leucine zipper domains. Soft Matter. 2007;3:99–107. doi: 10.1039/b610986a. [DOI] [PubMed] [Google Scholar]
- 37.Kopeček J, Tang A, Wang C, Stewart RJ. De novo design of biomedical polymers. Hybrids from synthetic macromolecules and genetically engineered protein domains. Macomol Symp. 2001;174:31–42. [Google Scholar]
- 38.Vandermeulen GWM, Klok HA. Peptide/protein hybrid materials: Enhanced control of structure and improved performance through conjugation of biological and synthetic polymers. Macromol Biosci. 2004;4:383–398. doi: 10.1002/mabi.200300079. [DOI] [PubMed] [Google Scholar]
- 39.Kopeček J. Smart and genetically engineered biomaterials and drug delivery systems. Eur J Pharm Sci. 2003;20:1–16. doi: 10.1016/s0928-0987(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Xu C, Kopečková P, Kopeček J. Hybrid hydrogels self-assembled from HPMA copolymers containing peptide grafts. Macromol Biosci. 2006;6:201–209. doi: 10.1002/mabi.200500208. [DOI] [PubMed] [Google Scholar]
- 41.Kopeček J. Swell gels. Nature. 2002;417:388–391. doi: 10.1038/417388a. [DOI] [PubMed] [Google Scholar]
- 42.Xu C, Kopeček J. Genetically engineered block copolymers: Imfluence of the length and structure of the coiled-coil block on hydrogel self-assembly. Pharmaceutical Res. doi: 10.1007/s11095-007-9343-z. submitted. [DOI] [PubMed] [Google Scholar]
- 43.Murakami Y, Maeda M. DNA-responsive hydrogels that can shrink or swell. Biomacromolecules. 2005;6:2927–2929. doi: 10.1021/bm0504330. [DOI] [PubMed] [Google Scholar]
- 44.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles inot macroscopic materials. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 45.Milam VT, Hiddessen AL, Crocker JC, Graves DJ, Hammer DA. DNA_driven assembly of biodisperse, micron-sized colloids. Langmuir. 2003;19:10317–10323. [Google Scholar]
- 46.Starr FW, Sciortino F. Model for assembly and gelation of four-armed DNA dendrimers. J Phys Condens Matter. 2006;18:L347–L353. doi: 10.1088/0953-8984/18/26/L02. [DOI] [PubMed] [Google Scholar]
- 47.Um SH, Lee JB, Kwon SY, Umbach CC, Luo D. Enzyme-catalyzed assembly of DNA hydrogel. Nature Mater. 2006;5:797–801. doi: 10.1038/nmat1741. [DOI] [PubMed] [Google Scholar]
- 48.Ding K, Alemdaroglu FE, Börsch M, Berger R, Herrmann A. Engineering the structural properties of DNA block copolymer micelles by molecular recognition. Angew Chem Int Ed. 2007;46:1172–1175. doi: 10.1002/anie.200603064. [DOI] [PubMed] [Google Scholar]
- 49.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–1360. [Google Scholar]
- 50.Eddington DT, Beebe DJ. Flow control with hydrogels. Adv Drug Deliv Rev. 2004;56:199–210. doi: 10.1016/j.addr.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opinion Biotechnol. 2003;14:551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 53.Nakabayashi N, Williams DF. Preparation of non-thrombogenic materials using 2-methacryloyloxyethyl phosphorylcholine. Biomaterials. 2003;24:2431–2435. doi: 10.1016/s0142-9612(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 54.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 55.Varghese S, Elisseeff JH. Hydrogels for musculoskeletal engineering. Adv Polym Sci. 2006;203:95–144. [Google Scholar]
- 56.Nayak S, Lyon LA. Soft nanotechnology with soft nanoparticles. Angew Chem Int Ed. 2005;44:7686–7708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]
- 57.Link JA, Mock ML, Tirrell DA. Non-canonical amino acids in protein engineering. Curr Opin Chem Biol. 2003;14:603–609. doi: 10.1016/j.copbio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Ryadnov MG, Woolfson DN. Engineering the morphology of a self-assembling protein fibre. Nature Mater. 2003;2:329–332. doi: 10.1038/nmat885. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi N, Zhang L, Chae BS, Pala CS, Furst EM, Kiick K. Growth factor mediated assembly of cell receptor-responsible hydrogels. J Am Chem Soc. 2007;129:3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng SY, Heilman S, Wasserman M, Archer S, Shuler ML, Wu M. A hydrogel-based microfluidic device for the studies of directed cell migration. Lab on a Chip. 2007 doi: 10.1039/b618463d. [DOI] [PubMed] [Google Scholar]
- 61.Seeman NC, Belcher AM. Emulating biology: Building nanostructures from the bottom up. Proc Natl Acad Sci USA. 2002;99 suppl. 2:6451–6455. doi: 10.1073/pnas.221458298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Condon A. Designed DNA molecules: principles and applicatins of molecular nanotechnology. Neture Rev Genetics. 2006;7:565–575. doi: 10.1038/nrg1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayres L, Vos MRJ, Adams PJHM, Shklyarevskiy, van Hest JCM. Elastin-based side-chain polymers synthesized by ATRP. Macromolecules. 2003;36:5967–5973. [Google Scholar]
- 64.Dong L, Agarwal AK, Beebe DJ, Jiang H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature. 2006;442:551–553. doi: 10.1038/nature05024. [DOI] [PubMed] [Google Scholar]
- 65.Check E. Scientists rethink approach to HIV gels. Nature. 2007;446:12. doi: 10.1038/446012a. [DOI] [PubMed] [Google Scholar]