Abstract
The mouse Slc39a8 gene encodes the ZIP8 transporter, which has been shown to be a divalent cation/HCO3− symporter. Using ZIP8 cRNA-injected Xenopus oocyte cultures, we show herein that: [a] ZIP8-mediated cadmium (Cd2+) and zinc (Zn2+) uptake have Vmax values of 1.8 ± 0.08 and 1.0 ± 0.08 pmol/oocyte/hour, and Km values of 0.48 ± 0.08 and 0.26 ± 0.09 μM, respectively; [b] ZIP8-mediated Cd2+ uptake is most inhibited by Zn2+, second-best inhibited by Cu2+, Pb2+ and Hg2+, and not inhibited by Mn2+ or Fe2+; and [c] electrogenicity studies demonstrate an influx of two HCO3− anions per one Cd2+ (or one Zn2+) cation, i.e. electroneutral complexes. Using Madin-Darby canine kidney (MDCK) polarized epithelial cells retrovirally-infected with ZIP8 cDNA and tagged with hemagglutinin at the C-terminus, we show that—similar to ZIP4—the ZIP8 eight-transmembrane protein is largely internalized during Zn2+ homeostasis, but moves predominantly to the cell surface membrane (trafficking) under conditions of Zn2+ depletion.
INTRODUCTION
Cadmium (Cd, Cd2+,) is a non-essential metal. Due to the growth of industrialization, the amount of Cd in our environment has increased dramatically. In combination with longer life expectancy, the rising levels of environmental Cd have teamed up to enhance the body's Cd burden: for example, the average renal accumulation of Cd in a cigarette smoker who has smoked at least two packs a day for 40 years is close to the threshold (∼30 μg/g wet weight of kidney) that is sufficient for causing overt renal failure (http://www.trace-elements.org.uk/cadmium.htm).
Cd has been classified by IARC as a “Category I” human lung carcinogen. Individuals at highest risk for Cd-induced lung cancer and chronic renal disease include cigarette smokers, those on a steady diet that is rich in high-fiber foods or contaminated shellfish, women having low body-iron stores, and malnourished populations [1-4].
In acute doses, Cd has been known for 80 years to cause damage to the central nervous system, lung, bone, gastrointestinal tract, liver, ovary, testis, placenta, and developing embryo [5; 6]. Chronic exposure to low Cd doses can cause renal proximal tubular metabolic acidosis and osteomalacia (renal Fanconi syndrome); Cd is eliminated very slowly from the body and thus accumulates as a total body burden—predominantly in the kidney—with age.
For many decades, Cd influx into mammalian cells has been assumed to take place before Cd-mediated disease occurs. Cd uptake by mammalian cultured cells has been shown to use Ca2+ channels [7-10]. SLC11A2 (DMT1) shows a preference for Fe2+, but also transports Pb2+ and Cd2+ [11] with protons [11]. Using SLC11A2 knockdown in human intestinal Caco-2 cells [12], proton-dependent Cd transport was demonstrated. Additional reports point out that SLC11A2 participates in Cd transport in renal distal tubular cells [13; 14] and in enterocytes [13; 15; 16]. Cd transport in Xenopus oocytes expressing human SLC11A2 shows Michaelis-Menten kinetics with a Km of 1.04 ± 0.13 μM [17].
Recent studies have shown for the first time a relationship between a specific genotype (allelic differences in the mouse Slc39a8 gene encoding the ZIP8 transporter) and specific phenotypes (risk of Cd-induced testicular necrosis; acute renal failure) [18; 19]. The ZIP8 transporter, which can be hijacked by Cd, undoubtedly transports one or more essential divalent cation(s) important in carrying out critical life functions. In mammalian cell culture, manganese (Mn) was shown to be the best inhibitor of ZIP8-mediated Cd uptake and has a low Km (2.2 μM) for ZIP8-mediated uptake [20]; however, zinc (Zn) could not be ruled out as another essential divalent cation that uses ZIP8, because of multiple interfering receptors known to be on the surface of mammalian cells.
ZIP8 is a Cd2+ or Mn2+/HCO3− symporter and has been localized to the apical surface of two cell types [20]: between the blood and vascular endothelial cells of the testis [18; 19], and between the glomerular filtrate and proximal tubular epithelial cells of the kidney [19]. In the present study, we examine the kinetics and electrogenicity of ZIP8-mediated Cd2+/HCO3− and Zn2+/HCO3− uptake in Xenopus oocytes, as well as trafficking of the ZIP8 protein in MDCK cultured cells as a function of extracellular Zn2+ concentration.
MATERIALS and METHODS
Chemicals
All divalent cations were purchased as chloride or acetate salts from Fisher Scientific (Pittsburgh, PA). Tetramethylammonium (TMA+) chloride, collagenase, and Chelex 100 were bought from Sigma (St. Louis, MO). 109CdCl2 and 65ZnCl2 have been described [20]. The ND-96 uptake medium (96 mM NaCl, 2.0 mM KCl, 1.0 mM MgCl2, 1.8 mM CaCl2, and 5.0 mM HEPES, pH 7.5) was prepared in our laboratory.
Cell cultures
Madin-Darby canine kidney (MDCK) cells cultured in Dulbecco's modified Eagle's medium (DMEM) has been described [18; 20].
Preparation of the ZIP8 cDNA-containing vector
Generation of this vector has been described [18].
Generation of ZIP8 capped RNA (cRNA) from cDNA-containing vector
The ZIP8 cDNA described above was excised from the pBluescript vector, using Bam HI and Sal I sites and ligated into the multiple-cloning site of pXFRM [21; 22], a specific Xenopus vector which was a generous gift of William F. Marzluff (University of North Carolina, Chapel Hill). For in vitro transcription, the plasmid was linearized with BspQ I. The cRNA was transcribed in vitro from the linearized cDNA template using the mMESSAGE mMACHINE SP6 kit (Ambion, Austin, TX), according to manufacturer's instructions. The size of the purified transcription product and its quantity were evaluated by gel electrophoresis. The cRNA was dissolved in RNase-free water and stored at −80°C until use.
Microinjection of cRNA into Xenopus oocytes
All frog experiments were approved by, and conducted in accordance with, the National Institutes of Health standards for the care and use of experimental animals and the University Cincinnati Medical Center Institutional Animal Care and Use Committee. Preparation of the Xenopus oocytes and cRNA microinjection were carried out exactly as described [23]. All assays were carried out 3 days after cRNA microinjection. Incubation medium was maintained at 22°C (pH 7.5), with five to ten oocytes per time-point, or per concentration-point. Protein content varies so much among oocytes (range = 266 to 1250 μg/oocyte); thus, all kinetics data are expressed “per oocyte”.
Divalent Cation Uptake and Km Determination
ND-96 medium always included 25 mM NaHCO3− (pH 7.5). Oocytes grown in ND-96 were otherwise treated with radiolabeled Cd2+ or Zn2+ as previously described [20].
Inhibition of Cd uptake by other metal ions
The competing divalent cation was added to the ND-96 medium simultaneously with addition of 0.25 μM Cd, but at concentrations 3, 10 or 30 times greater than that of Cd. Due to precipitation problems, Hg2+ and Pb2+ studies were carried out in ND-96 in which Cl− was replaced by gluconate ion. Fe2+ competition studies were performed in ND-96 having 1 mM ascorbic acid—in order to maintain reduced Fe2+. Incubation of the oocytes was for 60 min, following which oocytes were washed, and radioactivity determined.
Electrogenicity
The modified ND-96 medium (containing 25 mM NaHCO3−) was adjusted with TMA+ to maintain a constant 98-mM isosmolar replacement of monovalent cations: if K+ = 60 mM, then Na+ = 38 mM; if K+ = 20 mM, then Na+ = 38 mM and TMA+ = 40 mM; if K+ = 2 mM, then Na+ = 38 mM and TMA+ = 58 mM; if K+ = 1 mM, then Na+ = 38 mM and TMA+ = 59 mM). Cd or Zn uptake for 30 min by ZIP8 cRNA-injected oocytes was compared with that by water-injected oocytes at 1, 2, 20 and 60 mM K+ concentrations.
Delivery of the ZIP8 cDNA into MDCK Tet-off cells
Generation of the stable retrovirus-infected rvMDCK-LUC (which carries the non-ZIP luciferase cDNA control), rvMDCK-ZIP4ha, and rvMDCK-ZIP8ha cell lines has been described [20].
Detection of ZIPha protein levels on the cell surface
Antibody-detectable amounts of ZIP4ha and ZIP8ha on the cell surface were assessed by measuring the levels of anti-ha antibodies bound to the outside of rvMDCK-ZIP4ha and rvMDCK-ZIP8ha cells. These cells, plus rvMDCK-LUC control cells, were assessed by procedures detailed previously [24].
Statistical Analysis
Statistical significance between groups was determined a 4-way Student's t test. Statistical analyses were performed with the use of SAS statistical software (SAS Institute Inc., Cary, NC). The determinations of Km and Vmax values for ZIP-mediated metal uptake were determined using Sigma Plot (SPSS Inc., Chicago, IL).
RESULTS AND DISCUSSION
Cd and Zn uptake kinetics
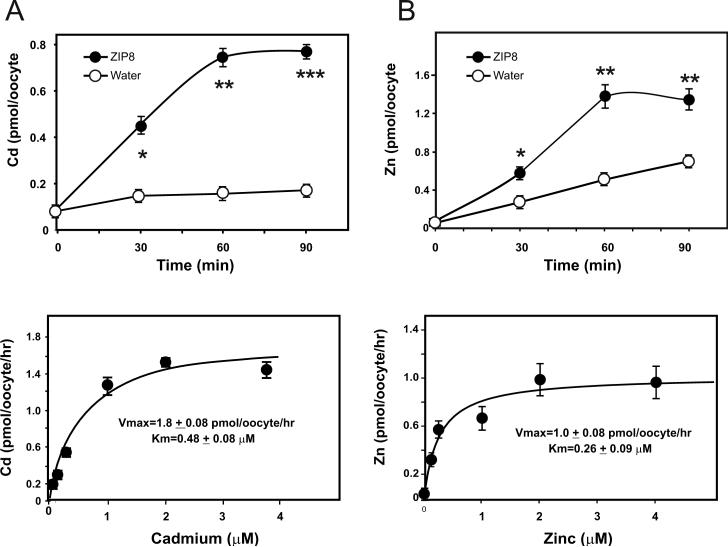
In Xenopus oocytes, we found that ZIP8-mediated Cd uptake was linear over 60 min of incubation time (Fig. 1A, top). We therefore used the 60-min time-point for examining Michaelis-Menten kinetics (Fig. 1A, bottom). Vmax values were estimated to be 1.8 ± 0.08 pmol/oocyte/hr, with a Km of 0.48 ± 0.08 μM. This Km value is ∼25% lower than the Km value of 0.62 μM found in MFF cultures in Hank's balanced salt solution (HBSS) medium [20]; as is commonly the case, Km values in Xenopus oocytes are lower than those in mammalian cell cultures—due to fewer numbers of interfering transporters on the Xenopus cell surface. Moreover, this Km value of 0.48 μM for ZIP8-mediated Cd transport in frog oocytes is at least twice lower than that of 1.04 μM reported for Cd transport in Xenopus oocytes expressing human SLC11A2 [17]. Clearly, human populations are usually exposed to environmental Cd levels that are extremely low, and Cd most likely would be transported by the system having the lowest Km for that metal. We therefore conclude that the ZIP8 transporter is more relevant than SLC11A2 for the influx of Cd from our environment.
FIG. 1.
Kinetics of cation uptake in Xenopus laevis oocytes. A, top, Cd uptake as a function of time in ZIP8 cRNA-injected versus water-injected oocytes. *P <0.01. **P <0.001. At bottom, Cd uptake as a function of its concentration in ZIP8 cRNA-injected minus water-injected oocytes; incubation time was 60 min. B, top, Zn uptake in ZIP cRNA-injected versus water-injected oocytes. *P <0.05. **P <0.01. At bottom, Zn uptake, determined as described in part A. Circles and brackets at top denote means ± S.E. Vmax and Km values are expressed as means ± S.E. Circles and brackets at bottom denote means ± S.D.
ZIP8-mediated Zn uptake was also linear over 60 min (Fig. 1B, top) and also demonstrated Michaelis-Menten kinetics (Fig. 1B, bottom), with a Vmax value of 1.0 ± 0.08 pmol/oocyte/hr and a Km of 0.26 ± 0.09 μM. Interestingly, Zn uptake in water-injected oocytes over the 90-min incubation period was about 4-fold greater than Cd uptake in water-injected oocytes; this finding suggests the existence of additional Zn transporters on the Xenopus oocyte cell surface that do cause some degree of interference. The Km value for Zn was almost twice lower than that for Cd, indicating that Zn is indeed an endogenous very-high-affinity substrate for ZIP8. As stated above, we were unable to determine a valid Km value for Zn in MFF cultures in HBSS medium [20]—most likely due to the excess numbers of interfering transporters on the mammalian cell surface.
Metal-mediated competitive inhibition of Cd uptake
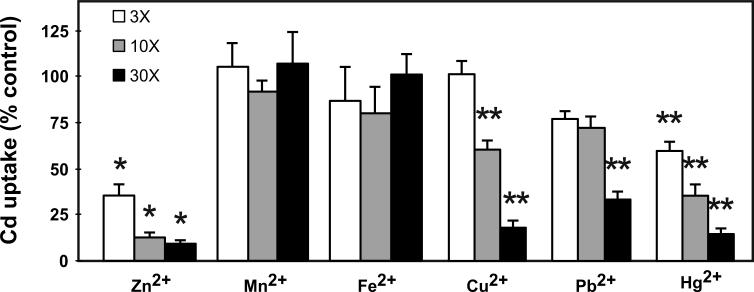
Studying six metals in Xenopus oocytes (Fig. 2), we found that Zn was the best inhibitor of ZIP8-mediated Cd influx. Curiously, Mn had been found to be a strong competitor of ZIP8-mediated uptake in MFF cultures in HBSS [20], whereas it was not a significant inhibitor in Xenopus oocytes; we have no explanation for this discrepancy.
FIG. 2.
Metal cation competition for Cd uptake in Xenopus oocytes. Non-labeled Cd was spiked with 109CdCl2 to make a final Cd concentration of 0.25 μM; the competing metal cations at concentrations of 0, 0.75, 2.5 or 7.5 μM were added at the same time as the Cd, and the oocytes were incubated at 20°C for 60 min, following which Cd accumulation was determined. *P <0.001. **P < 0.01).
Fe2+ also showed no significant inhibition of ZIP8-mediated Cd influx in frog oocytes (Fig. 2). Evolutionarily, the nearest neighbor to the Slc39a8 gene is the Slc39a14 gene, encoding ZIP14; this transporter has also been demonstrated to be a Cd2+/HCO3− symporter [25]. There was a recent study [26] indicating that ZIP14 mediates non-transferrin-bound Fe2+ into human HEK 293H cells, Sf9 insect cells, and AML12 mouse hepatocyte cultures. However, we did not find that Fe2+ competes for ZIP14-mediated Cd influx in MFF cultures [25].
Cu2+, Pb2+ and Hg2+ were also significant inhibitors of Cd uptake by ZIP8. There were specific reasons for testing these three metals. Why Cu2+? Whereas Zn was found to be the best competitive inhibitor of ZIP14-mediated Cd uptake, Cu2+ along with Mn2+ were tied for the second best inhibitor [25]. Why Hg2+ and Pb2+? The ZIP8 protein has been demonstrated to be localized on the apical surface of renal proximal tubular epithelial cells, and to participate in Cd uptake and Cd-mediated renal failure [19]. Along with Cd—Hg and Pb are known to cause renal proximal tubular acidosis (human renal Fanconi syndrome); the only other metals known to cause this human disease are uranium and platinum [27].
Electrogenicity studies
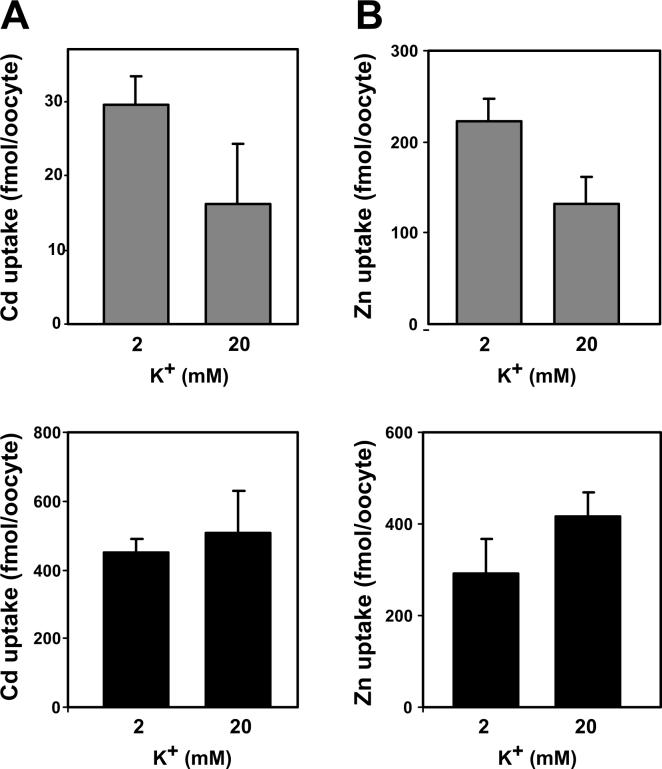
No significant difference in Cd uptake was seen in water-injected Xenopus oocytes, comparing 2 mM with 20 mM K+ in the incubation medium (Fig. 3A, top). Likewise, ZIP8-mediated Cd influx (Fig. 3A, bottom) was not statistically significantly different between 2 mM and 20 mM extracellular K+. We also found no difference in Cd uptake by water-injected oocytes at 1 versus 60 mM K+, nor did we find a difference in Cd uptake by ZIP8-injected oocytes at 1 versus 60 mM K+ (data not illustrated). We believe the effects we saw at 1 mM K+ and 60 mM K+ are probably early signs of oocytes toxicity—caused by even as little as 30 min of incubation at such adverse K+ concentrations.
FIG. 3.
Electrogenicity studies. A, top, Cd (0.5 μM) uptake by water-injected oocytes. At bottom, Cd uptake by ZIP8 cRNA-injected, minus water-injected oocytes. B, Zn (1.0 μM) uptake by water-injected oocytes (top) and by ZIP8 cRNA-injected, minus water-injected oocytes (bottom). Note the ordinates for each of the four panels are different. Incubation time 30 min.
For the membrane potential of a cell or a Xenopus oocyte, with regard to any cation,
where E = electrode potential, E0′ = the standard electrode potential, n = number of electrons transferred in the half-reaction, and 0.0591 represents the universal gas constant R (8.31451 joules K−1 mol−1) times T (temperature in °K) divided by the Faraday constant F (charge per one mole of electrons, which equals 9.6485309 × 104 coulombs mol−1). Using this Nernst equation and knowing the electrogenic experiments were carried out at pH 7.5 and 22°C, we calculated the intracellular charge to be −132, −114, −53, and −24 mV at extracellular K+ concentrations of 1, 2, 20 and 30 mM, respectively. Because Cd uptake is no more favorable with an electrochemical gradient of −53 mV instead of −114 mV (Fig. 3A), or even of the more extreme −132 mV instead of −24 mV (not shown), we are compelled to conclude that this complex is electroneutral, i.e. the cation/anion complex must include two HCO3− anions and one Cd2+ cation. Therefore, the complex that moves through the ZIP8-microinjected Xenopus oocyte membranes (or, for that matter, any mammalian cell membranes) must be Cd2+/[HCO3−]2, i.e. an electroneutral complex that traverses the membrane no more efficiently when the electrochemical gradient is very negative such as −132 mV or −114 mV or much less negative such as −53 mV or even −24 mV. These findings are actually consistent with those described for ZIP-mediated Cd uptake in MFF cells: no differences in Cd uptake were observed at various extremes of extracellular K+ concentrations [20].
If the rate of Cd uptake had been several-fold higher at −53 mV versus −114 mV, we would have concluded that the complex moving across the membrane is (Cd2+/[HCO3−]3)−, i.e. an electronegative complex. If the rate of Cd uptake had been several times lower at −53 mV than at −114 mV, we would have concluded that the complex moving across the membrane is [Cd2+/HCO3−]+, i.e. a positively-charged complex that moves across the membrane more readily when the electrochemical gradient is more negative such as −114 mV instead of −53 mV.
Just as with Cd, we found no statistically significant differences in Zn uptake in water-injected Xenopus oocytes (Fig. 3B, top), or in ZIP8-mediated Zn uptake (Fig. 3B, bottom), comparing 2 mM K+ with 20 mM K+ in the incubation medium. Likewise, no difference in ZIP8-mediated Zn influx was found at 1 mM versus 60 mM extracellular K+ concentrations (not shown).
Evidence for “trafficking”, dependent on extracellular Zn ion concentration
The ZIP4 transporter has previously been demonstrated to undergo trafficking—as a function of extracellular Zn concentrations [24]. Under Zn homeostasis, the majority of ZIP4 transmembrane-bound proteins was reported to be internalized; when the medium is depleted of Zn, a measurable increase was found in the amount of ZIP4 transmembrane-bound proteins on the cell surface [28-30]. Fig. 4 shows that we confirmed this effect on the ZIP4 transporter, and that the ZIP8 transporter also behaves similarly.
FIG. 4.
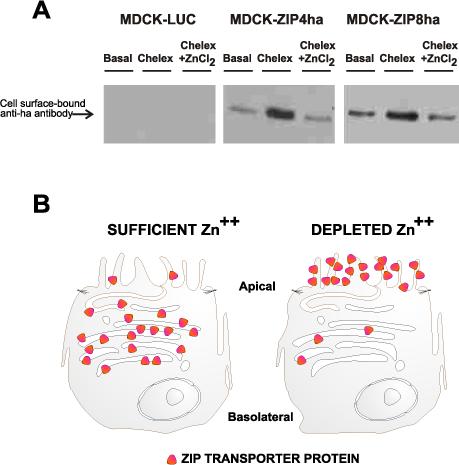
A, Western immunoblot to detect the relative amounts of ZIP4ha versus ZIP8ha protein on the surface of MDCK cells, carried out identically to that previously described [24]. Equal loading was confirmed by Coomassie staining (not shown). B, Schematic diagram showing location of the membrane-bound ZIP8 transporter under normal Zn homeostasis (left), compared with that under low zinc conditions (right).
This trafficking mechanism has biological significance: under comfortable conditions when extracellular Zn concentrations are physiological, there is no need for excess ZIP protein to be poised on the cell surface; under conditions of Zn depletion and therefore cell stress, there is much greater need for the ZIP protein to move to the cell surface so that larger amounts of Zn can be transported into the cell, if possible. The mechanism, or the “signal”, which determines where these membrane-bound ZIP transporters are located in the cell, is not well understood at this time [23]. We believe that ZIP trafficking might involve participation by the trans-Golgi network [31] and/or perhaps by phosphoinositides [32] in such cell regulation and membrane dynamics; clearly, movement of these eight-transmembrane transporter proteins from inside the cell, to the cell surface, and back again, is an intriguing phenomenon.
Concluding remarks
ZIP8 functions efficiently in ZIP8 cRNA-injected Xenopus oocytes, as shown by Cd and Zn uptake (Fig. 1). The most effective inhibitor of Cd influx was Zn (Fig. 2), which was not possible to demonstrate in MFF cultures [20]. Curiously, Mn—which was an effective competitor of Cd uptake in MFF cultures [20]—did not block Cd influx in Xenopus oocytes; reasons for this difference are not understood. Carrying out electrogenic experiments in Xenopus oocytes (Fig. 3), we demonstrate that ZIP8-mediated divalent cation movement across the membrane occurs as the Cd2+/[HCO3−]2 and Zn2+/[HCO3−]2 electroneutral complexes.
Finally, trafficking experiments in MDCK cultures (Fig. 4) show that the ZIP8 transporter is internalized during normal Zn homeostasis and is predominantly on the cell surface during Zn depletion. It would therefore logically follow that Cd influx into cells should be enhanced under conditions of hypozincemia. As stated above, it is well known that Cd uptake is increased in anemic and malnourished populations [1-4]; both anemia and poor nutrition are associated with inflammation and hypozincemia and, thus, this phenomenon of trafficking might help explain the observations as to why enhanced Cd uptake occurs under such disease conditions..
ZIP14 is evolutionarily most closely related, and is highly similar in function, to ZIP8 as compared with the other 12 family members [25]. ZIP8 and ZIP14 are Cd2+/HCO3− symporters, and in epithelial as well as endothelial cells, both these symporters are localized on the apical surface [20; 25]. Hence, we predict that ZIP14 will also transport Cd2+/[HCO3−]2 and Zn2+/[HCO3−]2 electroneutral complexes. As illustrated in Fig. 4, we also expect that ZIP14 will participate in the trafficking of Cd and Zn, as a function of extracellular metal concentration.
Acknowledgments
We thank our colleagues for many fruitful discussions and careful readings of this manuscript. We especially appreciate Marian Miller for expert help with graphics. Supported, in part, by NIH Grants R01 ES010416 (DWN.), R01 DK62809 (M.S.), and P30 ES06096 (TPD; DWN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jarup L. Hazards of heavy metal contamination. Br.Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 2.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand.J Work Environ.Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- 3.Jarup L. Cadmium overload and toxicity. Nephrol.Dial.Transplant. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- 4.Waalkes MP. Cadmium carcinogenesis. Mutat.Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 6.Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol.Appl.Pharmacol. 2003;186:163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 7.Hinkle PM, Osborne ME. Cadmium toxicity in rat pheochromocytoma cells: studies on the mechanism of uptake. Toxicol.Appl.Pharmacol. 1994;124:91–98. doi: 10.1006/taap.1994.1012. [DOI] [PubMed] [Google Scholar]
- 8.Olivi L, Bressler J. Maitotoxin stimulates Cd influx in Madin-Darby kidney cells by activating Ca-permeable cation channels. Cell Calcium. 2000;27:187–193. doi: 10.1054/ceca.1999.0115. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya I, Douglas WW. Calcium channels in rat melanotrophs are permeable to manganese, cobalt, cadmium, and lanthanum, but not to nickel: evidence provided by fluorescence changes in FURA-2-loaded cells. Endocrinology. 1992;131:1936–1941. doi: 10.1210/endo.131.4.1327724. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron PM, Jumarie C. Reciprocal inhibition of Cd2+ and Ca2+ uptake in human intestinal crypt cells for voltage-independent Zn-activated pathways. Biochim.Biophys.Acta. 2006;1758:702–712. doi: 10.1016/j.bbamem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D. Divalent metal transporter-1 in lead and cadmium transport. Ann.N.Y.Acad.Sci. 2004;1012:142–152. doi: 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- 12.Bannon DI, Abounader R, Lees PS, Bressler JP. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am.J Physiol Cell Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- 13.Park JD, Cherrington NJ, Klaassen CD. Intestinal absorption of cadmium is associated with divalent metal transporter-1 in rats. Toxicol.Sci. 2002;68:288–294. doi: 10.1093/toxsci/68.2.288. [DOI] [PubMed] [Google Scholar]
- 14.Olivi L, Sisk J, Bressler J. Involvement of DMT1 in uptake of Cd in MDCK cells: role of protein kinase. C Am J Physiol Cell Physiol. 2001;281:C793–C800. doi: 10.1152/ajpcell.2001.281.3.C793. [DOI] [PubMed] [Google Scholar]
- 15.Tallkvist J, Bowlus CL, Lonnerdal B. DMT1 gene expression and cadmium absorption in human absorptive enterocytes. Toxicol Lett. 2001;122:171–177. doi: 10.1016/s0378-4274(01)00363-0. [DOI] [PubMed] [Google Scholar]
- 16.Elisma F, Jumarie C. Evidence for cadmium uptake through NRAMP2: metal speciation studies with Caco-2 cells. Biochem.Biophys.Res.Commun. 2001;285:662–668. doi: 10.1006/bbrc.2001.5245. [DOI] [PubMed] [Google Scholar]
- 17.Okubo M, Yamada K, Hosoyamada M, Shibasaki T, Endou H. Cadmium transport by human NRAMP2 expressed in Xenopus laevis oocytes. Toxicol.Appl.Pharmacol. 2003;187:162–167. doi: 10.1016/s0041-008x(02)00078-9. [DOI] [PubMed] [Google Scholar]
- 18.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc.Natl.Acad.Sci.U.S.A. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, Miller ML, Stringer KF, Soleimani M, Richardson DD, Nebert DW. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am J Physiol Cell Physiol. 2007;292:C1523–C1535. doi: 10.1152/ajpcell.00409.2006. [DOI] [PubMed] [Google Scholar]
- 20.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol.Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 21.Falcone D, Andrews DW. Both the 5' untranslated region and the sequences surrounding the start site contribute to efficient initiation of translation in vitro. Mol.Cell Biol. 1991;11:2656–2664. doi: 10.1128/mcb.11.5.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erkmann JA, Wagner EJ, Dong J, Zhang Y, Kutay U, Marzluff WF. Nuclear import of the stem-loop binding protein and localization during the cell cycle. Mol.Biol.Cell. 2005;16:2960–2971. doi: 10.1091/mbc.E04-11-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+/HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis for proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2005;289:F61–F71. doi: 10.1152/ajprenal.00032.2005. [DOI] [PubMed] [Google Scholar]
- 24.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol.Chem. 2004;279:49082–49090. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- 25.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. J.Biol.Chem. 2008;283 doi: 10.1124/mol.107.043588. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. ZIP14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc.Natl.Acad.Sci.U.S.A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergeron M, Gougoux A. The renal Fanconi syndrome. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. McGraw-Hill; New York: 1989. pp. 2569–2580. [Google Scholar]
- 28.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J.Biol.Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum.Mol.Genet. 2004;13:563–571. doi: 10.1093/hmg/ddh049. [DOI] [PubMed] [Google Scholar]
- 30.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol.Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 31.McNiven MA, Thompson HM. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/science.1118133. [DOI] [PubMed] [Google Scholar]
- 32.Di PG, De CP. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]