Abstract
Interfacial anchoring interactions between aromatic amino acid residues and the lipid-water interface are believed to be important determinants for membrane protein structure and function. Thus, it is possible that molecules that partition into the lipid-water interface can influence membrane protein activity simply by interfering with these anchoring interactions. Here we tested this hypothesis by investigating the effects of 2,2,2-trifluoroethanol (TFE) on the interaction of a Trp-flanked synthetic transmembrane peptide (acetyl-GW2(LA)8LW2A-NH2) with model membranes of dimyristoylphosphatidylcholine. Two striking observations were made. First, using 2H nuclear magnetic resonance on acyl chain deuterated lipids, we found that addition of 4 or 8 vol % of TFE completely abolishes the ability of the peptide to order and stretch the lipid acyl chains in these relatively thin bilayers. Second, we observed that addition of 8 vol % TFE reduces the tilt angle of the peptide from 5.3° to 2.5°, as measured by 2H NMR on Ala-d4 labeled peptides. The “straightening” of the peptide was accompanied by an increased exposure of Trp to the aqueous phase, as shown by Trp-fluorescence quenching experiments using acrylamide. The observation of a reduced tilt angle was surprising because we also found that TFE partioning results in a significant thinning of the membrane, which would increase the extent of hydrophobic mismatch. In contrast to the Trp-flanked peptide, no effect of TFE was observed on the interaction of a Lys-flanked analog (acetyl-GK2(LA)8LK2A-NH2) with the lipid bilayer. These results emphasize the importance of interfacial anchoring interactions for membrane organization and provide new insights into how molecules such as TFE that can act as anesthetics may affect the behavior of membrane proteins that are enriched in aromatic amino acids at the lipid-water interface.
INTRODUCTION
Membrane proteins are involved in a large variety of cellular processes, where they fulfill many different functions. Most membrane proteins are embedded in the membrane with one or more hydrophobic segments that are in contact with the lipid acyl chains. It has been shown that the extent of matching between the length of these hydrophobic transmembrane segments and the hydrophobic thickness of the bilayer can influence the activity and/or structural properties of proteins and peptides in membranes (1–5). However, it is not only the consequences of hydrophobic mismatch itself that can result in modulation of membrane protein structure and function. A mismatch also results in a change in the positioning of the residues that flank the hydrophobic transmembrane segments with respect to the lipid-water interface. Thus, hydrophobic mismatch may in addition affect the behavior of membrane proteins by disturbing any “anchoring” interactions of these flanking residues with the interfacial region of the membrane. Such anchoring interactions may be particularly relevant for aromatic amino acids, which in virtually all integral membrane proteins are found to be preferentially positioned at the lipid-water interface (6–8). It was even suggested that interfacial anchoring interactions could dominate over effects of hydrophobic mismatch in the case of Trp-flanked transmembrane segments (9,10).
On a molecular level, relatively little is known about how interfacial anchoring interactions can influence properties of membrane proteins. A useful approach to gain insight into this is to use synthetic peptides that mimic the transmembrane segments of membrane proteins. Such peptides typically consist of an α-helical hydrophobic region, e.g., a sequence of alternating leucine and alanine, with variable length and with different flanking residues (9–14). Examples are the so-called WALP peptides, which are flanked on both sides with Trp, and the KALP peptides, which are flanked by lysine residues (15,16). By using these peptides, it was possible to demonstrate that interfacial interactions indeed play a role in membrane organization. For example, for the Trp-flanked WALP peptides, it was shown that a positive mismatch, i.e., the situation in which the hydrophobic length of the peptide is larger than the hydrophobic thickness of the bilayer, results in small but systematic increases in acyl chain order with increasing mismatch (9,17), whereas for the analogous Lys-flanked peptides, no significant stretching of the lipids was observed. In addition, both the extent and direction of tilt of these peptides in lipid bilayers were found to depend on the nature of the flanking residues (18,19).
The importance of interfacial anchoring interactions for membrane organization suggests that molecules that modify the physicochemical properties of the membrane-water interface may thereby also modify the behavior of integral membrane proteins. Candidate molecules that partition at the interface and therefore may interfere with interfacial anchoring include short-chain alcohols such as TFE. These molecules, which are known to have anesthetic properties, can strongly influence properties of membrane proteins. An example is the high potency of TFE and other small alcohols to dissociate oligomeric membrane proteins (20,21). As a possible mechanism for this effect, it was proposed that the partitioning of these alcohols in the interfacial region changes the packing properties of the lipids (22,23) or the lateral pressure profile across the lipid bilayer (20,21), thereby affecting the lipid-protein interactions. However, alternatively, it is possible that small alcohols act by interfering with the interfacial anchoring properties of membrane proteins. In this work, we investigated this possibility by analyzing how modification of the interface by addition of TFE affects peptide-lipid interactions for Trp-flanked WALP and Lys-flanked KALP peptides in zwitterionic model membranes. In particular, we have chosen the 23 amino-acid-long peptides WALP23 and KALP23 in di-C14:0-PC bilayers because these systems have been well characterized experimentally and because they form stable bilayers (9,10,18,19). One advantage of using these model peptides instead of a large natural membrane protein is that it allows detailed analysis of the effects of the alcohol on a molecular level using different biophysical approaches. An even more important advantage is that it facilitates interpretation of the results because the behavior of these single-span peptides is expected to be insensitive to changes in lateral pressure. This is because effects of lateral pressure are believed to be transmitted through changes in shape- or depth-dependent cross-sectional area of membrane proteins (24,25). In multispan proteins this can be accomplished by changes in tilt and rotation angles of individual transmembrane segments. However, for single-span peptides, the cross-sectional area will be largely unchanged as long as the peptide retains its α-helical conformation.
The results show that the presence of TFE strongly affects the membrane interaction of WALP peptides by interfering with their acyl chain ordering effect and by influencing their orientation in the membrane. In contrast, TFE does not affect the membrane interaction of analogous KALP peptides. These results provide new insights into the importance of interfacial anchoring interactions for the structure and function of membrane proteins and may help to explain the molecular mechanism by which small solutes such as alcohols may act as general anesthesia.
EXPERIMENTAL PROCEDURES
Materials
WALP23 and KALP23 (for amino acid sequence, see Table 1) were synthesized using Fmoc/tBu solid-phase peptide synthesis as described elsewhere (26). The peptides were purified by HPLC using a reverse-phase C4 column with an aqueous phosphoric acid/triethylamine buffer at pH 2.25. The purity of the peptides was generally higher than 95%. Purification and mass spectrometric analysis of the peptides were carried out as described elsewhere (26,27). Deuterated d4-Ala was obtained from Sigma Aldrich, and Fmoc was used to protect its amino functionality (28) before it was used in the synthesis. Deuterium-labeled peptides were isotopically labeled with one deuterium-labeled alanine residue at different positions in the transmembrane domain. 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (di-C14:0-PC) and 1,2-dimyristoyl-glycero-3-phosphocholine with a perdeuterated sn-2 chain (1-myristoyl-2-perdeuteriomyristoyl-sn-glycero-3-phosphocholine; di-C14:0-PC-d27) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL) and used without further purification. TFA and TFE were obtained from Merck (Darmstadt, Germany). Deuterium-depleted water was obtained from Cambridge Isotope Laboratories. All other chemicals were of analytical grade. Water was deionized and filtered with a Milli-Q Water purification system from Millipore (Bedford, MA).
TABLE 1.
Amino acid sequences of the peptides used
| Peptide | Design |
|---|---|
| Ac-WALP23-d4-Ala-NH2 | Acetyl-GWWLALALALALALALALALWWA-NH2* |
| Ac-KALP23-d4-Ala-NH2 | Acetyl-GKKLALALALALALALALALKKA-NH2* |
Underlined letters indicated in bold are positions where the peptides have been labeled with d4-Ala.
Methods
NMR sample preparation
Lipid stock solutions were prepared of ∼10 mM phospholipid in chloroform, and the exact concentrations of the phospholipid stocks were determined by a phosphorus assay (29).
For 2H NMR experiments on Ala-d4-labeled peptides and for 31P NMR measurements with unlabeled WALP23 and KALP23, samples were prepared as follows. An amount of 1 μmol of peptide was dissolved in 1 ml of TFE and dried to a film in a rotavapor twice to remove residual traces of TFA. The peptide films were dissolved in 1 ml of TFE, transferred to glass tubes, and mixed with lipid solutions containing 30 or 100 μmol of phospholipid to achieve peptide/lipid molars of 1:30 or 1:100 for 31P NMR and 2H NMR, respectively. The mixtures were stirred with a vortex mixer and dried to films under a nitrogen flow to evaporate solvents more efficiently. Traces of solvent in samples were further evaporated overnight under vacuum (0.5–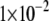 bar). The lipid-peptide films were hydrated in 500 μl of deuterium-depleted water, kept at room temperature for at least 1 h in a N2 atmosphere to equilibrate, and centrifuged 10–20 min at 2880 × g to collect the maximal amount of sample at the bottom of the tubes. Subsequently, the samples were lyophilized overnight. The lipid-peptide films were then hydrated in 100 μl of deuterium-depleted buffer (25 mM HEPES, 100 mM NaCl, pH 7.4), and the suspensions were transferred to NMR glass tubes. The tubes were airtight sealed under a N2 atmosphere with silicon stoppers. Samples were freeze-thawed at least 10 times to promote sample homogeneity.
bar). The lipid-peptide films were hydrated in 500 μl of deuterium-depleted water, kept at room temperature for at least 1 h in a N2 atmosphere to equilibrate, and centrifuged 10–20 min at 2880 × g to collect the maximal amount of sample at the bottom of the tubes. Subsequently, the samples were lyophilized overnight. The lipid-peptide films were then hydrated in 100 μl of deuterium-depleted buffer (25 mM HEPES, 100 mM NaCl, pH 7.4), and the suspensions were transferred to NMR glass tubes. The tubes were airtight sealed under a N2 atmosphere with silicon stoppers. Samples were freeze-thawed at least 10 times to promote sample homogeneity.
For the determination of acyl chain parameters by 2H NMR, the procedure was identical with the following exceptions. Unlabeled WALP23 or KALP23 (0.1 μmol) in 60 μl TFE was mixed to a lipid solution containing 3 μmol of di-C14:0-PC-d27 in 300 μl chloroform to achieve a peptide/lipid molar ratio of 1:30. The mixtures were vortexed and dried to a film with solvents evaporated under a constant N2 flow. The samples were vacuum-dried overnight, after which they were hydrated in 200 μl of deuterium-depleted buffer (25 mM TRIS, 100 mM NaCl, pH 7.4) and transferred to NMR glass tubes. The tubes were sealed under a N2 atmosphere with a silicon stopper and were freeze-thawed at least 10 times. The same procedures were used for the preparation of samples containing only the di-C14:0-PC-d27 suspensions.
To study the influence of TFE, all the samples were reopened after the initial NMR measurements. Next, 4, 8, 15, 30, or 50 vol % TFE was added, and the samples were resealed and freeze-thawed 10 times as described above.
To check whether any residual TFE from the sample preparation protocol could contribute to the measured effects, we compared 31P NMR and 2H NMR spectra of pure lipid systems prepared with and without TFE in the procedure. The similarity in the spectra confirmed that residual TFE did not significantly affect the NMR spectral shapes (data not shown).
NMR measurements
NMR experiments were carried out on a Bruker Avance 500-MHz NMR spectrometer. Samples were allowed to equilibrate at 40°C for at least 10 min before measurements. 31P NMR experiments were performed as described (18) on all samples used.
2H NMR experiments on deuterated peptides were performed at 76.78 MHz using a quadrupolar echo sequence as described previously (18). The measurements were performed with a 5.8-μs 90° pulse, an echo delay of 40 μs, a recycling delay of 100 ms, 1 MHz spectral width, and 4096 data points. Typically, between 200,000 and 1,000,000 scans were collected. Acquisition was started at the echo maximum and further processed by zero-filling to 16,384 data points, and using a 100-Hz exponential multiplication followed by Fourier transformation.
2H NMR experiments on di-C14:0-PC-d27 phospholipid samples were carried out using the same quadrupolar echo sequence with identical parameters as above, except that a spectral width of 500 kHz and a recycling time of 600 ms were used.
Calculation of peptide structural parameters
Quadrupolar splittings (Δνq, kHz) of seven or four labeled positions were measured from 2H NMR spectra of WALP23 and KALP23, respectively (Table 1). 2H NMR signals were assigned to the deuterons of the alanine side-chain methyl group as in previous work because the splitting of the backbone deuteron was not observed (30). The Δνq-values for unoriented samples were fitted to a model α-helix to determine the tilt (τ), rotation (ρ), and the labeled alanine side chain (ɛ‖) angles (18,19).
The tilt angle is defined as the angle between the peptide helical axis and the bilayer normal, and ɛ‖ is the angle between the peptide helix axis and the Cα-CβD3 bond vector. The angle ρ is the angle of rotation around the α-helical axis that is necessary to minimize the root mean-square deviation (RMSD, kHz) between experimental and simulated Δνq-values for discrete values of τ and ɛ‖ (17). The fitting procedure was based on the Δνq-values of the labeled positions as indicated in Table 1 using an in-house computer program written in Python 2.3. The errors in τ, ρ, and ɛ‖ were estimated with deviations ±0.4°, ±3°, and ±0.3°, respectively (19).
Analysis of 2H NMR spectra for determination of acyl chain parameters
The recorded 2H-NMR powder spectra were numerically deconvoluted to yield the θ = 0° spectra (31,32), resulting in so-called de-Paked spectra. Using the Peak-Fitting Module in Origin 7.5, we deconvoluted the de-Paked spectra to assign the peaks to carbon atoms along the sn-2 acyl chain. The quadrupolar splittings determined from the de-Paked spectra were used to calculate the order parameter, SCD(i), according to:
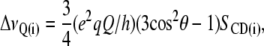 |
(1) |
where e2qQ/h = 167 kHz for θ = 0° (31). From the determined order parameters, we estimated the hydrocarbon thickness of one monolayer according to the method described in (33–35). To estimate the hydrocarbon thickness per monolayer the following equation was used:
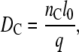 |
(2) |
where the factor nC is the number of carbons per acyl chain (i.e., 14 for di-C14:0-PC), and the maximum segmental projection l0, was 1.27 Å. The factor q was approximated from:
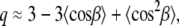 |
(3) |
described by Brown and co-workers (33). In Eq. 3, the first 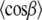 and second moments
and second moments 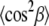 are given by:
are given by:
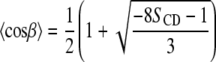 |
(4) |
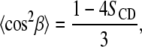 |
(5) |
where the order parameter, SCD of the plateau region is used to avoid complication of chain upturns, early terminations, and interdigitation (33–35).
Steady-state fluorescence
All fluorescence experiments were performed at 30°C using a QuantaMaster QM-1/2005 spectrofluorometer (Photon Technology International, Birmingham, NJ) in a quartz cuvette. The samples were excited at 295 nm, and emission spectra were collected between 300 and 400 nm. The bandwidths for both excitation and emission monochromators were 5 nm. Acrylamide quenching of tryptophan fluorescence was performed to check the accessibility of tryptophans in lipid bilayers as a function of TFE. Samples for fluorescence experiments were prepared as follows: di-C14:0-PC and WALP23 were mixed in TFE/chloroform at a peptide/lipid molar ratio of 1:100. The mixture was dried to a film that was rehydrated in buffer (25 mM TRIS, 100 mM NaCl, pH 7.4). The samples were freeze-thawed 10 times, extruded through a 200-nm membrane filter (Anotop 10, Whatman, Maidstone, UK), and diluted in 1.25 ml of buffer to achieve a final WALP23 concentration of 2.5 μM. The samples were treated with 4 or 8 vol % TFE. Acrylamide was added in aliquots from a 3 M stock solution to each sample up to a concentration of ∼70 mM. The Stern-Volmer equation was used to analyze the quenching data (36):
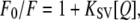 |
(6) |
where F0 is the Trp fluorescence in the absence of quencher and F is the observed fluorescence at the concentration [Q] of the quencher. KSV is the collisional quenching constant, which was determined from the slope of Stern-Volmer plots. As a control, similar experiments were performed for a l-Trp solution (10 μM). All data were corrected for inner filter effects as described previously (36).
RESULTS
Influence of TFE on phospholipid bilayer
Before studying how TFE influences the interaction between WALP and KALP peptides with lipid bilayers, we first analyzed the influence of this small alcohol on the properties of the lipids themselves. For this purpose, 31P NMR experiments were performed at 40°C on samples made of di-C14:0-PC in the absence of peptides at different TFE concentrations.
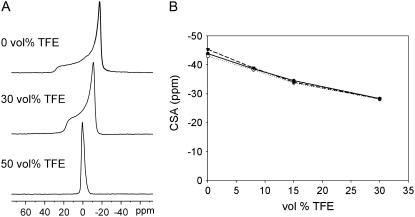
All spectra up to 30 vol % TFE showed an anisotropic pattern with a low field shoulder and a high field peak, which are typical of multilamellar vesicle (MLV) bilayers in the liquid crystalline phase (37). Selected spectra are shown in Fig. 1 A. As plotted in Fig. 1 B, the chemical shift anisotropy (CSA) decreases gradually on addition of TFE in the range of 0 to 30 vol % TFE. At 50 vol % TFE, vesicles are disrupted, resulting in an optically clear solution of di-C14:0-PC, which gives rise to an isotropic peak (Fig. 1 A).
FIGURE 1.
Influence of increasing concentration of TFE on 31P NMR CSA of di-C14:0-PC bilayers at 40°C. (A) Selected spectra of di-C14:0-PC multilamellar vesicles. (B) Graph of the 31P NMR CSA versus TFE vol % of di-C14:0-PC for pure lipids (▾), for lipids with WALP23 (•), and for lipids with KALP23 (○). The precision of the data is estimated to be ±0.3 ppm, based on measurements of duplicate samples.
The observed decrease in CSA may imply either a decrease in particle size, which would result in motional averaging from Brownian tumbling of the entire vesicles and lateral diffusion of the lipids (38) or a decreased order in the lipid headgroup region of the bilayers. To discriminate between these two possibilities, we performed 31P NMR experiments with flat-oriented bilayers of di-14:0-PC (18). In these systems the motional axis of the phospholipids is oriented parallel to the magnetic field, giving rise to a low-field 31P NMR peak. On addition of TFE, we observed an upfield shift of this peak (data not shown). In these systems averaging of the CSA by a decreased particle size is excluded because the motional axis of the phospholipids will remain parallel to the magnetic field. Therefore, the most logical explanation for the decreased CSA is that TFE affects the order in the headgroup region of the phospholipid bilayer.
We performed 31P NMR experiments similar to those above by adding TFE to MLV suspensions made of WALP23 or KALP23 in di-C14:0-PC in a peptide/lipid molar ratio of 1:30. All samples gave rise to typical “bilayer” spectra up to a TFE concentration of 30 vol %. Fig. 1 B shows that the presence of either KALP23 or WALP23 in di-C14:0-PC slightly reduces the CSA when no TFE is present. However, when TFE is added, the effect of peptides on the CSA is removed, and samples of pure lipids and lipids containing peptides behave similarly regardless of the amount of TFE that is added. This indicates that the presence of peptide does not interfere with the influence of TFE on the lipid headgroup organization.
Influence of TFE on the order in the phospholipid acyl chains
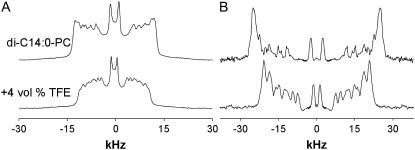
Recent work showed that the presence of TFE in a membrane increases the disorder of the acyl chains of unsaturated phospholipids (20). Here we investigated the effect on the saturated lipid di-C14:0-PC by performing 2H NMR on di-C14:0-PC-d27 (sn-2 chain deuterated) bilayers, as described in (17,27,33). Experiments were first performed in the absence of peptide. 2H NMR spectra of di-C14:0-PC-d27 multilamellar vesicles with and without TFE are shown in Fig. 2 A. The line shape and the quadrupolar splittings from the different labeling positions along the acyl chains for the pure lipid dispersion are in good agreement with results from a previous study of di-C14:0-PC-d27 (17,27,33). The addition of 4 vol % of TFE clearly reduces all the splittings, indicating an increased averaging of the signals from the acyl chains. In addition, some changes are observed in the 2H NMR line shape, which can be ascribed to a depth-dependent influence of TFE on the di-C14:0-PC bilayers.
FIGURE 2.
2H-NMR spectra of di-C14:0-PC-d27 at 40°C with or without 4 vol % TFE in TRIS buffer, pH = 7.4. (A) Original spectra. (B) De-Paked deuterium spectra obtained as described (32) using the GRAMS software.
To facilitate data analysis and assignment of the quadrupolar splittings that were difficult to resolve, the spectra were de-Paked (Fig. 2 B). The resulting spectra would correspond to aligned bilayers with their normals parallel to the magnetic field. Assuming a monotonic variation of the order parameters (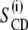 ) along the acyl chains, the peak assignment was based on the sequence of the labels along the acyl chain. The largest splittings were assumed to be from the labels closest to the headgroup region in the membranes.
) along the acyl chains, the peak assignment was based on the sequence of the labels along the acyl chain. The largest splittings were assumed to be from the labels closest to the headgroup region in the membranes.
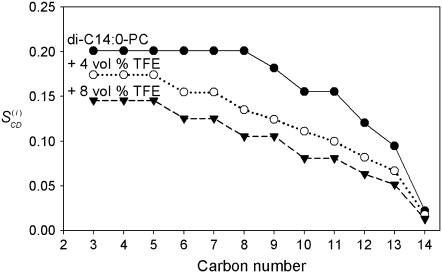
From the splittings, corresponding 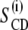 -values were calculated of the di-C14:0-PC-d27 lipids in the absence and presence of TFE (Fig. 3). A plateau region characterizes the order profile of the lipids in the absence of TFE, indicating a relatively high chain order near the bilayer interface. This is followed by a rapid decrease toward the end of the acyl chain in the core of the bilayer. As shown in Fig. 3, the order parameters are in general reduced by the addition of TFE. Note also that adding TFE shortens the plateau. Increasing the TFE concentration up to 8 vol % resulted in a further reduction of the order of the acyl chains.
-values were calculated of the di-C14:0-PC-d27 lipids in the absence and presence of TFE (Fig. 3). A plateau region characterizes the order profile of the lipids in the absence of TFE, indicating a relatively high chain order near the bilayer interface. This is followed by a rapid decrease toward the end of the acyl chain in the core of the bilayer. As shown in Fig. 3, the order parameters are in general reduced by the addition of TFE. Note also that adding TFE shortens the plateau. Increasing the TFE concentration up to 8 vol % resulted in a further reduction of the order of the acyl chains.
FIGURE 3.
Order parameter profiles in di-C14:0-PC-d27 bilayers at 40°C in TRIS buffer with pH = 7.4. Plotted values were calculated as described (31–33) for di-C14:0-PC-d27 (•), di-C14:0-PC-d27 + 4 vol % TFE (○), and di-C14:0-PC-d27 + 8 vol % TFE (▾).
Next, we used the order parameters to estimate the bilayer thickness as described previously (33–35). As shown in Table 2, the estimated bilayer thickness of di-C14:0-PC-d27 membranes at 40°C decreases from 25.0 Å to 21.2 Å on addition of 8 vol % TFE, showing that the TFE-induced disorder in the membrane results in a significant reduction of the bilayer thickness.
TABLE 2.
Estimation of the di-C14:0-PC bilayer thickness at 313 K deduced from order parameters
| Sample | Bilayer thickness* 2 × DC† (Å) |
|---|---|
| di-C14:0-PC | 25.0 (25.6‡ and 24.0§) |
| + 4% TFE | 23.4 |
| + 8% TFE | 21.2 |
| WALP23 | 26.4 |
| + 4% TFE | 23.2 |
| + 8% TFE | 21.6 |
| KALP23 | 25.2 |
| + 8% TFE | 21.0 |
Effects of peptide incorporation on lipid order in the presence of TFE
To investigate the influence of TFE on the interaction of the peptides with the lipids, we performed 2H NMR experiments on samples of WALP23 and KALP23 in di-C14:0-PC-d27. After recording the 2H NMR spectra of the lipid-peptide suspensions, further measurements were performed on the same samples with 4 or 8 vol % TFE added. Fig. 4 shows selected de-Paked deuterium spectra. Experiments without peptide in the membrane are included for comparison.
FIGURE 4.
De-Paked 2H-NMR spectra of di-C14:0-PC-d27 and di-C14:0-PC-d27 with WALP23 at 40°C with or without 4 vol % TFE in TRIS buffer, pH = 7.4. De-Paking the spectra was performed using the GRAMS software as described (30).
Introduction of WALP23 at a 1:30 peptide/lipid molar ratio increases the order in the acyl chains. This is illustrated by the increase of 2H NMR quadrupolar splittings with respect to those in the pure di-C14:0-PC-d27 suspension, which is in good agreement with previous observations (27). When 4% TFE is added, the quadrupolar splittings are reduced, but now the spectra are very similar in the absence and presence of peptide. Similar behavior was observed for 8 vol % TFE (spectra not shown).
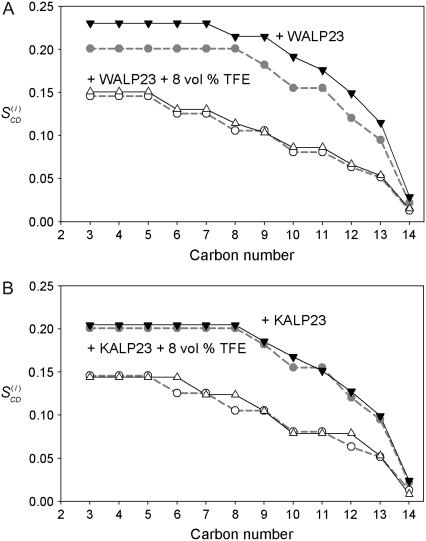
The loss of the ordering effect is also apparent from the order parameter profiles (Fig. 5 A). When no TFE is added, incorporation of the peptide results in a plot that is characterized by a plateau region with higher 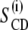 -values than for the pure di-C14:0-PC-d27 suspensions, indicating an increased order in the acyl chains. Toward the bilayer core, the order parameters of both types of samples converge gradually. In the presence of TFE, the order parameter profiles of di-C14:0-PC-d27 and di-C14:0-PC-d27/WALP23 samples look very similar.
-values than for the pure di-C14:0-PC-d27 suspensions, indicating an increased order in the acyl chains. Toward the bilayer core, the order parameters of both types of samples converge gradually. In the presence of TFE, the order parameter profiles of di-C14:0-PC-d27 and di-C14:0-PC-d27/WALP23 samples look very similar.
FIGURE 5.
Order parameter profiles in di-C14:0-PC-d27 bilayers at 40°C in TRIS buffer with pH = 7.4. The order parameters for di-C14:0-PC-d27 (dashed gray line, gray circle) and for di-C14:0-PC-d27 + 4 vol % TFE (dashed gray line, ○) are plotted for comparison issues in panels A and B. Plotted values for di-C14:0-PC-d27 + WALP23 (solid line, ▾) and di-C14:0-PC-d27 + WALP23 + 8 vol % TFE (solid line, ▵) are represented in panel A. Order parameter profiles for di-C14:0-PC-d27 + KALP23 (solid line, ▾), and di-C14:0-PC-d27 + KALP23 + 8 vol % TFE (solid line, ▵) are shown in panel B.
As shown in Table 2, the incorporation of WALP23 in di-C14:0-PC-d27 causes an estimated increase in bilayer thickness from 25.0 to 26.4 Å, in agreement with previous observations (27). Addition of TFE reduces the estimated bilayer thickness to values that are very similar to those obtained in the absence of peptide. These results clearly show that TFE dissipates the ordering effect and hence interferes with the lipid-peptide interactions in case of WALP23.
Previously, it was observed that KALP23 peptides, in contrast to WALP23, do not significantly influence lipid chain order in di-C14:0-PC (27). Our results show that the order parameter profiles (Fig. 5 B) or the bilayer thickness (Table 2) in the presence of TFE are also unaffected by KALP23.
Influence of TFE on tilt angle of WALP23 and KALP23
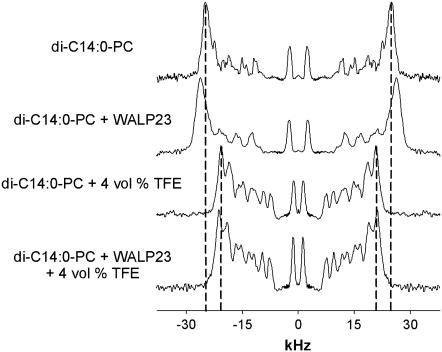
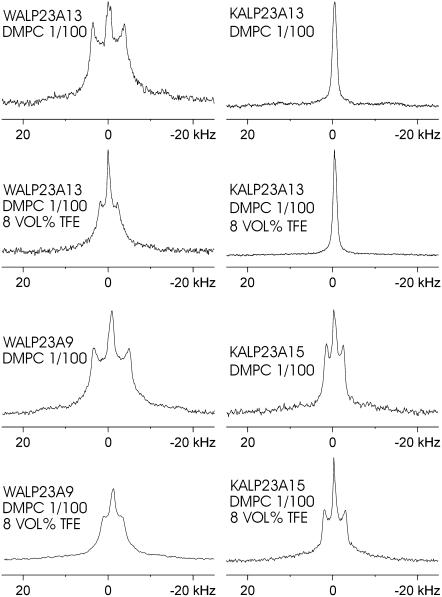
We next investigated how the presence of TFE affects properties of the peptides. In particular, we used 2H NMR on d4-Ala-labeled peptides to monitor effects of TFE on tilt angles of the peptides. Each peptide was labeled with a single d4-Ala as indicated in Table 1 for WALP23 and KALP23 at seven and four different positions, respectively. The labeling positions were chosen to enable precise determination of the tilt angles of the peptides in membrane bilayers and the direction in which they tilt, as represented by rotation angles of the α-helix axis with respect to the plane of tilt (18,19,30). Selected 2H NMR spectra are shown in Fig. 6 for two labeling positions of both the WALP23 and the KALP23, with 0 and 8 vol % TFE in the di-C14:0-PC suspensions. For both peptides in the absence of TFE, the spectra with corresponding quadrupolar splittings were in good agreement with earlier results (18,19). When 8 vol % TFE was added, changes in the quadrupolar splitting values could be observed for all the labels of WALP23 (Table 3). This effect was much less pronounced for KALP23, which was rather insensitive to the presence of TFE.
FIGURE 6.
2H NMR spectra for labeled alanines at positions 9 and 13 of WALP23 and 13 and 15 of KALP23, incorporated in di-14:0-PC without and with 8 vol % of TFE at a peptide/lipid ratio of 1:100. The isotropic peak in the middle of the spectra is assigned to residual deuterium in H2O.
TABLE 3.
Measured 2H NMR splittings of d4-Ala-labeled WALP23 and KALP23 peptides in unoriented PC bilayers with and without addition of TFE in kHz
| Peptide/phospholipid | TFE vol % | Labeled residue
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 7 | 9 | 11 | 13 | 15 | 17 | 19 | ||
| WALP23/di-14:0-PC | 0 | 4.3 | 0.7* | 6.8 | 0.9 | 7.5 | – | 6.9 | 1.0 |
| 8 | 2.2 | 0.0* | 3.5 | 0.2 | 4.0 | – | 3.8 | 1.7 | |
| KALP23/di-14:0-PC | 0 | 3.2 | 9.6 | – | – | 0.5† | 3.9 | – | – |
| 8 | 2.5 | 9.6 | – | – | 0.0† | 5.0 | – | – | |
The error of the splittings is estimated to be 0.2 kHz, based on measurements of duplicate samples.
Quadrupolar splittings that could not be resolved and for which an estimated value is given.
We calculated the tilt (τ), the rotation (ρ) and the labeled side chain angles (E‖) in the absence and the presence of TFE assuming a regular α-helical geometry of the peptides, as described (18,19,30). The results are shown in Table 4. When no TFE is added, a relatively small tilt angle of 5.3° is observed, in agreement with previous results (19). The presence of 8 vol % TFE results in a decrease of the tilt angle to 2.5° without a significant change of the rotation angle. In contrast, for KALP23 the addition of 8 vol % TFE changes neither the tilt angle nor the rotation angle.
TABLE 4.
Fit results using data from the four-labeled positions summarized in Table 3 for WALP23 and KALP23
| Fit parameters*
|
|||||
|---|---|---|---|---|---|
| Peptide/phospholipid | TFE (%) | Tilt angle | Rotation angle | ɛ‖ angle | RMSD (kHz) |
| WALP23/di-14:0-PC | 0 | 5.3 | 162 | 58.4 | 0.4 |
| 8 | 2.5 | 154 | 56.8 | 0.8 | |
| KALP23/di-14:0-PC | 0 | 7.8 | 278 | 58.5 | 0.4 |
| 8 | 7.5 | 286 | 58.7 | 0.3 | |
The estimated precisions (19) for the tilt angle τ, the rotation angle ρ, and ɛ‖ are ±0.4°, ±3°, and ±0.3°, respectively.
Table 4 also shows that the angle ɛ‖ of the labeled alanines of WALP23 decreases slightly when TFE is added. A similar effect was recently also observed for WALP23 and a Trp-flanked polyleucine analog of this peptide (WLP23) on reducing the bilayer thickness from di-C14:0-PC to di-C12:0-PC (19). In this case the reduction of ɛ‖ was proposed to be a possible indication of a reduction in the length of the backbone of Trp-anchored transmembrane peptides to help the system to adapt to mismatch when the phospholipid bilayers are too thin to accommodate the whole hydrophobic length of a transmembrane peptide. In the case of KALP23, ɛ‖ appears to be insensitive to the presence of TFE. This is in line with the previous observation that ɛ‖ in KALP23 is insensitive to bilayer thickness (19). These results might suggest that WALP23 senses mismatch more than KALP23 even though it does not react to it by tilting as much as KALP23.
Influence of TFE on the positioning of the tryptophans
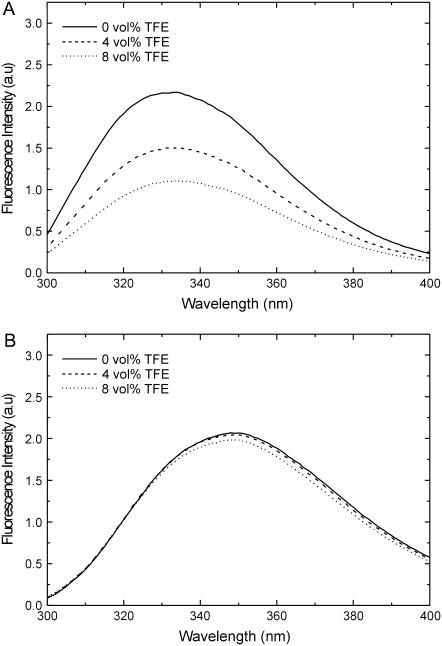
The deuterium NMR results suggest that WALP23 reduces its tilt angle when TFE is added, even though the bilayer gets thinner. To determine whether TFE has an influence on the positioning of tryptophans in lipid bilayers, we performed tryptophan fluorescence experiments. In di-C14:0-PC membranes, WALP23 exhibits an emission maximum at ∼334 nm, which is typical for positioning of tryptophans in the interfacial region (39). When TFE is added to samples containing large unilamellar vesicles composed of WALP23 and di-C14:0-PC in a 1/100 peptide/lipid molar ratio, the fluorescence intensity decreases (Fig. 7 A). In addition, a red shift of the emission spectrum (∼2 nm) is observed, indicating a more polar environment. This decrease in fluorescence intensity is not a direct effect of TFE because for free l-Trp in buffer, which shows an emission maximum at 350 nm in a more hydrophilic environment, neither the fluorescence intensity nor the wavelength of the emission maximum is affected by this solvent (Fig. 7 B).
FIGURE 7.
The effect of TFE on fluorescence emission spectra of WALP23 in di-C14:0-PC (A) and l-Trp in buffer (B). The peptide/lipid ratio of WALP23 in di-C14:0-PC was 1:100. The corrected spectra and the effects of 4 and 8 vol % TFE on the fluorescence intensities are shown.
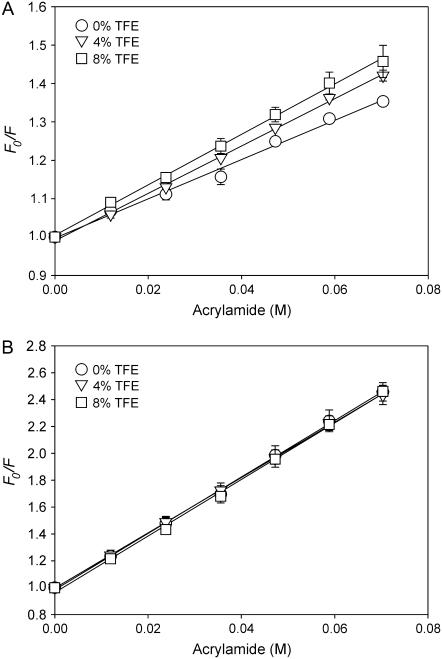
The effects of TFE on the local environment of Trp were further investigated by monitoring Trp accessibility using the aqueous quencher acrylamide. Fig. 8 A shows the Stern-Volmer quenching plots of WALP23 in di-C14:0-PC (1:100 molar ratio), in which F0/F is plotted against the acrylamide concentration in the absence and presence of TFE. Addition of 4 vol % TFE increased the quenching constant (KSV) from 5.2 ± 0.2 to 6.0 ± 0.1 M−1, and at 8 vol % it was further increased to 6.7 ± 0.5 M−1. A qualitatively similar effect was observed for a sample with a higher peptide/lipid molar ratio of 1:30 (data not shown). Again this was not a direct effect of TFE because experiments on l-Trp in buffer (Fig. 8 B) show no effect of TFE addition on acrylamide accessibility (KSV = 20.7 ± 1.4 M−1 in the absence of TFE and 20.5 ± 1.1 M−1 and 20.4 ± 0.4 M−1 in the presence of 4 and 8 vol% TFE, respectively). Thus, the fluorescence data are fully consistent with an increased exposure of Trp in WALP peptides to the aqueous environment and support our notion that TFE addition results in a more upright position of WALP peptides in a di-C14:0-PC bilayer.
FIGURE 8.
Stern-Volmer plots of Trp fluorescence quenching by acrylamide. Both WALP23 in di-C14:0-PC (A) or l-Trp in buffer (B) and were investigated with or without 4 or 8 vol % TFE. The WALP23:di-C14:0-PC ratio was 1:100. Mean ± SD from three experiments are given.
DISCUSSION
In this work, we have used NMR to investigate how TFE influences the structure of PC bilayers and how it affects the interactions of the lipids with peptides that mimic transmembrane segments of membrane proteins. The results show that TFE has drastic effects on membrane organization. We first discuss the consequences of the partitioning of TFE in the interfacial regions of the bilayer for the properties of the lipids. Next, we describe how TFE can affect the properties of the peptides by interfering with their interactions with the lipid-water interface. Finally, we propose a model for the mechanism by which TFE influences anchoring interactions, in particular of Trp-flanked peptides with the lipid-water interface.
Partitioning of TFE into the membrane-water interface and implications for the behavior of the lipids
In the first part of this study, we observed that the presence of TFE decreases the order of both the lipid headgroups and the acyl chains in PC bilayers. Similar disordering effects were previously also observed in different phospholipid bilayers for diverse short-chain alcohols such as ethanol (20,23). Like ethanol, TFE is amphiphilic and has an octanol-water partitioning coefficient and dielectric constant favorable for localizing in a relatively hydrophobic environment such as membrane-water interfaces (22,40–43). However, TFE has a stronger hydrogen-bond-donating hydroxyl group than ethanol (44) and a higher octanol-water partitioning coefficient, which may favor the distribution in the interface more than its nonfluorinated analog (41), explaining the larger efficacy of TFE to influence the order of the acyl chains as measured by 2H NMR (20) and, consequently, to reduce bilayer thickness (23,45). It is important to note that these effects of TFE on lipid packing imply that TFE also affects other properties of the membrane such as the lateral pressure profile. Moreover, the bilayer partioning of TFE, with its relatively large dipole moment of 2.52 D (46), will affect the dielectric properties of the interfacial region and the surface tension, thereby also affecting bilayer elastic properties such as the bending and compressibility moduli or its deformation energy. In principle, all these parameters can play an important role in lipid-peptide interactions (47–49 and references therein).
Effects of TFE on the behavior of transmembrane peptides
In the second part of our study, we analyzed the influence of TFE on the interaction of the peptides with lipid bilayers by 2H NMR and fluorescence techniques. From the NMR experiments, several striking results were obtained. First, we found that addition of TFE results in a complete loss of the acyl chain ordering effect of WALP23. A possible explanation for this is that TFE inhibits acyl chain adaptation by interfering with the anchoring interactions of Trp with the interface. Under conditions of positive mismatch, the peptide thus behaves similarly to a KALP peptide, which does not have strong interfacial anchoring interactions. The second striking observation is that the presence of TFE affects the tilt angle of WALP23 but not of KALP23, suggesting that this effect somehow also involves anchoring interactions with the interface. Finally, although the bilayer becomes more disordered and hence is expected to become thinner on TFE addition, WALP23 was found to tilt less. This result, based on 2H NMR, was supported by results from fluorescence quenching experiments, which showed an increased exposure of Trp to the aqueous environment on TFE addition. How can we understand this behavior?
In principle it is possible to explain the smaller tilt angle, as observed by the 2H NMR studies, by assuming that the presence of TFE in the lipid bilayer results in an increased mobility of WALP23 with large fluctuations around average tilt and rotation angles. This would lead to an increased motional averaging of the observed quadrupolar splittings and consequently to an underestimation of the “true” tilt angle. However, in that case one would also expect this apparent reduction of tilt angle to occur for KALP23. We found that the tilt angle of KALP23 is insensitive even to high concentrations of TFE (up to 30 vol %; data not shown). Moreover, if the presence of TFE indeed leads to increased motional averaging of WALP23, this should lead to a reduction of all the quadrupolar splittings, which is not observed, as illustrated by the effects of TFE on label 19 (see Table 3).
Thus, the intriguing question remains of why TFE affects the tilt angle of WALP23 and not of KALP23, and why WALP23 would tilt less if the bilayer becomes thinner. One likely explanation is that TFE actively drives WALP23 to adopt a more upright orientation because of packing constraints in the interfacial region. The bulky indole rings of Trp require much space in the tightly packed lipid-water interface, and partitioning of TFE into this region will reduce the available space even more. Such packing constraints are not present for KALP peptides, which require only the charged amine groups of the lysines to reach the hydrophilic surface of the membrane (9).
Another possible alternative to explain the decreased tilt involves interference of TFE with the interfacial anchoring interactions of Trp residues. Properties of the interfacial region that are important for anchoring interactions include the characteristic polarity gradient (50) and the electrostatic properties of the lipid-water interface, which offer possibilities for dipolar and quadrupolar interactions and for hydrogen bonding (9). Like ethanol, TFE will displace interfacial water molecules and thereby change the electrostatic properties and render the polarity gradient at the interface less steep by distributing in a gradient-like manner toward the exterior of the membrane. As a result of these changes, the preference for a specific orientation and localization of the indole groups may become lost, which ultimately may result in a reduction of the tilt angle.
In principle another possibility to explain the reduction of tilt could simply be that the presence of TFE, which results in a less hydrophilic environment at the interface, might reduce the penalty for mismatch, thereby allowing the peptide to be less tilted. However, in that case one might expect that the KALP peptides also become less tilted, which was not observed. Similarly, if the effect of TFE would be exerted indirectly by influencing other properties of the bilayer itself such as the curvature and the elastic properties of the membrane or the lateral pressure profile (47), we might expect that the reduction of tilt angle would be observed for both WALP23 and KALP23 peptides, which is not the case.
Biological implications
Like ethanol and other short-chain alcohols, TFE is believed to act as an anesthetic in a lipid-mediated way, although direct interactions with membrane proteins cannot be excluded (51–53). Recently, an attractive mechanism was postulated for a lipid-mediated effect, related to remodeling of the lateral pressure profile of the lipids in the bilayer, which in turn can influence the conformation of the transmembrane parts of membrane proteins (20,24). By using single-span α-helical transmembrane peptides, which are expected to be rather insensitive to changes in lateral pressure profile, we investigated here whether small alcohols can also act by simply interfering with interfacial anchoring properties of membrane proteins. We found that the effect of TFE on properties of transmembrane peptides depends specifically on the nature of the flanking membrane-anchoring residues, indicating that TFE indeed can influence transmembrane protein segments by disturbing their interactions with the lipid-water interface. Thus, although relatively high concentrations of TFE were used compared with clinically relevant concentrations (4 to 8 vol % in this study, corresponding to ∼0.6 to 1.1 M, versus an EC50 value of 24.3 mM) (54), our results suggest a new mechanism by which anesthetic molecules such as TFE may act on membrane proteins. An implication of these results is that membrane proteins that are rich in aromatic anchoring residues may be more efficiently affected by the presence of TFE or other small amphiphilic solutes, than proteins the transmembrane segments of which are flanked by charged residues such as Arg or Lys. It may also be interesting to study the influence of TFE on other types of anchoring interactions such as electrostatic interactions between charged flanking residues of model transmembrane peptides and anionic lipids (55).
Recently, it was observed that TFE and other small alcohols can influence the association of oligomeric membrane proteins in a way that is related both to their anesthetic potency and to their lipid-disturbing effect (20). Our results suggest that interference with anchoring properties of aromatic amino acids may be considered as an additional factor involved in the ability of these small alcohols to dissociate membrane protein complexes (21,56).
In conclusion, our results emphasize the importance of specific lipid-peptide interactions at the complex lipid-water interfacial region for the organization of proteins and lipids in membranes, and they shed new light on the possible roles of aromatic amino acids as flanking residues in membrane proteins.
Acknowledgments
We thank Hans Meeldijk and Loes Kroon-Batenburg for stimulating discussions about the influence of alcohols on the macroscopic structure of bilayers, and we thank Erica Kremer for her help in performing some of the control experiments. We acknowledge Ben de Kruijff for helpful discussions throughout the course of this work.
Abbreviations used: CSA, chemical shift anisotropy; PC, phosphatidylcholine; TFA, trifluoroacetic acid; TFE, 2,2,2-trifluoroethanol; tBu, tert-butyl; d4-Ala, deuterated l-alanine-d4; Fmoc, 9-fluorenylmethyloxycarbonyl; di-C14:0-PC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine; di-C14:0-PC-d27, 1-myristoyl-2-perdeuteriomyristoyl-sn-glycero-3-phosphocholine.
Editor: Thomas J. McIntosh.
References
- 1.Lee, A. G. 2003. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 1612:1–40. [DOI] [PubMed] [Google Scholar]
- 2.Dumas, F., M. C. Lebrun, and J. F. Tocanne. 1999. Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett. 458:271–277. [DOI] [PubMed] [Google Scholar]
- 3.Killian, J. A. 1998. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta. 1376:401–416. [DOI] [PubMed] [Google Scholar]
- 4.Park, S. H., and S. J. Opella. 2005. Tilt angle of a trans-membrane helix is determined by hydrophobic mismatch. J. Mol. Biol. 350:310–318. [DOI] [PubMed] [Google Scholar]
- 5.Ramamoorthy, A., S. K. Kandasamy, D.-K. Lee, S. Kidambi, and R. G. Larson. 2007. Structure, topology, and tilt of cell-signaling peptides containing nuclear localization sequences in membrane bilayers determined by solid-state NMR and molecular dynamics simulation studies. Biochemistry. 46:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landolt-Marticorena, C., K. A. Williams, C. M. Deber, and R. A. F. Reithmeier. 1993. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 229:602–608. [DOI] [PubMed] [Google Scholar]
- 7.von Heijne, G. 1994. Membrane proteins: from sequence to structure. Annu. Rev. Biophys. Biomol. Struct. 23:167–192. [DOI] [PubMed] [Google Scholar]
- 8.Reithmeier, R. A. F. 1995. Characterization and modeling of membrane proteins using sequence analysis. Curr. Opin. Struct. Biol. 5:491–500. [DOI] [PubMed] [Google Scholar]
- 9.de Planque, M. R. R., B. B. Bonev, J. A. A. Demmers, D. V. Greathouse, R. E. Koeppe II, F. Separovic, A. Watts, and J. A. Killian. 2003. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry. 42:5341–5348. [DOI] [PubMed] [Google Scholar]
- 10.Demmers, J. A. A., E. Van Duijn, J. Haverkamp, D. V. Greathouse, R. E. I. I. Koeppe II, A. J. R. Heck, and J. A. Killian. 2001. Interfacial positioning and stability of transmembrane peptides in lipid bilayers studied by combining hydrogen/deuterium exchange and mass spectrometry. J. Biol. Chem. 276:34501–34508. [DOI] [PubMed] [Google Scholar]
- 11.Davis, J. H., D. M. Clare, R. S. Hodges, and M. Bloom. 1983. Interaction of a synthetic amphiphilic polypeptide and lipids in a bilayer structure. Biochemistry. 22:5298–5305. [Google Scholar]
- 12.Webb, R. J., J. M. East, R. P. Sharma, and A. G. Lee. 1998. Hydrophobic mismatch and the incorporation of peptides into lipid bilayers: a possible mechanism for retention in the Golgi. Biochemistry. 37:673–679. [DOI] [PubMed] [Google Scholar]
- 13.Ren, J., S. Lew, J. Wang, and E. London. 1999. Control of the transmembrane orientation and interhelical interactions within membranes by hydrophobic helix length. Biochemistry. 38:5905–5912. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, Y. P., R. N. Lewis, R. S. Hodges, and R. N. McElhaney. 1995. Peptide models of helical hydrophobic transmembrane segments of membrane proteins. 2. Differential scanning calorimetric and FTIR spectroscopic studies of the interaction of Ac-K2-(LA)12-K2-amide with phosphatidylcholine bilayers. Biochemistry. 34:2362–2371. [DOI] [PubMed] [Google Scholar]
- 15.de Planque, M. R. R., and J. A. Killian. 2003. Protein-lipid interactions studied with designed transmembrane peptides: role of hydrophobic matching and interfacial anchoring. Mol. Membr. Biol. 20:271–284. [DOI] [PubMed] [Google Scholar]
- 16.Killian, J. A. 2003. Synthetic peptides as models for intrinsic membrane proteins. FEBS Lett. 555:134–138. [DOI] [PubMed] [Google Scholar]
- 17.de Planque, M. R. R., D. V. Greathouse, R. E. Koeppe II, H. Schafer, D. Marsh, and J. A. Killian. 1998. Influence of lipid/peptide hydrophobic mismatch on the thickness of diacylphosphatidylcholine bilayers. A 2H NMR and ESR study using designed transmembrane α-helical peptides and gramicidin A. Biochemistry. 37:9333–9345. [DOI] [PubMed] [Google Scholar]
- 18.Strandberg, E., S. Özdirekcan, D. T. S. Rijkers, P. C. A. van der Wel, R. E. Koeppe II, R. M. J. Liskamp, and J. A. Killian. 2004. Tilt angles of transmembrane model peptides in oriented and nonoriented lipid bilayers as determined by 2H solid-state NMR. Biophys. J. 86:3709–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Özdirekcan, S., D. T. S. Rijkers, R. M. J. Liskamp, and J. A. Killian. 2005. Influence of flanking residues on tilt and rotation angles of transmembrane peptides in lipid bilayers. A solid-state 2H NMR study. Biochemistry. 44:1004–1012. [DOI] [PubMed] [Google Scholar]
- 20.van den Brink-van der Laan, E., V. Chupin, J. A. Killian, and B. De Kruijff. 2004. Small alcohols destabilize the KcsA tetramer via their effect on the membrane lateral pressure. Biochemistry. 43:5939–5942. [DOI] [PubMed] [Google Scholar]
- 21.Spelbrink, R. E. J., A. Kolkman, M. Slijper, J. A. Killian, and B. De Kruijff. 2005. Detection and identification of stable oligomeric protein complexes in Escherichia coli inner membranes. J. Biol. Chem. 280:28742–28748. [DOI] [PubMed] [Google Scholar]
- 22.Feller, S. E., C. A. Brown, D. T. Nizza, and K. Gawrisch. 2002. Nuclear overhauser enhancement spectroscopy cross-relaxation rates and ethanol distribution across membranes. Biophys. J. 82:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry, J. A., and K. Gawrisch. 1994. Direct NMR evidence for ethanol binding to the lipid-water interface of phospholipid bilayers. Biochemistry. 33:8082–8088. [DOI] [PubMed] [Google Scholar]
- 24.Cantor, R. S. 1997. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 36:2339–2344. [DOI] [PubMed] [Google Scholar]
- 25.Cantor, R. S. 2002. Size distribution of barrel-stave aggregates of membrane peptides: influence of the bilayer lateral pressure profile. Biophys. J. 82:2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijkers, D. T. S., J. A. W. Kruijtzer, J. A. Killian, and R. M. J. Liskamp. 2005. A convenient solid phase synthesis of S-palmitoyl transmembrane peptides. Tetrahedron Lett. 46:3341–3345. [Google Scholar]
- 27.de Planque, M. R. R., J. A. W. Kruijtzer, R. M. J. Liskamp, D. Marsh, D. V. Greathouse, R. E. Koeppe II, B. de Kruijff, and J. A. Killian. 1999. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J. Biol. Chem. 274:20839–20846. [DOI] [PubMed] [Google Scholar]
- 28.Ten Kortenaar, P. B. W., B. G. van Dijk, J. M. Peters, B. J. Raaben, P. J. H. M. Adams, and G. I. Tesser. 1986. Rapid and efficient method for the preparation of Fmoc-amino acids starting from 9-fluorenylmethanol. Int. J. Pept. Protein Res. 27:398–400. [Google Scholar]
- 29.Rouser, G., S. Fleisher, and A. Yamamoto. 1970. Two-dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 5:494–496. [DOI] [PubMed] [Google Scholar]
- 30.Van der Wel, P. C. A., E. Strandberg, J. A. Killian, and R. E. Koeppe II. 2002. Geometry and intrinsic tilt of a tryptophan-anchored transmembrane α-helix determined by 2H NMR. Biophys. J. 83:1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis, J. H. 1983. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim. Biophys. Acta. 737:117–171. [DOI] [PubMed] [Google Scholar]
- 32.Sternin, E., M. Bloom, and A. L. Mackay. 1983. De-pake-ing of NMR spectra. J. Magn. Reson. 55:274–282. [Google Scholar]
- 33.Petrache, H. I., S. W. Dodd, and M. F. Brown. 2000. Area per lipid and acyl length distributions in fluid phosphatidylcholines determined by 2H NMR spectroscopy. Biophys. J. 79:3172–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henzler-Wildman, K. A., G. V. Martinez, M. F. Brown, and A. Ramamoorthy. 2004. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 43:8459–8469. [DOI] [PubMed] [Google Scholar]
- 35.Rajamoorthi, K., H. I. Petrache, T. J. McIntosh, and M. F. Brown. 2005. Packing and viscoelasticity of polyunsaturated ω-3 and ω-6 lipid bilayers as seen by 2H NMR and x-ray diffraction. J. Am. Chem. Soc. 127:1576–1588. [DOI] [PubMed] [Google Scholar]
- 36.Lackowicz, J. R. 1999. Principles of fluorescence spectroscopy, Kluwer Academic/Plenum, New York.
- 37.Seelig, J. 1978. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim. Biophys. Acta. 515:105–140. [DOI] [PubMed] [Google Scholar]
- 38.Burnell, E. E., P. R. Cullis, and B. de Kruijff. 1980. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P NMR lineshapes. Biochim. Biophys. Acta. 603:63–69. [DOI] [PubMed] [Google Scholar]
- 39.de Planque, M. R. R., E. Goormaghtigh, D. V. Greathouse, R. E. Koeppe II, J. A. W. Kruijtzer, R. M. J. Liskamp, B. de Kruijff, and J. A. Killian. 2001. Sensitivity of single membrane-spanning alpha-helical peptides to hydrophobic mismatch with a lipid bilayer: effects on backbone structure, orientation, and extent of membrane incorporation. Biochemistry. 40:5000–5010. [DOI] [PubMed] [Google Scholar]
- 40.Holte, L. L., and K. Gawrisch. 1997. Determining ethanol distribution in phospholipid multilayers with MAS-NOESY spectra. Biochemistry. 36:4669–4674. [DOI] [PubMed] [Google Scholar]
- 41.Abraham, M. H., H. S. Chadha, G. S. Whiting, and R. C. Mitchell. 1994. Hydrogen bonding. 32. An analysis of water-octanol and water-alkane partitioning and the Δlog P parameter of Seiler. J. Pharm. Sci. 83:1085–1100. [DOI] [PubMed] [Google Scholar]
- 42.Åkerlöf, G. 1932. Dielectric constants of some organic solvent-water mixtures at various temperatures. J. Am. Chem. Soc. 54:4125–4139. [Google Scholar]
- 43.Hong, D.-P., M. Hoshino, R. Kuboi, and Y. Goto. 1999. Clustering of fluorine-substituted alcohols as a factor responsible for their marked effects on proteins and peptides. J. Am. Chem. Soc. 121:8427–8433. [Google Scholar]
- 44.Mukherjee, L. M., and E. Grunwald. 1958. Physical properties and hydrogen-bonding in the system ethanol–2,2,2-trifluoro-ethanol. J. Phys. Chem. 62:1311–1314. [Google Scholar]
- 45.Lafleur, M., B. Fine, E. Sternin, P. R. Cullis, and M. Bloom. 1989. Smoothed orientational order profile of lipid bilayers by 2H-nuclear magnetic resonance. Biophys. J. 56:1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purohith, H. D., H. S. Sharma, and A. D. Vyas. 1975. Dielectric dispersion and relaxation mechanism in some trifluoro compounds at microwave frequencies. Bull. Chem. Soc. Jpn. 48:327–329. [Google Scholar]
- 47.Andersen, O. S., and R. E. Koeppe II. 2007. Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36:107–130. [DOI] [PubMed] [Google Scholar]
- 48.Lundbæk, J. A. 2006. Regulation of membrane protein function by lipid bilayer elasticity—a single molecule technology to measure the bilayer properties experienced by an embedded protein. J. Phys. Condens. Matter. 18:S1305–S1344. [DOI] [PubMed] [Google Scholar]
- 49.McIntosh, T. J., and S. A. Simon. 2006. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 35:177–198. [DOI] [PubMed] [Google Scholar]
- 50.White, S. H., and W. C. Wimley. 1998. Hydrophobic interactions of peptides with membrane interfaces. Biochim. Biophys. Acta. 1376:339–352. [DOI] [PubMed] [Google Scholar]
- 51.Meyer, H. H. 1899. Zur Theorie der Alkoholnarkose. Arch. Exp. Pathol. Pharmakol. 42:109–118. [Google Scholar]
- 52.Overton, E. 1901. Studien uber die Narkose, Zugleich ein Beitrag zur allgemeinen Pharmakologie. Gustav Fischer, Jena, Germany.
- 53.Dluzewski, A. R., M. J. Halsey, and A. C. Simmonds. 1983. Membrane interactions with general and local anaesthetics: a review of molecular hypotheses of anaesthesia. Mol. Aspects Med. 6:461–573. [DOI] [PubMed] [Google Scholar]
- 54.Krasowski, M. D., and N. L. Harrison. 2000. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br. J. Pharm. 129:731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kooijman, E. E., D. P. Tieleman, C. Testerink, T. Munnik, D. T. Rijkers, K. N. Burger, and B. de Kruijff. 2007. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J. Biol. Chem. 282:11356–11364. [DOI] [PubMed] [Google Scholar]
- 56.Akitake, B., R. E. Spelbrink, A. Anishkin, J. A. Killian, B. de Kruijff, and S. Sukharev. 2007. 2,2,2-Trifluoroethanol changes the transition kinetics and subunit interactions in the small bacterial mechanosensitive channel MscS. Biophys. J. 92:2771–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]