Abstract
Toll-like receptors are important in the activation of innate immunity, and CD40 is a molecule critical for many T and B cell responses. Whereas agonists for either pathway have been used as vaccine adjuvants, we show that a combination of Toll-like receptor (TLR)7 and CD40 agonists synergize to stimulate CD8+ T cell responses 10–20-fold greater than the use of either agonist alone. Antigen-specific CD8+ T cells elicited from combination CD40/TLR7 treatment demonstrated both lytic activities and interferon (IFN)γ production and an enhanced secondary response to antigenic challenge. Agonists for TLRs 2/6, 3, 4, and 9 also synergized with CD40 stimulation, demonstrating that synergy with the CD40 pathway is a property of TLR-derived stimuli in general. The CD8+ T cell expansion induced by CD40/TLR7 triggering was independent of CD4+ T cells, IFNγ, and IL-12 but dependent on B7-mediated costimulation and surprisingly on type I IFN. These studies provide the rational basis for the use of TLR and CD40 agonists together as essential adjuvants to optimize vaccines designed to elicit protective or therapeutic immunity.
Keywords: Toll like receptor, TLR7, CD40, CD8, T cell
Introduction
The magnitude and quality of the innate immune response exerts a profound impact on the ensuing adaptive immune response. Inflammatory cells and mediators generated as a result of initial tissue injury, infection, or necrotic death serve as initiators of a cascade of events that, when successful, culminates in the generation of productive T and B cell responses and long-term immunity. A family of receptors known as the Toll-like receptors (TLRs), named for their homology to molecules in Drosophila that serve functions in development and antimicrobial immunity (1), are critical to the ability of the cells of the innate immune system to respond to microbial and viral infections. Over the past few years, the macromolecules recognized by TLRs have been identified. Agonists for TLRs include the inflammatory mediators tri-acyl lipopeptides (TLR1), lipoteichoic acid (TLR2), dsRNA (TLR3), LPS (TLR4), flagellin (TLR5), diacyl lipopeptides (TLR6), imidazoquinolines (TLR7, TLR8), and CpGs (TLR9) (2). Activation of cells through TLRs elicits a variety of inflammatory cytokines and chemokines depending on the cell type and specific TLR being stimulated. As a testament to the importance of TLRs in immunity, TLR knockouts and knockouts of molecules critical to TLR signaling, such as MyD88 and TIRAP, result in the elimination of the majority of innate inflammatory mediators and a dramatic reduction in T and B cell responses (3–8).
The low molecular weight molecules known as imidazoquinolines or immune response modifiers (IRMs) have significant immunomodulatory capabilities and have been shown recently to be agonists for TLR7 in mouse and TLR7 and 8 in humans (9–11). Similar to other TLR agonists, IRMs such as imiquimod, resiquimod (R-848), and S-27609 (27609) induce a variety of cellular effects such as DC cytokine production, migration, and activation marker up-regulation, and B cell activation (12–15). Furthermore, IRMs induce significant amounts of type 1 IFN from the plasmacytoid DCs (9, 10, 16) in several species (15, 17, 18).
The central role played by TLRs in triggering innate immunity is mirrored by CD40 in controlling acquired immune responses. CD40, a TNFR superfamily member, is essential for a spectrum of cell-mediated immune responses and required for the development of T cell–dependent humoral immunity (19–21). The expression of CD40 on APCs (DCs, macrophages) and on B cells (19–23) provides an understanding for its profound impact on both arms of the acquired immune response. Stimulation through CD40 has been shown to induce the generation of CD4-independent CD8+ T cell responses (24–27). These reports speculated that CD40 agonists could potentially rescue failing CD4-dependent CD8+ T cell responses in some disease settings. Although data has supported the observation that CD40 has effects on long-term T cell survival (24, 28, 29), other data demonstrated that CD40 agonists alone are not sufficient to generate protective antitumor immunity or long-term immunity (30–32). In these cases, CD40 agonists used as a monotherapy have been shown to induce the deletion of antigen-specific T cells and cause the premature termination of humoral (32) and cellular (30, 31) immunity.
In the present study, we asked how the concomitant delivery of TLR and CD40 agonists enhanced antigen-specific, acquired immune responses. Although antigenic challenge in conjunction with either CD40 or TLR7 agonists alone elicited a minimal, though detectable, primary CD8+ T cell response, the combination of both agonists induced an exponential expansion of antigen-specific T cells. The combination of agonists induced heightened T cell expansion, high levels of lytic activity and cytokine production, and the development of a functional memory T cell pool. Interestingly, this synergy was a property of multiple TLR agonists including TLRs 2/6, 3, 4, and 9. Although the T cell expansion was not dependent on CD4+ cells or IL-12, IL-23, or IFNγ, synergy resulting from most, though not all, TLR agonists was dependent on type I IFN. Hence, the use of a CD40 agonistic antibody in conjunction with a low molecular weight TLR7 agonist can reconstitute all of the signals required to elicit profound acquired cell-mediated immunity.
Materials and Methods
Mice.
C57BL/6 (Ly5.1) mice were purchased from National Cancer Institute and Charles River Laboratories and housed under specific pathogen-free conditions. B6.129S1-Il12a tm1Jm, B6.129S1-Il12b tm1Jm, B6.129P2-Tnfrsf5 tm1Kik, B6.129S4-CD80 tm1Shr CD86 tm1Shr, B6.129S7-Ifng tm1Ts, and B6.129S2-CD4 tm1Mak mice were purchased from The Jackson Laboratory. B6.129-Abb Tm1N5 and B6/129 F1 mice were purchased from Taconic Farms Inc. MyD88 KO mice were a gift from Dr. Douglas T. Golenbock (University of Massachusetts Medical School, Worcester, MA) and were bred at Dartmouth College. IFNαβR KO mice were a gift from Dr. Philippa Marrack (National Jewish Medical and Research Center, Denver, CO) and were bred on site at 3M Pharmaceuticals.
Monoclonal Antibodies.
The following antibodies were purchased from BD Biosciences: anti–mouse CD8-APC (Ly-2), anti–mouse CD44-FITC (Pgp-1, Ly-24), and B220-Cy (RA3–6B2). CD40 (1C10 or FGK45) were produced by hybridomas that were grown in serum-free conditions. Ova-specific CD8 T cells were detected by H-2Kb–specific tetramers containing the SIINFEKL peptide, either made as described previously (33) (a gift from Dr. Lefrancois, University of Connecticut Health Center, Farmington, CT) or purchased from Beckman Coulter.
TLR Agonists.
The IRM 1-(4-amino-2-methyl-1H-imidazo[4,5-c]quinolin-1-yl)-2-methylpropan-2-ol hydrochloride (S-27609) was synthesized as described previously (15). It was reconstituted in water at 10 mg/ml and diluted in PBS for injection into mice. Other TLR agonists used were CpG 1826 (Invitrogen Life Technologies), LPS (Sigma-Aldrich), polyinosinic-polycytidylic acid (polyI:C) (Amersham Biosciences), Malp-2 (Alexis Biochemicals), and S-27609 (3M Pharmaceuticals).
Immunization.
6–12-wk-old female C57BL/6J mice were injected i.p., unless otherwise noted, with 0.5 mg whole ovalbumin (Sigma-Aldrich) with or without varying amounts of TLR agonists and/or 50 μg of 1C10 or FGK45 (anti-CD40). Where peptide injections are noted, mice were injected with anti-CD40 i.p. and then 4–6 h later injected i.v. with 100 mg SIINFEKL peptide and a given TLR agonist. Anti-CD40 was used at 50 μg per injection. Mice were immunized with a single injection i.p. and killed 6 d later unless otherwise noted.
Cell Preparation.
6 d after i.p. injections, spleens were removed and homogenized into single cell suspensions. RBCs were lysed using an ammonium chloride buffer followed by washing. Cells were resuspended in complete medium (SMEM [Biosource International], 10% heat-inactivated FBS [Hyclone], 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 1% PenStrep, and 1% l-glutamine [Sigma-Aldrich]). Cells were resuspended at 2–4 × 107 cells/ml.
Analysis of MHC Tetramer by Extracellular Staining and Flow Cytometry.
Cells were plated in duplicate in 96-well round-bottomed plates and stained with Kb/ovalbumin tetramer (33) for 1–2 h at room temperature (RT) or 37°C. Multiparameter analysis of tetramer-positive cells was afforded by staining cells (30 min at 37°C or RT) with anti-CD44–FITC, anti-Y3P–TNP, anti-B220–cychrome, and anti-CD8–APC. Cells were then washed in FACS® buffer, and four-color FACS® data was collected on a BD FACSCalibur flow cytometer and analyzed using CELLQUEST software. Analysis typically gated on CD8+, MHC class II− cells to assess tetramer staining.
Analysis of IFN-γ by Intracellular Staining and Flow Cytometry.
Cells were plated in 96-well round-bottomed plates and pulsed with SIINFEKL peptide as antigen (or without antigen as a control) in the presence of Golgi-plug (BD Biosciences) (brefeldin A) for 4–6 h in complete media at 37°C. Cells were stained using anti-CD4–cychrome and anti-CD8–FITC. After washing, cells were fixed using Cytofix (BD Biosciences) for ∼15 min. Cells were then washed and permeabilized using Perm/Wash (BD Biosciences). Intracellular staining for IFN-γ–APC was then performed according to the BD Biosciences protocol. Four-color FACS® data was collected on a BD FACSCalibur flow cytometer and analyzed using CELLQUEST software to quantify CD8+ T cells producing IFN.
In Vivo Cytotoxicity Assay.
Syngeneic splenocytes were labeled with either 0.5 or 5 μM CFSE for 15 min at 37°C and washed twice. CFSEhigh cells were subsequently pulsed with 50 μg/ml SIINFEKL peptide for 60 min at 37°C. CFSElow cells served as an internal control and therefore were not pulsed with peptide. Cells were mixed at a 1:1 ratio, and then 5 × 106 total cells were injected i.v. into mice challenged previously with combinations of antigen, TLR agonist, and CD40 as described above. 12–18 h later, splenocytes from each mouse were analyzed by FACS® to detect the presence of CFSE-labeled cells. The ratio of antigen-unpulsed, low CFSE cells to pulsed, high CFSE labeled cells was calculated as an indication of antigen-specific lytic activity.
Results
Concomitant Administration of TLR and CD40 Agonists Induce the Synergistic Expansion of Antigen-specific CD8+ T Cells.
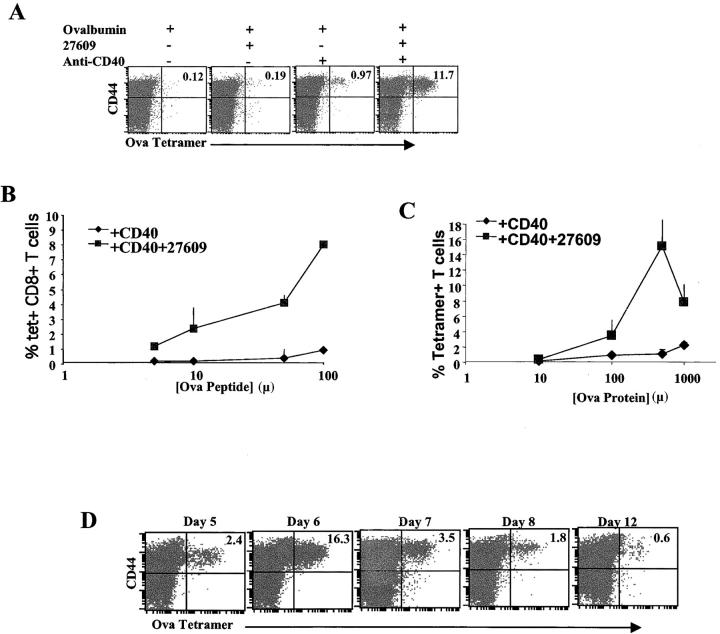
Previous studies have shown that CD40 triggering of DCs can enhance CTL activation and replace the need for T cell help (24–27). However, emerging data suggest that TLR triggering may be critical at optimizing CD40-induced maturation of DCs (34, 35). No studies thus far have exhaustively evaluated the impact of combined TLR and CD40 engagement on the CD8+-acquired immune response. To this end, mice were immunized with 500 μg soluble OVA protein combined with an agonistic anti-CD40 mAb and/or the TLR7 agonist, S-27609 (27609) (13) (Fig. 1 A). Expansion of antigen-specific CD8+ T cells in vivo was quantified by staining with an OVA H-2 Kb tetramer 6 d after immunization. As has been shown previously by Lefrancois et al., the administration of anti-CD40 and OVA can enhance the accumulation of tetramer-positive cells (24). Compared with the soluble OVA alone, modest enhancements in tetramer-positive CD8+ T cells appeared with OVA and anti-CD40 or S-27609 (Fig. 1 A). However, combined administration of OVA, anti-CD40, and S-27609 induced a synergistic accumulation of tetramer-positive cells. To determine whether the synergy between the TLR7 and CD40 would enhance CD8+ T cell responses at more limiting antigen doses as well, we immunized mice with decreasing amounts of ovalbumin peptide (Fig. 2 B) or protein (Fig. 2 C), and the CD8+ T cell response was assessed as before. As expected, cotriggering of CD40 and TLR7 induced a significant expansion of antigen-specific CD8+ T cells well above that (>10-fold) seen with antigen and CD40 alone even down to as little as 5 μg of peptide or 100 μg of protein.
Figure 1.
Anti-CD40 and the TLR7 agonist 27609 synergize to induce enhanced CD8+ T cell expansion. (A) C57BL/6 mice were immunized i.p. with 500 μg of whole ovalbumin protein, 50 μg of the anti-CD40 antibody FGK45, and 100 μg of 27609 in the combinations indicated above. Mice were killed 6 d after immunization, and spleen cells were isolated and stained with tetramer as described in Materials and Methods to identify ovalbumin-specific T cells. The data shown has been gated on all CD8+, B220− events. The percentages given in the top right quadrant are the percentage of tetramer staining cells out of total CD8+ T cells. (B and C) Mice were immunized as in A with increasing amounts of peptide i.v. (B) or whole ovalbumin (C) and anti-CD40 ± 27609 i.p. 6 d after priming, the spleen cells were stained and analyzed as in A for ovalbumin-specific T cells. Percentages given are tetramer staining cells out of total CD8+ T cells. Error bars represent SD of three mice per group. (D) Mice were immunized as in A with whole ovalbumin, anti-CD40, and 27609, and at the times indicated after priming, the spleen cells were removed and analyzed as in A. The data shown was gated on all live, CD8+, B220− events. The percentages given in the top right quadrant are the percentage of tetramer staining cells out of total CD8+ T cells.
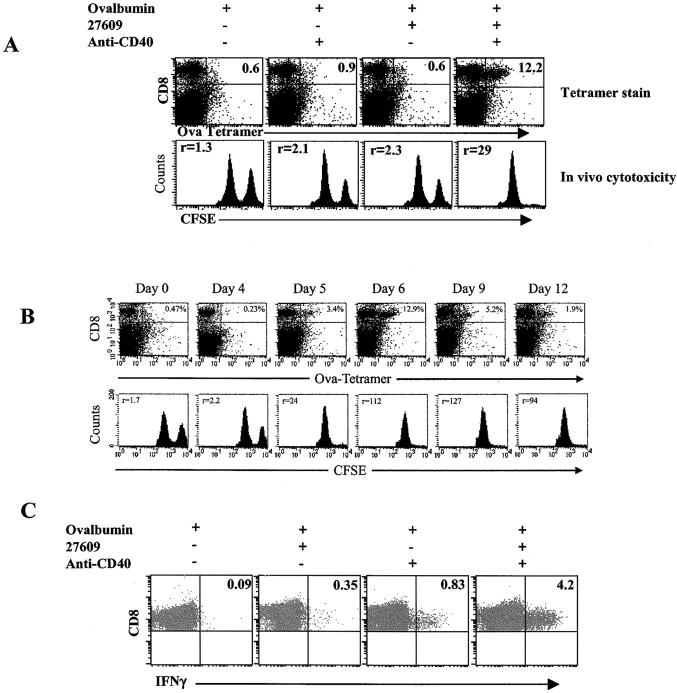
Figure 2.
CD40/TLR7 triggering induces functional CTL. (A) Mice primed with 500 μg whole ovalbumin ± CD40 and/or S-27609 were assessed by using an in vivo cytotoxicity assay as described in Materials and Methods. The number in the top left of the histograms indicates the ratio of nonantigen pulsed, low CFSE-labeled spleen cells to antigen pulsed, high CFSE-labeled spleen cells. (B) As in A, mice were immunized and analyzed by tetramer staining and in vivo cytotoxic activity at the time points indicated. (C) Cells from mice treated as in A (day 7 after priming) were incubated in the presence of brefeldin A with or without SIINFEKL peptide for 6 h at 37°. The cells were then stained for CD8, fixed, permeabilized, and stained for intracellular IFNγ as described in Materials and Methods. The data shown is gated on all CD8+ events. Numbers in the top right quadrant indicate the percentage of IFNγ+ cells out of the total CD8+ cells.
The kinetics and antigen dose response of the OVA-specific CD8+ T cell responses to combined CD40/TLR administration was evaluated. As seen in Fig. 1 D, combined TLR and CD40 triggering induces a rapid expansion in response to protein immunization followed by a contraction of antigen-specific CD8+ T cells. The peak accumulation of antigen-specific CD8+ T cells was observed on day 6 postadministration of antigen, TLR, and CD40 agonists. After the day 6 peak, a rapid contraction in CD8+ T cell numbers was observed by day 9. The magnitude of the CD8 response elicited by the combined triggering of CD40 and TLR is similar to that seen in antiviral responses, such as responses against LCMV (36). The time course of the response after peptide immunization was essentially indistinguishable from protein immunization (not depicted).
TLR7/CD40 Synergy Induces Functional CTL.
In addition to CD8+ T cell expansion, CTL killing activity and expression of IFNγ were evaluated. On days after immunization, in vivo CTL activity was measured (37–40). The in vivo CTL assay showed that cotriggering of CD40 and TLR7 induced significant lytic activity (Fig. 2 A). Detectable lytic activity was present in the immunized mice from day 5 through 12 (Fig. 2 B). Cotriggering of CD40/TLR7 also elicited intracellular expression of IFNγ (Fig. 2 C), though CD40 stimulation alone also induced detectable IFNγ production (Fig. 2 C) from the cells from this time point. IFNγ production was first detected on day 6 after priming (5–20% of Ag-specific T cells) and peaked by day 7–8 (60–90% of Ag-specific T cells) (not depicted and Fig. 1 C). Therefore, the data show that the combined actions of CD40 and TLR7 agonists resulted in antigen-specific T cell expansion and differentiation.
TLR/CD40 Triggering Elicits Long-Term, Antigen-specific CD8+ T Cell Immunity.
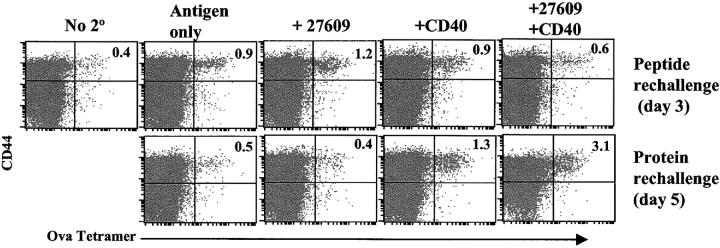
The fate of the TLR/CD40-induced T cells was evaluated to determine if the initial expansion and contraction of the tetramer-positive T cells lead to anergy or memory. Numerous models exist whereby dramatic T cell expansion is followed by an equally dramatic demise in T cell numbers and function, leaving any remaining cells in a hyporesponsive state of tolerance (30, 36, 41–44). Alternatively, productive responses present the same patterns of expansion and contraction, yet upon rechallenge, secondary expansion can be observed. Mice initially immunized with OVA, anti-CD40, and S-27609 were rechallenged 30 d after initial priming with combinations or CD40 and TLR7 agonists and either peptide (Fig. 3, top) or protein (Fig. 3, bottom). The secondary response was less reliant on costimulatory signals than the primary response, demonstrating a detectable increase in tetramer-staining cells in response to protein or peptide rechallenge alone. The addition of the TLR7 agonist to peptide rechallenge elicited a better (enhanced) response compared with peptide alone, which peaked on day 3 after rechallenge. In contrast, rechallenge with protein was enhanced when CD40 was added and the response peaked on day 5 after rechallenge. This difference between peptide and protein rechallenge in the time of the peak of the response likely reflects the additional time necessary for processing and presentation of the protein. The enhancement of the protein response with anti-CD40 is also consistent with the role of CD40 in augmenting crosspriming. CD40 did enhance the peptide response compared with peptide alone, but for reasons that are not clear, this response peaked on day 5 and was not any greater than the day 3 response to peptide and the TLR7 agonist (not depicted). In all cases, the secondary response was more rapid but lower than the peak of the primary response. This has been is seen in other experimental systems (41, 45, 46) and is probably due to the rapid clearance of the antigen by the high frequency of functional memory cells. In general, the data demonstrate that the primary response generated by immunization in the combined presence of CD40 and TLR7 agonists leads to the generation of functional memory CTLs.
Figure 3.
TLR/CD40 triggering produces long-term T cell immunity. (A) Mice were immunized i.p. with ovalbumin, anti-CD40, and S-27609 as in Fig. 1. 30 d later, the mice were rechallenged i.p. with either 100 μg SIINFEKL peptide or 500 μg ovalbumin protein ± 27609/CD40 as indicated. At days 3 and 5 after rechallenge, spleen cells were isolated and stained with tetramer as described in Materials and Methods. Peak responses for peptide or protein responses are shown (day 3 for peptide rechallenge and day 5 for protein rechallenge). The data shown was gated and analyzed as in Fig. 1 A. The data is representative of three experiments performed.
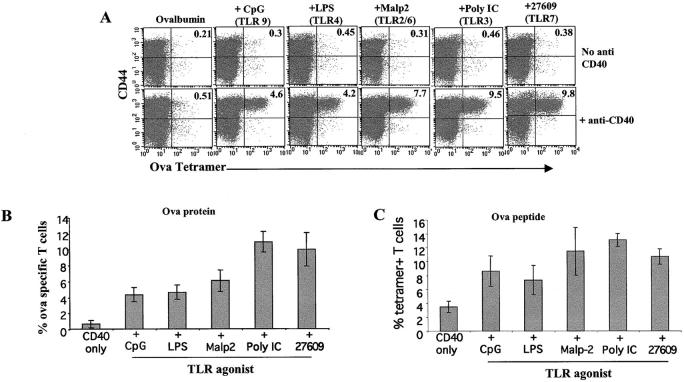
Synergy with CD40 in the Induction of CD8+ T Cell Proliferation and Differentiation Is a Property of Multiple TLR Agonists.
CD40 has been used in combination with a variety of stimuli and has been shown to enhance immune responses (28, 33, 47). We considered the possibility that the synergy we observed between TLR7 and CD40 was a property of TLR agonists in general and not necessarily of only TLR7 agonists. Mice were immunized with anti-CD40, ovalbumin protein (Fig. 4, A and B), or peptide (Fig. 4 C) and agonists for TLR 2/6, 3, 4, 7, or 9. 6 d after the administration of these agents, the frequency of OVA-specific T cells was determined as before. With respect to OVA-specific tetramer-positive CTL expansion, all TLR agonists demonstrated synergy with the CD40 agonist (Fig. 4 A), though each TLR agonists synergized to varying degrees with CD40 (Fig. 4, B and C). As with the TLR7 agonist, all of the TLR agonists elicited IFNγ production from the T cells as well (not depicted). Therefore, signals derived from a variety of TLRs are able to synergize with CD40 signaling to induce T cell expansion and differentiation.
Figure 4.
Synergy with CD40 in the induction of CD8+ T cell proliferation and differentiation is a property of multiple TLR agonists. (A) Mice were challenged i.p. with ovalbumin and the indicated TLR agonists (30 μg LPS as a TLR4 agonist, 100 μg CpG 1826 as a TLR9 agonist, 25 μg Malp-2 as a TLR2/6 agonist, 50 μg poly IC as a TLR3 agonist, and 100 μg 27609 as the TLR7 agonist), with or without anti-CD40. 6 d after challenge, spleens cells were isolated and stained with tetramer as described in Fig. 1. The data shown was gated and analyzed as in Fig. 1 A. (B and C) The average percentage of tetramer staining T cells and their SDs from three mice per treatment primed with whole protein (B) or peptide (C) were analyzed and calculated. The data shown is representative of three to eight experiments performed, depending on the TLR agonist.
TLR7/CD40 Triggering of CD8+ T Cell Expansion Is Independent of CD4 Cells, IFNγ, IL-12, or IL-23.
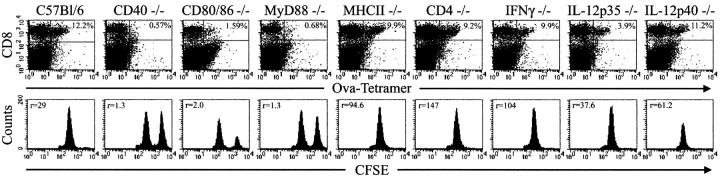
CD8+ T cell responses have been shown to be either dependent or independent of CD4+ T cells or cytokine-mediated help (48). To determine which cellular and molecular components influenced the magnitude of the CD8+ T cell response induced by CD40 and TLR7 agonists and antigen, a series of studies in genetically deficient mice were performed (Fig. 5). The magnitude of the CD8+ expansion or differentiation to lytic function induced by immunization with OVA and CD40 and TLR7 agonists was not diminished in either CD4 −/− or class II MHC −/− mice, demonstrating the helper independent nature of this form of priming. Additionally, neither IL-12, IL-23, nor IFNγ were required, as expansion in IL-12p35 −/− , IL-12p40 −/− , and IFNγ2/− mice, respectively, were similar to WT mice. Costimulation via CD28 was deemed critical as expansion in the CD80/CD86 −/− mice was ablated. Reaffirmation of the functional importance of CD40 and the TLR signaling pathway (2) was demonstrated by the lack of expansion of tetramer-positive cells in CD40−/− and MyD88−/− mice. Thus, other than classical B7-mediated costimulation, which is a property of T cell responses in general, the induction of primary immunity by TLR/CD40 cotriggering is independent of IL-12, IL-23 (49), IFNγ, and the presence of CD4 helper cells.
Figure 5.
TLR/CD40 triggering of CD8+ T cell expansion and effector function is largely dependent on costimulation via CD80/86 but independent of CD4 cells, IFNγ, IL-12, or IL-23. The indicated genetically deficient mice were immunized with 500 μg ovalbumin plus 50 μg anti-CD40 and 200 μg 27609 by i.p. injection. On day 6 postimmunization, in vivo lytic activity was measured as in Fig. 2 A, and splenocytes were isolated and analyzed as in Fig. 1 but using anti-CD8α PE and APC-labeled SIINFEKL/H-2Kb tetramers. Data is representative of at least three mice per group. Numbers in the top right quadrant of the dotplots indicates the percentage of tetramer+ CD8 T cells out of total CD8 T cells. The number in the top left of the histograms indicates the ratio of nonantigen pulsed, low CFSE-labeled spleen cells to antigen pulsed, high CFSE-labeled spleen cells.
Dependence on Type I IFN Varies with the Specific TLR Agonist Employed.
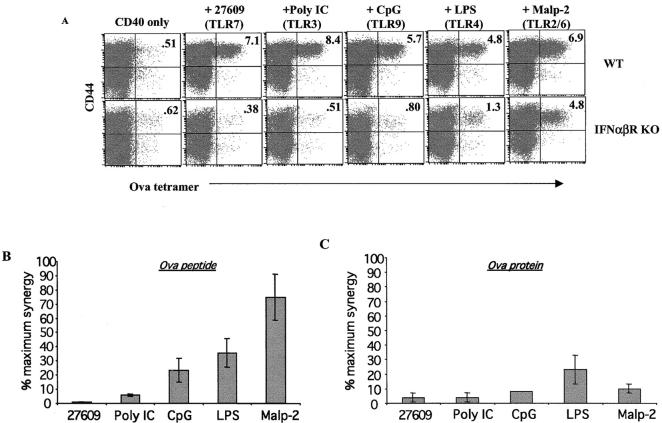
In previous experiments, it was observed that the TLR agonists that synergized with CD40 to produce the highest level of CD8+ T cell expansion (poly IC and 27609) were also potent inducers of type I IFN (IFNα/β) (Fig. 3). We sought to determine whether IFNα/β contributed to CD8+ T cell expansion by performing experiments in IFNα/β receptor knockout mice (IFNα/βR KO). Surprisingly, the synergy between CD40 and TLR7 was essentially abrogated in IFNα/βR KO mice with the CD8+ T cell expansion being reduced down to that seen in the presence of anti-CD40 alone (Fig. 6 A). However, the TLR agonists that induced the least IFNαβ appeared to induce synergy that was least affected by the loss of type I IFN signaling (Fig. 6, A–C). For example, in response to peptide immunization (Fig. 6, A and B) Malp-2 (TLR2/6 agonist) synergy with CD40 was only modestly affected in the IFNαβR KOs (60–90% of max), whereas LPS, CpG, polyI:C, and 27609 were increasingly affected (respectively) in the absence of the IFNαβ receptor. In contrast, synergy with all TLR agonists was all but abrogated when the mice were challenged with protein instead of peptide (Fig. 6 C). This is consistent with recent literature reporting that type I IFN facilitates crosspriming in APC (50, 51). However, the loss of synergy in response to priming with peptide (Fig. 6, A and B) demonstrates that for all TLR agonists but Malp-2, type I IFN plays a larger role than just enabling crosspriming. Thus, the data suggests that synergy between the TLR pathway and the CD40 pathway is best observed under conditions where the TLR agonist induces type I IFN and further that the CD8+ T cell expansion seen under these conditions is dependent on some aspect of type I IFN signaling.
Figure 6.
CD40 synergy with IFNα/β-inducing TLR agonists is critically dependent on type I IFN. Either wt control B6/129 F1 or IFNαβR KO mice were immunized with 100 μg of SIINFEKL peptide (A and B) or 500 μg ovalbumin protein (C), 50 μg anti CD40, and the indicated TLR agonist (30 μg LPS as a TLR4 agonist, 100 μg CpG 1826 as a TLR9 agonist, 25 μg Malp-2 as a TLR2/6 agonist, 50 μg poly IC as a TLR3 agonist, and 100 μg 27609 as the TLR7 agonist) as in Fig. 3. On day 6, spleen cells were stained with tetramer and analyzed as in Fig. 1 A. The legend above the figure indicates the TLR agonist used. (B and C) The percentage of tetramer staining cells in the mice from A after either peptide (B) or whole protein (C) challenge were calculated as the percentage of tetramer staining cells out of total CD8+ T cells. This percentage was then divided by the percentage of tetramer staining cells from wild-type mice challenged with the same TLR agonist. The data is expressed as the percentage of maximum synergy seen in the IFNαβR KO mice compared with the wild type for the given TLR agonist. The data shown is an average from three mice per treatment group, and error bars indicate the calculated SD. The data represents three experiments performed.
Discussion
The work presented here demonstrates that the concomitant signaling via TLR and CD40 results in a synergistic increase in expansion of antigen-specific CTL and their differentiation to effector cells. The combination of TLR7 and CD40 agonists synergized to stimulate heightened CTL proliferation and function with respect to IFNγ production and lytic activity. The high level of CD8+ T cell proliferation seen was neither abortive nor exhaustive (30, 36, 42, 43), since the mice generated secondary T cell expansion upon rechallenge with antigen with or without TLR7/CD40 agonists. These memory T cells expressed all of the hallmarks of a functional secondary response, i.e., responding more rapidly and with a reduced dependency on adjuvant compared with the primary response (46, 52–55). Of particular interest was the fact that TLR 2/6, 3, 4, and 9 agonists also were capable of synergizing with anti-CD40 to induce the expansion of OVA-specific CD8+ T cells, with the TLR agonists that generated higher levels of type I IFN tending to produce the highest levels of T cell expansion. Thus, some aspect of TLR and CD40 signaling must have a general point of intersection, an observation that is consistent with the biology of the receptors, i.e., APC could receive an initial activation stimulus through a TLR at the site of infection and after migration to the T cell zones of the lymphoid tissue receive a CD40 stimulus from antigen-specific T cells, effectively “confirming” the APC's activation and further reinforcing T cell expansion.
Previous studies have suggested that, at least in certain circumstances, CD40 stimulation could rescue defective or abortive CD8+ T cell proliferation and effector cell function. Indeed, these reports demonstrated that CD40 stimulation alone allowed normally CD4-dependent CD8+ T cell responses to proceed in the absence of CD4 help (24–27), implicating CD40 signaling as the primary mechanism of CD4 help for CTL proliferation. More recent reports have further suggested that at least in some disease and experimental models CD40 stimulation alone was necessary and sufficient to initiate long-lived CTL expansion and effector function (24, 28, 29). However, this is not the case in all systems and models tested. Both IL-12 production (34) and antitumor CTL responses (30) have been shown to be dependent on CD40 in combination with other bacterial/viral-derived stimuli. Indeed, some studies have demonstrated that CD40 stimulation alone resulted not in immunity but in deletion of antigen-specific T cells (30, 31) and termination of humoral responses (32). Our data demonstrates that although CD40 is able to induce CD8+ T cell immunity to a limited degree, this can be exponentially enhanced by the addition of a TLR agonist. Therefore, we would predict that the clinical efficacy of CD40 agonists could be dramatically improved if provided in conjunction with an appropriate antigen and TLR agonist, specifically a TLR7 agonist, as our data demonstrates.
CD40 is expressed on APCs (DCs, macrophages, B cells), and it is generally agreed that stimulation through CD40 plays a role in the activation of APCs to become competent to initiate CD8+ T cell proliferation. In contrast, a report from Bourgeois et al. has demonstrated that CD8+ T cells can express CD40 after activation and that this expression of CD40 on the T cells may play a greater role in CD8+ T cell proliferation than CD40 expression on the APC (56). Given this observation, it is possible that the synergy we demonstrate between TLR and CD40 agonists is due to direct stimulation of CD40 on the antigen-specific T cells themselves and not on the APC. However, a recent report from Lee et al. shows that CD8 responses to influenza are dependent on CD40 expression on the APC and that neither the APC nor the CD4 T cells directly stimulate CD8 T cells through CD40–CD40L interactions (57). This study showed through various bone marrow chimeras that CD40−/− CD8 T cells expanded as well as CD40 +/+ CD8 cells in host, strongly arguing against a functional role for CD40 expression on the CD8 T cells. However, we cannot as of yet rule out a role for direct stimulation of CD40 on the T cells in the CD8 + T cell expansion seen in our model system. Experiments are in progress to determine whether CD40 expression on APC or T cells is necessary for this synergistic induction of T cell expansion by the cotriggering of TLR/CD40.
Perhaps the most interesting feature of the observed synergy is its variable dependence on type I IFN. In general, the data is consistent with the interpretation that the degree of synergy is related to and dependent on the level of IFN induced by the TLR agonists. However, the TLR2/6 agonist Malp-2 (58, 59) appears to be somewhat of an exception to that rule. TLR2 agonists have been shown to induce little to no type I IFN (3, 60, 61), but Malp-2 demonstrated significant levels of synergy with CD40 nonetheless. Therefore, the data suggest that there are at least two ways that TLRs synergize with CD40: one mediated through type I IFN signaling and the other mediated through some aspect of Malp-2 signaling. We have demonstrated that for those TLR agonists that induce either IFNα or β, synergy with CD40 is increasingly dependent on type I IFN. However, it is currently unclear given the almost ubiquitous expression of the IFNαβ receptor, in which cell type(s) IFN signaling is necessary for producing this synergy. Interestingly the TLR/CD40-triggered CD8+ T cell expansion in the IFNαβR KO mice was reduced to a greater extent in response to whole protein antigen challenge, even when using Malp-2 as the TLR agonist. This further supports the identified role of type I IFN in crosspriming (50, 51) and demonstrates that at least some, though not all, aspects of TLR/CD40-induced synergy are mediated by the effects of IFN on antigen processing and presentation.
In conclusion, these studies underscore the powerful immune synergy that exists when receptors from both innate and acquired immunity are triggered. It is tempting to speculate that the TLR and CD40 signaling cascades evolved the means for cross talk and integration so as to control the magnitude, duration, and quality of the immune response. At this time, the basis for TLR/CD40 cross talk is not known. Studies are underway to determine if signaling derived from TLR and CD40 agonists are directed at the same or different cellular targets and whether signaling elements common to both cascades may play a role in communications between these two receptor systems.
Acknowledgments
The authors would like to thank Jody Lutterman and Dave Johnson for breeding and maintaining the mouse color, and John Vasilakos and W. Chad Kieper for help in reviewing the manuscript.
C.L. Ahonen and C.L. Doxsee contributed equally to this work.
Abbreviations used in this paper: IRM, immune response modifier; TLR, Toll-like receptor.
References
- 1.Medzhitov, R., P. Preston-Hurlburt, and C.A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5–11. [DOI] [PubMed] [Google Scholar]
- 3.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto, M., S. Sato, H. Hemmi, H. Sanjo, S. Uematsu, T. Kaisho, K. Hoshino, O. Takeuchi, M. Kobayashi, T. Fujita, et al. 2002. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 420:324–329. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R.T. Schoen, R. Medzhitov, E. Fikrig, and R.A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878–884. [DOI] [PubMed] [Google Scholar]
- 6.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. [DOI] [PubMed] [Google Scholar]
- 7.Horng, T., G.M. Barton, R.A. Flavell, and R. Medzhitov. 2002. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 420:329–333. [DOI] [PubMed] [Google Scholar]
- 8.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, S.J., J.M. Lindh, T.R. Riter, R.M. Gleason, L.M. Rogers, A.E. Fuller, J.L. Oesterich, K.B. Gorden, X. Qiu, and S.W. McKane. 2002. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 218:74–86. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 3:196–200. [DOI] [PubMed] [Google Scholar]
- 11.Jurk, M., F. Heil, J. Vollmer, C. Schetter, A.M. Krieg, H. Wagner, G. Lipford, and S. Bauer. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 12.Bishop, G.A., Y. Hsing, B.S. Hostager, S.V. Jalukar, L.M. Ramirez, and M.A. Tomai. 2000. Molecular mechanisms of B lymphocyte activation by the immune response modifier R-848. J. Immunol. 165:5552–5557. [DOI] [PubMed] [Google Scholar]
- 13.Doxsee, C.L., T.R. Riter, M.J. Reiter, S.J. Gibson, J.P. Vasilakos, and R.M. Kedl. 2003. The immune response modifier and TLR7 agonist S-27609 selectively induces IL-12 and TNFα production in CD11c+CD11b+CD8− dendritic cells. J. Immunol. 171:1156–1163. [DOI] [PubMed] [Google Scholar]
- 14.Ahonen, C.L., S.J. Gibson, R.M. Smith, L.K. Pederson, J.M. Lindh, M.A. Tomai, and J.P. Vasilakos. 1999. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell. Immunol. 197:62–72. [DOI] [PubMed] [Google Scholar]
- 15.Testerman, T.L., J.F. Gerster, L.M. Imbertson, M.J. Reiter, R.L. Miller, S.J. Gibson, T.L. Wagner, and M.A. Tomai. 1995. Cytokine induction by the immunomodulators imiquimod and S-27609. J. Leukoc. Biol. 58:365–372. [DOI] [PubMed] [Google Scholar]
- 16.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-{alpha} and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaca, K., J.M. Sharma, M.A. Tomai, and R.L. Miller. 1996. In vivo and in vitro interferon induction in chickens by S-28828, an imidazoquinolinamine immunoenhancer. J. Interferon Cytokine Res. 16:327–332. [DOI] [PubMed] [Google Scholar]
- 18.Tomai, M.A., S.J. Gibson, L.M. Imbertson, R.L. Miller, P.E. Myhre, M.J. Reiter, T.L. Wagner, C.B. Tamulinas, J.M. Beaurline, J.F. Gerster, et al. 1995. Immunomodulating and antiviral activities of the imidazoquinoline S-28463. Antiviral Res. 28:253–264. [DOI] [PubMed] [Google Scholar]
- 19.Aruffo, A., M. Farrington, D. Hollenbaugh, X. Li, A. Milatovich, S. Nonoyama, J. Bajorath, L.S. Grosmaire, R. Stenkamp, M. Neubauer, et al. 1993. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 72:291–300. [DOI] [PubMed] [Google Scholar]
- 20.Farrington, M., L.S. Grosmaire, S. Nonoyama, S.H. Fischer, D. Hollenbaugh, J.A. Ledbetter, R.J. Noelle, A. Aruffo, and H.D. Ochs. 1994. CD40 ligand expression is defective in a subset of patients with common variable immunodeficiency. Proc. Natl. Acad. Sci. USA. 91:1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renshaw, B.R., W.C. Fanslow, III, R.J. Armitage, K.A. Campbell, D. Liggitt, B. Wright, B.L. Davison, and C.R. Maliszewski. 1994. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 180:1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alderson, M.R., R.J. Armitage, T.W. Tough, L. Strockbine, W.C. Fanslow, and M.K. Spriggs. 1993. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J. Exp. Med. 178:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefrancois, L., J.D. Altman, K. Williams, and S. Olson. 2000. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J. Immunol. 164:725–732. [DOI] [PubMed] [Google Scholar]
- 25.Bennett, S.R., F.R. Carbone, F. Karamalis, R.A. Flavell, J.F. Miller, and W.R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 393:478–480. [DOI] [PubMed] [Google Scholar]
- 26.Ridge, J.P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 393:474–478. [DOI] [PubMed] [Google Scholar]
- 27.Schoenberger, S.P., R.E. Toes, E.I. van der Voort, R. Offringa, and C.J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480–483. [DOI] [PubMed] [Google Scholar]
- 28.Sotomayor, E.M., I. Borrello, E. Tubb, F.M. Rattis, H. Bien, Z. Lu, S. Fein, S. Schoenberger, and H.I. Levitsky. 1999. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 5:780–787. [DOI] [PubMed] [Google Scholar]
- 29.Diehl, L., A.T. den Boer, S.P. Schoenberger, E.I. van der Voort, T.N. Schumacher, C.J. Melief, R. Offringa, and R.E. Toes. 1999. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 5:774–779. [DOI] [PubMed] [Google Scholar]
- 30.Kedl, R.M., M. Jordan, T. Potter, J. Kappler, P. Marrack, and S. Dow. 2001. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc. Natl. Acad. Sci. USA. 98:10811–10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauri, C., L.T. Mars, and M. Londei. 2000. Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat. Med. 6:673–679. [DOI] [PubMed] [Google Scholar]
- 32.Erickson, L.D., B.G. Durell, L.A. Vogel, B.P. O'Connor, M. Cascalho, T. Yasui, H. Kikutani, and R.J. Noelle. 2002. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J. Clin. Invest. 109:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedl, R.M., W.A. Rees, D.A. Hildeman, B. Schaefer, T. Mitchell, J. Kappler, and P. Marrack. 2000. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 192:1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. [DOI] [PubMed] [Google Scholar]
- 35.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R.N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moskophidis, D., F. Lechner, H. Pircher, and R.M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 362:758–761. [DOI] [PubMed] [Google Scholar]
- 37.Barber, D.L., E.J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27–31. [DOI] [PubMed] [Google Scholar]
- 38.Byers, A.M., C.C. Kemball, J.M. Moser, and A.E. Lukacher. 2003. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J. Immunol. 171:17–21. [DOI] [PubMed] [Google Scholar]
- 39.Coles, R.M., S.N. Mueller, W.R. Heath, F.R. Carbone, and A.G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834–838. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez, J., S. Aung, W.L. Redmond, and L.A. Sherman. 2001. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J. Exp. Med. 194:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt, C.S., and M.F. Mescher. 1999. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J. Immunol. 163:2561–2567. [PubMed] [Google Scholar]
- 42.Kearney, E.R., K.A. Pape, D.Y. Loh, and M.K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1:327–339. [DOI] [PubMed] [Google Scholar]
- 43.Pape, K.A., R. Merica, A. Mondino, A. Khoruts, and M.K. Jenkins. 1998. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 160:4719–4729. [PubMed] [Google Scholar]
- 44.Shrikant, P., A. Khoruts, and M.F. Mescher. 1999. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 11:483–493. [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt, R.L., A. Khoruts, R. Merica, T. Zell, and M.K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105. [DOI] [PubMed] [Google Scholar]
- 46.Kedl, R.M., and M.F. Mescher. 1998. Qualitative differences between naive and memory T cells make a major contribution to the more rapid and efficient memory CD8+ T cell response. J. Immunol. 161:674–683. [PubMed] [Google Scholar]
- 47.Maxwell, J.R., J.D. Campbell, C.H. Kim, and A.T. Vella. 1999. CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. J. Immunol. 162:2024–2034. [PubMed] [Google Scholar]
- 48.Sprent, J., and M. Schaefer. 1990. Antigen-presenting cells for CD8+ T cells. Immunol. Rev. 117:213–234. [DOI] [PubMed] [Google Scholar]
- 49.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 50.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D.F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015. [DOI] [PubMed] [Google Scholar]
- 51.Cho, H.J., T. Hayashi, S.K. Datta, K. Takabayashi, J.H. Van Uden, A. Horner, M. Corr, and E. Raz. 2002. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J. Immunol. 168:4907–4913. [DOI] [PubMed] [Google Scholar]
- 52.Pihlgren, M., P.M. Dubois, M. Tomkowiak, T. Sjogren, and J. Marvel. 1996. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J. Exp. Med. 184:2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubey, C., M. Croft, and S.L. Swain. 1996. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J. Immunol. 157:3280–3289. [PubMed] [Google Scholar]
- 54.Byrne, J.A., J.L. Butler, and M.D. Cooper. 1988. Differential activation requirements for virgin and memory T cells. J. Immunol. 141:3249–3257. [PubMed] [Google Scholar]
- 55.Croft, M., L.M. Bradley, and S.L. Swain. 1994. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 152:2675–2685. [PubMed] [Google Scholar]
- 56.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 297:2060–2063. [DOI] [PubMed] [Google Scholar]
- 57.Lee, B.O., L. Hartson, and T.D. Randall. 2003. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J. Exp. Med. 198:1759–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 60.Toshchakov, V., B.W. Jones, P.Y. Perera, K. Thomas, M.J. Cody, S. Zhang, B.R. Williams, J. Major, T.A. Hamilton, M.J. Fenton, and S.N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 3:392–398. [DOI] [PubMed] [Google Scholar]
- 61.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 17:251–263. [DOI] [PubMed] [Google Scholar]