Abstract
Pretreatment of rodent hearts with platelet-derived growth factor (PDGF)–AB decreases myocardial injury after coronary occlusion. However, PDGF-AB cardioprotection is diminished in older animals, suggesting that downstream elements mediating and/or synergizing the actions of PDGF-AB may be limited in aging cardiac vasculature. In vitro PDGF-AB induced vascular endothelial growth factor (VEGF) and angiopoietin (Ang)-2 expression in 4-mo-old rat cardiac endothelial cells, but not in 24-mo-old heart cells. In vivo injection of young hearts with PDGF-AB increased densities of microvessels staining for VEGF and its receptor, Flk-1, and Ang-2 and its receptor, Tie-2, as well as PDGF receptor (PDGFR)–α. In older hearts, PDGF-AB–mediated induction was primarily limited to PDGFR-α. Studies in a murine cardiac transplantation model demonstrated that synergist interactions of PDGF-AB plus VEGF plus Ang-2 (PVA) provided an immediate restoration of senescent cardiac vascular function. Moreover, PVA injection in young rat hearts, but not PDGF-AB alone or other cytokine combinations, at the time of coronary occlusion suppressed acute myocardial cell death by >50%. However, PVA also reduced the extent of myocardial infarction with an age-associated cardioprotective benefit (4-mo-old with 45% reduction vs. 24-mo-old with 24%; P < 0.05). These studies showed that synergistic cytokine pathways augmenting the actions of PDGF-AB are limited in older hearts, suggesting that strategies based on these interactions may provide age-dependent clinical cardiovascular benefit.
Keywords: platelet-derived growth factor, vascular endothelial growth factor, angiopoietin, myocardial infarction, apoptosis
Introduction
Vascular dysfunction is associated with aging (1, 2) and is linked to an increased risk of cardiovascular events (3, 4). Recently, we demonstrated that impaired expression of the platelet-derived growth factor (PDGF)–AB heterodimer by cardiac microvascular endothelial cells (CMECs) and endothelial progenitor cells contributes to this vascular dysfunction and diminishes cardiac angiogenesis in older rodents (5, 6). Experimentally, intramyocardial delivery of PDGF-AB before coronary occlusion decreases the extent of myocardial injury (5). However, this protection is effective only when PDGF-AB is delivered before, but not at the time of, coronary occlusion. Moreover, this protection is greater in younger rodent hearts than in older hearts, suggesting that pathways mediating and/or synergizing with the cardiovascular actions of PDGF-AB may be limited with age.
Aging is also associated with significant dysregulation in the expression of vascular endothelial growth factor (VEGF; 6, 7) that is induced by PDGF-AB in young CMECs (8). Restoration of VEGF in older mice does not independently rescue senescent cardiac angiogenic function (5), suggesting that its role may be to augment the actions of other cytokines in PDGF-AB–mediated cardioprotection. To this end, the combination of PDGF-AB and VEGF has been reported recently to synergistically enhance the rapid formation of mature vascular networks (9). VEGF has also been shown to have cross-regulation and dependency with angiopoietins (Angs) in angiogenic regulation (10–12), suggesting that VEGF and Angs may have important roles in PDGF-AB–induced cardioprotection.
The decrease in infarction size after PDGF-AB injection may not be mediated solely by angiogenic induction. Papers have demonstrated that vascular cytokines have a profound effect in preventing apoptosis (13–15), which is responsible for a significant proportion of the early loss of myocytes and endothelial cells observed after acute coronary occlusion (16, 17). Experimental antiapoptotic therapies can reduce myocardial infarction size after coronary occlusion (18, 19), suggesting that a suppression of cardiac apoptosis may contribute to the cardioprotective actions of PDGF-AB pathways. Indeed, in the older rat heart, PDGF-AB can reverse the senescent proapoptotic enhancement in tumor necrosis factor receptor signaling to reduce mortality after acute coronary occlusion (20).
Here, we report that cardioprotection by PDGF-AB is mediated by the induction of cytokines and receptors, which act synergistically with PDGF-AB to rapidly suppress cardiac apoptosis and reduce the extent of myocardial injury in both young and old rodent hearts. However, senescent changes in these cytokine pathways downstream of PDGF-AB limit cardioprotection of the older rat heart.
Materials and Methods
Animals.
Studies using 4- and 24-mo-old F344 rats and neonatal and 18-mo-old C57BL/6 mice were performed in compliance with the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University.
Molecular Analysis of Age-associated PDGF Cytokine Pathways.
To define the potential age-associated changes in growth factor pathways downstream of PDGF-AB, CMECs were isolated from young (4-mo-old) and old (24-mo-old) rat hearts, as described previously (20). Cells treated with PDGF-AB and expression levels of the proangiogenic cytokines VEGF and Ang-2 were assessed by real time RT-PCR. In brief, rat hearts were harvested and digested using 0.2% collagenase and 0.005% DNase in 10 ml of complete media (DMEM with 10% FCS, 50 μg/ml of endothelial growth supplement [Sigma-Aldrich], 30 U/ml heparin, 100 U/ml penicillin, and 100 μg/ml streptomycin) for 45 min at 37°C. Endothelial cells were isolated by incubating the cell suspensions with anti-CD31 coated Dynabeads (Dynal) for 30 min at room temperature and collected with a magnetic particle collector. Cells were cultured up to four passages. 2 h before PDGF-AB treatment, the cells were fed with basic media (without serum and endothelial growth supplement) after which 50 ng/ml PDGF-AB (R&D Systems) was added to the wells. Total RNA was isolated at different time points (0, 15 min, 30 min, 1 h, 3 h, and 6 h; n = 3 wells/time point) using a Rneasy Mini Kit (QIAGEN). cDNA was prepared using Sensisript™ Reverse Transcriptase Kit (QIAGEN). Real-time PCR reactions were performed using 2 μl of cDNA with SYBR® green hot start PCR protocol (Applied Biosystems) with β-actin levels, determined from serial dilutions of cDNA to generate standard curves, and used as controls (95°C for a 600-s hot start, denaturing at 95°C for 30 s, and annealing at 60°C for 30 s and at 72°C for 30 s for a maximum of 40 cycles). All experiments were replicated with independent isolations of cells. The following primer pairs were used: β-actin, forward, 5′-GTCGTACCACTGGCATTGTC-3′, reverse, 5′-ACCCTCATAGATGGGCACAG-3′; Ang-2, forward, 5′-TCCGGCGAGGAGTCTAACTA-3′, reverse, 5′-AGCTGGAAAAGCAGAAGCTG-3′; and VEGF, forward, 5′-TGCCTACCTCACCTGTTTCC-3′, reverse, 5′-TCTGTCTGGCTGTCATCTGG-3′.
In Situ Analysis of PDGF Downstream Pathways.
To define the in vivo induction of protein patterns of growth factor pathways downstream of PDGF-AB, cardiac tissue was analyzed by immunostaining for VEGF, Ang-1, Ang-2, Flk-1, Flt-1, Tie-1, Tie-2, and PDGFR-α. Sets of young adult and old rats were anesthetized and underwent left intercostal thoracotomy and received intramyocardial injections of PDGF-AB or PBS (n = 3 per group). After identifying the left anterior descending artery (LAD), PDGF-AB (100 ng/50 μl in PBS) or PBS alone was injected through a 30-gauge needle (two 25 μl injections, 2 mm apart) in the mid–left ventricular anterior wall. The chest wall was closed, the lungs were inflated, the rats were extubated, and the tracheotomy was closed. Rats were killed 24 h after injection, and hearts were excised, fixed, and sectioned for immunohistochemistry staining. Rabbit or goat antibodies directed to VEGF, Ang-1, and Ang-2, Flk-1, Flt-1, Tie-1, Tie-2, and PDGFR-α (ABC Staining Kit; Santa Cruz Biotechnology, Inc.) were used as primary antibodies and visualized using the ABC staining method (DakoCytomation). Positive vessel numbers were assessed in sections at the mid–papillary level of each heart and all stained luminal structures in a total of six high-power fields (40×) per section were identified in a blinded analysis as described previously (5, 21, 22).
Senescent Cardiac Allograft Assay.
To test the physiological significance of the alterations in aging endothelial function, we used a cardiac allograft model, which allows the assessment of cardiac angiogenic potential in different age groups (5, 6). In this model, allograft neovascularization is mediated by host endothelial cells, which are recruited to the donor hearts and recapitulate the cardiac myocyte–endothelial cell communication in vivo (23). In brief, 18-mo-old C57BL/6 mice were anesthetized with 15 μg/ml avertin and a dose of 10 μl solution containing 0, 1, 3, 10, or 100 ng (n ≥ 20 for each group) of PDGB-AB was injected subcutaneously into each ear. C57BL/6 neonatal hearts were transplanted either at the time of, or 24 h after, injection into the subcutaneous pinnal tissue on the dorsal side of the ear. Allograft viability was scored by pinnal and transplant integrity 1 wk after engraftment, as we have described previously (5, 6). Additional sets of senescent hosts also were pretreated with subcutaneous pinnal injections of 3 ng PDGF-AB, 100 ng VEGF, and 3 ng PDGF-AB plus 100 ng VEGF, 100 ng Ang-2, or PBS alone (n ≥ 20 for each group) 24 h before transplantation. Transplantations at the time of injection were performed with sets of ears treated with VEGF, PDGF-AB, Ang-2, and PDGF-AB plus VEGF, PDGF-AB plus Ang-2, VEGF plus Ang-2), PDGF-AB plus VEGF plus Ang-2 (PVA; 100 ng of each cytokine), or PBS (n ≥ 10 for each group).
Concomitant Injection of VEGF and Ang-2 with PDGF-AB at the Time of Ligation.
To study the potential synergism between PDGF-AB and its downstream growth factors in the intact rat heart, combinations of growth factors were injected intramyocardially at the time of coronary occlusion. Sets of 4-mo-old rats were injected with combinations of PVA as well as PDGF-AB alone or PBS (100 ng of each in a total of 50 μl of PBS; n = 3 per group) as aforementioned, and the LAD was ligated just below the left atrial appendage with 8-0 nylon sutures. Pallor and regional wall motion abnormality of the left ventricle as well as ST segment elevation confirmed occlusion. These rats were killed 24 h after ligation. Hearts were excised and fixed, sections were analyzed for apoptotic cell death using TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP) staining (In Situ Cell Death Kit; Roche). In brief, slides were deparaffinized and rehydrated using histoclear and decreased grades of alcohol. After digestion in 10 μg/ml proteinase-k, slides were incubated in a mixture of TdT, dNTP, and dUDP-TMR for 1 h at 37°C and counterstained with 4′,6-diamidino-2-phenylindole (InnoGenex). Micrographs were taken using both low magnification (4×) and higher magnification (40×) using fluorescent microscopy. Apoptotic cell density in the anterior left ventricular wall was measured in 15 high-power fields/heart. The extent of cardiac tissue apoptosis was assessed on reconstructed 4× micrographs using National Institutes of Health Image J analyzing system and expressed as percentage of left ventricular area.
Additional sets of both 4- and 24-mo-old rats received injections of PVA combination, PDGF-AB alone, or PBS alone (n ≥ 5 for each group) at the time of LAD ligation. The LAD was ligated just below the left atrial appendage in 4-mo-old rats and 2 mm below the appendage in the old rats to avoid excess mortality in the older rats as described previously (20). These rats were killed 14 d after ligation, and sections at the mid–papillary level were stained by Masson's trichrome stain as a measure of the extent of myocardial injury as blinded analysis as described previously (5, 20).
Statistics.
Differences in positive vessel numbers and apoptotic cell numbers were analyzed by the Student's t test. The extent of myocardial infarction was compared using Fisher's test. A value of P < 0.05 was considered significant.
Results
Induction of VEGF and Ang-2 by PDGF Pretreatment.
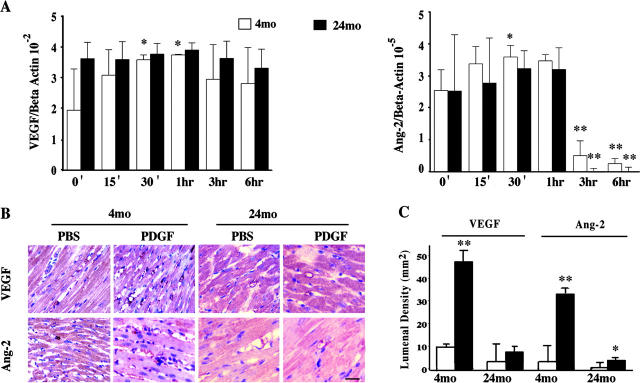
Real-time RT-PCR revealed that PDGF-AB induced significant increases in the expression of both VEGF and Ang-2 in the young, but not old, rat CMECs (Fig. 1 A). However, at the baseline, the older cells expressed higher levels of VEGF, similar to our previous findings with murine CMECs (5). In response to PDGF-AB, cytokine transcripts in the young CMECs showed an initial rise, peaking at between 30 min and 1 h, followed by a subsequent down-regulation of expression later in the time course. The older cells were also responsive to PDGF-AB, however, only with a negative regulation of both VEGF and Ang-2 levels. Immunohistological staining revealed in vivo induction of cardiac cytokines by PDGF-AB in the young hearts. Intramyocardial injection of PDGF-AB significantly increased the density of cardiac capillaries stained positive for VEGF and Ang-2 (but not Ang-1; not depicted) in the 4-mo-old rat hearts sections (Fig. 1, B and C). However, unlike the in vitro expression patterns of VEGF and Ang-2 in the older CMECs, the density of VEGF and Ang-2 positive vessels was significantly lower in the 24-mo-old rat hearts both in the control and PDGF-AB–treated sections compared with staining patterns in the young hearts. Indeed, PDGF-AB injection in the old hearts did not significantly alter VEGF density and resulted in only modest increases in Ang-2 patterns.
Figure 1.
PDGF-AB induction of cardiac microvascular endothelial proangiogenic cytokines. (A) Real-time RT-PCR of VEGF and Ang-2 in PDGF-AB treated 4- and 24-mo-old rat CMECs in vitro. (B) Representative immunohistochemical staining of VEGF or Ang-2 in cardiac capillaries in rat hearts injected with vehicle (PBS) or PDGF-AB 24 h before euthanization (n = 3, each). Bar = 10 μm. (C) Graph shows the densities of VEGF and Ang-2 positive capillaries in PDGF-AB– or PBS-treated hearts. *, P < 0.05; **, P < 0.005. Treatment versus control.
Based on the highly localized staining patterns of VEGF and Ang-2 in the young, but not old hearts, additional immunohistochemistry was performed to assess potential differences in receptors for these cytokines. Staining of sections of PBS-treated rat hearts revealed Flk-1, a VEGF receptor, and Tie-2, an Ang receptor, expression in the cardiac microvasculature of both age sets (Fig. 2) with approximately twofold higher density in the young hearts. PDGFR-α was present in the PBS-treated young hearts, but was significantly diminished in the aging control cardiac tissue. PDGF-AB significantly increased the density of all three receptors in the young hearts as well as PDGFR-α in the aging hearts without altering patterning of Flk-1 and Tie-2. Immunostaining of Flt-1, another VEGF receptor, and Tie-1, another Ang receptor, revealed sparse localization in sections of both ages (unpublished data).
Figure 2.
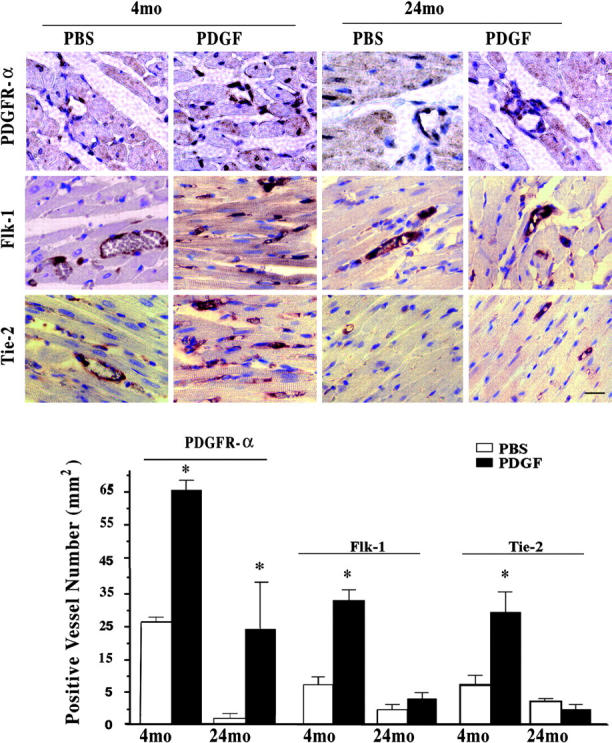
Age-associated induction of cytokine receptors by intramyocardial injection of PDGF-AB. Representative immunohistochemical staining of PDGFR-α, Flk-1, and Tie-2 in cardiac capillaries in rat hearts injected with vehicle (PBS) or PDGF-AB 24 h before euthanization in 4- and 24-mo-old hearts (n = 3, each). Bar = 10 μm. Graph shows the densities of PDGFR-α, Flk-1, and Tie-2 positive capillaries (mm2) in PDGF-AB– or PBS-treated hearts. *, P < 0.05. PDGF-AB versus PBS.
PDGF Pathway Synergism in Angiogenesis of Exogenous Cardiac Tissue
The potential cardiac vascular function of the cytokines in the PDGF-AB pathway was examined in a senescent cardiac allograft–pinnal transplant model. To detect synergistic interactions with downstream cytokines, a dose response curve of PDGF-AB restoration of cardiac vascularization was performed. These studies revealed that pinnal treatment the day before transplantation with 3 ng PDGF-AB rescued approximately half of the cardiac allografts in the aging mice (Fig. 3). Previously, we had demonstrated that VEGF was not sufficient to rescue cardiac viability in this model (5); however, the addition of 100 ng VEGF augmented the actions of the low dose PDGF-AB, fully restoring integrity of the transplanted cardiac allografts (Fig. 3, A and B). Administration of Ang-2 alone was also fully effective in restoring allograft integrity and so additional combinations were not studied (Fig. 3 C). Injection of the ears at the time of transplantation with combinations of cytokines demonstrated that VEGF and Ang-2 with or without PDGF-AB synergistically augmented senescent cardiac allograft viability, shifting the effective temporal window of pinnal treatment (Fig. 3 D).
Figure 3.
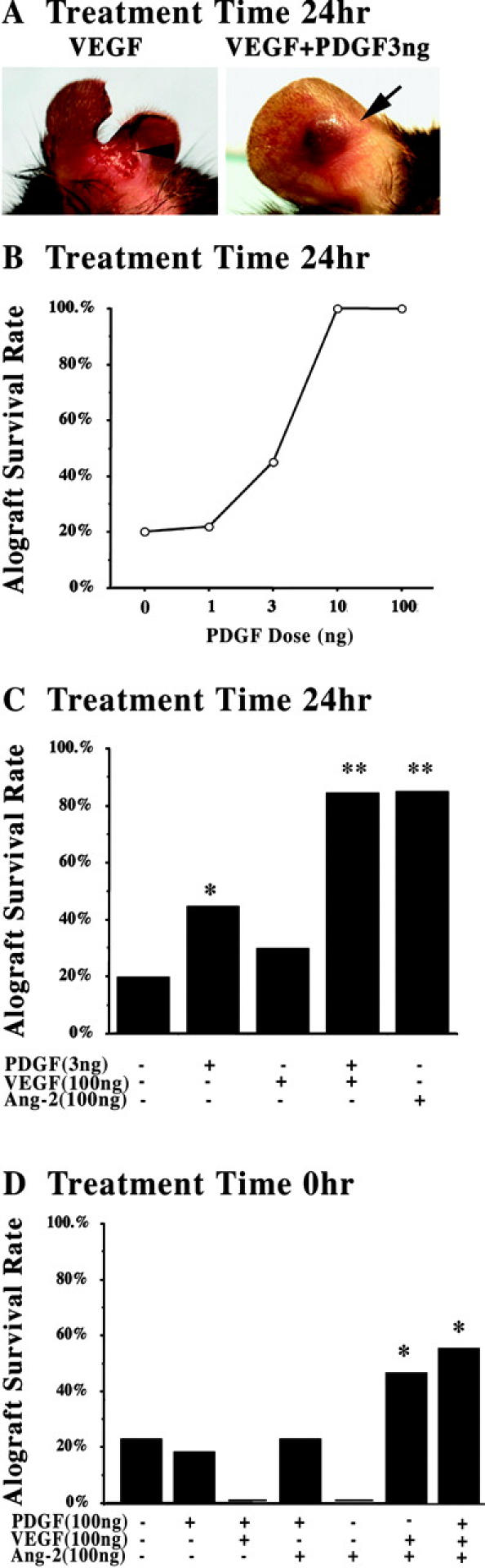
Cytokine synergism in restoration of senescent cardiac allografts. (A) Representative examples of neonatal cardiac transplants into 18-mo-old senescent hosts with pretreatment of VEGF (100 ng) or VEGF (100 ng) and PDGF-AB (3 ng). The arrowhead (left) indicates the site of necrotic loss of both allograft and host pinnal tissue beyond the transplant site in the majority of the VEGF-pretreated transplants. The arrow (right) indicates viable/intact cardiac transplants in the host pinnal tissue. (B) Dose response curve of PDGF-AB pretreatment in the restoration of cardiac allograft viability in 18-mo-old mice (n ≥ 20, per group). 10 ng and 100 ng of PDGF-AB restored a significant number of allografts compared with 0- and 1-ng doses. P < 0.005. (C) Assessment of VEGF and Ang-2 pretreatment and combined VEGF and PDGF-AB (n ≥ 20, per group). *, P < 0.05. PDGF-AB (3 ng) versus PBS. **, P < 0.01. Treatment versus PDGF-AB (3 ng) or PBS. (D) Assessment of cytokine combinations in the peri-transplantation of pinnal tissue at the time of cardiac allograft transplantation (n > 10, per group). *, P < 0.05. PDGF-AB plus VEGF plus Ang-2 or VEGF plus Ang-2 versus PBS and all other treatment groups.
Enhanced Cardioprotective Kinetics by Concomitant Delivery of PVA.
The synergistic actions of PDGF-AB pathway cytokines was confirmed in vivo in an acute myocardial infarction model with delivery of combinations of growth factors at the time of coronary occlusion. Results of cardiac TUNEL staining 24 h after LAD ligation demonstrated that only the triple combination of PVA limited cardiac cell death in the young rat heart, reducing both the area and density of myocardial cell apoptosis (Fig. 4).
Figure 4.
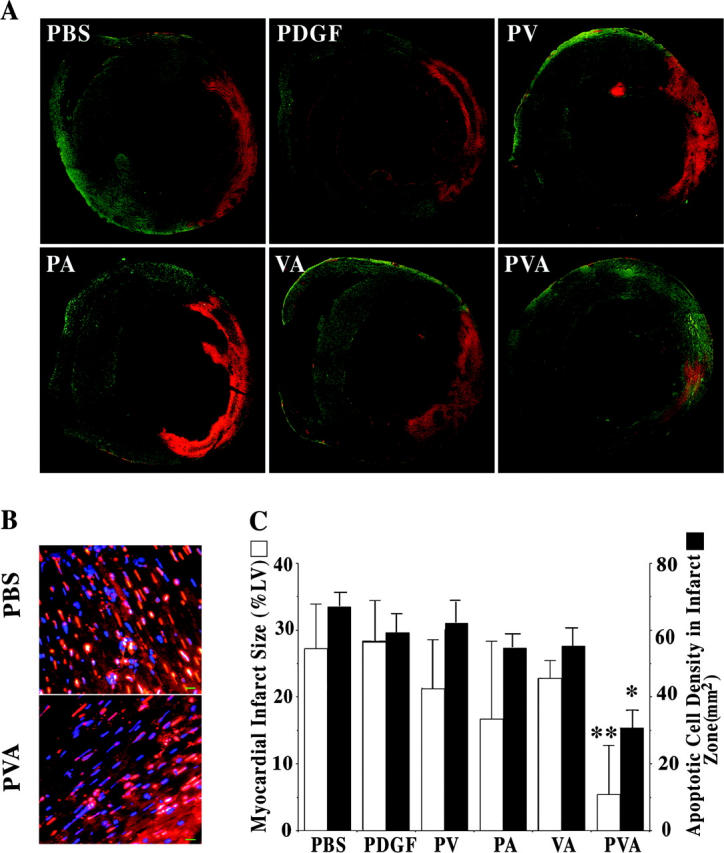
Synergistic suppression of cardiac apoptosis after coronary occlusion. TUNEL labeling of rat cardiac sections 24 h after injection with combinations of PDGF-AB, VEGF, and Ang-2 at the time of LAD ligation. (A) Reconstructed cross-section micrograph from lower magnification (4×) images for measurement of apoptotic area (percentage of left ventricular area). (B) High magnification (40×) TUNEL staining images used in the quantification of apoptotic cell density. Bar = 10 μm. (C) A graph is shown plotting mean apoptotic risk area size (white) and cell density (TUNEL+; black) in the sections of rat anterior left ventricular wall injected with PBS, PDGF-AB, PDGF-AB plus VEGF (PV), PDGF-AB plus Ang-2 (PA), VEGF plus Ang-2 (VA), or PDGF-AB plus VEGF and Ang-2 (PVA) (n = 3, each group) at the time of LAD ligation. *, P < 0.01; **, P < 0.005 PVA versus PBS and all other cytokine groups.
Based on the result of these acute cell death studies, the potential that the triple combination delivered peri-ligation was assessed 2 wk after myocardial infarction in 4- and 24-mo-old rat hearts. Using a more distal LAD occlusion to maintain 14 d postoperative survival in the older hearts (100% for both 4- and 24-mo-old rats), the control treatment groups did not demonstrate a statistically significant difference in the extent of myocardial infarction, though the older hearts trended to have larger areas of injury, similar to previous observations (5, 20). Injection of PVA at the time of coronary occlusion reduced the myocardial infarction size in both young and old rat hearts, with significantly greater benefit in the young hearts compared with old hearts treated with PVA relative to age-matched controls (4-mo-old reduction of 40%; 24-mo-old reduction of 24%; P < 0.05; Fig. 5).
Figure 5.
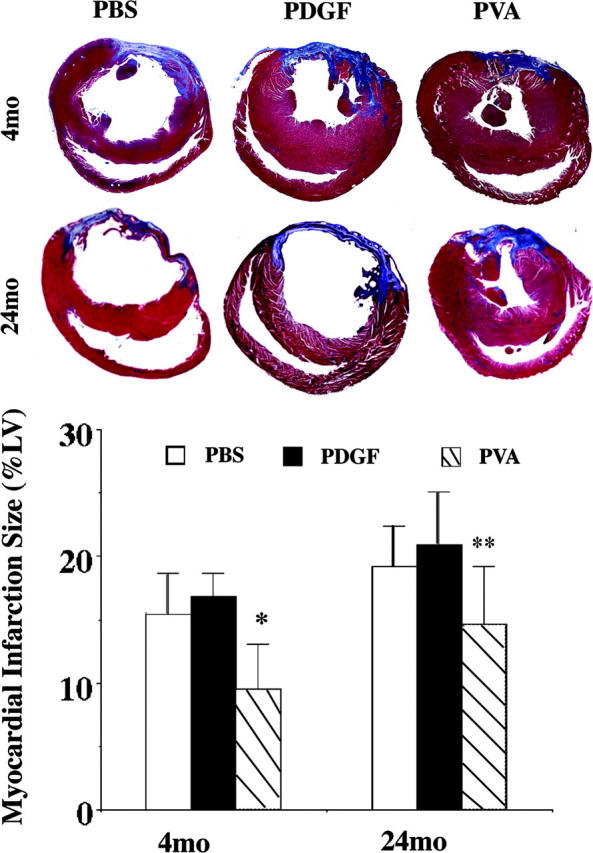
Age-associated cardioprotection by combined cytokine injection at the time of coronary occlusion. (A) Representative Masson's trichrome staining of rat hearts harvested 2 wk after peri-occlusion injections of PBS, PDGF-AB (P), or PDGF-AB plus VEGF and Ang-2 (PVA) and LAD ligation (n ≥ 5, each). Infarction area (blue stain) at the mid–papillary muscle level was significantly smaller in PVA peri-injection heart section than PBS- or PDGF-AB–injected sections. However, there is significant difference between 4- and 24-mo-old rats with PVA treatment. (B) Graph shows average myocardial infarction size (percentage of left ventricular area). *, P < 0.05. 4-mo-old PVA versus 4-mo-old PBS, 4-mo-old PDGF-AB, and 24-mo-old PVA. **, P < 0.05. 24-mo-old PVA versus 24-mo-old PBS and 24-mo-old PDGF-AB.
Discussion
In this paper, we have demonstrated that PDGF-AB can act synergistically with its downstream cytokines, VEGF and Ang-2, to promote cardioprotection in both young and old rodent hearts. We had shown previously that PDGF-AB treatment before coronary occlusion effectively limits the severity of myocardial infarction (5). Here, we have extended this finding and shown that the combination of PVA provides immediate benefit, suppressing cardiac cell death at the time of acute coronary occlusion and, thus, reducing myocardial injury within a therapeutically relevant time frame.
The combined actions of PDGF-AB with its induction of both VEGF and Ang-2 provide an important understanding of the cardioprotective effects beyond the proangiogenic signaling pathways attributed to these vascular cytokines. The role of VEGF in the augmentation of PDGF-AB as well as the independent actions of Ang-2 in the senescent cardiac allograft model support the well-documented role of these cytokines in the promotion of cardiac vascularization (24, 25). However, the immediate antiapoptotic actions of the growth factor combination demonstrate that the PDGF-AB–mediated protection from myocardial injury may be based primarily on the suppression of acute cell death of cardiac endothelial cells and myocytes. This antiapoptotic effect, in concert with the potential recruitment of cardiovascular progenitor/stem cells and the growth of new vascular supplies, acts to minimize the extent of myocardial infarction.
Previous works have demonstrated that vascular cytokines can have profound actions on apoptotic pathways. Indeed, although VEGF and Angs are potent angiogenic tyrosine kinase receptor growth factors (12, 26, 27), they have also both been shown to be strong protectors of endothelial cells from apoptosis (28, 29). Moreover, the widespread suppression in myocardial cell death suggests that PVA protection extends beyond the endothelial cells, which express receptors for these growth factors. The actions of PVA may be mediated though direct cell–cell communications in vascular cells (30, 31). The protection of local cardiac myocytes may involve the induction of other growth factors or paracrine pathways. To this end, PDGF and VEGF can induce the antiapoptotic factor survivin (32, 33), suggesting another potential mechanism by which the PVA treatment may promote autocrine and paracrine antiapoptotic pathways to protect the myocardium after coronary occlusion.
The paracrine actions of the triple growth factor combination may be mediated through subpopulations of resident endothelial cells as well as endothelial progenitor cells recruited into the cardiac microvasculature. Although cytokines have been well documented to mobilize populations of endothelial precursor cells from the bone marrow (34–36), the observation that PDGF-AB treatment markedly increases the number of PDGFR-α positive cardiac microvascular cells represents a novel finding. The mechanisms underlying this induction are as yet unclear, though they may involve the recruitment of PDGFR-α positive endothelial progenitor cells to the cardiac microvasculature. Previously, we demonstrated that endothelial progenitor cells expressing PDGF-B are recruited to foci of angiogenesis in the cardiac microvasculature (6), and the actions of PDGF-AB are mediated, in turn, by a subpopulation of PDGFR-α− cardiac endothelial cells (8). These findings, in conjunction with the present observations, suggest that PDGF may regulate positive feedback loops to maintain subpopulations of endothelial progenitor cells that govern its cardioprotective actions in the cardiac microvasculature. Indeed, impairment in this loop, with dysregulation of PDGF-B expression in the aging heart, correlates with the significantly diminished density of PDGFR-α in the senescent cardiac microvasculature. Reconstitution of this pathway through intratmyocardial injection of PDGF-AB results in increased PDGFR-α cell levels in the aging heart to those observed in the young cardiac tissue.
The role of vascular cytokines in the recruitment of specific subpopulations of endothelial progenitor cells may extend to the growth factors downstream from PDGF-AB in the heart. The increase in both VEGF and Ang-2 correlate with an augmentation in their respective receptors in the microvasculature of the young hearts after injection of PDGF-AB. The senescent impairment in VEGF and Ang-2 induction is associated with a lack of increase in Flk-1 and Tie-2 cells. However, treatment of the older hearts with the PVA combination provides significant cardioprotection, suggesting that the reconstitution of the downstream cytokines mediates the induction of their receptors in the cardiac microvascular, likely through the induction of endothelial progenitor cells. The diminished benefit of PVA in the older hearts may reflect the lower baseline in receptor populations in the hearts before treatment and acute coronary occlusion, suggesting that a more sustained approach to promoting PVA pathways may result in improved cardioprotection for the aging heart. Indeed, elucidation of the precise mechanisms governing the increase in these receptor subpopulations may provide approaches to further promote cardioprotective pathways in all age groups.
In addition to promoting vascular protection, PDGF-AB pathways could enhance the regeneration of cardiac myocytes after myocardial injury. The coincident recruitment of progenitors of cardiac endothelial cells and cardiac myocytes by systemic mobilization (37) suggests that PVA-stimulated pathways may also promote the cardiac homing and/or differentiation of cardiac myocyte progenitor and/or stem cells. Indeed, PDGF-AB–endothelial progenitor cell communication pathways may provide a foundation for the regeneration of cardiac myocytes in the injured heart. Previous studies have demonstrated the transdifferentiation capacity of endothelial cells (38). Moreover, recent papers have revealed that endothelial–cardiac myocyte interactions can promote the generation of additional cardiac myocytes from cultures of embryonic endothelial cells (39) and endothelial progenitor cells (40). These analyses suggest that signals such as PDGF-B, which is induced in the coculture of endothelial progenitor cells and cardiac myocytes (6), may mediate the induction of cardiac myocytes from cells recruited to the treated hearts. Indeed, PDGF isoforms have been demonstrated to promote the growth of embryonic cardiac myocytes, (41, 42) suggesting that PVA acting with or through cardiovascular stem and/or progenitor cells that are recruited to or reside within the heart (43) may promote the regeneration of cardiac tissue to reduce the extent of myocardial injury after coronary occlusion in both younger and older hearts.
The age-associated decrease in PVA-mediated cardioprotection may be due in part to senescent changes in the cardiac vasculature. In addition to alterations in angiogenic cytokine pathways that are critical in restoring vascular supply to compromised myocardial tissue, age-associated shifts in vascular function can enhance apoptotic signaling further and increase cardiac pathology in experimental models (20, 44). Furthermore, the senescent changes in VEGF and Ang-2 expression patterns, with higher levels of expression at baseline that are not increased by PDGF-AB, may be influenced by other age-dependent effects on gene regulation. Indeed, direct effects of aging on endothelial cells, as well as the alterations in the cardiac environment, including increased reactive oxygen species (45, 46) and shifts in hemostatic pathways (47, 48), may lead to a significant impairment in endothelial function in the old heart. The composite effect of these and potentially other age-associated physiologic actions may contribute to the decrease in cardioprotective cytokine pathways observed in vivo.
Overall, our findings provide a unique insight to the synergism of PDGF-AB pathways in the protection of the rodent heart from myocardial infarction. Future studies directed at identifying the set of antiapoptotic and proangiogenic elements, as well as other potential cardioprotective actions, including the recruitment of endothelial progenitor cells, may facilitate the development of novel clinical approaches to limit the extent of myocardial injury in both younger as well as older hearts.
Acknowledgments
The authors thank T. Dutta, A. Lazano, and D.S. Gidseg for contributions to the histological data collection.
This work was supported by the National Institutes of Health (grants AG19738, AG20918, and HL67839) and an American Federation for Aging Research-Paul Beeson Physician Faculty Scholar in Aging Research Award.
Abbreviations used in this paper: Ang, angiopoietin; CMEC, cardiac microvascular endothelial cell; LAD, left anterior descending artery; PDGF, platelet-derived growth factor; PVA, PDGF-AB plus VEGF plus Ang-2; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP; VEGF, vascular endothelial growth factor.
References
- 1.Kass, D.A. 2002. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail. Rev. 7:51–62. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta, E.G., and D. Levy. 2003. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 107:139–146. [DOI] [PubMed] [Google Scholar]
- 3.Halcox, J.P., W.H. Schenke, G. Zalos, R. Mincemoyer, A. Prasad, M.A. Waclawiw, K.R. Nour, and A.A. Quyyumi. 2002. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 106:653–658. [DOI] [PubMed] [Google Scholar]
- 4.Gokce, N., J.F. Keaney, Jr., L.M. Hunter, M.T. Watkins, Z.S. Nedeljkovic, J.O. Menzoian, and J.A. Vita. 2003. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J. Am. Coll. Cardiol. 41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 5.Edelberg, J.M., S.H. Lee, M. Kaur, L. Tang, N.M. Feirt, S. McCabe, O. Bramwell, S.C. Wong, and M.K. Hong. 2002. Platelet-derived growth factor-AB limits the extent of myocardial infarction in a rat model: feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation. 105:608–613. [DOI] [PubMed] [Google Scholar]
- 6.Edelberg, J.M., L. Tang, K. Hattori, D. Lyden, and S. Rafii. 2002. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ. Res. 90:E89–E93. [DOI] [PubMed] [Google Scholar]
- 7.Rivard, A., L. Berthou-Soulie, N. Principe, M. Kearney, C. Curry, D. Branellec, G.L. Semenza, and J.M. Isner. 2000. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J. Biol. Chem. 275:29643–29647. [DOI] [PubMed] [Google Scholar]
- 8.Edelberg, J.M., W.C. Aird, W. Wu, H. Rayburn, W.S. Mamuya, M. Mercola, and R.D. Rosenberg. 1998. PDGF mediates cardiac microvascular communication. J. Clin. Invest. 102:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson, T.P., M.C. Peters, A.B. Ennett, and D.J. Mooney. 2001. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19:1029–1034. [DOI] [PubMed] [Google Scholar]
- 10.Holash, J., P.C. Maisonpierre, D. Compton, P. Boland, C.R. Alexander, D. Zagzag, G.D. Yancopoulos, and S.J. Wiegand. 1999. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 284:1994–1998. [DOI] [PubMed] [Google Scholar]
- 11.Ray, P.S., T. Estrada-Hernandez, H. Sasaki, L. Zhu, and N. Maulik. 2000. Early effects of hypoxia/reoxygenation on VEGF, ang-1, ang-2 and their receptors in the rat myocardium: implications for myocardial angiogenesis. Mol. Cell. Biochem. 213:145–153. [DOI] [PubMed] [Google Scholar]
- 12.Patan, S. 2000. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 50:1–15. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet, P., M.G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, et al. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 98:147–157. [DOI] [PubMed] [Google Scholar]
- 14.Neufeld, G., T. Cohen, S. Gengrinovitch, and Z. Poltorak. 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB Journal January. 13:9–22. [PubMed] [Google Scholar]
- 15.Kim, I., J.H. Kim, S.O. Moon, H.J. Kwak, N.G. Kim, and G.Y. Koh. 2000. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 19:4549–4552. [DOI] [PubMed] [Google Scholar]
- 16.Green, A.M., and N.D. Steinmetz. 2002. Monitoring apoptosis in real time. Cancer J. 8:82–92. [DOI] [PubMed] [Google Scholar]
- 17.Hinescu, M.E. 2001. Cardiac apoptosis: from organ failure to allograft rejection. J. Cell. Mol. Med. 5:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs, P., I. Bak, L. Szendrei, M. Vecsernyes, E. Varga, I.E. Blasig, and A. Tosaki. 2001. Non-specific caspase inhibition reduces infarct size and improves post-ischaemic recovery in isolated ischaemic/reperfused rat hearts. Naunyn Schmiedebergs Arch. Pharmacol. 364:501–507. [DOI] [PubMed] [Google Scholar]
- 19.Zou, Z., S. Sasaguri, K.G. Rajesh, and R. Suzuki. 2002. dl-3-Hydroxybutyrate administration prevents myocardial damage after coronary occlusion in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 283:H1968–H1974. [DOI] [PubMed] [Google Scholar]
- 20.Cai, D., M. Xaymardan, J.M. Holm, J. Zheng, J.R. Kizer, and J.M. Edelberg. 2003. Age-associated impairment in TNF-alpha cardioprotection from myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 285:H463–H469. [DOI] [PubMed] [Google Scholar]
- 21.Brooks, P.C., R.A. Clark, and D.A. Cheresh. 1994. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 264:569–571. [DOI] [PubMed] [Google Scholar]
- 22.Dellian, M., B.P. Witwer, H.A. Salehi, F. Yuan, and R.K. Jain. 1996. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am. J. Pathol. 149:59–71. [PMC free article] [PubMed] [Google Scholar]
- 23.Aird, W.C., J.M. Edelberg, H. Weiler-Guettler, W.W. Simmons, T.W. Smith, and R.D. Rosenberg. 1997. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J. Cell Biol. 138:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsunaga, T., D.C. Warltier, J. Tessmer, D. Weihrauch, M. Simons, and W.M. Chilian. 2003. Expression of VEGF and angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 285:H352–H358. [DOI] [PubMed] [Google Scholar]
- 25.Silvestre, J.S., and B.I. Levy. 2002. Angiogenesis therapy in ischemic disease. Arch. Mal. Coeur Vaiss. 95:189–196. [PubMed] [Google Scholar]
- 26.Breier, G. 2000. Functions of the VEGF/VEGF receptor system in the vascular system. Semin. Thromb. Hemost. 26:553–559. [DOI] [PubMed] [Google Scholar]
- 27.Risau, W. 1998. Development and differentiation of endothelium. Kidney Int. Suppl. 67:S3–S6. [DOI] [PubMed] [Google Scholar]
- 28.Spyridopoulos, I., E. Brogi, M. Kearney, A.B. Sullivan, C. Cetrulo, J.M. Isner, and D.W. Losordo. 1997. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha: balance between growth and death signals. J. Mol. Cell. Cardiol. 29:1321–1330. [DOI] [PubMed] [Google Scholar]
- 29.Kim, I., S.O. Moon, C.Y. Han, Y.K. Pak, S.K. Moon, J.J. Kim, and G.Y. Koh. 2001. The angiopoietin-tie2 system in coronary artery endothelium prevents oxidized low-density lipoprotein-induced apoptosis. Cardiovasc. Res. 49:872–881. [DOI] [PubMed] [Google Scholar]
- 30.Risau, W., and I. Flamme. 1995. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 11:73–91. [DOI] [PubMed] [Google Scholar]
- 31.Dejana, E., M.G. Lampugnani, O. Martinez-Estrada, and G. Bazzoni. 2000. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int. J. Dev. Biol. 44:743–748. [PubMed] [Google Scholar]
- 32.Blanc-Brude, O.P., J. Yu, H. Simosa, M.S. Conte, W.C. Sessa, and D.C. Altieri. 2002. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat. Med. 8:987–994. [DOI] [PubMed] [Google Scholar]
- 33.Conway, E.M., F. Zwerts, V. Van Eygen, A. DeVriese, N. Nagai, W. Luo, and D. Collen. 2003. Survivin-dependent angiogenesis in ischemic brain: molecular mechanisms of hypoxia-induced up-regulation. Am. J. Pathol. 163:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hattori, K., S. Dias, B. Heissig, N.R. Hackett, D. Lyden, M. Tateno, D.J. Hicklin, Z. Zhu, L. Witte, R.G. Crystal, et al. 2001. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 193:1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalka, C., H. Masuda, T. Takahashi, W.M. Kalka-Moll, M. Silver, M. Kearney, T. Li, J.M. Isner, and T. Asahara. 2000. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA. 97:3422–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwaguro, H., J. Yamaguchi, C. Kalka, S. Murasawa, H. Masuda, S. Hayashi, M. Silver, T. Li, J.M. Isner, and T. Asahara. 2002. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 105:732–738. [DOI] [PubMed] [Google Scholar]
- 37.Orlic, D., J. Kajstura, S. Chimenti, F. Limana, I. Jakoniuk, F. Quaini, B. Nadal-Ginard, D.M. Bodine, A. Leri, and P. Anversa. 2001. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA. 98:10344–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipton, B.H., K.G. Bensch, and M.A. Karasek. 1991. Microvessel endothelial cell transdifferentiation: phenotypic characterization. Differentiation. 46:117–133. [DOI] [PubMed] [Google Scholar]
- 39.Condorelli, G., U. Borello, L. De Angelis, D. Sirbella, M. Coletta, R. Galli, G. Balconi, A. Follenzi, G. Frati, M.G. Cusella De Angelis, et al. 2001. Cardiomyocytes induce endothelial cells to transdifferentiate into cardiac muscle: implications for myocardium regeneration. Proc. Natl. Acad. Sci. USA. 98:10733–10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badorff, C., R.P. Brandes, R. Popp, S. Rupp, C. Urbich, A. Aicher, I. Fleming, R. Busse, A.M. Zeiher, and S. Dimmeler. 2003. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 107:1024–1032. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu, T., K. Kinugawa, A. Yao, Y. Sugishita, K. Sugishita, K. Harada, H. Matsui, O. Kohmoto, T. Serizawa, and T. Takahashi. 1999. Platelet-derived growth factor induces cellular growth in cultured chick ventricular myocytes. Cardiovasc. Res. 41:641–653. [DOI] [PubMed] [Google Scholar]
- 42.Price, R.L., S.T. Haley, T.A. Bullard, E.C. Goldsmith, D.G. Simpson, T.E. Thielen, M.J. Yost, and L. Terracio. 2003. Effects of platelet-derived growth factor-AA and -BB on embryonic cardiac development. Anat. Rec. 272A:424–433. [DOI] [PubMed] [Google Scholar]
- 43.Beltrami, A.P., L. Barlucchi, D. Torella, M. Baker, F. Limana, S. Chimenti, H. Kasahara, M. Rota, E. Musso, K. Urbanek, et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 114:763–776. [DOI] [PubMed] [Google Scholar]
- 44.Higami, Y., and I. Shimokawa. 2000. Apoptosis in the aging process. Cell Tissue Res. 301:125–132. [DOI] [PubMed] [Google Scholar]
- 45.Matz, R.L., C. Schott, J.C. Stoclet, and R. Andriantsitohaina. 2000. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res. 49:11–18. [PubMed] [Google Scholar]
- 46.Yu, B.P., and H.Y. Chung. 2001. Oxidative stress and vascular aging. Diabetes Res. Clin. Pract. 54:S73–S80. [DOI] [PubMed] [Google Scholar]
- 47.Hatake, K., E. Kakishita, I. Wakabayashi, N. Sakiyama, and S. Hishida. 1990. Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke. 21:1039–1043. [DOI] [PubMed] [Google Scholar]
- 48.Harris, N.R., and R.E. Rumbaut. 2001. Age-related responses of the microcirculation to ischemia-reperfusion and inflammation. Pathophysiology. 8:1–10. [DOI] [PubMed] [Google Scholar]