Abstract
The tumor necrosis factor family members BAFF and APRIL induce Ig isotype switching in human B cells. We analyzed the ability of BAFF and APRIL to induce isotype switching in murine B cells to IgG1, IgA, and IgE. APRIL and BAFF each engage two receptors, transmembrane activator and calcium-modulator and cytophilin ligand interactor (TACI) and B cell maturation antigen (BCMA), on B cells. In addition, BAFF engages a third receptor on B cells, BAFF-R. To determine the role of these receptors in isotype switching, we examined B cells from mice deficient in TACI, BCMA, and BAFF-R. The results obtained indicate that both TACI and BAFF-R are able to transduce signals that result in isotype switching.
Class switch recombination (CSR) in B cells requires two signals (1). The first is normally delivered by cytokines, which target specific CH genes for transcription; the second is delivered in the case of T-dependent (TD) antigens by interaction of CD40 on B cells with its ligand CD40L on activated T cells. CSR is severely impaired in patients and mice deficient in CD40L or CD40 (2, 3), although low levels of IgG and variable levels of IgA are still detected in serum. Exposure to LPS derived from Gram-negative bacteria may account for some of this residual CSR in mice, but not in humans since LPS does not activate CSR in human B cells. EBV infection triggers CSR in human B cells independently of CD40L and CD40 (4) and may contribute to residual CSR in humans with CD40L and CD40 deficiency.
B cell–activating factor of the TNF family (BAFF) and A proliferation–inducing ligand (APRIL) are two TNF family members that have been shown to activate CSR in human B cells (5) and hence may contribute to residual CSR in CD40L and CD40 deficiency. BAFF is expressed mainly by monocytes and dendritic cells. APRIL is expressed in a large variety of tissues that include monocytes/macrophages, dendritic cells, and activated T cells. APRIL and BAFF both bind to two receptors, B cell maturation antigen (BCMA) and transmembrane activator and calcium-modulator and cytophilin ligand interactor (TACI), which are members of the TNF receptor family. BCMA is exclusively expressed on B cells, whereas TACI is expressed on B cells and activated T cells. A third receptor, BAFF receptor (BAFF-R), that is unique for BAFF is expressed mainly on B cells but also on T cells (6). To identify the receptors that are involved in the induction of Ig class switching by BAFF and APRIL, we ascertained that these ligands activate CSR in mouse B cells and then examined their activity on B cells from TACI-, BCMA-, and BAFF-R–deficient mice.
Results and Discussion
BAFF and APRIL activate IgG1, IgA, and IgE isotype switching in mouse B cells
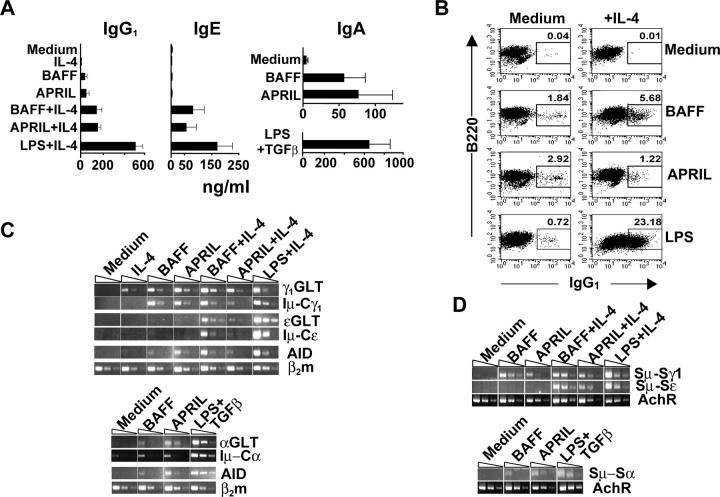
We examined the capacity of BAFF and APRIL to induce IgG1, IgA, and IgE switching in mice. Splenic B cells from CD40−/− mice were negatively sorted and consisted of >96% sIgM+sIgD+, 3–6% CD11b+, and undetectable CD3+ cells. APRIL and BAFF induced IgG1, IgA but no detectable IgE synthesis in these cells (Fig. 1 A). IL-4 enhanced the induction of IgG1 synthesis by BAFF and APRIL and synergized with these two ligands to induce IgE synthesis. As expected, B cells synthesized large amounts of IgG1 and IgE in response to LPS + IL-4, and TGFβ synergized with LPS to induce IgA switching. Neutralization of TGFβ had no effect on IgA secretion in response to BAFF and APRIL (unpublished data). Failure to block induction of IgA secretion by αTGFβ suggests that BAFF and APRIL induce αgerm line transcripts (GLTs) independently of TGFβ, or they induce TGFβ, but not all of it is accessible to neutralization by the antibody. IL-6 neutralization had no effect on IgG1 or IgA induction by BAFF or APRIL (unpublished data). IL-10 neutralization partially inhibited IgG1 secretion by BAFF (∼40%) and APRIL (∼60%) and IgA secretion by these ligands (∼10 and ∼30%, respectively). As another measure of CSR, we examined the induction of expression of surface IgG1. There were virtually no sIgG1+ cells in the negatively sorted B cells (Fig. 1 B). APRIL and BAFF alone and with IL-4 induced IgG1 surface expression in these B cells. Together these results suggest that APRIL and BAFF activate CSR in murine B cells.
Figure 1.
Induction of CSR by BAFF and APRIL in negatively sorted B cells from CD40 − / − mice. (A) IgG1, IgA, and IgE synthesis in negatively sorted B cells. Results represent mean and SD of at least three experiments. (B) Surface expression of IgG1 in negatively sorted B cells. Numbers represent the percentage of sIgG1+ cells. (C) Semiquantitative RT-PCR analysis of the expression of γ1, α, and ɛGLT, Iμ-Cγ1, Iμ-Cα, Iμ-Cɛ, and AID transcripts. (D) Sμ→Sɛ, Sμ→Sα, and Sμ→Sγ1 deletional switch recombination measured by DC-PCR. Dividing lines are used to group different dilutions (1:1, 1:3, and 1:9) of cDNA from B cells cultured in the same experiment with various stimuli. All samples were loaded contiguously in the same gel, except for cDNAs from cells stimulated with LPS + IL-4 and LPS + TGFβ which were loaded in noncontiguous lanes of the gel. Results in B, C, and D are representative of three experiments.
CSR has been linked to cell division (7). APRIL- and BAFF-induced proliferation of negatively sorted B cells in a [3H]thymidine uptake assay and of splenic B220+ B cells in a 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) dye dilution assay (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20032000/DC1). Induction of CSR by APRIL and BAFF was not due to contamination with endotoxin because the preparations used contained <1 endotoxin U/μg protein; and polymyxin B, which inhibits LPS activation (8), failed to inhibit induction of IgG1 synthesis by APRIL and BAFF (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20032000/DC1).
Molecular events involved in CSR include expression of GLTs, expression of the gene for activation-induced deaminase (AID), followed by deletional switch recombination and expression of Iμ-CH transcripts. APRIL and BAFF induced γ1GLT and αGLT, but no detectable ɛGLT, in negatively sorted B cells from CD40−/− mice (Fig. 1 C). APRIL, and to a lesser extent, BAFF induced AID gene expression. APRIL and BAFF synergized with IL-4 in inducing ɛGLT. Digestion circularization (DC)–PCR analysis revealed that APRIL and BAFF induced Sμ→Sγ1 and Sμ→Sα but not Sμ→Sɛ deletional switch recombination (Fig. 1 D). IL-4 synergized with APRIL and BAFF to induce Sμ→Sɛ deletional switch recombination and to up-regulate Sμ→Sγ1 recombination. Consistent with the results of Ig synthesis, APRIL and BAFF induced Iμ-Cγ1 and Iμ-Cα but not Iμ-Cɛ transcripts, unless IL-4 was added (Fig. 1 C). Since we used negatively sorted B cells from CD40−/− mice, these results indicate that APRIL and BAFF induce CSR in naive B cells.
Our findings extend previous results on human B cells positively sorted for IgD expression (5). Our observation that BAFF and APRIL activate CSR in B cells from CD40−/− mice definitively establishes that CSR mediated by these ligands is independent of CD40L–CD40 interactions (Fig. 1). In the case of human B cells, BAFF/APRIL induction of secretion of the switched isotypes requires additional signals that include cross-liking of the B cell receptor and the cytokines, such as IL-10 and IL-15 (5). One possibility is that mouse B cells endogenously produce cytokines that support secretion of the switched Ig. This is supported by our observation that neutralizing αIL-10 antibody inhibited BAFF- and APRIL-driven IgG1 and IgA secretion by mouse B cells. Another possibility is that mouse B cells survive better in culture to the stage where they are able to secrete Igs.
TACI mediates class switching by APRIL
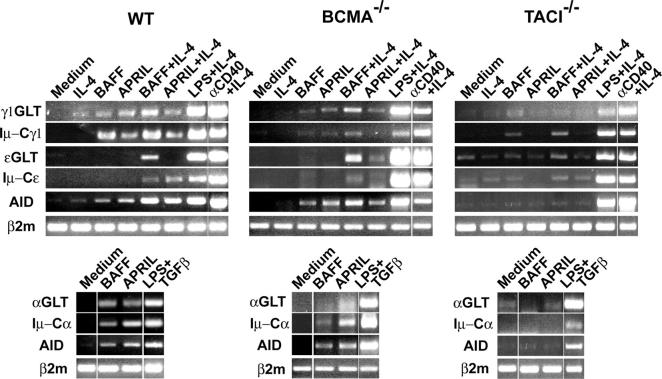
We next examined negatively sorted splenic B cells from mice that lack BCMA or TACI. B cells from WT mice were used as controls with results similar to those obtained with CD40−/− B cells. BCMA−/− B cells synthesized IgG1, IgA, and IgE in response to APRIL and BAFF in amounts that were not significantly different from those secreted by WT B cells (Fig. 2, A and B). Intact CSR in BCMA−/− B cells was confirmed by examination of molecular events involved in CSR to IgG1, IgA, and IgE (Fig. 3).
Figure 2.
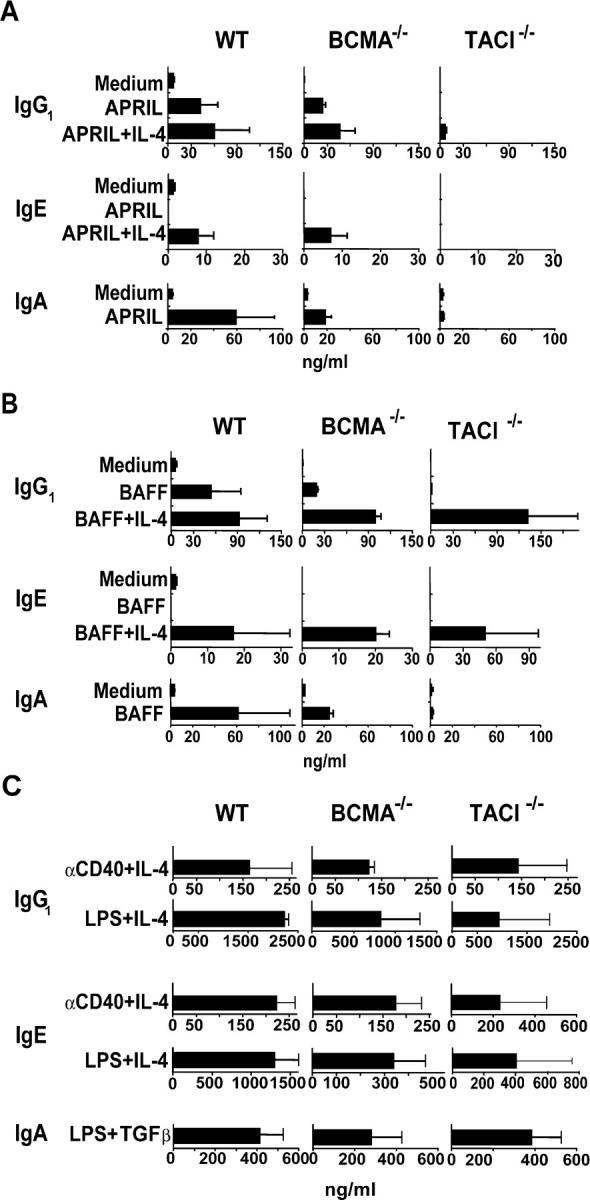
Role of TACI and BCMA in inducing IgG1, IgA, and IgE synthesis. Negatively sorted B cells from WT, TACI−/−, and BCMA−/− mice were stimulated with (A) APRIL, (B) BAFF, and (C) αCD40 and LPS as controls. Results represent mean and SD of three experiments.
Figure 3.
Molecular events involved in IgG1, IgA, and IgE switching in negatively sorted B cells from WT, TACI −/−, and BCMA −/− mice. Dividing lines are used to demarcate noncontiguous parts of the same gel Results are representative of three experiments.
TACI−/− B cells virtually failed to synthesize IgG1, IgA, and IgE in response to APRIL (Fig. 2 A). This was not due to an intrinsic defect in CSR because they synthesized IgG1 and IgE in response to LPS + IL-4 and αCD40 + IL-4 and IgA in response to LPS + TGFβ (Fig. 2 C). Examination of molecular events confirmed the inability of APRIL to activate CSR in TACI−/− B cells (Fig. 3). In some experiments, CHGLT and AID were faintly detected in unstimulated B cells from TACI−/− mice. This may be related to the B cell activation observed in these mice in vivo (9). However, these faint CHGLT and AID transcripts were not up-regulated by APRIL. These results suggest that APRIL induction of CSR is mediated by TACI.
Both TACI and BAFF-R mediate class switching by BAFF
In contrast to their total inability to class switch in response to APRIL, TACI−/− B cells synthesized IgG1 and IgE in response to BAFF + IL-4 in amounts that were not significantly different from those secreted by normal B cells (Fig. 2 B). This was confirmed by the presence of γ1 and ɛGLTs, AID, and mature Iμ-Cγ1 and Iμ-Cɛ (Fig. 3) and was observed in the presence of polymyxin B (unpublished data). BCMA engagement by BAFF cannot account for the ability of BAFF to induce CSR in TACI−/− B cells because these cells are unable to undergo CSR in response to APRIL, which has a higher affinity for BCMA in mice than BAFF (6). Thus, induction of CSR by BAFF in TACI−/− B cells indicates that BAFF-R engagement can activate CSR and suggests that BAFF may use both TACI and BAFF-R to induce CSR. We cannot rule out the possibility that BCMA synergizes with BAFF-R in mediating BAFF-induced CSR in TACI−/− B cells.
Negatively sorted TACI−/− B cells stimulated with BAFF consistently failed to secrete IgA (Fig. 2 B), and we were unable to detect in these B cells induction of molecular events involved in IgA switching, including expression of αGLT (Fig. 3). Since intracellular signaling by TACI differs from that triggered by BAFF-R (6), it is possible that TACI signals are indispensable for the activation of the Iα promoter and induction of IgA switching.
APRIL induction of CSR is independent of BAFF–BAFF-R interaction
Given the observation that BAFF-R engagement can activate CSR, it was important to rule out the possibility that APRIL-mediated switching involved, in addition to TACI, engagement of BAFF-R by BAFF which may be endogenously made by B cells. Unstimulated B cells expressed small amounts of BAFF mRNA as assessed by RT-PCR (Fig. S3, available at http://www.jem.org/cgi/content/full/jem. 20032000/DC1). Stimulation with APRIL or APRIL + IL-4 caused no detectable increase in BAFF mRNA expression. More importantly, we examined the ability of APRIL to induce isotype switching in B cells from A/WySnJ mice, which carry a mutation in BAFF-R (10). These mice have very few peripheral B cells with a decreased proportion of mature CD23+ B cells. To examine CSR under culture conditions similar to those used for WT, BCMA−/−, and TACI−/− B cells (i.e., same cell number and density), we examined B cells from pooled splenocytes of 4 A/WySnJ mice. APRIL and BAFF induced IgG1 and IgA secretion in B cells from these mice (Fig. S3). These results suggest that APRIL-mediated CSR does not involve autocrine BAFF–BAFF-R interactions and that TACI engagement is sufficient to induce CSR. We cannot rule out the possibility that TACI synergizes with a putative APRIL-specific receptor to cause CSR.
Binding of TRAF2 and/or TRAF3 is essential for CD40-mediated CSR, whereas TRAF6 is important in plasma cell differentiation (11, 12). TACI, like CD40, recruits TRAF 2, 5, and 6 (6). This may explain its ability to activate CSR. BAFF-R binds TRAF3 but no other TRAF protein (6). TRAF3 may be important for CSR induced by the EBV protein LMP-1 (13). It is possible that CSR induced by BAFF in TACI−/− B cells involves a cooperative interaction between BAFF-R and BCMA, which recruits TRAF1, 2, and 3 proteins (6). The fact that BCMA fails to activate CSR may be due to the fact that the majority of BCMA has a low surface density and most of it is intracellular (6). Alternatively, non-TRAF signals may be important for CSR but may not be delivered by BCMA.
A clue to the physiological role of APRIL- and BAFF-mediated CSR is provided by results obtained on mice deficient in these ligands and their receptors. BAFF−/− and BAFF-R−/− mice are severely deficient in B cells (6) and not informative. BCMA−/− mice have normal serum Ig levels and normal antibody responses (14). This is consistent with our data that B cells from these mice switch normally in response to BAFF and APRIL. TACI−/− mice have low serum IgA and deficient antibody responses to immunization with type II T-independent antigens (15, 16). This is consistent with the failure of B cells from these mice to secrete IgA in response to BAFF and APRIL. We have shown that APRIL−/− mice have a selective IgA deficiency and decreased serum IgA antibody responses to mucosal immunization with TD antigen (17). This suggests that APRIL and BAFF play nonredundant roles in IgA switching in vivo. Since serum IgA levels are normal in CD40−/− mice (11, 18), APRIL–BAFF–TACI interactions play an important role in physiologic IgA switching and could be manipulated therapeutically to enhance antibody responses to oral vaccines.
MATERIALS AND METHODS
Mice
CD40−/−, BCMA−/−, and TACI−/− mice were described previously (14, 16, 19). A/WySnJ mice that carry a mutation in BAFF-R were purchased from Jackson Laboratories. All mice were kept in a specific pathogen-free animal facility.
In vitro isotype switching
Spleen cells from CD40−/−, BCMA−/−, and TACI−/− mice were labeled with a cocktail of biotin-conjugated mAbs to CD43, CD11b, Thy1.2, CD138, IgG1, IgG2a, IgG2b, IgG3, IgA, and IgE and negatively sorted with Streptavidin magnetic beads (Dynal). B cells were cultured at 106/ml in RPMI containing 10% FCS, l-glutamine, and 50 μM 2-ME (complete medium). For Ig synthesis, B cells were cultured in complete medium alone or in the presence of 1 μg/ml sAPRIL (R&D Systems), 1 μg/ml sBAFF (Alexis), IL-4 (50 μg/ml; R&D Systems), TGFβ (R&D Systems), 10 μg/ml LPS (Sigma-Aldrich), or 1 μg/ml αCD40 (BD Biosciences). Neutralizing antibodies IL-6, IL-10, and TGFβ (R&D Systems) were used as suggested by the manufacturer. After 6 d, supernatants were assayed for IgA, IgE, and IgG1 by ELISA (11), and genomic DNA was prepared for DC-PCR.
IgG1 surface expression
B cells stimulated for 6 d as above were stained with αB220-FITC and αIgG1 biotin–conjugated mAbs followed by staining with PE-conjugated Streptavidin and FACS analyis.
RT-PCR for GLT, AID, and post switch transcripts (Iμ-CH)
RNA was extracted from 4-d-cultured B cells using TRIzol (Invitrogen) and was reverse transcribed by Supercript II RT (Invitrogen). PCR primers used for γ1, ɛ, and αGLT, Iμ-Cγ1, Iμ-Cɛ, Iμ-Cα, AID, and β2-microglobulin were as described previously (11, 20). All PCR reactions were performed on three dilutions of cDNA (1:1, 1:3, and 1:9) for semiquantitative evaluation. Amplified products were separated on agarose gel and stained with ethidium bromide.
DC-PCR
Genomic DNA isolated from cultured B cells on day 6 was digested with EcoRI, circularized, and used as template for PCR using primers as reported previously for Sμ-Sγ1, Sμ-Sα, Sμ-Sɛ, and the nicotinic acetylcholine receptor β unit (11, 21). All PCR reactions were performed on three dilutions of circularized DNA (1:1, 1:3, and 1:9) for semiquantitative evaluation.
Online supplemental material
Figs. S1–S3 show additional analysis of BAFF- or APRIL-stimulated B cells. Supplemental Materials and methods describe CFSE staining, [3H]thymidine incorporation assay, polymycin B treatment, RT-PCR for BAFF mRNA, and isolation of Igm+ and IgD+ B cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20032000/DC1.
Acknowledgments
This work was supported by National Institutes of Health grants AI31136 and AI31541, the March of Dimes, and the Wallace Fund.
The authors have no conflicting financial interests.
References
- 1.Geha, R., H. Jabara, and S. Brodeur. 2003. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 3:721–732. [DOI] [PubMed] [Google Scholar]
- 2.Gulino, A.V., and L.D. Notarangelo. 2003. Hyper IgM syndromes. Curr. Opin. Rheumatol. 15:422–429. [DOI] [PubMed] [Google Scholar]
- 3.Castigli, E., R. Fuleihan, N. Ramesh, E. Tsitsikov, A. Tsytsykova, and R.S. Geha. 1995. CD40 ligand/CD40 deficiency. Int. Arch. Allergy Immunol. 107:37–39. [DOI] [PubMed] [Google Scholar]
- 4.Jabara, H., S. Brodeur, and R. Geha. 2001. Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J. Clin. Invest. 107:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litinskiy, M.B., B. Nardelli, D.M. Hilbert, B. He, A. Schaffer, P. Casali, and A. Cerutti. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay, F., P. Schneider, P. Rennert, and J. Browning. 2003. BAFF and APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 21:231–264. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkin, P.D., J.H. Lee, and A.B. Lyons. 1996. B cell differentiation and isotype switching is related to division cycle number. J. Exp. Med. 184:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duff, G.W., and E. Atkins. 1982. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J. Immunol. Methods. 52:333–340. [DOI] [PubMed] [Google Scholar]
- 9.Seshasayee, D., P. Valdez, M. Yan, V.M. Dixit, D. Tumas, and I.S. Grewal. 2003. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 18:279–288. [DOI] [PubMed] [Google Scholar]
- 10.Thompson, J.S., S.A. Bixler, F. Qian, K. Vora, M.L. Scott, T.G. Cachero, C. Hession, P. Schneider, I.D. Sizing, C. Mullen, et al. 2001. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 293:2108–2111. [DOI] [PubMed] [Google Scholar]
- 11.Jabara, H., D. Laouini, E. Tsitsikov, E. Mizoguchi, A. Bhan, E. Castigli, F. Dedeoglu, V. Pivniouk, S. Brodeur, and R. Geha. 2002. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 17:265–276. [DOI] [PubMed] [Google Scholar]
- 12.Ahonen, C., E. Manning, L. Erickson, B. O'Connor, E. Lind, S. Pullen, M. Kehry, and R. Noelle. 2002. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat. Immunol. 3:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie, P., B.S. Hostager, and G.A. Bishop. 2004. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 199:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu, S., and K.P. Lam. 2001. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 21:4067–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan, M., H. Wang, B. Chan, M. Roose-Girma, S. Erickson, T. Baker, D. Tumas, I.S. Grewal, and V.M. Dixit. 2001. Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2:638–643. [DOI] [PubMed] [Google Scholar]
- 16.von Bulow, G.U., J.M. van Deursen, and R.J. Bram. 2001. Regulation of the T-independent humoral response by TACI. Immunity. 14:573–582. [DOI] [PubMed] [Google Scholar]
- 17.Castigli, E., S. Scott, F. Dedeoglu, P. Bryce, H. Jabara, A.K. Bhan, E. Mizoguchi, and R.S. Geha. 2004. Impaired IgA class switching in APRIL-deficient mice. Proc. Natl. Acad. Sci. USA. 101:3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castigli, E., F.W. Alt, L. Davidson, A. Bottaro, E. Mizoguchi, A.K. Bhan, and R.S. Geha. 1994. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc. Natl. Acad. Sci. USA. 91:12135–12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollander, G.A., E. Castigli, R. Kulbacki, M. Su, S.J. Burakoff, J.C. Gutierrez-Ramos, and R.S. Geha. 1996. Induction of alloantigen-specific tolerance by B cells from CD40-deficient mice. Proc. Natl. Acad. Sci. USA. 93:4994–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563. [DOI] [PubMed] [Google Scholar]
- 21.Zelazowski P, Carrasco D, Rosas FR, Moorman MA, Bravo R, and S. CM. 1997. B cells genetically deficient in the c-Rel transactivation domain have selective defects in germline CH transcription and Ig class switching. J Immunol. 159:3133-3139. [PubMed] [Google Scholar]