Abstract
Malaria parasites within red blood cells digest host hemoglobin into a hydrophobic heme polymer, known as hemozoin (HZ), which is subsequently released into the blood stream and then captured by and concentrated in the reticulo-endothelial system. Accumulating evidence suggests that HZ is immunologically active, but the molecular mechanism(s) through which HZ modulates the innate immune system has not been elucidated. This work demonstrates that HZ purified from Plasmodium falciparum is a novel non-DNA ligand for Toll-like receptor (TLR)9. HZ activated innate immune responses in vivo and in vitro, resulting in the production of cytokines, chemokines, and up-regulation of costimulatory molecules. Such responses were severely impaired in TLR9−/− and myeloid differentiation factor 88 (MyD88)−/−, but not in TLR2, TLR4, TLR7, or Toll/interleukin 1 receptor domain–containing adaptor-inducing interferon β−/− mice. Synthetic HZ, which is free of the other contaminants, also activated innate immune responses in vivo in a TLR9-dependent manner. Chloroquine (CQ), an antimalarial drug, abrogated HZ-induced cytokine production. These data suggest that TLR9-mediated, MyD88-dependent, and CQ-sensitive innate immune activation by HZ may play an important role in malaria parasite–host interactions.
Malaria infection is still the major cause of disease and mortality in humans, especially in the tropical regions of the world. Due to a complex life cycle and rapid polymorphism of parasite antigens, host–parasite interactions and resulting innate immune responses to malaria parasites are still poorly understood despite the urgent necessity of effective immunotherapeutic interventions (1, 2). Robust innate immune activation, including proinflammatory cytokine production in response to malaria parasites and/or their metabolites released from ruptured infected red blood cells, has been linked to the major symptoms such as high fever (3). Recent evidence suggests that Toll-like receptors (TLRs) are involved in the innate immune responses to a variety of pathogens, including Plasmodium (4, 5). In murine malaria infection, myeloid differentiation factor 88 (MyD88), an essential adaptor molecule for cytokine induction through TLRs, was shown to be critical for IL-12 induction by Plasmodium berghei parasites that cause liver injury (6). A recent study has shown that Plasmodium falciparum blood-stage parasites activate human plasmacytoid DCs as well as murine DCs through MyD88- and TLR9-dependent pathways, whereas the responsible molecule(s) is yet unidentified (7).
Hemozoin (HZ), known as a malaria pigment, is a detoxification product of heme molecules persisting in the food vacuoles of Plasmodium parasite (8, 9). Intracellular HZ is released into the circulation during schizont rupture and phagocytosed by myeloid cells, which results in the concentration of HZ in the reticulo-endothelial system (9). It has been shown that HZ purified from P. falciparum activates macrophages to produce proinflammatory cytokines, chemokines, and nitric oxide and enhances human myeloid DC maturation (10, 11). These studies prompted us to study the molecular mechanism(s) through which HZ activates the innate immune system, which may improve our understanding of malaria parasite–host interactions. We found that both in vivo and in vitro, HZ purified from P. falciparum activates murine immune cells that are mediated by TLR9 and dependent on MyD88. Importantly, such activation was inhibited by chloroquine (CQ), a common antimalarial drug.
Results and Discussion
Purified HZ from P. falciparum activates murine spleen and DCs through MyD88-dependent pathway
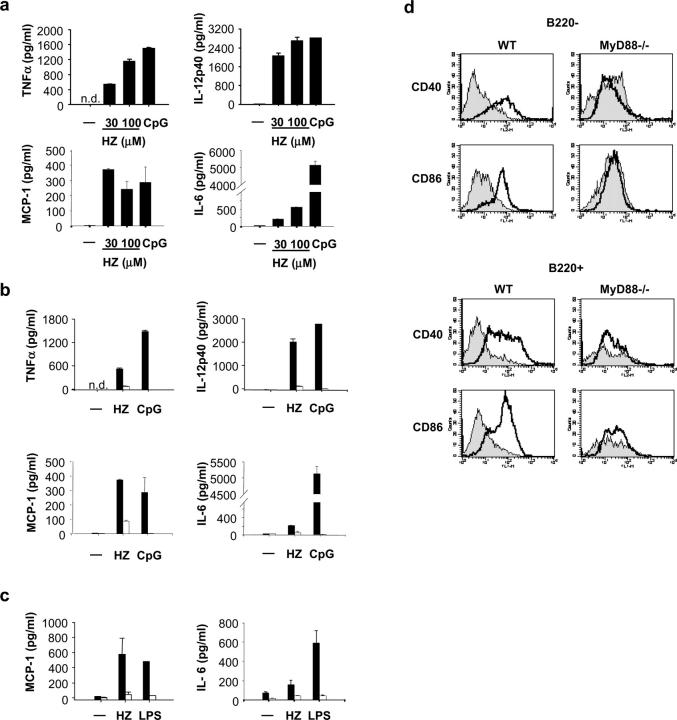
To examine whether HZ purified from P. falciparum activates the murine immune system, spleen cells and DCs were stimulated in vitro with purified HZ, and proinflammatory cytokine production in the culture supernatant was measured by ELISA. Flt3 ligand–induced bone marrow–derived DCs (FL-DCs) produced large amounts of TNFα, IL-12p40, monocyte chemoattractant protein (MCP)-1, and IL-6 in response to HZ in a dose-dependent manner to a similar extent to that of CpG oligodeoxynucleotide (ODN; Fig. 1 a). To investigate possible roles of TLRs in HZ-induced innate immune activation, mice lacking MyD88, an essential adaptor molecule for cytokine inductions mediated by most TLRs, were used (5). FL-DCs from MyD88−/− mice showed severely impaired levels of TNFα, IL-12p40, MCP-1, and IL-6 production upon stimulation with HZ (Fig. 1 b). Similarly, MyD88−/− spleen cells showed impaired responses to produce MCP-1, IL-6, TNFα, IL-12p40, and IFN-inducible protein 10 in response to HZ (Fig. 1 c and not depicted). Additionally, HZ stimulated both CD11c+ B220+ plasmacytoid and CD11c+ B220− myeloid DC subsets of FL-DCs to up-regulate the expression of CD40 and CD86, which were abrogated in both FL-DC subsets in MyD88−/− mice (Fig. 1 d).
Figure 1.
Purified HZ from P. falciparum activates proinflammatory responses through MyD88-dependent pathway. (a) FL-DCs from wild-type (WT) mice were stimulated with 30 and 100 μM of purified HZ for 24 h. ELISA was performed to measure TNFα, IL-12p40, MCP-1, or IL-6 production in the supernatants. As a control, 3 μM CpG DNA (D35) was used. (b) FL-DCs and (c) spleen cells from wild-type (solid bars) and MyD88−/− mice (open bars) were stimulated with 30 μM HZ and 3 μM CpG DNA (D35) or 100 ng/ml LPS for 24 h. Production of TNFα, IL-12p40, MCP-1, and IL-6 was measured by ELISA in the culture supernatant. (d) The myeloid DC (CD11c+ B220−) and plasmacytoid DCs (CD11c+ B220+) were analyzed for CD40 and CD86 expression by flow cytometry. The shaded area represents nonstimulated cells, and the solid line represents stimulated cells with HZ. Results represent the mean ± SD of duplicate cultures and are representative of at least five independent experiments. n.d., not detected.
HZ activation of innate immune responses is Toll/IL-1 receptor domain–containing adaptor-inducing IFNβ (TRIF) independent
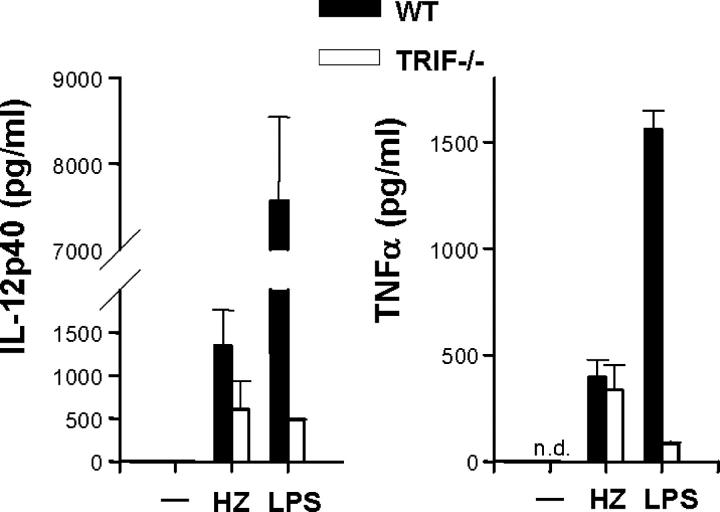
To confirm that HZ-induced innate immune activation is solely dependent on MyD88, mice lacking TRIF, an essential adaptor molecule for the MyD88-independent pathway, were used (5). In contrast to MyD88−/− mice, FL-DCs from TRIF−/− mice responded to HZ and produced TNFα and IL-12p40 (P < 0.05, TRIF−/− vs. media) comparable to that of wild-type mice (P > 0.05; Fig. 2). LPS-induced TNFα and IL-12p40 was impaired in TRIF−/− FL-DCs, suggesting that extensively purified HZ from P. falciparum cultures is not contaminated with LPS. These data clearly demonstrate that HZ activates a proinflammatory response in mice via MyD88, indicating that one of the MyD88-dependent TLRs might be involved in the recognition of HZ.
Figure 2.
HZ-induced DC activation is TRIF independent. FL-DCs from wild-type (WT) and TRIF−/− mice were incubated with 30 μM HZ and 100 ng/ml LPS for 24 h, after which supernatants were assayed by ELISA for TNFα or IL-12p40 production. Results are the mean ± SEM of four independent experiments (n = 4 mice). P > 0.05, HZ (WT) versus HZ (TRIF−/−). n.d., not detected.
HZ activation of innate immune responses is TLR9-dependent
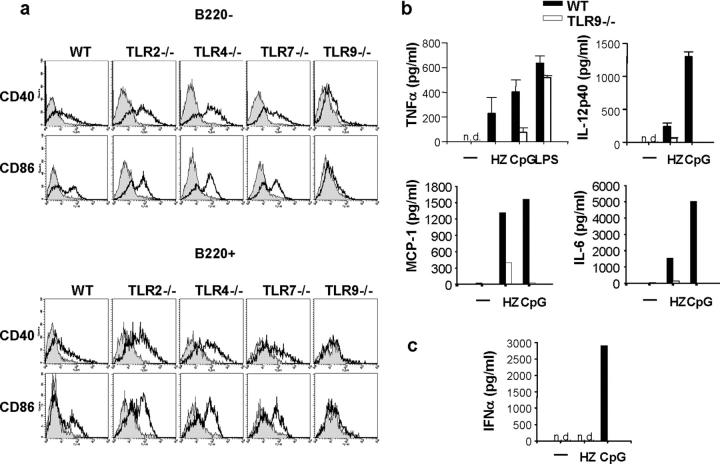
Further experiments were performed using spleen cells and DCs obtained from TLR2−/−, TLR4−/−, TLR7−/−, or TLR9−/− mice to examine whether HZ-induced innate immune activations are impaired or altered. HZ stimulated FL-DCs in wild-type, TLR2−/−, TLR4−/−, and TLR7−/− mice to up-regulate CD40 and CD86 in both CD11c+ B220+ plasmacytoid and CD11c+ B220− myeloid DC subsets (Fig. 3 a). In contrast, both subsets of FL-DCs derived from TLR9−/− mice failed to up-regulate CD40 and CD86 in response to HZ (Fig. 3 a). Similarly, FL-DCs derived from wild-type, TLR2−/−, TLR4−/−, and TRL7−/− mice, but not from TLR9−/− mice, produced TNFα, IL-12p40, MCP-1, and IL-6 in response to HZ (Fig. 3 b and not depicted). It is of note that HZ did not induce IFNα by FL-DCs, suggesting that the HZ-induced cytokine profile is similar to that of the K-type CpG ODN (also known as B type), but distinct from that of the D-type ODN (also know as A-type) or known natural DNA ligands for TLR9 such as bacterial or viral DNA (Fig. 3 c; reference 12). Nevertheless, these data clearly demonstrate that TLR9 and MyD88 are critical for HZ-induced activation of murine spleen cells and DCs.
Figure 3.
TLR9 mediates HZ-induced innate immune activation. (a) The myeloid (CD11c+ B220−) and plasmacytoid DCs (CD11c+ B220+) from wild-type (WT) and TLR2−/−, TLR4−/−, TLR7−/−, or TLR9−/− mice were analyzed for CD40 and CD86 expression by flow cytometry. The shaded area represents nonstimulated cells, and the solid line represents stimulated cells with HZ. Wild-type and TLR9−/− cells were incubated with the indicated stimuli and analyzed for (b) TNFα, IL-12p40, MCP-1, or IL-6 production by spleen cells and (c) IFNα production by FL-DCs by ELISA. Results are representative of at least five independent experiments. n.d., not detected.
HZ activation of proinflammatory cytokines is MyD88/TLR9 dependent in vivo
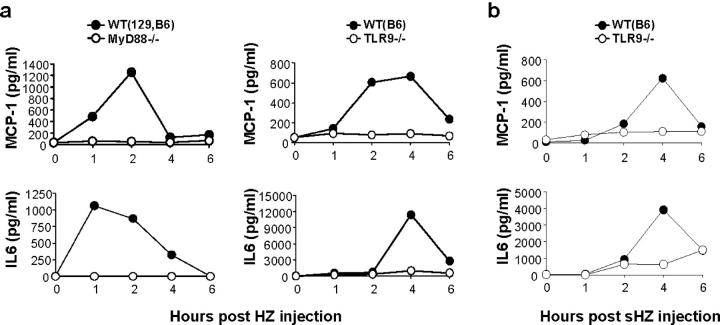
To further confirm that HZ activation is mediated by TLR9 and dependent on MyD88 in vivo, purified P. falciparum HZ was injected i.p. into wild-type and MyD88−/− or TLR9−/− mice, and serum cytokine productions were monitored. HZ injection significantly increased serum levels of MCP-1 and IL-6 in wild-type mice, which peaked between 1 and 4 h and declined within 6 h (Fig. 4 a). In contrast, such increases were completely abrogated in MyD88−/− and TLR9−/− mice. After 6 h, the cytokine levels declined in wild-type mice. These data clearly demonstrate that HZ-induced proinflammatory responses were mediated by TLR9 and MyD88 both in vitro and in vivo.
Figure 4.
Serum cytokine productions by HZ and synthetic HZ (β-hematin) in vivo are dependent on MyD88 and TLR9. MyD88−/−, TLR9−/−, or wild-type (WT) mice were injected i.p. with either 1,500 μg of (a) purified P. falciparum HZ or (b) synthetic HZ (sHZ). Serum levels of MCP-1 and IL-6 were measured by ELISA at the indicated time points.
Synthetic HZ (β-hematin, synthesized from monomeric heme in laboratory conditions) is structurally similar to HZ formed naturally by parasites (13, 14) and free of contaminant derived from parasites or culture. To examine whether synthetic HZ also activates the innate immune system in a TLR9-dependent manner, wild-type or TLR9−/− mice were injected with synthetic HZ, and then IL-6 and MCP-1 production in sera was monitored. Synthetic HZ injection into wild-type mice resulted in the production of MCP-1 and IL-6 in serum peaked at 4 h (Fig. 4 b). In contrast, such responses were abrogated in TLR9−/− mice (Fig. 4 b). These data suggest that synthetic HZ as well as natural HZ purified from P. falciparum stimulates the innate immune system in mice, excluding the contribution of the other possible contaminant(s) to HZ-induced, TLR9-mediated innate immune activation.
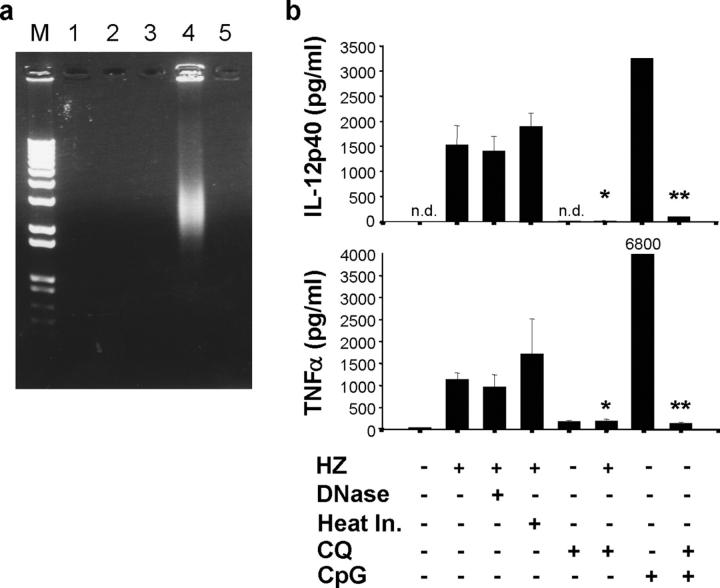
To further exclude any possible contaminants in or during HZ preparation from the P. falciparum culture, a series of analyses was also performed to determine the purity of HZ in addition to the extensive purification method described above (11). DNA or RNA was not detectable in HZ or DNase-treated HZ solution (1 mM) by either ethidium bromide–stained agarose gel (Fig. 5 a) or with a spectrophotometer. DNase treatment or heat inactivation had no effect on HZ-induced DC production of TNFα and IL-12p40 (Fig. 5 b). Furthermore, genomic DNA and RNA isolated from P. falciparum did not activate innate immune responses significantly, including the production of TNFα and IL-12 (unpublished data and reference 15). No protein or lipid was detected in the HZ solution (1 mM) by all of the methods we tested. Taken together, these data strongly suggest that HZ-induced, TLR9-mediated innate immune activation is not due to the other contaminants.
Figure 5.
HZ is free of DNA, and HZ-induced proinflammatory cytokine production is DNase and heat resistant, but CQ sensitive. (a) HZ was run on an agarose gel and ethidium bromide stained to detect possible DNA contamination. M, marker; lane 1, HZ solution (1 mM, 5 μl); lane 2, heat-inactivated HZ (1 mM, 5 μl); lane 3, DNase-treated HZ (1 mM, 5 μl); lane 4, P. falciparum crude extract (5 μl from packed 100 ml of culture containing 4.5% parasitemia); lane 5, DNase-treated crude extract (5 μl). (b) FL-DCs from C57/B6 mice was incubated with either 30 μM HZ or with DNase treatment, heat inactivation, and/or 10 μM CQ for 24 h. Supernatants were then collected and measured for TNFα or IL-12p40 by ELISA. As a control, 3 μM CpG ODN (D35) was used. The value of TNFα by D35 is shown. Results are representative of one of three independent experiments performed in duplicate (mean ± SD). *, P < 0.05, HZ versus HZ plus CQ; **, P < 0.05, CpG ODN versus CpG ODN plus CQ. n.d., not detected.
HZ-induced innate immune activation is CQ sensitive
The antimalarial drug CQ has been shown to inhibit proinflammatory responses during malaria infection in addition to endo/lysosomal maturations and malaria HZ crystal formations, whereas the exact mechanism of the antimalarial effects of CQ is still under debate (8, 16). Recent evidence suggests that CQ also inhibits TLR9-mediated innate immune activation (17). To examine the effect of CQ on the HZ-induced proinflammatory responses, FL-DCs were stimulated with HZ in the presence of CQ. HZ-induced TNFα and IL-12p40 productions were diminished by CQ (Fig. 5 b), suggesting that in addition to previously known mechanisms of CQ inhibition of malaria pathogenesis, CQ may also inhibit HZ-induced, TLR9-mediated innate immune responses during malaria infection, possibly contributing to its therapeutic effects. Although further studies are needed, it is possible that in addition to its ability to inhibit HZ formation, CQ may also interfere with the interaction of HZ with TLR9 or inhibit the maturation of HZ-containing phagosome, thereby inhibiting the following innate immune activation.
It has been shown that TLR9 recognizes CpG motifs in microbial DNA or self-DNA–chromatin complex with specific IgG, whereas a non-DNA molecule(s) as a TLR9 ligand has not been reported (18, 19). This work provides the first evidence of a non-DNA ligand as recognized by TLR9. HZ is a crystal form of polymerized heme (ferriprotoporphyrin IX) produced in the food vacuole of parasites during degradation of hemoglobin from red blood cells. Once captured by phagocytes such as macrophages and DCs, HZ accumulates in the phagosome where TLR9 can be recruited from the endoplasmic reticulum via PI3 kinases (20, 21), indicating that TLR9 may recognize HZ in phagosome. HZ is also extremely hydrophobic, which may contribute to its immunostimulatory effect according to the hypothesis proposed recently (22). It is of interest that HZ is originally derived from hemoglobin in the host red blood cells, but modified by parasites from free heme (toxic to parasite) into HZ (nontoxic to parasite), self-molecules (heme) become active “non-self” molecules in the innate immune system during malaria infection.
The physiological role of HZ-induced, TLR9-mediated innate immune activation in malaria infection and the host defense against it is currently under investigation. Preliminary results suggest that innate immune responses to malaria parasite are dependent on multiple factors in the host, including TLRs as well as Plasmodium. In particular, it is important to study whether TLR-mediated innate immune activation during malaria infection contributes to host-protective immunity or to malarial immune escape mechanism. It is of our interest as well to study the molecular mechanisms by which TLR9 discriminates between CpG DNA and HZ. Nonetheless, these findings clearly demonstrate that HZ, heme metabolite during malaria infection, activates the innate immune system via a TLR9-mediated, MyD88-dependent, and CQ-sensitive pathway, which may open a key to further understanding of malaria parasite–host interactions in innate immunity.
Materials and Methods
Mice
Mutant mice (MyD88-, TRIF-, TLR2-, TLR4-, TLR7-, and TLR9-deficient) either on a 129/Ola × C57/BL6 or C57/BL6 background were generated as described previously (23–27). Age-matched groups of wild-type and mutant mice were used for the experiments. For the in vivo studies, 1,500 μg HZ purified from P. falciparum culture and synthetic HZ (β-hematin) were injected i.p. (28) into wild-type, MyD88−/− or TLR9−/− mice. Serum was collected from tails at 1, 2, 4, and 6 h for the cytokine ELISA.
Reagents
Synthetic CpG ODNs D35 (29) were synthesized and purchased from Hokkaido System Science. LPS from Salmonella minnesota Re-595, hemin chloride, and CQ was purchased from Sigma-Aldrich. DNase (DNase-I) was purchased from Invitrogen.
Preparation of HZ and synthetic HZ (β-hematin)
HZ (nonsoluble crystalloid structure) was purified from P. falciparum (3D7 strain)–infected erythrocytes as described previously (11, 13, 14). In brief, after saponin lysis of erythrocytes, parasites were sonicated and washed seven to eight times in 2% SDS. The pellet was incubated with 2 mg/ml proteinase-K at 37°C overnight. The pellet was then washed three times in 2% SDS and incubated in 6 M urea for 3 h at room temperature on a shaker. After three to five washes in 2% SDS and then in distilled water, the HZ pellet was resuspended in distilled water and sonicated again before use. In some experiments, HZ was either heat inactivated at 95°C for 15 min or treated with 100 U/ml DNase-I for 1 h as described previously (30). DNase-I treatment was successful in removing genomic DNA completely from the P. falciparum crude extract (Fig. 5 a). Quantification of nucleic acid and protein was performed by using a spectrophotometer or by ethidium bromide staining in agarose gel, BCA (Bio-Rad Laboratories), or the pyrogallol red method (Wako Pure Chemical Industries, Ltd.). Total lipid was measured by TLC using the Bligh-Dyer method (Toray Research Center) or by an enzymatic method using the Iatron LQ (Mitsubishi Kagaku Iatron Inc.). Synthetic HZ (β-hematin) was purified from hemin chloride using the protocol of Jaramillo et al. (28) based on the acetic acid treatment and alkaline bicarbonate wash. To avoid endotoxin contaminations, all solutions were prepared using endotoxin-free PBS or distilled water. Endotoxin levels measured by LAL assays (Bio-Whittaker) were <0.001 EU for each nmole HZ used.
Quantification of HZ or synthetic HZ (β-hematin)
The concentration of HZ or synthetic HZ was determined by depolymerizing heme polymers in 20 mM sodium hydroxide/2% SDS solution for 2 h at room temperature, and then the OD was read at 400 nm (14). The molar extinction coefficient for heme is 105 at 400 nm and 25 μg P. falciparum HZ equals 29 nmole of heme content (14, 28).
Cells
Single cell suspensions of spleen cells (5 × 105 cells/well) were cultured in complete RPMI 1640 medium supplemented with 10% FCS for 48 h. FL-DCs (105 cells/well) were generated by culturing bone marrow cells with Flt3-ligand (100 ng/ml; PeproTech) for 8–9 d in DMEM medium containing 10% FCS. Cells were stimulated in the presence of the indicated stimuli and supernatants were collected for cytokine ELISA.
Cytokine ELISA.
Mouse TNFα, IL-12p40, MCP-1, IL-6 (R&D Systems), and IFNα (PBL Biomedical Laboratories) were measured either from the supernatants or the serum by ELISA according to the manufacturer's instructions.
Flow cytometric analysis of costimulatory molecule expressions.
Cell surface molecule expression of stimulated cells was measured as described previously (21). In brief, stimulated cells were washed with ice cold PBS, fixed, and stained with FITC-, PE-, CyChrome-, and APC-labeled antibodies in the presence of anti-CD16 antibody for 30 min at room temperature. Stained cells were washed, resuspended in PBS/0.1% BSA/0.1% NaN3, and analyzed by FACSCalibur followed by analysis using CELLQuest software (Becton Dickinson). All antibodies were obtained from Becton Dickinson.
Statistical analysis
Statistically significant differences were analyzed by using Student's t test. P < 0.05 was considered significant.
Acknowledgments
We thank T. Mitamura, N. Arisue, N. Palacpac, and other members of the Horii lab for helpful discussions and support. We also thank all members of our laboratory, especially Y. Torii and Y. Fujita for generous support.
The authors have no conflicting financial interests.
C. Coban and K.J. Ishii contributed equally to this work.
References
- 1.Miller, L.H., and S.L. Hoffman. 1998. Research toward vaccines against malaria. Nat. Med. 4:520–524. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson, M.M., and E.M. Riley. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4:169–180. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski, D., C.A. Bate, I.G. Scragg, P. Beattie, I. Udalova, and J.C. Knight. 1997. The malarial fever response–pathogenesis, polymorphism and prospects for intervention. Ann. Trop. Med. Parasitol. 91:533–542. [DOI] [PubMed] [Google Scholar]
- 4.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 5.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 6.Adachi, K., H. Tsutsui, S. Kashiwamura, E. Seki, H. Nakano, O. Takeuchi, K. Takeda, K. Okumura, L. Van Kaer, H. Okamura, et al. 2001. Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J. Immunol. 167:5928–5934. [DOI] [PubMed] [Google Scholar]
- 7.Pichyangkul, S., K. Yongvanitchit, U. Kum-arb, H. Hemmi, S. Akira, A.M. Krieg, D.G. Heppner, V.A. Stewart, H. Hasegawa, S. Looareesuwan, et al. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 172:4926–4933. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan, D.J. 2002. Theories on malarial pigment formation and quinoline action. Int. J. Parasitol. 32:1645–1653. [DOI] [PubMed] [Google Scholar]
- 9.Arese, P., and E. Schwarzer. 1997. Malarial pigment (haemozoin): a very active ‘inert’ substance. Ann. Trop. Med. Parasitol. 91:501–516. [DOI] [PubMed] [Google Scholar]
- 10.Sherry, B.A., G. Alava, K.J. Tracey, J. Martiney, A. Cerami, and A.F. Slater. 1995. Malaria-specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP-1 alpha, and MIP-1 beta) in vitro, and altered thermoregulation in vivo. J. Inflamm. 45:85–96. [PubMed] [Google Scholar]
- 11.Coban, C., K.J. Ishii, D.J. Sullivan, and N. Kumar. 2002. Purified malaria pigment (hemozoin) enhances dendritic cell maturation and modulates the isotype of antibodies induced by a DNA vaccine. Infect. Immun. 70:3939–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinman, D.M. 2004. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 4:249–258. [DOI] [PubMed] [Google Scholar]
- 13.Slater, A.F., W.J. Swiggard, B.R. Orton, W.D. Flitter, D.E. Goldberg, A. Cerami, and G.B. Henderson. 1991. An iron-carboxylate bond links the heme units of malaria pigment. Proc. Natl. Acad. Sci. USA. 88:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan, D.J., Jr., I.Y. Gluzman, D.G. Russell, and D.E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA. 93:11865–11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koski, G.K., K. Kariko, S. Xu, D. Weissman, P.A. Cohen, and B.J. Czerniecki. 2004. Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J. Immunol. 172:3989–3993. [DOI] [PubMed] [Google Scholar]
- 16.Fitch, C.D. 2004. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci. 74:1957–1972. [DOI] [PubMed] [Google Scholar]
- 17.Macfarlane, D.E., and L. Manzel. 1998. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J. Immunol. 160:1122–1131. [PubMed] [Google Scholar]
- 18.Wagner, H. 2004. The immunobiology of the TLR9 subfamily. Trends Immunol. 25:381–386. [DOI] [PubMed] [Google Scholar]
- 19.Krieg, A.M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709–760. [DOI] [PubMed] [Google Scholar]
- 20.Latz, E., A. Schoenemeyer, A. Visintin, K.A. Fitzgerald, B.G. Monks, C.F. Knetter, E. Lien, N.J. Nilsen, T. Espevik, and D.T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190–198. [DOI] [PubMed] [Google Scholar]
- 21.Ishii, K.J., F. Takeshita, I. Gursel, M. Gursel, J. Conover, A. Nussenzweig, and D.M. Klinman. 2002. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J. Exp. Med. 196:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seong, S.Y., and P. Matzinger. 2004. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4:469–478. [DOI] [PubMed] [Google Scholar]
- 23.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1999. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 26.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
- 28.Jaramillo, M., I. Plante, N. Ouellet, K. Vandal, P.A. Tessier, and M. Olivier. 2004. Hemozoin-inducible proinflammatory events in vivo: potential role in malaria infection. J. Immunol. 172:3101–3110. [DOI] [PubMed] [Google Scholar]
- 29.Gursel, M., D. Verthelyi, I. Gursel, K.J. Ishii, and D.M. Klinman. 2002. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J. Leukoc. Biol. 71:813–820. [PubMed] [Google Scholar]
- 30.Ishii, K.J., K. Suzuki, C. Coban, F. Takeshita, Y. Itoh, H. Matoba, L.D. Kohn, and D.M. Klinman. 2001. Genomic DNA released by dying cells induces the maturation of APCs. J. Immunol. 167:2602–2607. [DOI] [PubMed] [Google Scholar]