Abstract
After vaccination of melanoma patients with MAGE antigens, we observed that even in the few patients showing tumor regression, the frequency of anti-vaccine T cells in the blood was often either undetectable or <10−5 of CD8 T cells. This frequency being arguably too low for these cells to be sole effectors of rejection, we reexamined the contribution of T cells recognizing other tumor antigens. The presence of such antitumor T cells in melanoma patients has been widely reported. To begin assessing their contribution to vaccine-induced rejection, we evaluated their blood frequency in five vaccinated patients. The antitumor cytotoxic T lymphocyte (CTL) precursors ranged from 10−4 to 3 × 10−3, which is 10–10,000 times higher than the anti-vaccine CTL in the same patient. High frequencies were also observed before vaccination. In a patient showing nearly complete regression after vaccination with a MAGE-3 antigen, we observed a remarkably focused antitumoral response. A majority of CTL precursors (CTLp's) recognized antigens encoded by MAGE-C2, another cancer-germline gene. Others recognized gp100 antigens. CTLp's recognizing MAGE-C2 and gp100 antigens were already present before vaccination, but new clonotypes appeared afterwards. These results suggest that a spontaneous antitumor T cell response, which has become ineffective, can be reawakened by vaccination and contribute to tumor rejection. This notion is reinforced by the frequencies of anti-vaccine and antitumor CTLs observed inside metastases, as presented by Lurquin et al. (Lurquin, C., B. Lethé, V. Corbière, I. Théate, N. van Baren, P.G. Coulie, and T. Boon. 2004. J. Exp. Med. 201:249–257).
Antitumor CTL clones derived from the blood or from metastases of melanoma patients by in vitro stimulation with autologous tumor cells have been found to recognize several classes of antigens. First, there are those encoded by the “cancer-germline” genes, such as the MAGE, BAGE, GAGE, and LAGE families (1–5). These genes are expressed in a large fraction of tumors of various histological types and therefore code for shared antigens. These antigens are strictly tumor specific because the cancer-germline genes are silent in normal adult tissues with the exception of male germ cells, which do not carry HLA molecules and therefore cannot present antigens to T cells. Three subfamilies of MAGE genes are expressed in tumors: MAGE-A, which codes for most of the antigens identified so far, MAGE-B, and MAGE-C. A second major class of tumor antigens is encoded by genes that are mutated in tumor cells. These antigens are also strictly tumor specific, but in most cases they were observed only in a single tumor with notable exceptions, such as one encoded by a mutated CDK4 (6). In a third class are antigens encoded by melanocytic differentiation genes, such as tyrosinase, Melan-A MART-1, or gp100 Pmel17 (7–10).
Antigens encoded by MAGE genes have been used for small-scale therapeutic vaccination trials of metastatic melanoma patients with detectable disease. The vaccines consisted of either antigenic peptides, a protein, a recombinant poxvirus of the ALVAC-type encoding two antigenic peptides, or dendritic cells pulsed with an antigenic peptide (11–14). In the peptide, protein, and ALVAC-MAGE trials, some evidence of tumor regression was observed in ∼20% of the patients. In the trials involving dendritic cells, the rates of tumor regression were not clearly different from those observed with other vaccine modalities (13).
Our initial work suggested that most vaccinated patients, including those who displayed tumor regression, did not have anti-vaccine T cells in the blood at frequencies >10−4 of CD8 T cells. Therefore, we developed a sensitive approach aimed at detecting low CD8 T cell responses. It is based on in vitro restimulation of PBMCs with the antigenic peptide followed by labeling with tetramers. To evaluate CTL precursor (CTLp) frequencies, these mixed lymphocyte–peptide cultures (MLPCs) are performed under limiting dilution conditions. The tetramer-stained cells of the positive microcultures are cloned, the lytic specificity of the clones is verified, and their diversity is analyzed by TCR sequencing (15). This approach enables us to detect CTL responses as low as 8 × 10−7 of the CD8 T cells (16).
We have focused our efforts on the detection of CTLs recognizing the antigenic peptide MAGE-3168-176, which is encoded by the gene MAGE-A3 and presented by HLA-A1. In patients vaccinated with a recombinant canarypox virus of the ALVAC-type coding for the MAGE-3.A1 peptide, anti–MAGE-3.A1 CTL responses were found in 3 out of 4 patients who showed tumor regression and in 1 out of 11 who did not (15–17). Among seven patients vaccinated with MAGE-3.A1 peptide who showed evidence of tumor regression, only one had a detectable CTL response (16). Even among those vaccinated patients who showed a CTL response, most had a low frequency of anti–MAGE-3.A1 CTLs in the blood, ranging between 10−6 and 10−5 of CD8 T cells (15, 17).
These results clearly contradicted our initial view on antitumoral vaccination, namely that massive anti-vaccine CTL responses would be required for tumor rejection. Moreover, they raised the possibility that the low frequency of anti-vaccine CTLs would be insufficient to provide all the specific effector cells that lyse the tumor cells. Therefore, we set out to examine whether CTLs directed against other antigens present on the tumor might provide additional effectors for the tumor rejections observed after vaccination. As a first step, we show here the presence of high levels of such antitumor CTLs in the blood of vaccinated patients, and we describe the antigens recognized by these CTLs in one patient. In Lurquin et al. (18), which appears in this issue of JEM, we describe the distribution of anti-vaccine and other antitumor CTLs inside the metastases of this patient.
Results
High frequencies of antitumor CTLp's in the blood of melanoma patients
We selected five metastatic melanoma patients who had been vaccinated with MAGE antigens (Table I). They were selected because it had been possible to derive a permanent cell line from their tumor cells. We set up mixed lymphocyte–tumor cell cultures (MLTCs) to estimate the blood frequencies of CTLp's directed specifically against the tumor. Purified CD8 blood T cells were stimulated with irradiated autologous tumor cells in medium containing IL-2, IL-4, and IL-7. These stimulations were performed in limiting dilution conditions with 300–4,000 lymphocytes per microculture (19). After three weekly stimulations, the lytic activity of the microcultures was tested against the autologous tumor cells, autologous EBV-transformed B cells, and NK target K562. We considered as positive those microcultures that lysed tumor cells considerably more than EBV-B and K562 cells. We will refer to the lytic effectors detected in these MLTCs as “antitumor” CTLs to distinguish them from the “anti-vaccine” CTLs, which recognize the vaccine antigen. Precise criteria and representative results are shown in Fig. 1.
Table I.
Frequencies of antitumor CTLp's in vaccinated melanoma patients
| Antitumor CTLp frequencyb
|
|||||
|---|---|---|---|---|---|
| Patient | Vaccine antigens | Tumor regressiona | Anti-vaccine CTLp frequencyb | Before | After |
| CP64 | MAGE-3.A1c | + | 5 × 10−5 | 6 × 10−5 | 6 × 10−4 |
| EB81 | MAGE-3.A1dMAGE-1.A1 | + | 3 × 10−6
<3 × 10−7 |
3 × 10−4 | 3 × 10−3 |
| CP67 | MAGE-3.A1dMAGE-1.A1 | + | 3 × 10−7
<3 × 10−7 |
5 × 10−4 | 10−4 |
| BB132 | MAGE-3.A1dMAGE-1.A1 | − | 10−7
<3 × 10−7 |
2 × 10−3 | 2 × 10−3 |
| LB2269 | MAGE-4.A2eMAGE-10.A2 | − | <10−7
5 × 10−5 f |
6 × 10−4 | 6 × 10−4 |
Some evidence of tumor regression observed after vaccination as detailed in Materials and Methods.
Among blood CD8 lymphocytes. The anti-vaccine CTLp frequencies were measured by in vitro restimulation of PBMCs with the antigenic peptide in limiting dilution conditions followed by labeling with tetramers. Before and After stands for before and after vaccination.
15 injections of 300 µg of peptide without adjuvant.
Three injections of ALVAC-MAGE followed by three injections of peptides without adjuvant.
Three injections of 300 µg of each peptide with rIL-12.
A similar frequency was already present before vaccination.
Figure 1.
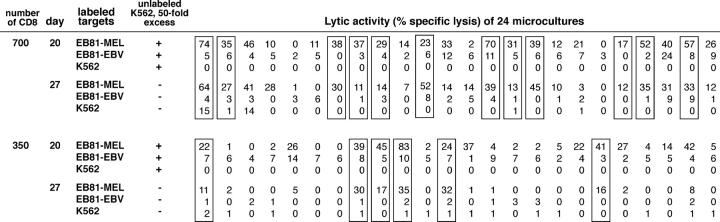
Limiting dilution analysis of antitumor CTLp's in patient EB81. Lytic patterns obtained at days 20 and 27 with limiting dilution microcultures set up with 700 and 350 blood CD8 lymphocytes and stimulated with irradiated autologous tumor cells, IL-2, IL-4, and IL-7 on days 0, 7, 14, and 21. Microcultures deemed to contain specific antitumor T cells are boxed. The lytic activity of aliquots of the microcultures was measured against autologous tumor cells EB81-MEL, autologous EBV-transformed B cells (EB81-EBV), and K562. On day 20 only, effector cells and 51Chromium-labeled targets were incubated in the presence of a 50-fold excess of unlabeled K562 to quench the lytic activity of NK-like effectors. Microcultures were considered to contain antitumor CTLs if they satisfied on day 20 as well as on day 27 the following criteria: lysis of tumor >10% and lysis of EBV-B and K562 lower than one third of the lysis of tumor.
For all five patients, antitumor CTLp's were found at high frequencies, i.e., from 10−4 to 3 × 10−3 of the CD8 T cells in the blood after vaccination (Table I). These frequencies were considerably higher than that of the anti-vaccine CTLs, ranging from 12- to 20,000-fold higher (Table I and Fig. 2). Antitumor CTLp's were already present at similar high frequencies before vaccination (Table I). In two patients who showed evidence of tumor regression, including patient EB81 (Fig. 2), the frequencies of antitumor CTLp's were ∼10-fold higher after vaccination. But this was not observed in the other regressor patient and therefore may not be significant. The level of antitumor CTLp's found either before or after vaccination was not higher in the three regressors than in the two progressor patients.
Figure 2.
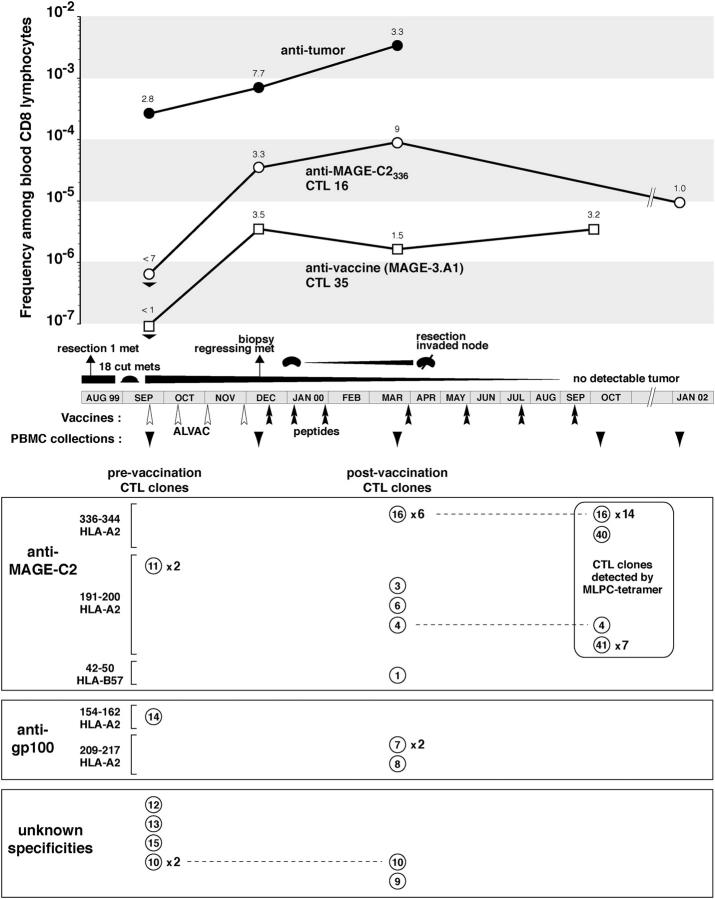
Frequencies and target antigens of antitumor CTLs from patient EB81. The clinical evolution of the patient and the frequencies of antitumor and anti-vaccine CTLp's are indicated in the top. Frequencies of antitumor CTLp's were measured by MLTC with a tumor cell line derived from the invaded lymph node, whereas those of anti–MAGE-3.A1 and anti–MAGE-C2336–344 CTLp's were measured by clonotypic PCR. The bottom panels represent antitumor CTL clones with different numbers for each TCR sequence and the occurrence of repeated clones. Most of these CTL clones were derived from lymphocytes collected in September 1999 and March 2000 and were stimulated in autologous MLTC. Additional CTL clones were derived from lymphocytes collected in October 2000 by stimulation with MAGE-C2 peptides and identification of the positive microcultures with the appropriate tetramer. For peptide MAGE-C2336–344, 15 additional CTL clones were obtained. 14 turned out to be the CTL 16 clonotype, whereas one, CTL 40, had another TCR. A similar experiment performed with MAGE-C2191–200 revealed a new highly repeated clonotype, CTL 41.
Antigens recognized by antitumor CTLs of patient EB81
To establish the relevance of the antitumor T cells observed at such high frequencies, we set out to identify their target antigens. We focused our efforts on patient EB81, who had shown complete regression of a large number of cutaneous metastases. Stable CTL clones were derived from microcultures of postvaccination lymphocytes that displayed a high level of antitumor lytic activity and did not lyse EBV-B cells and K562 (Fig. 3). 15 CTL clones were derived from independent microcultures. We were able to identify the antigens recognized by 13 of these 15 CTL clones. Remarkably, these antigens were encoded by only two genes: MAGE-C2, a cancer-germline gene, and gp100 Pmel17, a melanocytic differentiation gene (20, 21).
Figure 3.
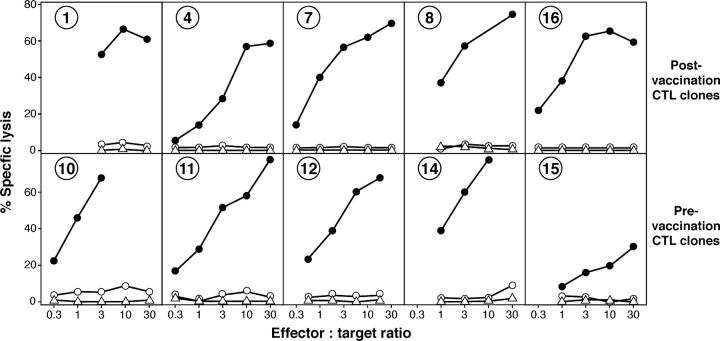
Lytic activity of antitumor CTL clones of patient EB81. Target cells included autologous melanoma cells EB81-MEL (•), autologous EBV-B cells (○), and K562 cells (▵).
10 of the 15 CTL clones recognized antigens encoded by MAGE-C2 (Fig. 2). CTL clone 16 recognized the MAGE-C2336–344 peptide ALKDVEERV on HLA-A2. T cell receptor sequencing indicated that this clonotype was repeated six times among the 15 CTL clones. Another peptide, MAGE-C2191–200 LLFGLALIEV, was recognized by three CTL clones, each having a different TCR. The detailed identification of these antigens is described elsewhere (22). Finally, one CTL clone recognized a third peptide, MAGE-C242–50 ASSTLYLVF, on HLA-B57. All of the clones recognized peptide-pulsed targets with half-maximal lysis at peptide concentrations between 20 and 100 nM, the usual range for such CTLs. Thus, five different clonotypes recognizing three different MAGE-C2 antigenic peptides constituted the majority of the antitumor CTL clones derived from the blood of patient EB81. Two additional clonotypes were found, using HLA-A2 tetramers, in blood collected 7 mo later (Fig. 2). 3 of the 15 antitumor CTL clones recognized an antigenic peptide encoded by gp100, namely gp100209–217 ITDQVPFSV, which is presented by HLA-A2 (21). Two had the same TCR.
These results show that the massive antitumor CTL response of patient EB81 is directed against antigens that belong to the main known classes of tumor antigens. The antitumor CTL response of this patient was remarkably focused on MAGE-C2 antigens, targeting three different peptides with seven different clonotypes, whereas no CTL directed against other antigens encoded by cancer-germline genes was observed. This is not due to the lack of expression of other cancer-germline genes. Although MAGE-C2 appeared to have the highest expression, several other cancer-germline genes, such as MAGE-A3, MAGE-A6, and LAGE-1, were expressed at similar levels in tumor samples and in the tumor cell line (Table II). It is worth noting in Table II that the gene expression profile of the melanoma cell line, which was used to derive all of the antitumor CTLs of patient EB81, shows a good match with those of the autologous tumor samples.
Table II.
Expression of cancer-germline and melanocytic differentiation genes in tumor cells from patient EB81
| Before vaccination
|
After vaccination
|
||||
|---|---|---|---|---|---|
| cut. met. | cut. met. | lymph node | cell line | ||
| Real-time
|
Semiquantitative RT-PCR
|
||||
| MAGE-A | |||||
| 1 | 0 | − | − | ± | + |
| 2 | 507 | ± | + | − | +++ |
| 3 | 3559 | ++ | + | + | +++ |
| 4 | 0 | − | − | − | − |
| 6 | 2320 | + | +++ | ++ | +++ |
| 8 | − | − | − | ± | |
| 9 | − | − | − | + | |
| 10 | 1159 | + | ++ | ± | + |
| 11 | 2 | − | ± | − | − |
| 12 | 25 | − | + | ± | ++ |
| MAGE-B | |||||
| 1 | 0 | − | − | − | − |
| 2 | 0 | − | − | − | ± |
| MAGE-C | |||||
| 1 | ± | ± | + | ++ | |
| 2 | 3941 | +++ | +++ | +++ | +++ |
| LAGE | |||||
| 1 | 527 | + | +++ | + | ++ |
| 2 | 1 | − | ± | − | − |
| Tyrosinase | 29742 | +++ | +++ | +++ | +++ |
| Melan-AMART-1 | 31442 | ++ | +++ | ++ | +++ |
| gp100 | 27220 | ± | ++ | ++ | +++ |
Tumor samples included a cutaneous metastasis (cut. met.) resected before vaccination, in August 1999, a cutaneous metastasis biopsied in December 1999, at the time of tumor regression, and an invaded lymph node resected 4 mo later while regression of cutaneous metastases carried on. The EB81-MEL tumor cell line was derived from the lymph node metastasis. The results of the real-time PCR experiments are expressed as number of mRNA/1,000 cells, considering 10 pg total RNA per cell and an efficiency of reverse transcription of 25%. The other results are expressed as +++, ++, +, or ± if the amount of the amplified product is equal or greater than that obtained with 1:1, 1:3, 1:9, and 1:27 dilutions of the reference RNA used for each gene. Lower levels of expression are scored negative. Reference RNA is extracted from tumor cell lines expressing the relevant genes.
Presence of anti–MAGE-C2 and anti-gp100 CTLs before vaccination
We observed that a spontaneous CTL response against MAGE-C2 had already occurred in patient EB81 before vaccination. Eight independent antitumor CTL clones were obtained from the prevaccination lymphocytes (Fig. 2). Two recognized the MAGE-C2191–200 peptide (Fig. 2). Both carried the same TCR. Another CTL clone recognized gp100154–162.
Considering the presence of CTLs directed against MAGE-C2 and gp100 both before and after vaccination, we wondered whether individual CTL clonotypes also had a constant presence. Some clonotypes were clearly present in the blood before and after vaccination. An example is anti–MAGE-C2191–200 CTL clone 11. As shown in Lurquin et al. (18), its frequency among blood CD8 T lymphocytes was evaluated with a clonotypic PCR. It was 9 × 10−6 before vaccination and 3 × 10−5 after vaccination. On the other hand, some clonotypes found at high frequency after vaccination were not detectable at all before vaccination. An example is anti–MAGE-C2336–344 CTL 16. Its frequency in the blood before vaccination was <7 × 10−7, whereas after vaccination it reached 9 × 10−5 (Fig. 2). Similar observations were made with the anti-gp100 CTL. CTL 7 appeared after vaccination, whereas CTL 14 was already present before.
A more complete description of the frequencies of various CTL clonotypes before and after vaccination, not only in the blood but also inside metastases, is provided in Lurquin et al. (18).
Discussion
It is well known that tumor-specific T lymphocytes can be obtained from the blood and from tumor-infiltrating lymphocytes of cancer patients, even at the time of tumor progression. It is with these tumor-specific T cells that most of the tumor antigens that are used today for vaccination have been identified. It is generally assumed that such antitumor T cells are ineffective at rejecting the tumor either because their frequency is too low, because the tumor cells were selected to escape recognition (23), or because these lymphocytes are functionally deficient (24, 25). It is important to note, however, that this state of functional tolerance is reversible in vitro and that adoptive transfer of antitumor T cells restimulated and amplified in vitro can show clinical efficacy (26, 27).
Because of the low or even undetectable frequency of anti-vaccine T cells in patients who show tumor rejection after vaccination, we wished to evaluate a possible contribution of other antitumor T cells as specific effectors for the destruction of tumor cells, which occurs occasionally after vaccination. All of five vaccinated melanoma patients were found to have circulating antitumor CTLp's at frequencies that were 10–10,000-fold higher than those of the anti-vaccine CTLp's. These results do not contradict previous studies that suggested that antitumor CTLp's were present in the blood of some melanoma patients at frequencies in the range of 10−4 to 10−3 (19, 28, 29).
To progress in the exploration of the contribution of these antitumor T cells, we felt that it was necessary to ascertain rigorously their tumor specificity by defining their target antigens. In one patient, we obtained a synthetic view of the target antigens recognized by the antitumor T cells, providing us with completely characterized CTL clonotypes that could be tracked in blood samples collected at various times and, as shown in Lurquin et al. (18), also in metastases. The antigens recognized by the antitumor CTLs of patient EB81 turned out to belong to the main classes of tumor antigens that have been defined previously. A majority of antitumor CTL clones recognized antigens encoded by MAGE-C2, a cancer-germline gene. Others recognized an antigen encoded by gp100, a melanocytic differentiation gene. As shown in Lurquin et al. (18), another antitumor CTL clone of patient EB81 recognized an antigen encoded by an ubiquitously expressed gene that had undergone a point mutation in the tumor.
A high frequency of antitumor CTLs was already present in all five patients before vaccination. In patient EB81, antigens encoded by MAGE-C2 and gp100 were targets of antitumor T cells found both before and after vaccination. Several anti–MAGE-C2 clonotypes were found at frequencies equal or greater than 10−5 in the blood before vaccination (see Lurquin et al. [18]). Their cumulative frequency was ∼7 × 10−5. It is noteworthy that in face of such a high prevaccination T cell response, the tumor had not lost the expression of the MAGE-C2 target antigens.
In conclusion, it appears that the melanoma patients who are being vaccinated have already mounted a strong spontaneous T cell response against the types of antigens used in the vaccines. At the time of vaccination, these highly frequent T cells are clearly ineffective in halting tumor progression. This is not due to a lack of presence of these cells inside the tumors, as shown in Lurquin et al. (18). We also show in this report that after vaccination, antitumor CTLs are highly concentrated in the tumors. This reinforces the possibility that they constitute the main component of the effectors that cause tumor rejection.
One might regard the unexpected observation of tumor rejection in the face of a low anti-vaccine CTL response as a minor issue, fading away with the use of better vaccines. In our view, this opinion neglects the uncomfortable fact that up to now no vaccination modality has stood out as producing a superior frequency of tumor rejection, even though higher frequencies of anti-vaccine CTLs are observed in regressing patients with certain modalities, such as dendritic cell vaccination. Recruitment of other antitumoral T cells by the anti-vaccine T cell may therefore be a general feature of any successful vaccination procedure. A possible consequence might be that the functional properties enabling anti-vaccine T cells to do so matter more than their number. Elucidating what these functional properties are and how to generate them might be key to improving anti-tumoral vaccination strategies.
MATERIALS AND METHODS
Patients
The clinical study protocol was approved by the Ethics Review Committee of the Faculty of Medicine of the Catholic University of Louvain. Five metastatic melanoma patients with measurable disease at the time of vaccination were included in this study. Patient CP64 received 15 injections of 300 μg of peptide MAGE-3.A1 without adjuvant. He mounted a monoclonal CTL response to the vaccine antigen (15) and showed an about threefold volume reduction of a cutaneous metastasis 10 cm in diameter. However, other metastases progressed or appeared during the same period of time and the patient died from tumor progression 10 mo after the onset of vaccination. Patients EB81, CP67, and BB132 received four injections of ALVAC-MAGE, a recombinant poxvirus containing two minigenes encoding peptide MAGE-1.A1 (EADPTGHSY) and MAGE-3.A1 (EVDPIGHLY), followed by three booster vaccinations with 300 μg of each peptide. Patient EB81 had ∼20 cutaneous metastases on her right leg at the onset of vaccination. By the third ALVAC vaccination, several of these nodules had flattened, and all cutaneous metastases subsequently disappeared slowly. An inguinal adenopathy appeared 2 mo after the first vaccination and was resected. It was invaded by a large metastatic nodule. The EB81-MEL tumor cell line was derived from this invaded node. The patient has remained disease-free for >4 yr since the resection of this lymph node metastasis. Patient CP67 had cutaneous metastases on the left arm and hand and an axillary lymph node metastasis. All of these lesions regressed during vaccination over a period of 7 mo. Then new nodules appeared on the left arm and axillary areas. Patient BB132 displayed no evidence of tumor regression. The last patient of this study, LB2269, received three vaccinations 3 wk apart with 300 μg of peptide MAGE-4.A2 (GVYDGREHTV) and 300 μg of peptide MAGE-10.A2 (GLYDGMEHL) injected subcutaneously and intradermally. On the day of each vaccination, and 2 and 4 d later, 4 μg rhIL-12 (Genetics Institute) was injected subcutaneously at the vaccine site. The patient had lymph node metastases at the onset of vaccination, and no tumor regression was observed.
Cells
Melanoma cell lines, EBV-transformed B cells, and K562 were cultured in Iscove's medium (Life Technologies) supplemented with 10% FCS, 116 mg/L l-arginine, 36 mg/L l-asparagine, and 216 mg/L l-glutamine. MLTC and MLPC experiments and culture of CTL clones were performed in Iscove's medium as described above but with human serum instead of FCS.
Analysis of antitumor CTLs with MLTC
PBMCs isolated by Lymphoprep (Nycomed) density gradient centrifugation were cryopreserved in Iscove's medium supplemented with 10% human serum and 10% DMSO. Blood CD8+ lymphocytes were purified from thawed PBMCs by sorting on a FACS Vantage (Becton Dickinson) or with magnetic beads (Miltenyi Biotec). Lymphocytes were seeded at 200–2,000 cells/well with 48 or 96 wells/cell number in V-bottom microwells (Nunc) and stimulated by the addition of irradiated (100 Gy) autologous tumor cells (3,000 cells/well) pretreated with 50 U/ml IFN-γ (Roche Diagnostics) in a final volume of 100 μl Iscove's medium supplemented with 10% human serum, 100 μM 1-methyl-l-tryptophan, 20 U/ml IL-2, 10 ng/ml IL-4, and 5 ng/ml IL-7. The microcultures were restimulated each week by the addition of irradiated tumor cells and the same cytokine cocktail. Around day 21, aliquots collected from all microcultures were used as effector cells in a lysis assay. 51Chromium-labeled target cells included IFN-γ–treated autologous tumor cells, autologous EBV-transformed B cells, and K562. Effectors and targets were incubated for 4 h in the presence of a 50-fold excess of unlabeled K562 to quench the activity of NK-like lytic effectors. Usually a second lysis assay was performed around day 28 in the same conditions but in the absence of unlabeled K562 cells.
To derive antitumor CTL clones from microcultures displaying a high level of lytic activity, effector cells were cloned by limiting dilution and stimulated with 3,000 irradiated IFN-γ–treated EB81-MEL cells and 30,000 irradiated allogenic LG2-EBV–transformed B cells as feeder cells in 200 μl medium containing 50 U/ml IL-2. CTL clones that lysed specifically the tumor cells were restimulated each week with tumor cells and feeder cells as described above.
MLPCs
MLPCs were performed as described previously (17). In brief, PBMCs from patient EB81 were incubated for 60 min at room temperature with antigenic peptide MAGE-C2191 or MAGE-C2336 at 20 μM, washed, and distributed at 2 × 105 cells /microwell in 0.2 ml of medium containing 20 U/ml IL-2, 10 ng/ml IL-4, and 10 ng/ml IL-7. On day 7, 50% of the medium was replaced by fresh medium containing 20 μM of peptide, IL-2, IL-4, and IL-7. Tetramer labeling was performed on day 14 using a control tetramer as described previously (17).
TCR analysis
Total RNA from CTL clones was extracted with the TriPure reagent (Roche Diagnostics). Reverse transcription was performed as described previously (17). cDNA served as template for PCR amplifications using panels of Vα- or Vβ-specific upstream primers and one downstream Cα or Cβ primer. PCR products were purified and sequenced to obtain a complete identification of the CDR3 region. Frequency estimations using clonotypic PCR on PBMCs were based on limiting dilution analysis as described in Lurquin et al. (18).
Gene expression analysis
Expression of cancer-germline and melanocytic differentiation genes was assessed by reverse transcription and conventional PCR amplification. Gene expression levels were evaluated semiquantitatively by the intensity of the bands obtained by separating PCR products in agarose gel. Expression of all of these genes in the prevaccination metastasis of patient EB81 was also measured by real-time quantitative PCR based on TaqMan methodology using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Sequences of primers and probes are available from the authors and will be described elsewhere.
Acknowledgments
We thank C. Muller, T. Aerts, and M.-C. Letellier-Przysiuda for excellent technical assistance; S. Depelchin for editorial assistance; and B. Van den Eynde, P. van der Bruggen, and A. Van Pel for comments on the manuscript.
This work was supported by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programing, and by grants from the Fonds J. Maisin (Belgium), the Fédération Belge contre le Cancer (Belgium), the Fondation Salus Sanguinis (Belgium), the Fonds National de la Recherche Scientifique (Belgium), and the Fortis Banque Assurances and VIVA (Belgium).
The authors have no conflicting financial interests.
Abbreviations used: CTLp, CTL precursor; MLPC, mixed lymphocyte–peptide culture; MLTC, mixed lymphocyte– tumor cell culture.
C. Germeau and W. Ma contributed equally to this work.
F. Schiavetti's present address is Glaxo-SmithKline Bio, 1330 Rixensart, Belgium.
References
- 1.Chomez, P., O. De Backer, M. Bertrand, E. De Plaen, T. Boon, and S. Lucas. 2001. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 61:5544–5551. [PubMed] [Google Scholar]
- 2.Boël, P., C. Wildmann, M.-L. Sensi, R. Brasseur, J.-C. Renauld, P. Coulie, T. Boon, and P. van der Bruggen. 1995. BAGE, a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 2:167–175. [DOI] [PubMed] [Google Scholar]
- 3.De Backer, O., K.C. Arden, M. Boretti, V. Vantomme, C. De Smet, S. Czekay, C.S. Viars, E. De Plaen, F. Brasseur, P. Chomez, et al. 1999. Characterization of the GAGE genes that are expressed in various human cancers and in normal testis. Cancer Res. 59:3157–3165. [PubMed] [Google Scholar]
- 4.Lethé, B., S. Lucas, L. Michaux, C. De Smet, D. Godelaine, A. Serrano, E. De Plaen, and T. Boon. 1998. LAGE-1, a new gene with tumor specificity. Int. J. Cancer. 76:903–908. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y.-T., M.J. Scanlan, U. Sahin, Ö. Türeci, A.O. Gure, S. Tsang, B. Williamson, E. Stockert, M. Pfreundschuh, and L.J. Old. 1997. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA. 94:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel, T., M. Hauer, J. Schneider, M. Serrano, C. Wölfel, E. Klehmann-Hieb, E. De Plaen, T. Hankeln, K.-H. Meyer zum Büschenfelde, and D. Beach. 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 269:1281–1284. [DOI] [PubMed] [Google Scholar]
- 7.Brichard, V., A. Van Pel, T. Wölfel, C. Wölfel, E. De Plaen, B. Lethé, P. Coulie, and T. Boon. 1993. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 178:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulie, P.G., V. Brichard, A. Van Pel, T. Wölfel, J. Schneider, C. Traversari, S. Mattei, E. De Plaen, C. Lurquin, J.-P. Szikora, et al. 1994. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 180:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami, Y., S. Eliyahu, K. Sakaguchi, P.F. Robbins, L. Rivoltini, J.R. Yannelli, E. Appella, and S.A. Rosenberg. 1994. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2–restricted tumor infiltrating lymphocytes. J. Exp. Med. 180:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker, A.B.H., M.W.J. Schreurs, A.J. de Boer, Y. Kawakami, S.A. Rosenberg, G.J. Adema, and C.G. Figdor. 1994. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 179:1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchand, M., N. van Baren, P. Weynants, V. Brichard, B. Dréno, M.-H. Tessier, E. Rankin, G. Parmiani, F. Arienti, Y. Humblet, et al. 1999. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer. 80:219–230. [DOI] [PubMed] [Google Scholar]
- 12.Marchand, M., C.J.A. Punt, S. Aamdal, B. Escudier, W.H.J. Kruit, U. Keilholz, L. Håkansson, N. van Baren, Y. Humblet, P. Mulders, et al. 2003. Immunization of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: clinical report. Eur. J. Cancer. 39:70–77. [DOI] [PubMed] [Google Scholar]
- 13.Thurner, B., I. Haendle, C. Roder, D. Dieckmann, P. Keikavoussi, H. Jonuleit, A. Bender, C. Maczek, D. Schreiner, P. von den Driesch, et al. 1999. Vaccination with MAGE-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 190:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuler-Thurner, B., E.S. Schultz, T.G. Berger, G. Weinlich, S. Ebner, P. Woerl, A. Bender, B. Feuerstein, P.O. Fritsch, N. Romani, and G. Schuler. 2002. Rapid induction of tumor-specific type I T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulie, P.G., V. Karanikas, D. Colau, C. Lurquin, C. Landry, M. Marchand, T. Dorval, V. Brichard, and T. Boon. 2001. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc. Natl. Acad. Sci. USA. 98:10290–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonchay, C., P. van der Bruggen, T. Connerotte, T. Hanagiri, P. Coulie, D. Colau, S. Lucas, A. Van Pel, K. Thielemans, N. van Baren, and T. Boon. 2004. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc. Natl. Acad. Sci. USA. 101:14631–14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karanikas, V., C. Lurquin, D. Colau, N. van Baren, C. De Smet, B. Lethé, T. Connerotte, V. Corbière, M.-A. Demoitié, D. Liénard, et al. 2003. Monoclonal anti-MAGE-3 CTL responses in melanoma patients displaying tumor regression after vaccination with a recombinant canarypox virus. J. Immunol. 171:4898–4904. [DOI] [PubMed] [Google Scholar]
- 18.Lurquin, C., B. Lethé, V. Corbière, I. Théate, N. van Baren, P.G. Coulie, and T. Boon. 2004. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J. Exp. Med. 201:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulie, P.G., M. Somville, F. Lehmann, P. Hainaut, F. Brasseur, R. Devos, and T. Boon. 1992. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int. J. Cancer. 50:289–297. [DOI] [PubMed] [Google Scholar]
- 20.Lucas, S., E. De Plaen, and T. Boon. 2000. MAGE-B5, MAGE-B6, MAGE-C2 and MAGE-C3: four new members of the MAGE family with tumor-specific expression. Int. J. Cancer. 87:55–60. [PubMed] [Google Scholar]
- 21.Kawakami, Y., S. Eliyahu, C. Jennings, K. Sakaguchi, X. Kang, S. Southwood, P.F. Robbins, A. Sette, E. Appella, and S.A. Rosenberg. 1995. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 154:3961–3968. [PubMed] [Google Scholar]
- 22.Ma, W., C. Germeau, N. Vigneron, A.-S. Maernoudt, S. Morel, T. Boon, P.G. Coulie, and B. Van den Eynde. 2004. Two new tumor-specific antigenic peptides encoded by gene MAGE-C2 and presented to cytolytic T lymphocytes by HLA-A2. Int. J. Cancer. 109:698–702. [DOI] [PubMed] [Google Scholar]
- 23.Khong, H.T., and N.P. Restifo. 2002. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 3:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, P.P., C. Yee, P.A. Savage, L. Fong, D. Brockstedt, J.S. Weber, D. Johnson, S. Swetter, J. Thompson, P.D. Greenberg, et al. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5:677–685. [DOI] [PubMed] [Google Scholar]
- 25.Zippelius, A., P. Batard, V. Rubio-Godoy, G. Bioley, D. Lienard, F. Lejeune, D. Rimoldi, P. Guillaume, N. Meidenbauer, A. Mackensen, et al. 2004. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 64:2865–2873. [DOI] [PubMed] [Google Scholar]
- 26.Dreno, B., J.M. Nguyen, A. Khammari, M.C. Pandolfino, M.H. Tessier, S. Bercegeay, A. Cassidanius, P. Lemarre, S. Billaudel, N. Labarrière, and F. Jotereau. 2002. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol. Immunother. 51:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudley, M.E., J. Wunderlich, P.F. Robbins, J.C. Yang, P. Hwu, D.J. Schwartzentruber, S.L. Topalian, R. Sherry, N.P. Restifo, A.M. Hubicki, et al. 2002. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 298:850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herr, W., T. Wölfel, M. Heike, K.H. Meyer zum Büschenfelde, and A. Knuth. 1994. Frequency analysis of tumor-reactive cytotoxic T lymphocytes in peripheral blood of a melanoma patient vaccinated with autologous tumor cells. Cancer Immunol. Immunother. 39:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzocchi, A., F. Belli, L. Mascheroni, C. Vegetti, G. Parmiani, and A. Anichini. 1994. Frequency of cytotoxic T lymphocyte precursors (CTLp) interacting with autologous tumor via the T-cell receptor: limiting dilution analysis of specific CTLp in peripheral blood and tumor-invaded lymph nodes of melanoma patients. Int. J. Cancer. 58:330–339. [DOI] [PubMed] [Google Scholar]