Abstract
Interleukin (IL)-23 is a heterodimeric cytokine composed of a unique p19 subunit, and a common p40 subunit shared with IL-12. IL-12 is important for the development of T helper (Th)1 cells that are essential for host defense and tumor suppression. In contrast, IL-23 does not promote the development of interferon-γ–producing Th1 cells, but is one of the essential factors required for the expansion of a pathogenic CD4+ T cell population, which is characterized by the production of IL-17, IL-17F, IL-6, and tumor necrosis factor. Gene expression analysis of IL-23–driven autoreactive T cells identified a unique expression pattern of proinflammatory cytokines and other novel factors, distinguishing them from IL-12–driven T cells. Using passive transfer studies, we confirm that these IL-23–dependent CD4+ T cells are highly pathogenic and essential for the establishment of organ-specific inflammation associated with central nervous system autoimmunity.
IL-23 plays a pivotal role in the establishment and maintenance of organ-specific inflammatory autoimmune diseases. In particular, we have demonstrated that IL-23–deficient (IL-23p19−/−) mice are resistant to experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis (CIA; references 1, 2), and IBD (unpublished data), highlighting an important role of this cytokine in autoimmune pathogenesis. Although IL-23 shares a common p40 subunit with IL-12 (3), these cytokines have divergent activities. IL-12 is an important factor for the differentiation of naive T cells into IFN-γ–producing Th1 cells, is essential for antimicrobial responses (4, 5), and serves as a suppressor of human B cell tumors (6). IL-23–deficient mice display normal immune responses to acute microbial infections such as Toxoplasma gondii. (7). However, IFN-γ−/− (8), IFN-γR−/− (9), IL-12Rβ2−/− (10), and IL-12p35−/− mice (11, 12), which all lack critical components of the Th1–IFN-γ pathway, are highly susceptible to inflammatory autoimmune diseases, questioning whether IFN-γ–producing T cells have an essential role in autoimmune pathogenesis. In this paper, we demonstrate that IL-23 promotes a T cell population characterized by the production of IL-17, IL-17F, TNF, IL-6, and other additional novel factors. Upon adoptive transfer to naive recipient mice, this IL-23–dependent T cell subset invades the target organ and can promote the development of organ-specific autoimmune inflammation.
Results
Differential cytokine expression of central nervous system (CNS)–infiltrating CD4+ T cells
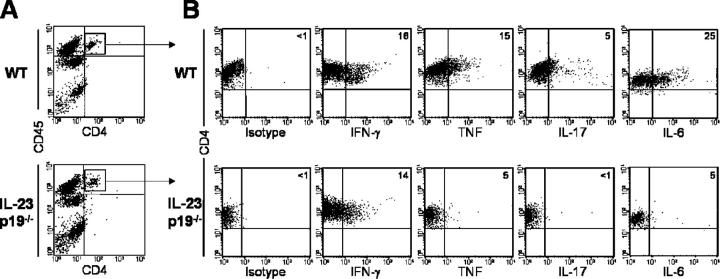
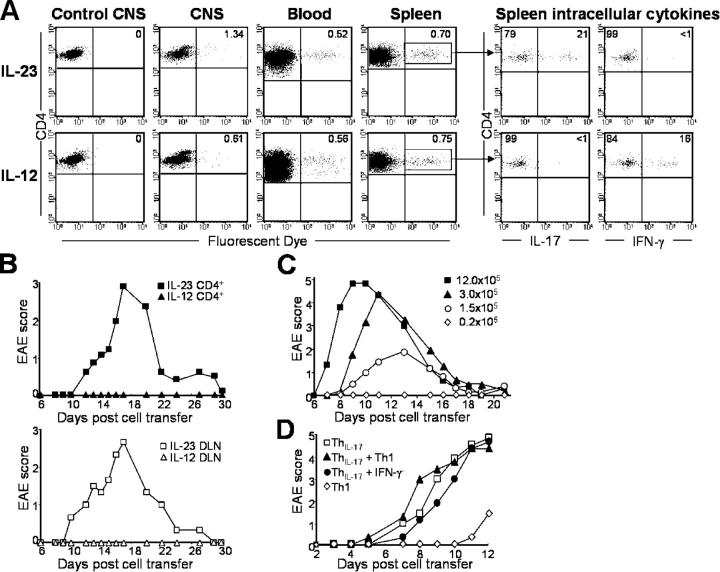
To study the role of IL-23 in autoimmune inflammatory disease pathogenesis, we used a CNS autoimmune model because the target organ is normally free of activated macrophages and CD4+ T cells (13); therefore, we can monitor the infiltration of inflammatory cells into the CNS during disease development. Just before disease onset, myelin-oligodendrocyte glycoprotein peptide (MOG)35-55–primed WT mice exhibited a large influx of CNS-invading immune cells (Fig. 1 A). Surprisingly, although IL-23–deficient mice (IL-23p19−/−) were completely EAE resistant, comparable numbers of immune cells invaded the CNS (Fig. 1 A). In vitro stimulation of the CNS-invading CD4+ cells showed equivalent number of MOG-specific, IFN-γ–producing cells in both the IL-23p19−/− and WT mice (Fig. 1 B), suggesting that IL-23 is not required for T cell migration across the blood–brain barrier or for Th1 cell development. In addition, the presence of Th1 cells capable of producing IFN-γ within the CNS of IL-23–deficient mice is not sufficient to induce EAE. Intracellular cytokine analysis also detected IL-6, IL-17, and TNF in the CNS-infiltrating CD4+ T cells from WT mice. In contrast, CD4+ T cells isolated from IL-23p19−/− mice displayed reduced IL-6 and undetectable IL-17 production (Fig. 1 B). TNF production was also markedly reduced in the IL-23p19−/− cells, consistent with a previous paper suggesting that IL-23 is required for TNF production by CNS-infiltrating T cells (12). Ex vivo analysis of draining LN (DLN) cells from immunized WT and IL-23p19−/− mice reflected the CNS data, with similar IFN-γ levels, but no IL-17 production by the IL-23p19−/− cells (2, 14, 15). These results suggest that IL-23 is not required for Th1 development, but is essential for the development of autoantigen-specific, IL-17–producing CD4+ T cells.
Figure 1.
T cells and inflammatory macrophages enter the CNS of MOG-immunized IL-23p19 −/− mice. (A) Phenotypic analysis of CNS-infiltrating cells in the brains of WT and IL-23p19−/− mice at day 7 (before EAE onset) after immunization with MOG/CFA. (B) Intracellular cytokine staining of day 7 CNS-infiltrating cells from WT and IL-23p19−/− mice after 18 h of stimulation with 100 μg/ml MOG peptide, all plots are gated on live CD4+ T cells. Data are representative of five separate experiments.
IL-23 drives an IL-17–producing T cell population
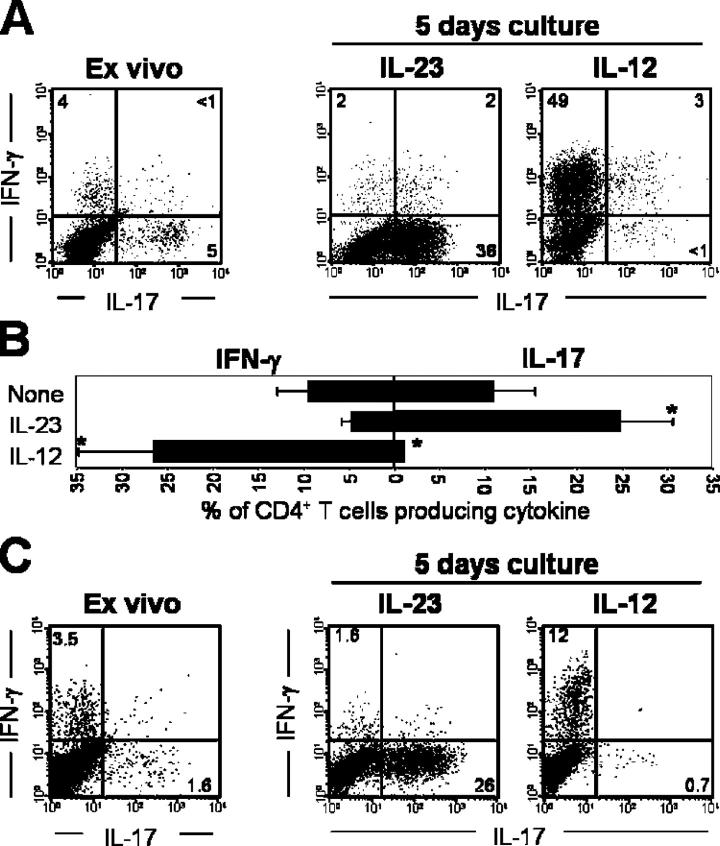
To confirm our in vivo data, next we cultured antigen-primed DLN cells in vitro with IL-23 to analyze cytokine production and proinflammatory gene expression. DLN cells isolated from SJL mice immunized with proteolipid protein peptide (PLP)139-151 were cultured in the presence of PLP, plus rIL-23, rIL-12, or medium alone. Immediately after ex vivo isolation, CD4+ PLP-specific IFN-γ–producing (Th1) cells and IL-17–producing (ThIL-17) cells were identified in the isolated DLN cells after PMA restimulation (Fig. 2 A). Administration of rIL-23 promoted the expansion of ThIL-17 cells, and reduced the growth of Th1 cells after 5 d of culture (Fig. 2, A and B). In contrast, addition of rIL-12 promoted Th1 but reduced ThIL-17 growth (Fig. 2 A and B). These results are consistent with papers suggesting that IL-23 is required for the development and expansion of IL-17–producing CD4+ T cells (2, 16, 17). Both ThIL-17 and Th1 cells also produced TNF (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20041257/DC1) and were characterized as CD4+ CD62Llo CD44hi, indicative of activated T cells; however, the ThIL-17 cells displayed lower CD45RB expression (Fig. S1 B). ThIL-17 CNS-infiltrating T cells isolated from myelin antigen-immunized mice are also CD62Llo CD44hi CD45RBlo (unpublished data). DLN cells taken from IL-12p40−/− mice, which lack both IL-12 and IL-23, could still be driven in culture with rIL-23 or rIL-12 to generate ThIL-17 or Th1 cells, respectively (Fig. 2 C). This suggests that IL-12 and IL-23 are not required during in vivo priming for subsequent in vitro generation of IL-23 responsive ThIL-17 cells.
Figure 2.
IL-23 promotes the expansion of IL-17 producing CD4 + (ThIL-17) cells. (A) Intracellular IL-17 and IFN-γ production by CD4+ DLN cells isolated from PLP-immunized WT SJL mice, either immediately after ex vivo isolation, or cultured with PLP139-151 peptide plus either rIL-12, rIL-23, or no added cytokine for 5 d. All plots are gated on live CD4+ T cells. (B) Mean percentage (±1 SD of error) of CD4+ T cells producing IFN-γ or IL-17 in response to 5 d of culture with IL-12 or IL-23. *, Student's unpaired t test, P < 0.02, averaged from eight separate experiments. (C) Intracellular IL-17 and IFN-γ production by CD4+ DLN cells isolated from MOG-immunized p40−/− C57BL/6 either immediately after ex vivo isolation or cultured for 5 d with rIL-12 or rIL-23. All intracellular staining samples were restimulated with PMA/ionomycin for 4 h before analysis. All plots are gated on live CD4+ T cells and are representative of three independent experiments.
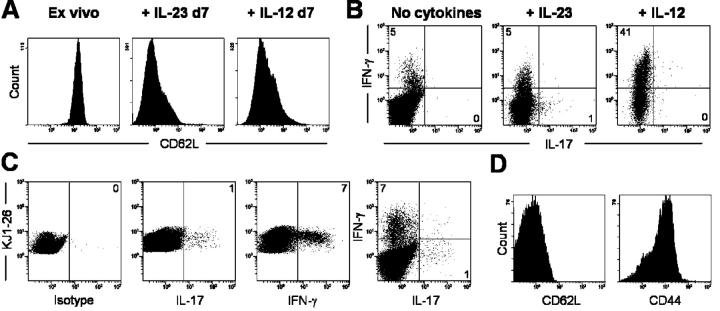
To address whether ThIL-17 cells can be generated in vitro from naive T cells, without in vivo priming, we isolated TCR-transgenic naive CD4+ T cells from DO11.10 Tg × Rag−/− mice, and stimulated them with anti-CD3/anti-CD28 in the presence of rIL-12 or rIL-23. By day 7, the cultured cells were all CD62Llo, indicative of an activated phenotype (Fig. 3 A). Upon restimulation with PMA/ionomycin, a small number of these activated T cells produced IL-17 in the IL-23–driven culture. These results suggest that a subpopulation of naive T cells can respond to IL-23 upon in vitro activation. In contrast, IL-12–driven cells produced high levels of IFN-γ and no IL-17 in vitro (Fig. 3 B). The naive T cells are biased toward IFN-γ production after anti-CD3/anti-CD28 stimulation. In the absence of exogenous cytokines, a small number of T cells producing IFN-γ (∼5%) were present after anti-CD3/anti-CD28 stimulation (Fig. 3 B). The same frequency of IFN-γ producers were found in the IL-23 driven cultures, suggesting that during primary in vitro stimulation, IL-23 is unable to affect IFN-γ levels. As seen with SJL DLN cultured cells, three to four rounds of in vitro IL-23 stimulation lead to reduction of IFN-γ producers (unpublished data).
Figure 3.
IL-23 drives a small population of naive T cells to produce IL-17 after in vitro activation. (A) Surface expression of CD62L on naive splenic DO11.10 × Rag−/− T cells, either analyzed immediately after isolation (ex vivo) or on day 7 after in vitro anti-CD3/anti-CD28 stimulation plus IL-12 or IL-23. (B) Intracellular cytokine staining of CD4+ DO11.10 × Rag−/− T cells on day 7 after in vitro anti-CD3/anti-CD28 stimulation plus IL-12 or IL-23. Cells were restimulated with PMA/ionomycin for 4 h before intracellular staining. (C) Naive DO11.10 × Rag−/− T cells were parked in BALB/c recipients and OVA/CFA immunized. DLN cells were restimulated, PMA/ionomycin and intracellular stained for IL-17 and IFN-γ or (D) surface stained for CD44 and CD62L. All plots are gated on CD4+ KJ1-26+ cells. Results are representative of two experiments.
Next, we parked purified naive DO11.10 Tg × Rag−/− CD4+ T cells into BALB/c hosts. After OVA323-339/CFA immunization, DLN cells were isolated 4 d later and analyzed immediately. A small subset of KJ1-26+ T cells were capable of producing IL-17 and were characterized as CD62Llo and CD44hi (Fig. 3, C and D). These results suggest that subcutaneous OVA323-339/CFA priming, which induces IL-23 expression in the DLN (not depicted) and also induces IL-17–producing T cells (Fig. 3 C). Both Th1 and ThIL-17 cells were present in the KJ1-26+ OVA-primed DLN, indicating that both Th1 and ThIL-17 cells can be generated from naive T cells. However, it is likely that additional factors are required to enhance IL-23 responsiveness.
Gene expression pattern induced by IL-12 versus IL-23
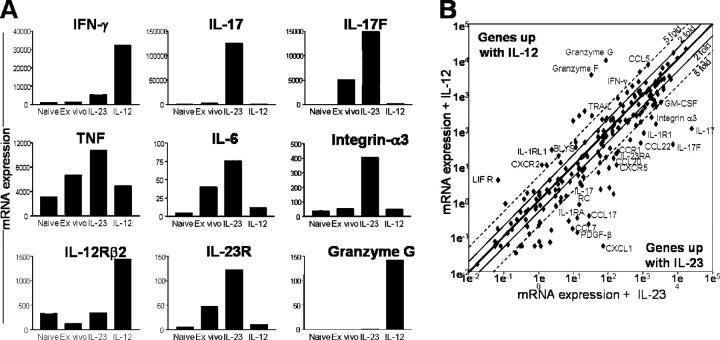
Next, we compared the gene expression profile of rIL-23– or rIL-12–stimulated PLP-specific CD4+ T cells by real-time PCR to study the functional characteristics of these different cell populations. Both IL-12– and IL-23–driven cells expressed constitutive levels of IL-12Rβ1 (unpublished data). IL-12 drives high expression of IL-12Rβ2, but little IL-23R, consistent with the ability of these cells to respond to IL-12 (Fig. 4 A). IFN-γ and granzyme G expression was also significantly increased. Conversely, IL-23 drives high mRNA expression of IL-23R, but little IL-12Rβ2, consistent with the ability of these cells to respond to IL-23 (Fig. 4 A). IL-17, IL-17F, IL-6, and TNF RNA levels were also significantly elevated; however, IFN-γ and granzyme G expression were dramatically reduced in the IL-23 driven cultures (Fig. 4 A). Integrin-α3, which enhances cellular migration within the target organ during inflammation (18), was also up-regulated in the IL-23–driven cells. These results suggest that the IL-23–driven T cells may play an important role in organ-specific inflammation, but not immune surveillance, via the classical IL-12–dependent IFN-γ pathway. Further analysis of >200 lymphoid and myeloid-specific genes by real-time PCR, comparing total (lymphoid and myeloid) DLN cells cultured with rIL-23 or rIL-12 showed that ∼80% of the genes displayed comparable expression profiles (Fig. 4 B). Of the remaining genes showing divergence, IL-12 elevated expression of many host defense genes such as granzyme F, granzyme G, TRAIL, BLYS, IFN-γ, TRAIL R2, and FASL. However, IL-23 induced, in addition to IL-17 and IL-17F, elevated expression of genes such as CCL7, CCL17, CCL20, CCL22, CCR1, and GM-CSF. We are currently characterizing the molecular basis of the cellular responses to IL-12 versus IL-23 by microarray analysis. Preliminary data identifies that IL-12 specifically induced 306 genes (greater than fivefold), comprised of many cytotoxicity and host defense genes. In contrast, IL-23 specifically induced 162 genes (greater than fivefold), including numerous novel expression sequence tags with unknown functions in addition to a 34-fold increase of IL-17 (unpublished data). Together, these results suggest that IL-12, not IL-23, drives development of T cell–expressing host defense genes. The precise in vivo function of IL-23 is currently unclear. Its capacity to induce IL-17 and other proinflammatory factors appears important for neutrophil recruitment (17) and granuloma formation (19). However, when dysregulated, it causes inflammatory responses often associated with autoimmunity.
Figure 4.
Gene expression analysis of IL-23 versus IL-12 driven T cells. (A) Quantitative analysis of mRNA expression levels of selected genes, comparing sorted CD4+ DLN cells isolated from PLP-immunized mice, either not cultured (ex vivo) or cultured with rIL-23 or rIL-12 for 10 d. (B) Quantitative analysis of mRNA expression levels of >200 selected genes, comparing total DLN cells isolated from PLP-immunized mice, cultured with either rIL-23 or rIL-12 for 10 d. All samples were activated with PMA/ionomycin for 4 h before RNA extraction and Taqman analysis. Results are normalized to a housekeeping gene, ubiquitin, and representative of two similar experiments.
ThIL-17 cells are highly encephalogenic
To compare the capacity of Th1 versus ThIL-17 cells to induce EAE pathology, we transferred IL-23– or IL-12–expanded PLP-specific cells to naive recipient mice. To confirm that both IL-23– and IL-12–cultured cells were viable in vivo and could cross the blood–brain barrier, cells were fluorescently labeled before passive transfer. 3 d after transfer, similar frequencies of labeled cells were detected in the blood, spleen, and CNS, regardless of whether they were cultured with IL-23 or IL-12, suggesting both Th1 and ThIL-17 cells can survive in vivo and traffic to the CNS. In addition, the transferred cells also remained stable with respect to their IFN-γ or IL-17 production in vivo (Fig. 5 A). Labeled cells were still detectable 6 d after transfer, but with decreased mean fluorescence intensity in both groups, indicative of normal proliferation/division of the cells (unpublished data).
Figure 5.
IL-17–producing ThIL-17 cells induce EAE. (A) PLP-primed DLN cells cultured with rIL-23 or rIL-12 for 10 d, labeled with CFSE (peak MFI = 4,654) and transferred (5 × 106 cells/mouse) into recipient WT SJL mice. Cells were tracked at day 3 after immunization, with further gating on splenic CFSE+ CD4+ cells to determine IFN-γ and IL-17 production by intracellular staining. (B) PLP-primed DLN cells cultured with either rIL-23 (squares) or rIL-12 (triangles) for 10 d, and transferred into WT SJL mice as either purified CD4+ cells (3 × 106 cells/mouse; n = 10/group; top), or as total DLN cells (7 × 106 cells/mouse; n = 3/group; bottom), with mean EAE scores recorded. (C) Mean EAE scores of WT recipient mice (n = 5/group) passively transferred with graded numbers of ThIL-17 cells: 1.2 × 106 ThIL-17 (closed squares), 3 × 105 ThIL-17 (closed triangles), 1.5 × 105 ThIL-17 (open circles), or 2.0 × 104 ThIL-17 (open diamonds). Results are summarized from two separate experiments. (D) Mean EAE scores of WT recipient mice (n = 5/group) passively transferred with either 5 × 105 ThIL-17 cells (open squares), 5 × 105 Th1 cells (open diamonds), or cotransferred with ThIL-17 plus Th1 cells (5 × 105 of each; closed triangles), or ThIL-17 plus sorted IFN-γ− cells (5 × 105 ThIL-17 plus 2 × 105 IFN-γ− cells; closed circles). (C–D) ThIL-17 cell numbers are calculated from intracellular cytokine staining analysis, with ∼3-5 × 106 total DLN cells passively transferred per recipient mouse.
Next, we determined the encephalogenicity of ThIL-17 versus Th1 cell populations. Mice that received either IL-23–driven ThIL-17 cells (either as purified CD4+ T cells or total DLN containing ∼3 × 105 ThIL-17 cells) showed severe clinical signs of EAE (Fig. 5 B). However, mice that received an equivalent number of IL-12–driven Th1 cells showed no clinical signs of EAE, suggesting that Th1 cells are less efficient at inducing EAE (Fig. 5 B). When graded numbers of ThIL-17 were transferred into naive SJL mice, ThIL-17 cell number directly correlated with disease severity, regardless of the number of Th1 cells present (Fig. 5 C). To determine whether the IFN-γ− cells might negatively regulate EAE, next we cotransferred FACS-sorted IFN-γ− cell population or Th1 cells with ThIL-17 cells to recipient mice. Neither IFN-γ− cells nor Th1 cells could reduce the ThIL-17-mediated EAE pathology (Fig. 5 D), suggesting that the inability of Th1 cells to induce EAE was not due to regulatory components in the IL-12–driven cultures. In addition, we noted that short-term IL-12–driven cell populations (7 d) could contain a small number of contaminating IL-17–producing cells that can induce mild EAE (Fig. 5 D). These results demonstrate that IL-17–producing T cells are more pathogenic than IFN-γ–producing T cells.
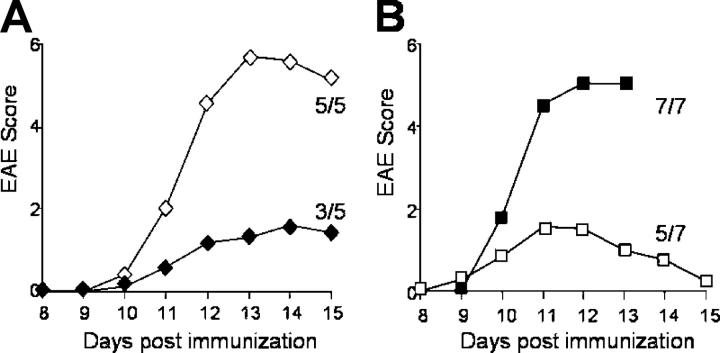
To test whether IL-17 production directly contributes to EAE severity, PLP-immunized WT SJL mice were treated with neutralizing antibodies against IL-17 (Fig. 6 A). Anti–IL-17 treatment resulted in partial protection from EAE, consistent with a recent paper suggesting that IL-17–deficient mice are resistant to actively induced EAE and CIA (20), and confirming that the IL-23–IL-17 pathway is important in inflammatory autoimmune diseases. However, the observed partial protection indicates that other factors induced by IL-23, including IL-6, IL-17F, and TNF, are also important in EAE pathogenesis. Indeed, the highly intense disease induced by passive transfer of ThIL-17 cells (3–5 × 105 cells/recipient) cannot be blocked by anti–IL-17 antibody treatment of recipient mice (unpublished data), suggesting that multiple factors produced by these exceptionally pathogenic ThIL-17 cells must be neutralized before inhibiting their actions. In addition, IL-17 may also have a role in the initial expansion of the ThIL-17 cells as suggested by a recent paper showing a role for IL-17 during immune priming (21). It was previously unclear whether the classical Th1-type/IFN-γ–mediated immune response is essential for induction or maintenance of autoimmune disease. Treatments with IFN-γ lead to disease exacerbation in human MS (22), and transgenic mice overexpressing IFN-γ in the CNS showed increased oligodendrocyte death leading to demyelinating diseases (23, 24). However, despite the potential toxic effects of IFN-γ in the CNS, mice that lack critical components of the Th1–IFN-γ pathway are highly susceptible to EAE (8–12). Consistent with this, treatment of PLP-immunized mice with neutralizing antibodies against IFN-γ demonstrated intensified disease leading to >75% mortality (Fig. 6 B), indicating that loss of IFN-γ results in disease exacerbation.
Figure 6.
IL-17, not IFN-γ, contributes to EAE severity. (A) WT SJL mice actively immunized with PLP emulsified in CFA, and treated at day 7 with 200 μg anti–IL-17 (closed diamonds) or isotype control (open diamonds), n = 5/group. (B) WT SJL mice actively immunized with PLP emulsified in CFA (using suboptimal immunization strategy to give weaker disease), and treated at days 0 and 7 with 200 μg anti–IFN-γ (closed squares) or isotype control (open squares), n = 7/group. Plots are representative of three similar experiments.
Discussion
In this paper, we have identified a pathogenic T cell population critical for autoimmune inflammation. This IL-23–mediated immune response is not associated with IFN-γ or IL-4 production. IL-23 promotes the development and expansion of activated CD4+ cells that produce IL-17, IL-17F, IL-6, and TNF upon antigen-specific stimulation. Genetic analysis of these ThIL-17 cells identified a unique expression pattern of proinflammatory cytokines and other novel factors. This data, plus the observation that IFN-γ–producing cells, are present in EAE-resistant, IL-23–deficient mice, suggesting that the IL-23–driven immune response is independent of the IFN-γ–Th1 pathway. IL-17 production was undetectable in CD4+ T cells from IL-23–deficient mice (derived from either CNS or LN), suggesting that IL-23 is essential for the development of autoantigen-specific T cell production of IL-17. By in vitro expansion of antigen-primed DLN cells with rIL-23 and passive transfer studies, we confirm that these IL-23–dependent T cells are highly pathogenic, and essential for the establishment of organ-specific inflammation associated with autoimmunity.
In key published EAE passive transfer papers (25, 26), PLP-primed DLN cells were cultured with rIL-12 for 4 d before transfer of 20–30 × 106 cells into recipient mice. Both Th1 and ThIL-17 cells can be found in antigen-primed DLN cells immediately after isolation; however, they reduce over time when cultured with IL-23 or IL-12, respectively. 4 d in the presence of rIL-12 is not sufficient time for the complete loss of IL-17 production; therefore, the cells were cultured for 10–14 d with either IL-12 or IL-23 before passive transfer. Our data indicates that the ThIL-17 cells are far more efficient than Th1 cells at inducing EAE; as a result, we transferred ∼10-fold fewer cells in comparison to the published reports.
A recent paper demonstrated that antigen-primed IFN-γ+ versus IFN-γ− cells have differential in vivo survival potentials (27). The IFN-γ+ cells were short-lived and performed immediate effector functions, whereas the IFN-γ− cells had increased durability and possessed long-term memory function. This is consistent with our work demonstrating the existence of an IFN-γ− IL-17+–producing T cell population. In addition, some but not all IFN-γ− cells could produce IFN-γ at a later stage after strong antigenic stimulation in the presence of IL-12 (27), suggesting a shared a precursor lineage. In our studies, antigen-primed activated T cells are responsive to both IL-12 and IL-23. In short-term in vitro assays, CD62Lhi naive T cells appear to predominately respond to IL-12 and are biased toward IFN-γ production after anti-CD3 and anti-CD28 stimulation (Fig. 3, A and B, and reference 3). In vitro activation of naive T cells in the presence of IL-23 could induce the generation of a small population of IL-17–producing T cells; however, during this short-term culture, IL-23 was unable to affect residual IFN-γ production. It is likely that during in vivo activation of naive T cells, an array of cytokines and cellular interactions are required to optimally induce IL-23 responsiveness. For example, naive T cells from autoimmune-prone DBA-1 mice can be driven to produce IL-17 when alternatively stimulated via ICOS (28).
The in vivo function of IL-23 remains to be determined, but appears to be clearly associated with IL-17, IL-17F, IL-6, and TNF production. IL-17 is an important effector cytokine during inflammation. IL-17–deficient mice are resistant to CIA and EAE (20), and therapeutic treatment with an IL-17 antagonist antibody can reverse ongoing CIA (29), consistent with the notion that IL-17 functions during the effector phase of an inflammatory response. In addition, IL-17 has been found in many human autoimmune diseases, including multiple sclerosis (30), rheumatoid arthritis (31), and psoriasis (32). IL-17–producing T cells have also been identified in the synovial fluid of patients with Lyme arthritis (33), suggesting involvement of IL-17 in infection-induced immunopathology. IL-17 receptors that bind both IL-17 and IL-17F are ubiquitously expressed in a broad range of cell types including myeloid and endothelial cells. Engagement of IL-17 and/or IL-17F on these cell types promotes expression of IL-1, IL-6, IL-8, TNF, and ICAM-1, which are all critical factors that drive inflammation (34).
IL-6 is predominately produced by myeloid cells and is an important factor promoting inflammatory responses. The production of IL-6 by ThIL-17 but not Th1 cells is intriguing and may explain the hyperencephalogenicity of ThIL-17 cells. IL-6 produced by LPS-activated dendritic cells can turn off regulatory T cell (T reg cell) function, allowing effector T cell activation (35). It is tempting to speculate that in the absence of microbe-activated dendritic cells, the IL-6–producing ThIL-17 cells may have the potential to directly inhibit the action of T reg cell function within the target organ during autoimmune inflammation. The role of TNF in chronic inflammatory diseases is well recognized (36). Interestingly, TNF is produced by both Th1 and ThIL-17 cells after in vitro stimulation, suggesting that TNF may play a role in both IL-12 and IL-23 linked immune responses. Thus, the cellular mechanism of action of IL-23 in autoimmunity is the expansion of self-reactive IL-17, IL-17F, TNF, and IL-6–producing T cells. Importantly, this IL-23–mediated immune response has a different gene expression pattern than IL-12–driven T cell responses. Understanding the molecular basis for the differential gene expression pattern of the IL-23–dependent T cell population could provide additional therapeutic targets for the treatment of inflammatory autoimmune diseases.
Materials and Methods
Mice
IL-23p19−/− (IL-23–deficient mice) and their WT controls were generated on a mixed B6 × 129 background using approaches described previously (1).
SJL/J and IL-12p40–deficient (p40−/− C57BL/6) mice were obtained from The Jackson Laboratory. All animal procedures were approved by the DNAX IACUC committee, in accordance with AAALAC guidelines.
EAE
EAE was induced in female mice at 8–12 wk and clinically assessed as described previously (1), using 100 μg MOG35-55 per mouse for B6 × 129 and C57BL/6 strains, or 80 μg PLP139-151 (PLP) per mouse for SJL strains and emulsified in CFA. Where required, brain- and spinal cord–infiltrating cells were isolated from MOG-immunized WT and IL-23p19−/− mice at day 7 (before EAE onset; reference 1), and cultured with 100 μg/ml MOG for 18 h (plus Golgi-Plug for final 4 h) before intracellular cytokine staining.
Intracellular cytokine flow cytometry.
Cells were surface stained with anti–CD4-FITC in the presence of Fc blocking antibodies. Cells were washed, fixed, permeabilized with Cytofix/Cytoperm buffer and intracellular stained with antibodies against IFN-γ/TNF/IL-17/IL-6/IL-4, and analyzed with a FACScalibur flow cytometer. For DLN and naive T cell intracellular cytokine staining, samples were restimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of Golgi-plug for 4 h, before surface staining with combinations of antibodies against CD4/CD45RB/CD62L/CD44 and intracellular cytokine staining with combinations of antibodies against IFN-γ/IL-17/TNF/IL-4. All antibodies and staining buffers were purchased from BD Biosciences.
DLN cultures and passive transfer
Inguinal, axillary, and branchial LNs were harvested from SJL mice 9 d after antigen immunization. DLN cells were cultured in complete RPMI 1640 media containing 10% FCS, supplemented with Pen-Strep, l-glutamine, Hepes, sodium pyruvate, and 2-ME, in the presence of 20 μg/ml PLP139-151 for an initial 5 d, plus addition of either 10 ng/ml rIL-12 or 10 ng/ml rIL-23. Media was replaced on days 5 and 8 and supplemented with 2 ng/ml IL-2 (BD Biosciences), plus rIL-23 or rIL-12. At day 5, cells initially cultured with IL-12 were rested without IL-12 for 3 d to prevent excessive apoptosis. As required, day 10 cultured DLN cells were positively selected and enriched using CD4+ microbeads (Miltenyi Biotec) with >95% purity. For passive transfer, SJL DLN cells (either total DLN or CD4+ MACS enriched) were harvested, washed in RPMI 1640, and injected i.v. into naive WT female SJL mice (see Fig. 5 for culture conditions and cell number in each experiment). The frequency of ThIL-17 and Th1 cells was calculated by intracellular flow cytometry performed in parallel for each sample. For IL-12p40−/− cultures, DLN cells were harvested from p40−/− mice at day 9 after MOG immunization and cultured with 100 μg/ml MOG35-55 peptide plus 10 ng/ml rIL-12 or 10 ng/ml rIL-23 for 5 d before analysis.
Naive T cell cultures and adoptive transfer.
DLN and spleen cells were isolated from naive DO11.10 Tg × Rag−/− mice, and CD4+ cells were purified by MACS. For culture, 106 naive CD4+ spleen cells/ml were activated for 3 d with 10 μg/ml of plate-bound anti-CD3 and 1 μg/ml anti-CD28 with 10 ng/ml rIL-12 or 10 ng/ml rIL-23. Cultured cells were rested for 4 d before restimulation with PMA/ionomycin in the presence of Golgi-plug for intracellular cytokine staining on day 7. For adoptive transfer, pooled naive CD4+ MACS-purified spleen and LN cells from DO11.10 Tg × Rag−/− mice were pooled and injected into recipient BALB/c mice, with each mouse receiving ∼5 × 106 CD4+ KJ1-26+ cells. Recipient mice were immunized with 20 μg OVA323-339 peptide emulsified in CFA on day 2, and the DLN cells were harvested on day 6 and immediately analyzed for intracellular cytokine staining after PMA/ionomycin restimulation. Where required, cells were surface stained with combinations of fluorescently labeled antibodies KJ1-26, CD4, CD62L, and CD44.
Gene expression analysis.
RNA was extracted from DLN cells isolated from naive SJL non–PLP-primed control mice (unprimed), and as total DLN or CD4+ MACS-enriched cells from PLP-immunized mice. These cells were either not cultured (ex vivo) or cultured with rIL-23 or rIL-12 for 10 d. All samples were activated with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) for 4 h before cDNA preparation. mRNA expression was quantitated by real-time PCR as described previously (2).
Fluorescent dye staining and tracking.
SJL DLN cells cultured for 10 d with either IL-23 or IL-12 were labeled with CFSE (Molecular Probes), transferred i.v. into naive recipient mice (5 × 106 total cells/mouse). At day 3 after transfer, spleens were removed and homogenized, red blood cells were lysed, and mononuclear cells were surface stained with anti–CD4-APC and intracellular stained with anti–IL-17 or anti–IFN-γ as described before. Blood was collected and lysed (RBC lysis buffer; Sigma-Aldrich), and mononuclear cells from brains and spinal cord tissue were prepared as described previously (1) and surface stained with anti–CD4-APC.
Sorting of IFN-γ+ and IFN-γ DLN cells
IFN-γ+ and IFN-γ− cells were sorted from PLP-primed SJL DLN cells cultured with rIL-12 for 10 d, after 3 h stimulation with anti-CD3/anti-CD28, using anti-CD45 anti–IFN-γ bispecific antibody-capture matrix kit (Miltenyi Biotec) as described previously (27).
Antibody treatment of EAE.
WT SJL mice were treated with 200 μg i.p anti–IL-17 (clone 18H10, rat IgG1), or treated with anti–IFN-γ (clone XMG1.2, rat IgG1) or relevant isotype controls, immunized with PLP139-151 as described before. Both neutralizing antibodies were generated at DNAX (with <4 EU/mg of mAb); however, they are also commercially available from BD Biosciences, Southern Biotechnology Associates, Inc., and Upstate Biotechnology.
Online supplemental material
Fig. S1 demonstrates that both ThIL-17 and Th1 cells produce TNF, but not IL-4, and are phenotypically characterized as CD4+CD44hiCD62Llo indicative of activated T cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20041257/DC1.
Acknowledgments
We acknowledge C. Murphy, E. Bowman, and M. Oft for critical reading of the paper. We also thank A. O'Garra, R. Coffman, and A. Sher for scientific discussions.
This work was fully supported by Schering-Plough Corporation. The authors have no other conflicting financial interests.
Abbreviations used: CIA, collagen-induced arthritis; CNS, central nervous system; DLN, draining LN; EAE, experimental autoimmune encephalomyelitis; MOG, myelin-oligodendrocyte glycoprotein peptide; PLP, proteolipid protein peptide.
References
- 1.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 2.Murphy, C.A., C.L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R.A. Kastelein, J.D. Sedgwick, and D.J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 4.O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 10:542–550. [DOI] [PubMed] [Google Scholar]
- 5.Shtrichman, R., and C.E. Sanuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251–259. [DOI] [PubMed] [Google Scholar]
- 6.Airoldi, I., E. Di Carlo, B. Banelli, L. Moserle, C. Cocco, A. Pezzolo, C. Sorrentino, E. Rossi, M. Romani, A. Amadori, and V. Pistoia. 2004. The IL-12Rbeta2 gene functions as a tumor suppressor in human B cell malignancies. J. Clin. Invest. 113:1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Lieberman, L.A., F. Cardillo, A.M. Owyang, D.M. Rennick, D.J. Cua, R.A. Kastelein, and C.A. Hunter. 2004. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J. Immunol. 173:1887–1893. [DOI] [PubMed] [Google Scholar]
- 8.Ferber, I., S. Brocke, C. Taylor-Edwards, W. Ridgway, C. Dinisco, L. Steinman, D. Dalton, and C. Fathman. 1996. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156:5–7. [PubMed] [Google Scholar]
- 9.Willenborg, D.O., S. Fordham, C.C. Bernard, W.B. Cowden, and I.A. Ramshaw. 1996. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157:3223–3227. [PubMed] [Google Scholar]
- 10.Zhang, G., B. Gran, S. Yu, J. Li, I. Siglienti, X. Chen, M. Kamoun, and A. Rostami. 2003. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J. Immunol. 170:2153–2160. [DOI] [PubMed] [Google Scholar]
- 11.Becher, B., B.G. Durell, and R.J. Noelle. 2002. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 110:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gran, B., G. Zhang, S. Yu, J. Li, X. Chen, E. Ventura, M. Kamoun, and A. Rostami. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 169:7104–7110. [DOI] [PubMed] [Google Scholar]
- 13.Hickey, W.F. 1991. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1:97–105. [DOI] [PubMed] [Google Scholar]
- 14.Ghilardi, N., N. Kljavin, Q. Chen, S. Lucas, A.L. Gurney, and F.J. De Sauvage. J. 2004. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 172:2827–2833. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 16.Aarvak, T., M. Chabaud, P. Miossec, and J.B. Natvig. 1999. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 162:1246–1251. [PubMed] [Google Scholar]
- 17.Happel, K.I., M. Zheng, E. Young, L.J. Quinton, E. Lockhart, A.J. Ramsay, J.E. Shellito, J.R. Schurr, G.J. Bagby, S. Nelson, and J.K. Kolls. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada, Y., E.A. Wayner, W.G. Carter, and M.E. Hemler. 1988. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J. Cell. Biochem. 37:385–393. [DOI] [PubMed] [Google Scholar]
- 19.Holscher, C., R.A. Atkinson, B. Arendse, N. Brown, E. Myburgh, G. Alber, and F. Brombacher. 2001. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 167:6957–6966. [DOI] [PubMed] [Google Scholar]
- 20.Nakae, S., A. Nambu, K. Sudo, and Y. Iwakura. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173–6177. [DOI] [PubMed] [Google Scholar]
- 21.Nakae, S., S. Saijo, R. Horai, K. Sudo, S. Mori, and Y. Iwakura. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 100:5986–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panitch, H.S., R.L. Hirsch, A.S. Haley, and K.P. Johnson. 1987. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1:893–895. [DOI] [PubMed] [Google Scholar]
- 23.Renno, T., V. Taupin, L. Bourbonniere, G. Verge, E. Tran, R. De Simone, M. Krakowski, M. Rodriguez, A. Peterson, and T. Owens. 1998. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Mol. Cell. Neurosci. 12:376–389. [DOI] [PubMed] [Google Scholar]
- 24.Vartanian, T., Y. Li, M. Zhao, and K. Stefansson. 1995. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol. Med. 1:732–743. [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard, J.P., K.E. Waldburger, and S.J. Goldman. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 181:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal, B.M., and E.M. Shevach. 1996. IL-12 unmasks latent autoimmune disease in resistant mice. J. Exp. Med. 184:771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, C.Y., J.R. Kirman, M.J. Rotte, D.F. Davey, S.P. Perfetto, E.G. Rhee, B.L. Freidag, B.J. Hill, D.C. Douek, and R.A. Seder. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852–858. [DOI] [PubMed] [Google Scholar]
- 28.Nurieva, R.I., P. Treuting, J. Duong, R.A. Flavell, and C. Dong. 2003. Inducible costimulator is essential for collagen-induced arthritis. J. Clin. Invest. 111:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Lubberts, E., M.I. Koenders, B. Oppers-Walgreen, L. van den Bersselaar, C.J. Coenen-de Roo, L.A. Joosten, and W.B. van den Berg. 2004. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50:650–659. [DOI] [PubMed] [Google Scholar]
- 30.Matusevicius, D., P. Kivisakk, B. He, N. Kostulas, V. Ozenci, S. Fredrikson, and H. Link. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5:101–104. [DOI] [PubMed] [Google Scholar]
- 31.Ziolkowska, M., A. Koc, G. Luszezykiewicz, K. Ksiezopolska-Pietrzak, E. Klimczak, H. Chwalinska-Sadowska, and W. Maslinski. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832–2838. [DOI] [PubMed] [Google Scholar]
- 32.Teunissen, M.B., C.W. Koomen, R. De Waal Malefyt, E.A. Wierenga, and J.D. Bos. 1998. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111:645–649. [DOI] [PubMed] [Google Scholar]
- 33.Infante-Duarte, C., H.F. Horton, M.C. Byrne, and T. Kamradt. 2000. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 165:6107–6115. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal, S., and A. Gurney. 2002. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 71:1–8. [PubMed] [Google Scholar]
- 35.Pasare, C., and R. Medzhitov. 2003. Toll pathway–dependent blockade of CD4+CD25+ T cell–mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 36.Feldmann, M. 2002. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2:364–371. [DOI] [PubMed] [Google Scholar]