Abstract
Sphingosine-1-phosphate receptor 1 (S1P1) was recently shown to be required for lymphocyte egress from lymphoid organs. Here we have examined the relationship between S1P1 abundance on the cell and egress efficiency. Using an integrin neutralization approach to separate the processes of entry and exit, we show that pertussis toxin treatment reduces lymphocyte egress from lymph nodes. Retrovirally mediated S1P1 overexpression is sufficient to reduce B cell accumulation in the splenic white pulp and to promote egress of activated T cells from lymph nodes, whereas S1P1 +/ −cells have reduced lymph node exit efficiency. Furthermore, lymphocyte S1P1 is down-regulated in the blood, up-regulated in lymphoid organs, and down-regulated again in the lymph. We propose that cyclical ligand-induced modulation of S1P1 on circulating lymphocytes contributes to establishing their lymphoid organ transit time.
After entering a secondary lymphoid organ from the blood, naive lymphocytes travel to separate subcompartments where they survey for antigen. In the absence of antigen encounter, the cells leave the organ via the efferent lymphatics or in the case of the spleen, via the red pulp. Timely egress ensures that the cells travel rapidly to further lymphoid organs to continue their antigen-surveillance process. The molecular mechanisms of lymphocyte entry into secondary lymphoid organs have been well characterized over the past several decades; however, the molecules involved in lymphocyte exit are just starting to be realized.
Integrins are essential for lymphocyte entry into peripheral lymphoid organs (1–3). The integrin αLβ2 (LFA-1) plays a major role and β2-deficient lymphocytes exhibit ∼50% reduced homing to peripheral lymph nodes. α4 integrins, α4β1 and α4β7, account for the remaining integrin requirement during entry, and when both αLβ2 and α4 integrins are blocked with neutralizing antibodies, lymphocyte entry into lymph nodes is inhibited by >98% (1). Integrin function during entry into lymphoid organs is controlled at least in part through the actions of chemokine receptors and associated Gαi signals (3, 4). Despite the well-established requirement for integrins during entry from blood into lymphoid organs, it is unclear whether integrins are involved during egress.
Recently, lymphocyte egress from secondary lymphoid organs and thymus was found to depend on expression of sphingosine-1-phosphate receptor 1 (S1P1) within the lymphocyte (5, 6). The S1P1 ligand, S1P, is present at high concentrations (100–300 nM) within blood and body fluids (7, 8). S1P1 couples to Gαi and stimulates a variety of events within cells in response to S1P, including chemotaxis (9). The Gαi requirement for T cell egress from the thymus has been suggested to reflect the role of Gαi downstream of S1P1 (6, 10). The requirement of Gαi for lymphocyte entry into lymph nodes and splenic white pulp is well established, but whether Gαi signaling is required for egress of lymphocytes from secondary lymphoid organs has been unclear.
The immunosuppressant drug, FTY720, blocks egress of lymphocytes from lymph nodes, Peyer's patches, and thymus (11–13). In vivo, FTY720 is phosphorylated and in vitro analysis established that FTY720-phosphate is an agonist for S1P1 and three of the four other S1P receptors (14, 15). However, after exposure to FTY720 for several hours, transfected cell lines exhibit prolonged down-regulation of S1P1, and it has been suggested that the egress-blocking activity of FTY720 can be explained by its S1P1 down-modulating activity on lymphocytes (6, 16). Although the inhibitory effects of FTY720 on egress from lymph nodes and Peyer's patches have been firmly established, its effects on recirculating lymphocytes in the spleen have been less clear as spleen lymphocyte numbers decrease after treatment (11, 17), and the drug causes a shift in viral-specific CTLs from spleen to lymph nodes (18).
In this study we have examined the relationship between S1P1 abundance in the cell and transit through lymphoid tissues. We first established that α4 and β2 integrins are not required for lymphocyte exit and we then used integrin neutralization as an approach to block entry and study requirements for exit. We find that pertussis toxin (PTX) treatment causes a reduction in lymphocyte exit, establishing that Gαi signaling has a positive role in egress. S1P1 overexpression in activated B cells reduces their accumulation in the splenic white pulp and restores the ability of activated T cells to egress from lymph nodes. Reciprocally, S1P1 +/− cells are shown to have reduced egress efficiency. Finally, we establish that S1P1 receptor levels are down-regulated on lymphocytes during transit through the blood and lymph and up-regulated within the lymphoid organs. We propose that cyclical modulation of S1P1 on circulating lymphocytes influences their transit time through lymphoid organs.
Results
α4 and β2 integrins are not essential for egress from secondary lymphoid tissues
To test whether integrins were required during lymphocyte egress from lymph nodes, we examined the effect of treating mice with a combination of α4 and αL neutralizing antibodies. Within 20 min of treatment, surface integrins on circulating lymphocytes were saturated with the antibodies and entry into lymph nodes was blocked (not depicted). When mice were treated for 26 h there was an 80–90% decrease in lymph node cell number compared with control treated mice (Fig. 1 A). By contrast, lymphocyte numbers in blood increased, consistent with continued release of cells from lymphoid organs in the absence of further entry (not depicted). Analysis of cells within lymph nodes revealed that integrin saturation with neutralizing antibodies was not achieved for 8–10 h, in agreement with studies showing that antibodies are poorly able to penetrate lymph nodes (19). However, lymph node cell numbers continued to decrease between 14 and 26 h of antibody treatment, indicating that exit was still able to occur after all lymphocyte α4 and αL integrins had been neutralized (Fig. 1 A).
Figure 1.
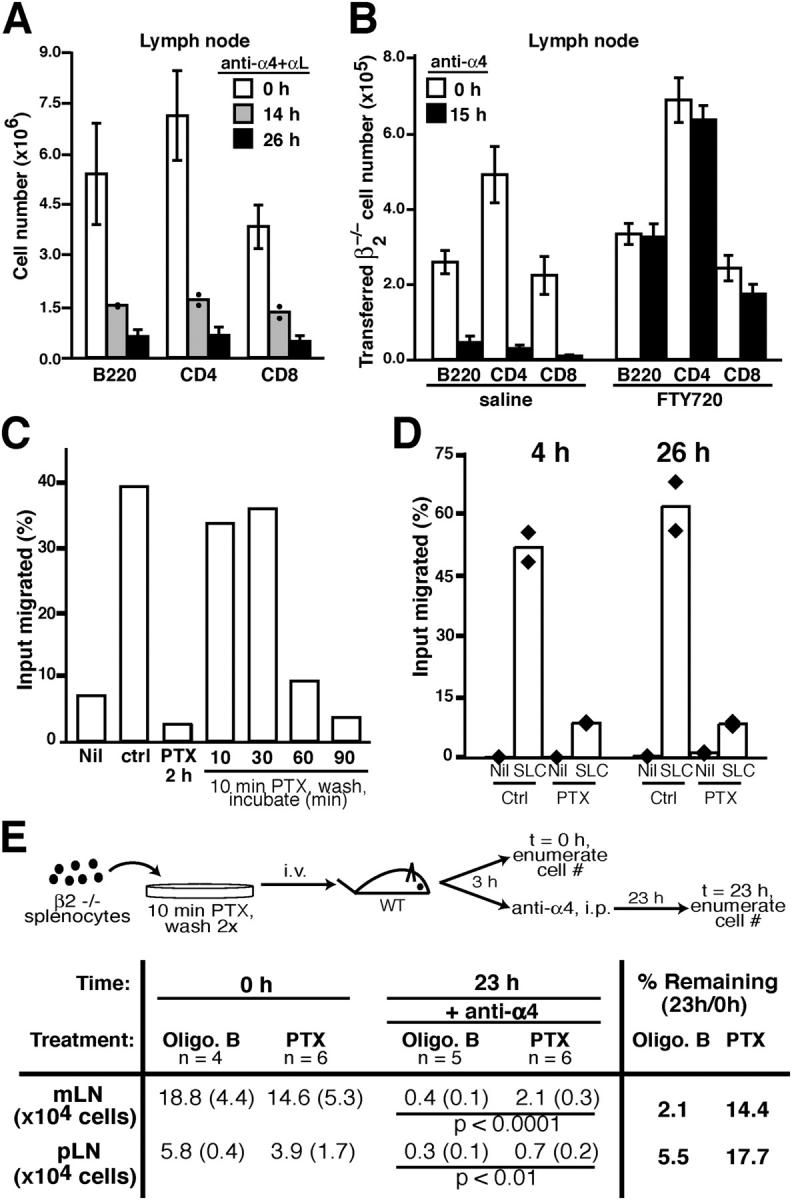
Lack of integrin requirement but role for Gαi during lymphocyte egress. (A) Effect of treatment with a combination of α4 and αL neutralizing antibodies for the indicated times on B220+ B cell numbers, and CD4 and CD8 T cell numbers in mesenteric lymph nodes. Each bar represents the mean (± SD) for at least five mice per group. (B) Egress of β2−/− cells from lymph nodes. CFSE-labeled β2−/− splenocytes were transferred into wild-type (WT) mice, and after 32 h, the mice were treated with saline or FTY720 for 6 h and then either analyzed (0 h) or treated with α4 antibodies to block further entry of transferred β2−/− cells and analyzed after 15 h. Panels show numbers of transferred β2−/− cells in mesenteric lymph nodes. Bars show mean (± SD) numbers of transferred B220, CD4, and CD8 cells from mesenteric lymph nodes of the indicated mice. Data are pooled from two experiments with at least four animals in each group. (C) Determination of the time needed for PTX-mediated inhibition of chemotaxis. Wild-type splenocytes were treated with PTX or saline (ctrl) for 2 h or treated with PTX for 10 min, washed, and then further incubated for the indicated times. All cells were then subjected to chemotaxis assays with 0.3 μg/ml CXCL12 or no chemokine (nil). The data depict the percent of input CD4 T cells that migrated and are representative of two experiments. (D) Persistence of PTX inhibitory effects in vivo. CD45.1+ splenocytes were treated for 10 min with 2 ng/ml PTX or saline, washed twice, and then adoptively transferred into CD45.2+ wild-type mice. 4 or 26 h later, recipient lymph node cells were harvested and subjected to chemotaxis assays. Bars show means of duplicates for CD45.1+ CD4 T cell chemotaxis to 1 μg/ml CCL21 (SLC) and are representative of two experiments. (E) PTX sensitivity of lymphocyte exit from lymph nodes. CFSE-labeled β2−/− splenocytes were treated with oligomer B (Oligo. B) or PTX for 10 min, washed twice, and then adoptively transferred into wild-type mice. After 3 h, mice were analyzed (0 h) or treated with α4 antibodies for 23 h, and then analyzed for numbers of transferred cells as indicated. Mean (± SD) values are shown for the indicated number of animals. Two-tailed student's t test was performed between the indicated (underlined) groups. The percent of transferred cells remaining (% Remaining) within each type of lymph node was calculated by dividing the number of cells recovered at 23 h by the number recovered at 0 h.
As a further approach to specifically test the β2 integrin requirement during exit, β2−/− splenocytes were adoptively transferred into wild-type recipient mice, allowed to equilibrate for 32 h, and then the mice were treated with neutralizing anti-α4 antibodies (Fig. 1 B). α4 neutralization on its own has only a weak inhibitory effect on lymph node entry of wild-type cells but causes an almost complete block in entry of β2−/− cells (1). A significant (∼95%) decrease occurred in the number of transferred β2−/− cells in lymph nodes 15 h after entry was blocked, whereas the number of endogenous cells remained similar over the course of the treatment (Fig. 1 B and not depicted).
To test whether FTY720-mediated sequestration of lymphocytes within lymph nodes was integrin dependent, recipients of β2−/− cells were treated with FTY720 6 h before treatment with α4 neutralizing antibodies. 15 h later, the number of β2−/− cells in the FTY720-treated group had not changed in contrast to the 20-fold decrease in β2−/− cell numbers in mice not treated with the drug (Fig. 1 B). These observations establish that β2 and α4 integrins are not required for T and B lymphocyte egress from lymph nodes or for FTY720-induced sequestration of cells.
Gαi inhibition reduces lymphocyte exit from lymph nodes
To examine whether Gαi signaling contributes to lymphocyte exit, we tested the effect of treatment with PTX, an enzyme that ADP-ribosylates and inactivates Gαi (20). Because Gαi is essential for lymphocyte entry into lymphoid organs, it was not possible to use the conventional method of Gαi inactivation by 100–200 ng/ml PTX treatment for 1–2 h before cell transfer. In vivo PTX treatment was also considered a problematic approach as Gαi function is critical in many cell types in addition to lymphocytes; for example, functioning to help maintain vascular integrity (9). Instead, we developed an approach where cells are pulsed with PTX for 10 min to load them with the toxin and then transferred to wild-type recipients. Immediately after such pulse loading, we found that minimal inhibition of Gαi function had occurred, but partial inhibition was evident after 60 min and almost complete inhibition after 90 min as assessed by chemotactic responsiveness to CXCL12 (Fig. 1 C). During initial tests to track PTX-pulsed cells in vivo after transfer, we observed lymphocytosis of endogenous cells, indicating that PTX was leaking from the treated cells and having trans effects (not depicted). Therefore, we performed titration experiments and found that a 10-min treatment with 2 ng/ml PTX was adequate to cause a substantial (∼75%) inhibition of CCL21 chemokine responsiveness after 4 h (Fig. 1 D), while having minimal effects on endogenous lymphocytes (not depicted). The inhibitory effect in the transferred cells was still evident after 26 h (Fig. 1 D). The same experiment performed at the high PTX dose resulted in ∼95% inhibition in the CCL21 response (not depicted).
To determine the contribution of Gαi signaling to lymphocyte exit, β2−/− cells were pulsed with 2 ng/ml PTX or, as a control, the non-ADP ribosylating oligomer B subunit of PTX, washed and transferred to wild-type recipient mice. After 3 h, these animals were treated with anti-α4 antibodies to block any further entry of β2−/− cells (Fig. 1 E). Enumeration 23 h later revealed that although the number of CD4 T cells decreased between 0 and 23 h, 14% of the transferred PTX-treated cells remained in the mesenteric lymph node, whereas only 2% of the oligomer B control-treated cells remained (Fig. 1 E). Similar findings were made for peripheral lymph nodes (Fig. 1 E) as well as for CD8 T cells and B cells (not depicted). Experiments were also performed with cells pulsed with the high dose of PTX (or oligomer B) and yielded ∼22% PTX-treated CD4 T cells remaining compared with ∼6% oligomer B–treated cells in peripheral lymph nodes (not depicted). These results indicate that Gαi signaling contributes to the efficiency of lymphocyte exit from lymph nodes.
S1P1 overexpression reduces B cell localization in the splenic white pulp
To better understand the role of S1P1 during lymphocyte recirculation, we tested whether S1P1 overexpression was sufficient to promote lymphocyte egress from secondary lymphoid tissues. Activated B cells were transduced with a retrovirus containing a Flag-tagged S1P1 insert and a human CD4 (hCD4) reporter, or with a control retrovirus lacking the S1P1 insert, and transferred into wild-type recipient mice. Transferred B cells were recovered from the blood and spleen 1 d later, but insufficient numbers homed into lymph nodes for analysis, possibly due to their activated state. S1P1-transduced cells isolated ex vivo from the spleen responded robustly to S1P in chemotaxis assays compared with vector-transduced cells (Fig. 2 A). To examine whether S1P1 overexpression led to altered cell trafficking within the spleen, immunohistochemical analysis was performed on recipient spleens isolated 15 h after cell transfer. In contrast to the predominantly follicular distribution of vector control cells, most of the S1P1-overexpressing cells were localized in the red pulp (Fig. 2, B and D). To determine whether this effect on cell distribution was reversible, recipient mice were treated with FTY720. Although the treatment did not appear to alter the splenic compartmentalization of vector-transduced cells (not depicted), it was sufficient to cause relocalization of S1P1-overexpressing cells into the white pulp (Fig. 2, C and D). FTY720 treatment led to reduced S1P chemotactic responsiveness of the S1P1-overexpressing cells (Fig. 2 E). Furthermore, Flag-S1P1 surface levels were decreased (Fig. 2 F), extending previous in vitro findings that FTY720 exposure causes down-modulation of S1P1 in cell lines (6, 16). These results establish that elevated S1P1 expression reduces accumulation of B cells in the splenic white pulp, possibly by favoring white pulp exit over entry, and that in vivo treatment with FTY720 causes S1P1 down-modulation. By contrast with these findings for B cells, S1P1-transduced activated T cells were not altered in their homing to the splenic T zone (not depicted). The basis for this difference between transduced B and T cells is not clear, although it is notable that B and T cells have differing requirements for entry into the splenic white pulp (2, 21). However, the activated T cells were able to enter lymph nodes in greater numbers than the B cells, permitting experiments to test effects of S1P1 overexpression on lymph node egress.
Figure 2.
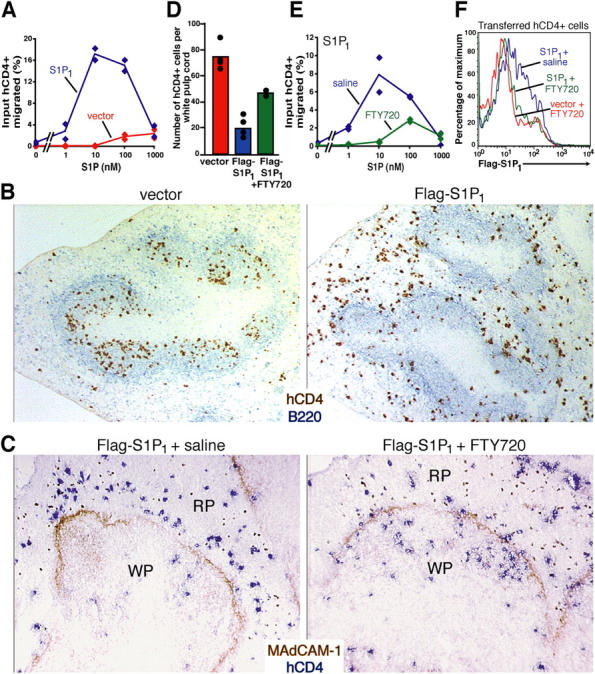
S1P1 overexpression reduces B cell localization in the splenic white pulp. (A) S1P chemotactic responsiveness of Flag-S1P1 and vector-transduced B cells isolated from recipient spleens 15 h after transfer, shown as percent of input hCD4+ cells of the indicated type that migrated. (B) Immunohistochemical analysis of spleen sections from wild-type mice that had received either vector or Flag-S1P1–transduced cells 15 h earlier. Transferred transduced B cells were detected by hCD4 staining (brown) and endogenous B cells detected by B220 staining (blue). Objective magnification of 5. (C–E) Effects of FTY720 treatment on S1P1-overexpressing B cell distribution. Flag-S1P1–and vector-transduced B cells were transferred into wild-type mice for 15 h and the mice were then treated with saline or FTY720 for a further 6 h. (C) Immunohistochemical analysis of spleen sections stained to detect transferred B cells (hCD4, blue) and the marginal sinus-lining cells (MAdCAM-1, brown). The latter marker delineates the boundary between the red pulp (RP) and white pulp (WP). Objective magnification of 10. (D) Quantitation of the number of hCD4+ cells per white pulp cord in the groups shown in B and C. The number of transferred cells in at least four white pulp cords of similar size were enumerated in each type of recipient. (E) S1P chemotactic responsiveness of Flag-S1P1–transduced cells from transfer recipients treated with saline or FTY720 for 6 h as indicated. (F) Flow cytometric analysis for Flag epitope staining on hCD4+ Flag-S1P1– or vector-transduced cells from spleens of transfer recipients treated for 6 h with saline or FTY720 as indicated. The data in A–E are representative of at least three experiments.
Exit of activated T cells from lymph nodes is restored by S1P1 overexpression
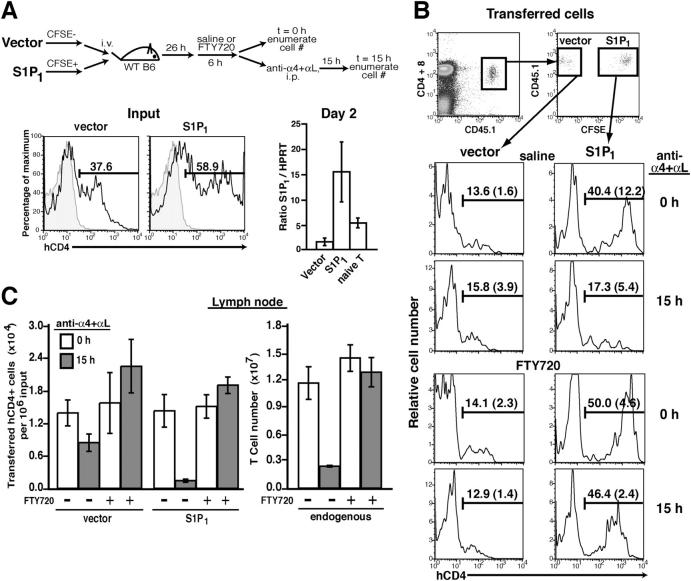
After activation, T cells down-regulate S1P1 mRNA and are retained in lymph nodes for a period of 2–3 d (6, 22). To determine whether S1P1 down-regulation is sufficient to explain the retention of activated T cells, we tested the effect of restoring S1P1 expression by retroviral gene transduction (Fig. 3 A). Analysis of the input cells before adoptive transfer showed that S1P1 transduction was more efficient than transduction with the control virus and hCD4 expression was higher (Fig. 3 A). The basis for this difference was not clear but it was stably maintained after the cells were transferred to recipient mice. S1P1 transcripts were 10–20-fold more abundant in S1P1-transduced cells than in the vector control cells and about threefold more abundant than in naive T cells (Fig. 3 A). CD45.1+ carboxyfluorescein succinimidyl ester (CFSE)− vector and CD45.1+ CFSE+ Flag-S1P1-transduced T cells were cotransferred into CD45.2+ wild-type recipient mice, and after a 1-d equilibration period (time = 0 h), further lymph node entry was blocked by treating with integrin-neutralizing antibodies. After 15 h, the frequency of hCD4+ cells amongst the transferred vector-transduced cells remained similar to the 0-h time point, indicating that transduction with the empty vector did not alter the trafficking of the cells (Fig. 3 B). As expected, few of the activated vector-transduced cells exited the lymph node during the 15 h, decreasing less than twofold in number, compared with a sixfold decrease in the number of endogenous naive T cells over this period (Fig. 3, B and C). By contrast, the hCD4+ S1P1-expressing cells exhibited a significant decrease in frequency amongst the transferred cells 15 h after entry was blocked (Fig. 3 B), and their egress efficiency was increased to a level exceeding that of the endogenous naive cells (Fig. 3 C). To further test whether the greater reduction in cell number of the S1P1-overexpressing group was due to increased egress, some of the recipient mice were treated with FTY720 before integrin neutralization. In these animals, S1P1 overexpression had no effect on cell frequency or number over time (Fig. 3, B and C). These results demonstrate that S1P1 overexpression is sufficient to promote exit of activated T lymphocytes from lymph nodes.
Figure 3.
Exit of activated T cells from lymph nodes is restored by S1P1 overexpression. (A) Upper diagram shows the experimental strategy. Lower left panels, hCD4 expression on activated CD45.1+ T cells that had been transduced with vector or S1P1-containing retrovirus, immediately before transfer (Input) to recipient mice. Lower right panel, quantitative PCR analysis of total S1P1 transcript levels in vector or S1P1-transduced hCD4+-purified T cells after 48 h in vitro culture, or in naive T cells shown relative to HPRT. (B) Vector- and S1P1-transduced cells were cotransferred into wild-type recipients and after 26 h the mice were treated with saline or FTY720 for 6 h followed by anti-integrin antibodies. Transferred cells were identified by staining for CD45.1 and CD4 plus CD8, as indicated in the upper panels. The frequencies of transferred vector- (CFSE−) or S1P1- (CFSE+) transduced hCD4+ T cells in mesenteric lymph nodes as a proportion of the transferred cells were determined at time 0 and 15 h after integrin neutralization. Frequencies are the mean (± SD) values of at least five mice per group and are pooled from four experiments. The frequency of hCD4+ cells in the S1P1-transduced population at 15 h in α4 and αL antibody-treated recipients was significantly reduced compared with the frequency present at 0 h (P < 0.005; Student's t test). (C) Number of transferred vector- and S1P1-transduced cells and endogenous cells in the mesenteric lymph nodes at time 0 (white bars) and 15 h (gray bars) after anti-integrin antibody treatment with or without FTY720 treatment as indicated. Bars represent the mean (± SD) value for three mice per group except for the integrin-neutralization groups where there were two mice per group (here the error bars represent the range). Data are representative of two experiments.
S1P1 heterozygous cells are impaired in their ability to exit lymph nodes
As another approach to test the sensitivity of lymphocyte egress to S1P1 levels, we asked whether egress efficiency was altered in S1P1 heterozygous cells. By flow cytometric analysis, surface S1P1 levels on S1P1 +/− cells were reduced compared with wild-type cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20041509/DC1). S1P1 +/+ and S1P1 +/− cells were cotransferred into wild-type recipients and allowed 32 h for equilibration in the animal. The mice were then treated with neutralizing α4 and αL antibodies to block further cell entry into lymph nodes, or with saline as a control, and transferred cell numbers were enumerated in lymph nodes either at this time (time = 0 h) or 15 h later (time = 15 h). As in the experiments shown in Fig. 3 C, total lymph node cell numbers decreased approximately sixfold after the antibody treatment (not depicted). Flow cytometric analysis of peripheral lymph nodes showed that although the frequency of transferred wild-type (CFSElo) cells did not change substantially over time, indicating that they were leaving the tissue at the same rate as the endogenous cells, the frequency of S1P1 +/− (CFSEhi) cells increased, indicating less efficient exit (Fig. 4, A and B). Similar findings were made for mesenteric lymph nodes (Fig. 4 B). We also observed that the frequency of mature L-selectinhi CD4 and CD8 single positive cells was increased in the thymus of S1P1 +/− mice compared with littermate controls, suggesting that thymic egress was reduced (not depicted). These results indicate that a partial reduction in S1P1 abundance in the cell impairs egress efficiency from lymphoid organs.
Figure 4.
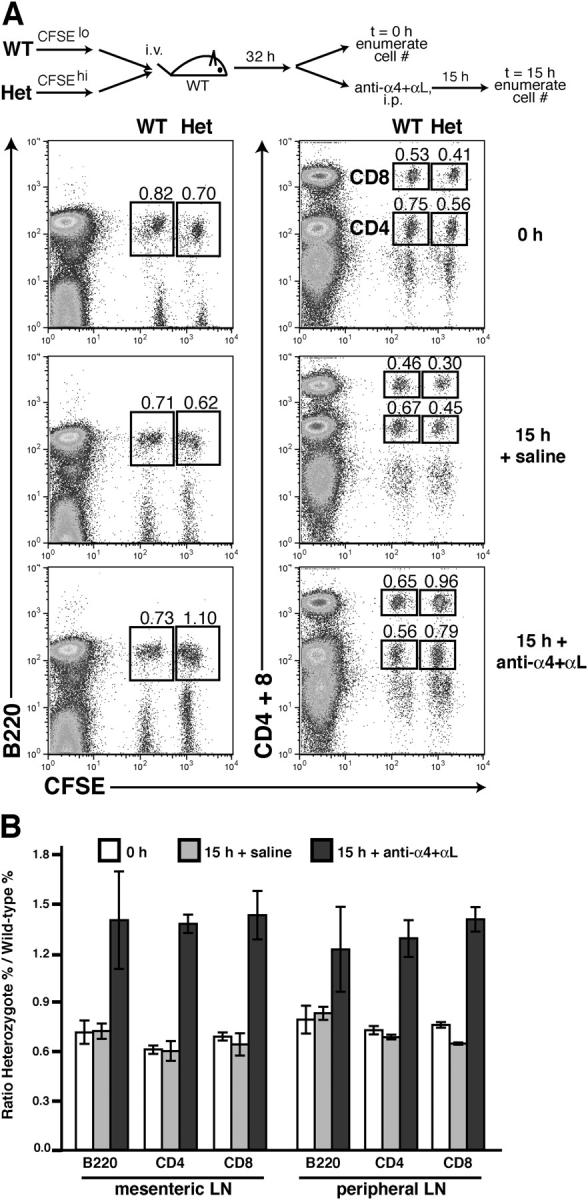
Reduced exit efficiency of S1P1 heterozygous cells. (A) S1P1 wild-type and heterozygote splenocytes were labeled with low or high amounts of CFSE, respectively, cotransferred into wild-type mice, and equilibrated for 32 h, followed by 0 or 15 h of α4 plus αL antibody or saline treatment. Gates show the frequencies of cotransferred S1P1 wild-type (CFSElo) and heterozygote (CFSEhi) B220+ cells (left) and CD4+ and CD8+ cells (right) recovered from a pool of brachial and inguinal (peripheral) lymph nodes as a percent of total lymph node cells. The data shown are from one experiment that is representative of three experiments with three mice per group. (B) Ratio of S1P1 heterozygote and wild-type cotransferred cells within the mesenteric and peripheral lymph nodes (LN) at 0 (white bars) or 15 h of saline treatment (gray bars) or 15 h of α4 plus αL antibody treatment (black bars). Bars show the mean (± SD) for three mice per group in one experiment. Representative of three similar experiments.
S1P1 surface levels modulate cyclically during lymphocyte recirculation
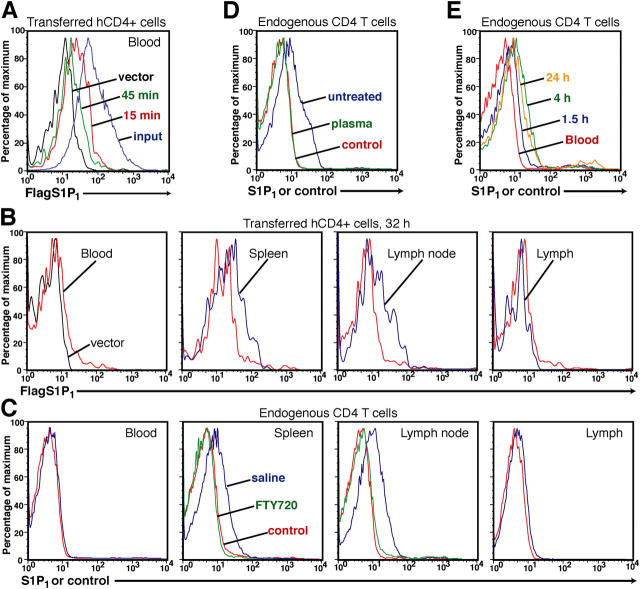
Because plasma contains high (100–300 nM) amounts of S1P, we tested the possibility that S1P1 is down-regulated on cells circulating in the blood compared with cells within lymphoid organs. We first examined surface expression of Flag-S1P1 on transferred retrovirally transduced cells. Within 15 min of transfer, Flag-S1P1 levels were reduced on cells within the blood, and after 45 min, the cells had lost most of their surface staining (Fig. 5 A). To test whether surface expression was restored after cells migrated into lymphoid organs, transfer recipients were analyzed after 32 h, a time point when retrovirally transduced cells are present in spleen, lymph nodes, and lymph as well as blood. Flag-S1P1–transduced T cells in the blood exhibited minimal Flag staining compared with the vector control, whereas cells isolated from spleen and lymph nodes showed up-regulation compared with cells in the blood (Fig. 5 B). Flag-S1P1 levels were down-regulated again on cells present in the lymph (Fig. 5 B).
Figure 5.
S1P1 surface levels modulate cyclically during recirculation. (A) Flow cytometric analysis of cell surface Flag epitope on hCD4+ Flag-S1P1–transduced B cells before transfer (input, blue) or in the blood of recipient mice 15 (15 min, red) or 45 min (45 min, green) after transfer, compared with cells transduced with empty vector (vector, black). (B) Flow cytometric analysis of Flag levels on Flag-S1P1–transduced T cells during lymphocyte recirculation. Flag staining of Flag-S1P1– (blue) transduced cells (hCD4+) in the blood, spleen, peripheral lymph nodes, and lymph compared with staining of vector transduced cells (left, black) or to Flag-S1P1–transduced cells in the blood (red). Individual histograms are from different experiments and are each representative of four experiments. (C) Flow cytometric analysis of endogenous S1P1 levels on CD4 L-selectinhi T cells in the indicated tissues of untreated or FTY720-treated mice. Data are representative of more than six untreated mice for blood, spleen, and lymph node, three treated mice for spleen and lymph node, and for four pools of lymph representing two or three mice. (D) Flow cytometric analysis of endogenous S1P1 levels on lymph node CD4+ L-selectinhi T cells after 45 min of incubation with medium (untreated) or a 1/20 dilution of mouse plasma (plasma). Data are representative of three experiments. (E) Flow cytometric analysis of S1P1 levels on transferred CD4+ L-selectinhi T cells isolated from recipient lymph nodes at the indicated times after transfer. Data are representative of four similar experiments (five mice total). In C–E, control refers to cells stained with an irrelevant rabbit antiserum.
Using a newly generated anti–mouse S1P1 antiserum, we were able to confirm these findings for endogenous S1P1 on naive CD4 L-selectinhi T cells, showing that S1P1 was expressed on the surface of cells in spleen and lymph nodes but was down-regulated on cells from blood or lymph (Fig. 5 C). The specificity of the antiserum for S1P1 was confirmed by analysis of mature single positive thymocytes from S1P1-deficient fetal liver chimeras (Fig. S1). Consistent with the above findings, treatment of mice with FTY720 caused down-modulation of S1P1 on lymph node cells (Fig. 5 C) and mature thymocytes (Fig. S1). Furthermore, if lymph node cells were incubated in vitro with mouse plasma, they rapidly down-regulated S1P1 (Fig. 5 D). Finally, when S1P1 abundance was measured on adoptively transferred splenocytes after entry into lymph nodes, S1P1 was partially up-regulated by 1.5 h and fully up-regulated by 4 h compared with cells that had been transferred 24 h previously (Fig. 5 E). Taken together, these observations demonstrate that S1P1 modulates cyclically in cell surface levels as lymphocytes recirculate from the blood into secondary lymphoid organs and then into the blood or lymph.
Discussion
The above findings established that S1P1 overexpression was sufficient to reduce B cell accumulation in the splenic white pulp and to overcome retention of activated T cells in lymph nodes, whereas S1P1 heterozygous deficiency in naive lymphocytes was associated with reduced lymph node egress. Furthermore, wild-type lymphocytes were found to down-modulate S1P1 during transit through lymph and blood and a transferred cohort of cells required more than an hour to fully up-regulate the receptor upon entering a lymphoid tissue. These findings suggest that there is normally a delay between when a cell enters a lymphoid tissue and when it regains full egress competence (Fig. 6), a delay that might help ensure the cell dwells in the tissue and does not immediately undergo the exit program. To our knowledge, S1P1 is the first example of a receptor that is down-modulated on cells circulating in the blood or lymph and then reexpressed on cells that migrate into tissues.
Figure 6.
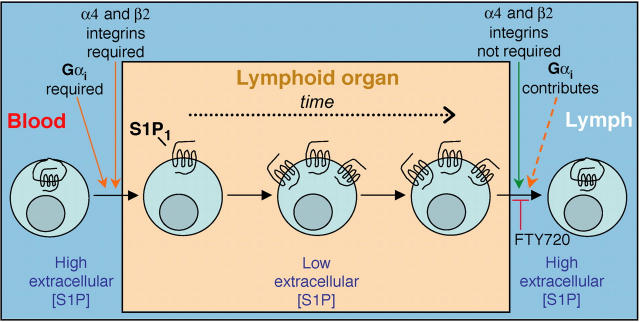
Summary model relating requirements for lymph node egress and possible effect of cyclical S1P1 expression on lymphocyte recirculation. Lymphocytes in the blood, which has abundant extracellular S1P, have little surface S1P1. After migration into a lymphoid organ in an integrin- and Gαi-dependent manner, the cells begin to up-regulate S1P1, perhaps because extracellular S1P concentrations are no longer adequate to cause receptor down-modulation. Only when S1P1 is up-regulated do the cells become fully egress competent and the time required for up-regulation may help ensure that the cells dwell in the tissue for a period of hours. Additional undefined factors are also anticipated to influence lymphoid organ transit time. Egress occurs in a manner that does not require α4 and β2 integrins but Gαi contributes to this process, perhaps acting downstream of S1P1. Exit sites are suggested to have high S1P levels, and S1P1 signaling is essential for egress to occur. By down-modulating S1P1, FTY720 can block the egress step. Once in the lymph, S1P1 is down-regulated, most likely due to the abundance of S1P, and the cycle begins again. Transit through the splenic white pulp is suggested to involve a similar cycle of events except that egress is into the blood. The reduced S1P1 levels on circulating lymphocytes may also help ensure that S1P does not antagonize chemokine signaling required for lymphoid organ entry.
Lymphocyte exit from lymph nodes and Peyer's patches is thought to involve reverse transmigration across lymphatic endothelial cells (23–25). The lack of an essential role for α4 or β2 integrins during lymphocyte egress from lymph nodes is consistent with in vitro studies showing that reverse transmigration does not require α4 or β2 integrins (26). A study tracking lymphocyte migration through rat Peyer's patches also concluded that α4 integrins were not required for egress (23) and in a recent report, egress of activated T cells from lymph nodes was not blocked by integrin neutralizing antibodies (27). Moreover, we failed to observe release of β2−/− cells from lymph nodes of FTY720-treated mice after 15 h of α4 antibody treatment, providing evidence that integrin-mediated adhesion is not involved in mediating FTY720-induced lymphocyte sequestration. The finding that lymph node egress is partially PTX sensitive is consistent with the S1P1 requirement during this step as Gαi is the only defined Gα signaling partner for this receptor (9). Lymphocyte egress from the thymus is also sensitive to the enzymatic subunit of PTX and depends on Gαi2 and S1P1 (5, 6, 10). However, it is notable that we did not observe a complete block in lymph node egress even after PTX exposures that were sufficient to inhibit lymph node entry. One explanation for these observations may be that the PTX treatment failed to inactivate all of the Gαi molecules and a smaller fraction of total cellular Gαi may be needed during exit than during entry. Alternatively, S1P1 may signal in part via PTX insensitive G proteins, a possibility supported by evidence that S1P1 can activate Rho, a small G protein usually thought to be downstream of Gα12/13 rather than Gαi (9). Our experiments also do not exclude the possibility that the reduced egress of PTX-treated cells is due to a reduced ability to migrate to exit sites, perhaps in response to chemokines, rather than being due to an effect on exit itself. In a recent report where mice were treated in vivo with PTX, it was concluded that lymphocyte egress was not affected (27). However, the interpretation of these experiments is complicated by the evidence that Gαi-coupled receptors are important for vascular integrity (9, 28) and might be required in a variety of cell types for maintaining lymphoid tissue function.
Most of the lymphocytes in the spleen are localized within the white pulp but as many as one third of the cells are in the nonlymphoid red pulp, a region through which blood filters from open-ended arterioles and returns to the vascular system via venous sinuses (29, 30). Due to the presence of these separate compartments and the property that lymphocyte entry and exit both occur via the blood, it has been difficult to study the process of lymphocyte egress from the spleen. Based on the finding that splenic B and T cell numbers decrease during the first hours after FTY720 treatment, it has been suggested that the drug does not block splenic egress and therefore that S1P receptors may not be involved in this process (11, 17, 18, 31). However, these studies tracked total spleen cell numbers and did not exclude the possibility that cells left the nonlymphoid red pulp but were blocked from exiting the white pulp. Our finding that S1P1 overexpression decreases B cell localization in the splenic white pulp and promotes their accumulation in the red pulp, and that FTY720 treatment can overcome this effect, provides firm evidence that S1P1 receptor function is involved in regulating B cell trafficking between the white and red pulps. This extends the recent finding that transferred S1P1-deficient lymphocytes accumulate in the splenic white pulp while being depleted from the red pulp and blood compared with wild-type cells (6). A unique feature of the spleen compared with other secondary lymphoid organs is that entry into and exit from the lymphoid area occur at common locations, the marginal sinus, and marginal zone bridging regions (2, 32). S1P1-overexpressing B cells were reduced in number within the splenic white pulp even when analyzed 4 h after transfer (not depicted). Therefore, we consider it likely that S1P1 overexpression causes newly arriving cells to immediately take the exit route into the red pulp. Marginal zone B cells were recently shown to express higher amounts of S1P1 than follicular cells and their lodgment in the marginal zone was S1P1 dependent (33). As well as being necessary, our present findings suggest that the high expression of S1P1 in marginal zone B cells might be sufficient to antagonize their migration into follicles.
In previous studies, it has been found that T cell activation is associated with a rapid and marked down-regulation of S1P1 and this has been suggested to provide an explanation for activation-induced T cell retention in lymphoid tissues (6, 22). Our finding that selective restoration of S1P1 by retroviral gene transduction promotes egress of activated cells provides evidence that S1P1 down-modulation is sufficient to account for the retention of activated T cells. The finding that the transduced cells overexpressed S1P1 transcripts by about threefold compared with naive T cells and left the lymph nodes to a slightly greater extent than naive T cells in a 15-h assay suggests that endogenous S1P1 expression levels may limit the rate of naive T cell exit.
In vitro studies with transfected cells have demonstrated that S1P1 is rapidly down-modulated after exposure to 10–100 nM S1P (34, 35). Our finding that S1P1 is rapidly down-modulated on circulating lymphocytes and on isolated lymph node cells exposed to plasma is in agreement with these findings because blood S1P concentrations are estimated to be between 100 and 300 nM (7, 36). Measurements of total tissue S1P levels in spleen and other organs indicate concentrations that typically exceed 1pmol S1P per mg wet weight of tissue (7). However, because all cell types generate S1P intracellularly during normal sphingolipid metabolism and only some cell types are able to export S1P, much of the total tissue S1P is likely to be intracellular (8). The observation that S1P1 surface levels recover after lymphocyte migration from blood into spleen and lymph nodes provides evidence that extracellular S1P concentrations within the lymphoid tissues are lower than in plasma. Reciprocally, the down-modulation of surface S1P1 on cells within lymph argues that S1P concentrations are higher in lymph than in lymph nodes. Taken together, these findings favor the view that egress from lymphoid tissues is promoted by cells sensing the higher S1P levels present in lymph (for lymph node and Peyer's patch cells) or blood (for spleen cells). Additional factors must act to ensure that lymphocytes exit lymph nodes and Peyer's patches via lymphatics rather than blood vessels.
In summary, we propose that S1P1 down-modulation on circulating cells helps ensure that after entry into a lymphoid organ, the cells are not able to immediately undergo the exit program (Fig. 6). In vitro studies in transfected cells indicate that after down-modulation, cells require more than 2 h to fully reexpress the receptor on the cell surface (34, 35) and our transfer studies suggest that it may take more than an hour for full S1P1 up-regulation to occur on naive T lymphocytes after entry into a lymphoid organ. The finding that S1P1 heterozygosity is associated with reduced exit efficiency provides evidence that exit will not be maximal until cells have fully up-regulated their surface receptor levels. This delay in acquiring full egress competence may help ensure that newly entering cells are drawn into the parenchyma of the tissue by lymphoid chemokines rather than undergoing immediate exit, thereby contributing to establishing the half-day to 1-d residence time of naive lymphocytes within lymphoid tissues (37–39). We anticipate that additional factors such as responsiveness to lymphoid chemokines also contribute to establishing the transit time. Based on in vitro evidence that S1P can reduce lymphocyte chemokine responses (40), it can also be suggested that S1P1 down-modulation on cells within the blood ensures that S1P does not antagonize lymphoid organ entry.
Materials and Methods
Mice and adoptive cell transfer.
CD45.2 C57Bl/6 (B6) and CD45.1 B6 mice were from the National Cancer Institutes or a colony maintained at the University of California, San Francisco, and β2−/− (41) B6 mice were from The Jackson Laboratory. IgHEL transgenic B6 mice express IgMa and IgDa specific for hen egg lysozyme. For adoptive transfers, mice were injected with ∼1–3 × 107 cells in ∼0.3 ml medium. In some experiments, cells were labeled with CFSE (Molecular Probes) as described previously (42). Protocols were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Antibodies and treatments.
The anti-αL (clone M17/4, rat IgG2a) hybridoma was from American Type Culture Collection and the anti-α4 (clone PS/2, rat IgG2b) hybridoma was provided by D. Erle (University of California, San Francisco, San Francisco, CA). Antibodies were administered intraperitoneally at 100 μg per mouse in PBS. FTY720 (Novartis) was administered intraperitoneally at 1 mg/kg in saline. Cells were treated with PBS, 2–200 ng/ml oligomer B, or PTX (Sigma-Aldrich) for the indicated times at 37°C as described previously (30), washed twice in warm RPMI 1640, 2% FCS, and 10 mM Hepes, and then assayed or transferred to recipient mice. S1P1-specific antiserum was generated by immunizing rabbits according to standard procedures (AnimalPharm Services) with a glutathione-S-transferase fusion protein containing the first 49 amino acids of mouse S1P1. The antiserum was affinity purified using sepharose beads coupled to a mannose binding protein fusion containing the same segment of S1P1. An affinity-purified antiserum raised against the cytoplasmic domain of mouse BAFF was used as a control.
Epitope-tagged S1P1.
Full-length murine S1P1 was inserted into the MSCV2.2 retroviral vector in frame with a preprolactin leader sequence and Flag epitope and upstream of an internal ribosomal entry site and a cytoplasmic domain truncated hCD4 (42). In the vector control, a second copy of the truncated hCD4 was inserted in place of the preprolactin leader, Flag epitope and S1P1. Retrovirus-containing culture supernatant was generated using the Bosc23-packaging cell line and primary B cell retroviral infection was with IgHEL transgenic B cells as described previously (42). Primary T cell retroviral infection was performed by incubating total splenocytes at ∼107 per ml for 20–24 h on 24-well plates precoated for 2–3 h at 37°C with 3 μg/ml anti-CD3 and 0.5 μg/ml anti-CD28 (BD Biosciences). Activated T cells were spin infected at 2,450 RPM in a Beckman Coulter Allegra 6R centrifuge for 2 h at 25°C as described previously (42), and then cultured in vitro for 24 h before transfer to recipient mice or incubated in vitro for 48 h. In vitro–incubated transduced T cells were purified by autoMACS (Miltenyi Biotec) by depleting CD11b+, Mac-1+, B220+, I-Ab+, and Gr-1+ cells, and RNA was isolated using QIAGEN RNeasy kits treated with DNaseI, and real-time quantitative PCR was performed for total S1P1 mRNA relative to HPRT by using SYBR Green (Applied Biosystems). The primers used for quantitative PCR were S1P1 forward CCTTCATCCGGATCGTATCT, S1P1 reverse TGCTGCGGCTAAATTCCATG, HPRT forward AGGTTGCAAGCTTGCTGGT, and HPRT reverse TGAAGTACTCATTATAGTCAAGGGCA.
Immunohistochemical and flow cytometric analysis.
7-μm cryostat sections were fixed and stained as described previously (42) using the following antibodies: B220, MAdCAM-1, and hCD4-biotin (BD Biosciences). Amplification of the hCD4 signal was performed by using the Tyramide Signal Amplification Biotin System (NEN Life Science Products) by first quenching endogenous peroxidase activity by incubating sections in 0.045% H2O2 for 15 min at room temperature and then adhering to the manufacturer's protocol. Enumeration of transferred cells in white pulp cords were performed as described previously (2). Flow cytometric analysis was performed on a FACSCalibur (Becton Dickinson). Transferred transduced B cells were identified flow cytometrically by staining for CD45.1, B220, and hCD4. Transferred transduced T cells were identified by staining for CD45.1, CD4 and CD8, and hCD4. CD4 and CD8 antibodies were typically combined in the same channel and were distinguished by the more intense fluorescence of the CD8 cells. All monoclonal antibodies used were from BD Biosciences. Flag levels were detected by staining with the M2-Flag antibody (Sigma-Aldrich). CFSE-labeled cells were detected by staining for B220, CD4, or CD8. Integrin saturation with anti-αL and anti-α4 antibodies was determined as described previously (2). For detecting endogenous S1P1, cells were incubated with affinity purified rabbit anti-S1P1 (∼20 μg/ml) for 1.5 h followed by anti–rabbit IgG biotin (BD Biosciences) and lastly with CD45.2-PE, L-selectin–FITC, CD4-PerCP (BD Biosciences), and streptavidin-APC (Molecular Probes). Thymocyte populations were distinguished by CD4, CD8, and L-selectin staining. Blood lymphocytes were prepared by lysing red blood cells twice on ice with a tris ammonium chloride solution. Mouse plasma was prepared by diluting nine parts whole blood into one part acid-citrate-dextrose solution (Sigma-Aldrich), spinning at 83 g for 10 min, removing the clear, top fraction, and spinning at 630 g for 10 min and retaining the supernatant.
Chemotaxis assays.
Cells were tested for transmigration across uncoated 5-μm transwell filters (Corning Costar Corp.) for 3 h to S1P (Sigma-Aldrich), CXCL12 (SDF-1; PeproTech), or CCL21 (SLC; R&D Systems), or medium in the bottom chamber and were enumerated by flow cytometry (43).
Online supplemental material
Fig. S1 shows flow cytometric analysis of S1P1 expression on thymocytes from 8-wk reconstituted S1P1 −/− or S1P1 +/+ fetal liver chimeras that had been generated as described previously (6), from saline- or FTY720-treated wild-type mice and from S1P1 +/− mice. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20041509/DC1.
Acknowledgments
We thank Matthew Lesneski, Caroline Low for help with the mouse colony, Mehrdad Matloubian and Takaharu Okada for excellent discussions, Volker Brinkmann for providing FTY720, and Chris Allen, Guy Cinamon, Kenji Kabashima, and Susan Schwab for critical reading of the manuscript.
C.G. Lo was supported by the University of California, San Francisco, Boyer Program in the Biological Sciences (Tetrad Program in Cell Biology) and is currently supported by a University of California, San Francisco, Chancellor's Fellowship. J.G. Cyster is a Howard Hughes Medical Institute Assistant Investigator. This work was supported in part by National Institutes of Health grant number AI45073.
The authors have no conflicting financial interests.
Abbreviations used: CFSE, carboxyfluorescein succinimidyl ester; hCD4, human CD4; PTX, pertussis toxin; S1P1, sphingosine-1-phosphate receptor 1.
References
- 1.Berlin-Rufenach, C., F. Otto, M. Mathies, J. Westermann, M.J. Owen, A. Hamann, and N. Hogg. 1999. Lymphocyte migration in lymphocyte function–associated antigen (LFA)-1–deficient mice. J. Exp. Med. 189:1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo, C.G., T.T. Lu, and J.G. Cyster. 2003. Integrin dependence of lymphocyte entry into the splenic white pulp. J. Exp. Med. 197:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Andrian, U.H., and T.R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878. [DOI] [PubMed] [Google Scholar]
- 4.Butcher, E.C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209–253. [DOI] [PubMed] [Google Scholar]
- 5.Allende, M.L., J.L. Dreier, S. Mandala, and R.L. Proia. 2004. Expression of the sphingosine-1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279:15396–15401. [DOI] [PubMed] [Google Scholar]
- 6.Matloubian, M., C.G. Lo, G. Cinamon, M.J. Lesneski, Y. Xu, V. Brinkmann, M.L. Allende, R.L. Proia, and J.G. Cyster. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360. [DOI] [PubMed] [Google Scholar]
- 7.Pyne, S., and N.J. Pyne. 2000. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 349:385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel, S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4:397–407. [DOI] [PubMed] [Google Scholar]
- 9.Hla, T. 2003. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol. Res. 47:401–407. [DOI] [PubMed] [Google Scholar]
- 10.Chaffin, K.E., and R.M. Perlmutter. 1991. A pertussis toxin sensitive process controls thymocyte emigration. Eur. J. Immunol. 21:2565–2573. [DOI] [PubMed] [Google Scholar]
- 11.Chiba, K., Y. Yanagawa, Y. Masubuchi, H. Kataoka, T. Kawaguchi, M. Ohtsuki, and Y. Hoshino. 1998. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 160:5037–5044. [PubMed] [Google Scholar]
- 12.Rosen, H., G. Sanna, and C. Alfonso. 2003. Egress: a receptor-regulated step in lymphocyte trafficking. Immunol. Rev. 195:160–177. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann, V., J.G. Cyster, and T. Hla. 2004. FTY720: Sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 4:1019–1025. [DOI] [PubMed] [Google Scholar]
- 14.Mandala, S., R. Hajdu, J. Bergstrom, E. Quackenbush, J. Xie, J. Milligan, R. Thornton, G.J. Shei, D. Card, C. Keohane, et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296:346–349. [DOI] [PubMed] [Google Scholar]
- 15.Brinkmann, V., and K. Lynch. 2002. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr. Opin. Immunol. 14:569–575. [DOI] [PubMed] [Google Scholar]
- 16.Graler, M.H., and E.J. Goetzl. 2004. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J. 18:551–553. [DOI] [PubMed] [Google Scholar]
- 17.Honig, S.M., S. Fu, X. Mao, A. Yopp, M.D. Gunn, G.J. Randolph, and J.S. Bromberg. 2003. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J. Clin. Invest. 111:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinschewer, D.D., A.F. Ochsenbein, B. Odermatt, V. Brinkmann, H. Hengartner, and R.M. Zinkernagel. 2000. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J. Immunol. 164:5761–5770. [DOI] [PubMed] [Google Scholar]
- 19.Gretz, J.E., C.C. Norbury, A.O. Anderson, A.E. Proudfoot, and S. Shaw. 2000. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192:1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ui, M. 1988. The multiple biological activities of pertussis toxin. In Pathogenesis and Immunity in Pertussis. A.C. Wardlaw and R. Parton, editors. John Wiley & Sons, London. 121–146.
- 21.Ohl, L., G. Henning, S. Krautwald, M. Lipp, S. Hardtke, G. Bernhardt, O. Pabst, and R. Forster. 2003. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 197:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graeler, M., and E.J. Goetzl. 2002. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 16:1874–1878. [DOI] [PubMed] [Google Scholar]
- 23.Tsuzuki, Y., S. Miura, M. Suematsu, I. Kurose, T. Shigematsu, H. Kimura, H. Higuchi, H. Serizawa, H. Yagita, K. Okumura, and H. Ishil. 1996. alpha 4 integrin plays a critical role in early stages of T lymphocyte migration in Peyer's patches of rats. Int. Immunol. 8:287–295. [DOI] [PubMed] [Google Scholar]
- 24.Young, A.J. 1999. The physiology of lymphocyte migration through the single lymph node in vivo. Semin. Immunol. 11:73–83. [DOI] [PubMed] [Google Scholar]
- 25.Azzali, G. 2003. Structure, lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol. Rev. 195:178–189. [DOI] [PubMed] [Google Scholar]
- 26.Muller, W.A., and G.J. Randolph. 1999. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J. Leukoc. Biol. 66:698–704. [DOI] [PubMed] [Google Scholar]
- 27.Arnold, C.N., E.C. Butcher, and D.J. Campbell. 2004. Antigen-specific lymphocyte sequestration in lymphoid organs: lack of essential roles for alpha(L) and alpha(4) integrin-dependent adhesion or Galpha(i) protein-coupled receptor signaling. J. Immunol. 173:866–873. [DOI] [PubMed] [Google Scholar]
- 28.Allende, M.L., and R.L. Proia. 2002. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim. Biophys. Acta. 1582:222–227. [DOI] [PubMed] [Google Scholar]
- 29.Bowdler, A.J., editor. 1990. The Spleen. Structure, Function and Clinical Significance. Chapman and Hall Medical, London. 515 pp.
- 30.Cyster, J.G., and C.C. Goodnow. 1995. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J. Exp. Med. 182:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanna, M.G., J. Liao, E. Jo, C. Alfonso, M.Y. Ahn, M.S. Peterson, B. Webb, S. Lefebvre, J. Chun, N. Gray, and H. Rosen. 2004. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 279:13839–13848. [DOI] [PubMed] [Google Scholar]
- 32.Brelinska, R., C. Pilgrim, and I. Reisert. 1984. Pathways of lymphocyte migration within the periarterial lymphoid sheath of rat spleen. Cell Tissue Res. 236:661–667. [DOI] [PubMed] [Google Scholar]
- 33.Cinamon, G., M. Matloubian, M.J. Lesneski, Y. Xu, C. Low, T. Lu, R.L. Proia, and J.G. Cyster. 2004. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 5:713–720. [DOI] [PubMed] [Google Scholar]
- 34.Liu, C.H., S. Thangada, M.J. Lee, J.R. Van Brocklyn, S. Spiegel, and T. Hla. 1999. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 10:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohno, T., A. Wada, and Y. Igarashi. 2002. N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 16:983–992. [DOI] [PubMed] [Google Scholar]
- 36.Yatomi, Y., Y. Ozaki, T. Ohmori, and Y. Igarashi. 2001. Sphingosine 1-phosphate: synthesis and release. Prostaglandins Other Lipid Mediat. 64:107–122. [DOI] [PubMed] [Google Scholar]
- 37.Ford, W.L., and S.J. Simmonds. 1972. The tempo of lymphocyte recirculaiton from blood to lymph in the rat. Cell Tissue Kinet. 5:175–189. [DOI] [PubMed] [Google Scholar]
- 38.Smith, M.E., and W.L. Ford. 1983. The recirculating lymphocyte pool of the rat: a systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 49:83–94. [PMC free article] [PubMed] [Google Scholar]
- 39.Westermann, J., Z. Puskas, and R. Pabst. 1988. Blood transit and recirculation kinetics of lymphocyte subsets in normal rats. Scand. J. Immunol. 28:203–210. [DOI] [PubMed] [Google Scholar]
- 40.Graeler, M., G. Shankar, and E.J. Goetzl. 2002. Cutting edge: suppression of T cell chemotaxis by sphingosine 1-phosphate. J. Immunol. 169:4084–4087. [DOI] [PubMed] [Google Scholar]
- 41.Scharffetter-Kochanek, K., H. Lu, K. Norman, N. van Nood, F. Munoz, S. Grabbe, M. McArthur, I. Lorenzo, S. Kaplan, K. Ley, et al. 1998. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 188:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reif, K., E.H. Ekland, L. Ohl, H. Nakano, M. Lipp, R. Forster, and J.G. Cyster. 2002. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 416:94–99. [DOI] [PubMed] [Google Scholar]
- 43.Ngo, V.N., H.L. Tang, and J.G. Cyster. 1998. Epstein-Barr virus–induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J. Exp. Med. 188:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]