Abstract
Cow's milk allergy in children is often of short duration, which makes this disorder an interesting clinical model for studies of tolerance to dietary antigens. Here, we studied T cell responses in 21 initially allergic children who, after a milk-free period of >2 mo, had cow's milk reintroduced to their diet. Children who outgrew their allergy (tolerant children) had higher frequencies of circulating CD4+CD25+ T cells and decreased in vitro proliferative responses to bovine β-lactoglobulin in peripheral blood mononuclear cells (PBMCs) compared with children who maintained clinically active allergy. No significant difference in proliferative activity stimulated by the polyclonal mitogen phytohemagglutinin was observed between the two groups. Depletion of CD25+ cells from PBMCs of tolerant children led to a fivefold increase in in vitro proliferation against β-lactoglobulin. This suggests that tolerance is associated with the appearance of circulating CD4+CD25+ regulatory T (Treg) cells that are capable of suppressing the effector T cells generated 1 wk after reintroduction of cow's milk. The suppressive function of the CD4+CD25+ Treg cells was shown to be partly cell contact dependent. Collectively, our study provides human data to suggest that mucosal induction of tolerance against dietary antigens is associated with the development of CD4+CD25+ Treg cells.
Keywords: human, oral tolerance, regulatory T cells, food allergy, cytokines
Introduction
The usual immune response to harmless gut antigens is generation of local and systemic immunological tolerance known as “oral tolerance.” This mechanism may be explained by different T cell events such as anergy, clonal deletion, and induction of regulatory T (Treg) cells. For ethical reasons, the existence of oral tolerance in humans is mainly supported by circumstantial evidence. Thus, healthy individuals have hardly any hyperactivated T cells in their gut mucosa, very little mucosal IgG production, and only low levels of serum IgG antibodies to food antigens (1). Moreover, nasal antigen application or feeding experiments induced peripheral down-regulation of T cell responses in healthy individuals (2, 3).
Decreased robustness of mucosally induced tolerance in “westernized” populations is suggested by the recent striking rise of atopic diseases (4), and some 5–8% of children up to 3 yr of age now develop food allergy. This disorder is associated with serious health consequences and may unfortunately also be the starting point for an “allergic march,” i.e., the development of subsequent atopic diseases in the airways. Therefore, it is of great importance to understand the immunological mechanisms involved in break of oral tolerance against dietary antigens.
Indirect evidence exists to suggest that the development of food allergy may be controlled by CD4+CD25+ Treg cells. Patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, or X-linked autoimmunity-allergic dysregulation syndrome lack this T cell subset, and they often develop food allergic reactions and increased specific IgE antibody levels accompanied by severe eczema (5, 6). Further support for a role of CD4+CD25+ Treg cells in mediating oral tolerance has been obtained from feeding experiments in mice (7–11). Other T cell subsets possibly involved in oral tolerance, at least in animals, are Th3 cells which produce TGF-β, Tr1 cells which produce IL-10, and TGF-β and NK T cells (12–14). However, it is not firmly established whether all these subsets represent separate cell lineages or different maturational phenotypes.
A few years after the discovery of naturally occurring CD4+CD25+ Treg cells in mice, a similar T cell subset has been identified in human peripheral blood, thymus, LNs, and cord blood (15). In mice, the CD4+CD25+ Treg cells reportedly play a role in the control of immune responses to infectious agents, transplants, and cancer as well as in graft-versus-host reactions (16–21), whereas in humans CD4+CD25+ Treg cells have been shown to be involved in regulating cancer immunity (22–24). These human cells are typically anergic upon TCR stimulation (25), although recent studies in mice suggest that Treg cells can be expanded both in vitro and in vivo when interacting with the appropriate APC type (26). This might explain why a constant number of CD4+CD25+ Treg cells is maintained in the circulation.
Common phenotypic markers of CD4+CD25+ Treg cells are CD45RO, L-selectin (CD62L), CTLA-4 (CD152), CD25, and glucocorticoid-induced tumor necrosis factor receptor (27–30), although the same phenotypic profile can also be displayed by other T cell subsets. Therefore, only functional assays can firmly identify CD4+CD25+ Treg cells. Nevertheless, recent studies in mice and humans have demonstrated that a novel transcription-repressor protein, FOXP3, is exclusively expressed by naturally occurring and induced CD4+CD25+ Treg cells (31–38).
Current evidence suggests that human CD4+CD25+ Treg cells employ several mechanisms to suppress immune responses, for instance, via direct cell contact or indirectly by reducing the antigen-presenting capacity of APCs (39). In addition, at least in mice, suppressive cytokines appear to be involved in the effector function of Treg cells (40, 41). It remains an open question whether one or more of these down-regulatory properties may be acquired by any naive CD4+ T cell in a normal peripheral immunostimulatory process.
Cow's milk allergy in children is often a disease of relatively short duration, which makes it an interesting clinical model for the development of oral tolerance. Using depletion of CD25+ cells as a read-out test for the suppressive effect that the Treg subset exerts on effector T cells, we were able to show that children who had outgrown their allergy (i.e., are now tolerant against cow's milk allergens), developed a population of CD4+CD25+ T cells with regulatory function in their peripheral blood 1 wk after an in vivo milk challenge. This subset was numerically and functionally reduced in children who remained allergic to cow's milk. These results suggested mucosal induction of Treg cells in response to cow's milk proteins or, alternatively, that centrally generated Treg cells had become activated and expanded in children with outgrown food allergy.
Materials and Methods
Patient Characteristics and Selection Criteria
A total number of 21 children (median age 24 mo, range 6–56 mo) previously diagnosed as having cow's milk allergy were included consecutively in the study as they routinely visited the out-patient clinic just before starting a trial of reintroduction of cow's milk and cow's milk products into the diet after at least 2 mo (median 6 mo, range 2–11 mo) on a strictly cow's milk-free diet. All families were routinely informed by a nutrition physiologist and given an information brochure about how to achieve a diet free from cow's milk products.
The original allergy diagnosis had been made at a median age of 5 mo (range 2–18 mo) based on the following criteria: persistent gastroenterological symptoms without, or in combination with, extraintestinal symptoms (see next paragraph) reported by the parents, followed by total disappearance of these symptoms on cow's milk-free diet for a period of at least 1 mo, and then followed by reappearance of similar symptoms by at least two open milk challenges observed by the parents (see next paragraph). Children with a history of anaphylactic symptoms or evidence of cow's milk-specific IgE antibodies were excluded to make the test group as homogeneous as possible.
The patients had demonstrated one or more of the following symptoms (number of cases in parenthesis): bloody and usually loose stools (n = 4), diarrhea (n = 8), failure to thrive (n = 5), eczema (n = 2), vomiting or regurgitation (n = 5), excessive crying (n = 5), and anemia (n = 1). The symptoms had been observed by the parents from a median age of 3 mo (range 2 wk–12 mo). Where suspected, lactose intolerance was excluded by routine lactose challenge test (H2 breath test).
Cow's Milk Challenge Procedure
The reintroduction of cow's milk into the diet was done gradually, increasing the amount of milk or cow's milk-based formula to at least 100 ml/day during the first two days, continuing until the parents experienced unacceptable symptoms or until a routine evaluation was performed at the out-patient clinic after 1 wk. All these clinical evaluations were performed by the same pediatrician, and 8 children (median age 24 mo, range 16–45 mo) were deemed to display persistently active cow's milk allergy, whereas 13 children (median age 24 mo, range 9–49 mo) had outgrown their allergy since they were now found to be tolerant to the milk challenge. A blood sample was taken just before and 1 wk after the start of cow's milk reintroduction.
Ethics
The study was approved by the local Regional medical ethics committee (East Norway, Blindern, pb1139, Norway), and informed consent was obtained from the parents.
Cell Isolation
PBMCs were obtained from heparinized blood by Lymphoprep centrifugation (Nycomed Pharma). Cells were washed and finally diluted in RPMI-1640 medium supplemented with 10% heat-inactivated pooled human serum, 1% l-glutamine, and 1% gentamycin (GIBCO BRL).
Proliferation Assay
PBMCs (105) were cultured in 96-well round-bottom tissue culture plates (200 μl/well) and stimulated with phytohemagglutinin (PHA; 10 μl, 100 μg/ml) or β-lactoglobulin (β-LG; 10 μl, 20 mg/ml) from cow's milk (L0130; Sigma-Aldrich). Radioactive thymidine (3H-Tdr, 1 mCi/well, TRK 120; Amersham Biosciences) was added to the wells 6 d after the initiation of stimulation. The cells were harvested 12–16 h later. Incorporation of 3H-Tdr was determined in a β-counter.
Depletion of CD25+ Cells
An Ab-coated microbead isolation procedure was used for depletion of CD25+ cells, according to the manufacturer's instruction (Miltenyi Biotec). Briefly, 20 μl of CD25-specific beads were suspended together with 5 × 106–107 PBMCs and incubated for 15 min on ice, followed by two washes. LD columns were prepared as recommended by the manufacturer and used for magnetic depletion. The effluent PBMC samples contained <0.5% CD25+ cells as determined by flow cytometry (see below). Unrelated microbeads, i.e., specific for mouse CD5 (Miltenyi), were used to prepare control samples of PBMCs.
Antibodies
The following mouse mAbs with specificity for human molecules were used for immunostaining of PBMCs: FITC- and PE-conjugated anti-CD25 (IgG1, clone 2A3; Becton Dickinson); biotin-conjugated anti–CTLA-4 (IgG1, clone BNI3.1; Becton Dickinson); PerCp anti-CD3 (IgG1) and biotin-conjugated anti-CCR4 (IgG1, clone 1G1; Becton Dickinson); FITC- and APC-conjugated anti-CD4 (IgG2a, clone EDU-2; Diatec); FITC- and PE-conjugated anti-CD8 (IgG2a, clone UCH-T4; Diatec); PE-conjugated anti-CD45RO (IgG2a, clone UCHL-1; LeukoSite Inc.); anti-CCR9 (IgG2b, clone 3C3; LeukoSite); anti-α4β7 (IgG1, Act 1; courtesy of Dr. A.I. Lazarovits, University of Western Ontario, London, Ontario, Canada); and PE/FITC-conjugated streptavidin (Southern Biotechnology). Secondary reagents were goat anti–mouse IgG2b-PE (Southern Biotechnology) and goat anti–mouse IgG1-PE (Catalog Laboratories).
Flow Cytometric Analysis and Cell Sorting
Immunofluorescence staining was performed after washing the cells with PBS plus 0.5% (wt/vol) BSA (Sigma-Aldrich). Cells were incubated for 20 min on ice with a pretitrated optimal dilution of each mAb. Cells were washed again and then analyzed by flow cytometry (FACS Caliber; Becton Dickinson). Immunostained subsets of T cells were sorted with FACS® Vantage SE (Becton Dickinson).
Transwell Experiments
Transwell experiments were performed in 24-well plates with a 0.4-μm pore size (Costar Inc.). FACS®-sorted CD4+CD25− cells (4 × 105) were added to the lower chamber. FACS-sorted CD4+CD25+ cells (105) with a purity ≥99% were added either directly to the lower chamber (together with the CD4+CD25− subset) or placed in the upper transwell chamber. After 48 h of stimulation with anti-CD3/CD28 beads (106 beads/well; Dynalbiotech), the cells in the lower chambers were transferred to a 96-well plate and radioactive thymidine was added for 12–16 h. Incorporation of 3H-Tdr was determined in a β-counter.
Cytokine Analysis
PBMCs (5 × 105) were cultured in flat 24-well plates (1 ml/well) and stimulated with PHA (50 μg/ml) or β-LG (1 mg/ml) or remained unstimulated in medium alone for 96 h. The supernatants were collected and frozen at −70°C until analysis by the following assays.
TGF-β.
An ELISA was performed in round-bottom wells coated overnight with a pretitrated optimal concentration of mAb to human TGF-β in its inactive complex (anti–TGF-β1-LAP; 2 μg/ml, clone 27235.1; RD System Europe Ltd.). The supernatants and standard recombinant TGF-β (RD System Europe) were serially diluted in PBS-Tween and incubated overnight at 4°C. A biotinylated mouse mAb to human TGF-β1-LAP (125 ng/ml, clone 27240; RD System Europe) was added to the wells and incubated overnight at 4°C. The wells were than incubated with a Streptavidin-horseradish peroxidase conjugate (RD System Europe) for 20 min in room temperature. Finally, TMB (Microwell Peroxidase Substrate System; KLP) was added for 30 min at room temperature, and the reaction was then stopped with 2 M HCl. The incubations were performed in humid chambers, with intervening washes (3 × 200 μl PBS containing 0.05% Tween).
IL-10.
Human Th1/Th2 Cytokine Cytometric Bead Array kit was used (550749; Becton Dickinson). The assay was performed according to the manufacturer's recommendation.
Statistics.
Shapiro-Francia W test was used for normality distribution test. Unpaired and paired two-tailed Student's t tests were used for statistical analyses. (Statview software designed for Macintosh computers.)
Results
This study was designed to reveal possible immunoregulatory differences between PBMCs obtained from children showing outgrown compared with active cow's milk allergy. The former group had presumably been subjected to oral tolerance induction against milk allergens. All of the included children had been on a cow's milk-free diet for at least 2 mo before the experimental milk challenge was performed to distinguish clinically between the two groups. Various immunological variables were examined both before and after this in vivo exposure to cow's milk via the gut for up to 1 wk. To evaluate milk-induced immune stimulation and regulation, restimulation in vitro was performed with the major milk allergen bovine β-LG. In addition, we performed polyclonal stimulation of PBMCs with PHA to obtain a robust base-line response as a reference for functional Treg cell induction (see Discussion).
Peripheral blood from the two groups of children was analyzed with regard to the frequency of CD4+ T cells expressing CD25 and the modulation of this subset caused by the in vivo milk challenge. The percentage of CD4+ CD25+ T cells was fairly similar in the allergic and tolerant groups before this challenge. After the in vivo milk exposure, however, the tolerant group tended to show an increased frequency of circulating CD25+ cells (P = 0.07), whereas the number was significantly reduced (P < 0.01) in the allergic children (Fig. 1 A). Within this human T cell subset, the fraction with a high level of CD25 has been reported to be responsible for a down-regulatory function (39), but we did not detect differences in the immunostaining intensity between the two groups of children at either time points. Nevertheless, the CD4+CD25+ T cell subset profile was more distinct in the FACS® histogram 1 wk after the in vivo milk challenge for children with outgrown allergy than for those remaining allergic (Fig. 1 A). Also, comparison between the tolerant and allergic group with regard to the fraction of CD4+CD25+ T cells before and after the in vivo milk challenge revealed a significant difference (P < 0.03) in the modulation of this subset relative to the prechallenge levels; although only one out of seven of the allergic group showed an increased frequency of the CD25+ subset, this was the case for five out of ten of the tolerant group (Fig. 1 B). Notably, the percentage of CD3+CD4+ cells showed a similar range for both groups, both before (tolerant group: median 39.9%, range 28.7–56.3%; allergic group: median 43.9, range 36.5–57.2%) and after (tolerant group: median 42.5%, range 34.1–60.2%; allergic group: median 40.1%, range 24.9–56.3%) the challenge.
Figure 1.
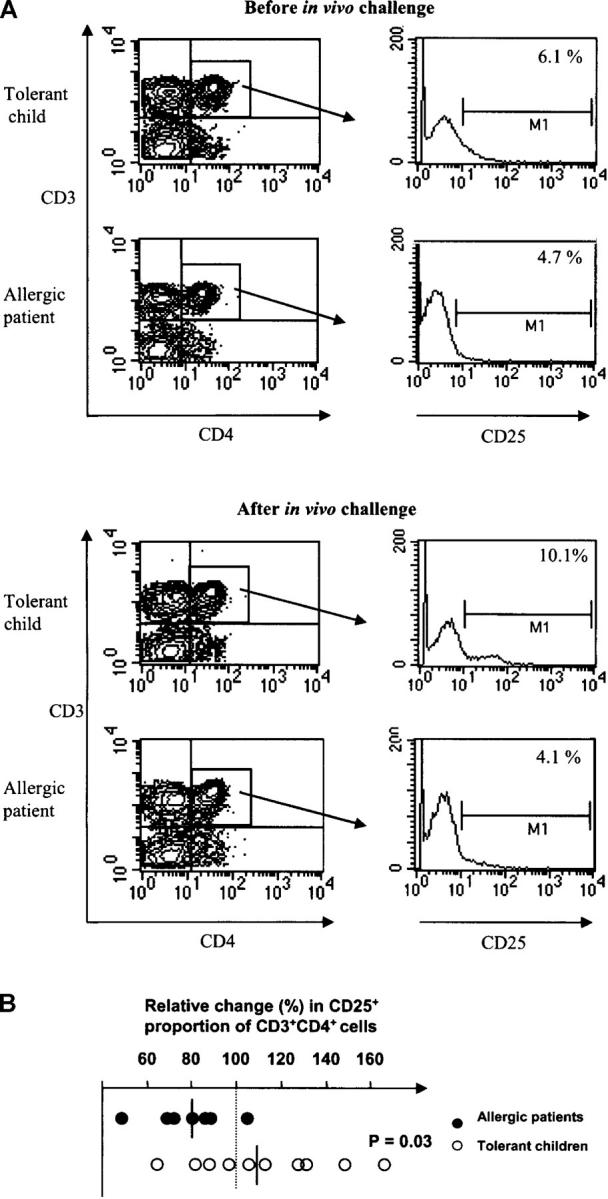
Frequency of circulating CD4+CD25+ T cells before and 1 wk after in vivo challenge with cow's milk. (A) PBMCs were purified from blood before and after the challenge, immunostained with mAbs, and then analyzed with FACS® Caliber. The M1 gates were set in accordance with isotype- and concentration-matched mAb controls. One representative experiment is shown for the tolerant group (n = 10) and one for the allergic group (n = 7). (B) Increase or decrease of circulating CD4+CD25+ T cells 1 wk after in vivo exposure to cow's milk normalized in relation to the prechallenge levels (set to 100%, dotted line). Student's t test was used for statistical analysis.
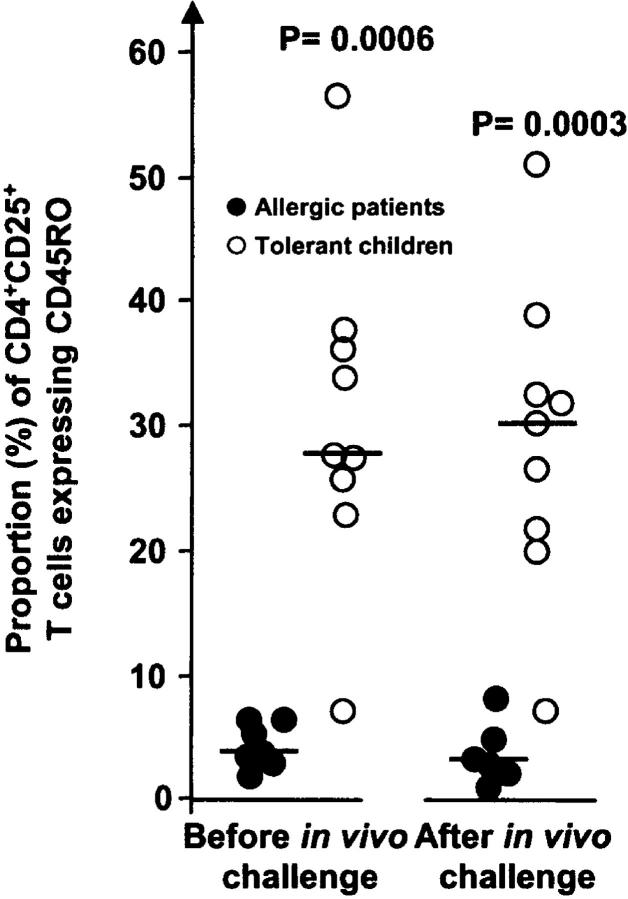
The increase of allergy in industrialized societies might reflect an immature immune system due to reduced microbial exposure (4, 42). Therefore, we investigated the maturation state of the CD4+CD25+ T cells by examining their CD45RO expression. Approximately 30% of the CD4+CD25+ T cells in the tolerant children were positive for this marker in contrast to only 5% in the allergic group; these values remained unchanged after the in vivo milk challenge (Fig. 2). PBMCs were also immunostained with mAbs to α4β7, CCR9, CCR4, CD8, surface CTLA-4, CD40L, and CD45RA, but we did neither reveal any differences between the two groups of children nor an effect of in vivo milk challenge on these markers (unpublished data).
Figure 2.
Expression of the maturation marker CD45RO (%) on circulating CD4+CD25+ T cells before and 1 wk after in vivo challenge with cow's milk. PBMCs were immunostained with mAbs, and CD3+CD4+CD25+ cells were gated to determine the frequency of CD45RO+ cells within this T cell subset. •, children with active allergy; ○, tolerant children (outgrown allergy). Student's t test was used for statistical analysis.
Interestingly, there was no significant difference between the two groups of children in T cell proliferation induced in vitro by β-LG when PBMCs collected before the in vivo milk challenge were tested (Fig. 3 A). By contrast, PBMCs obtained 1 wk after introduction of this challenge showed significantly more proliferative activity against β-LG for patients with active allergy compared with tolerant children (Fig. 3 A). Conversely, no difference in proliferation at either time points was revealed by in vitro stimulation of PBMCs with PHA (Fig. 3 B).
Figure 3.
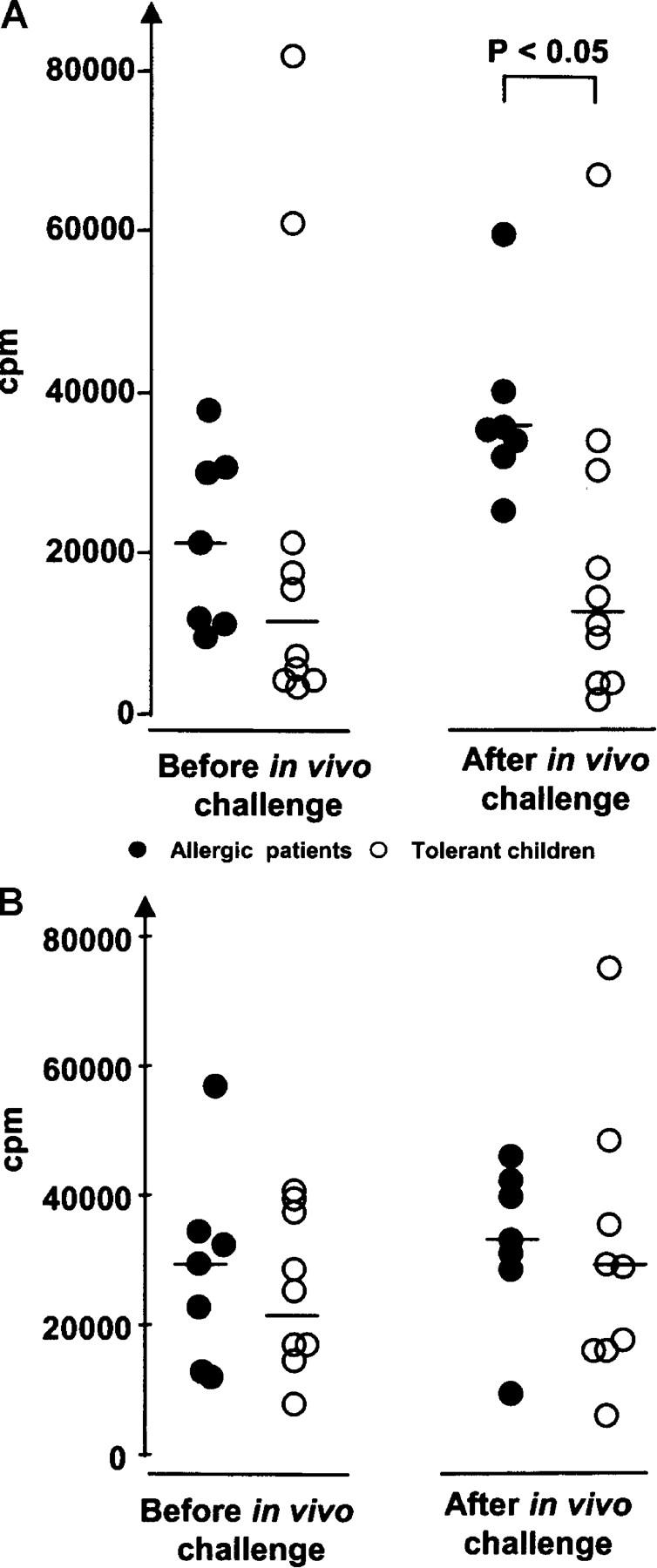
Proliferation of PBMCs. (A) PBMCs were purified from samples obtained before and 1 wk after in vivo challenge with cow's milk and then stimulated in vitro with bovine β-LG. Radioactive thymidine (3H-Tdr) was added 6 d after addition of β-LG to the PBMC cultures. The cells were harvested 12–16 h later. Incorporation of 3H-Tdr was determined in a β-counter. •, children with active allergy; ○, tolerant children (outgrown allergy). (B) Similar samples and procedure as in A, but stimulation was performed with PHA. Student's t test was used for statistical analysis.
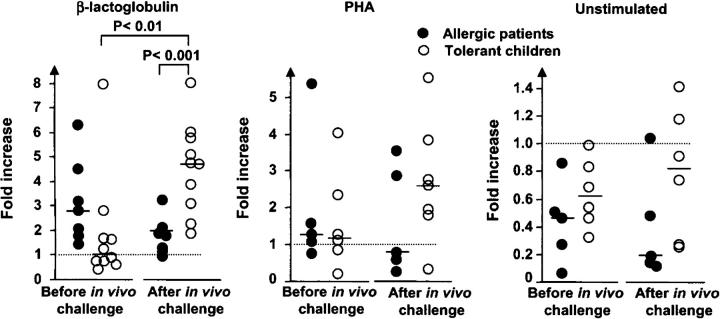
Although CD25 expression on CD4+ T cells is associated with a regulatory function, it is not an exclusive marker for Treg cells but can, instead, indicate a productive effector function. To investigate whether the CD4+CD25+ subset in our PBMC cultures possessed regulatory capacity, we removed CD25+ T cells from the samples and performed in vitro stimulation with either β-LG or the mitogen PHA (Fig. 4). Depleted PBMCs purified from blood obtained before in vivo milk challenge tended to show increased proliferation in the allergic group, perhaps reflecting a higher proliferative activity in these children (Fig. 4 A), but no statistically significant difference was obtained. 1 wk after the milk challenge, depleted PBMC cultures showed, on average, an almost fivefold increase in the proliferative activity against β-LG in the tolerant group compared with only twofold in the allergic group (P < 0.001), and there was moreover a significant increase (P < 0.01) compared with the proliferative level after depletion before the in vivo challenge. (Note that the actual proliferation of undepleted PBMCs stimulated with β-LG is shown in Fig. 3 A.) These results most likely reflected that, after a long period of milk-restricted diet, most β-LG specific memory T cells were probable residing in the gut, whereas few were circulating. However, after the oral milk challenge specific T cells would become activated and expanded in GALT and subsequently appear in the circulation before homing back to the gut. Also, PHA tended to elicit augmented proliferation after depletion of CD25+ cells from PBMCs collected from the tolerant children 1 wk after the challenge, whereas no such enhancement was seen in unstimulated cultures (Fig. 4).
Figure 4.
Fold increase or decrease in proliferation after depletion of CD25+ cells from PBMCs purified from blood before and 1 wk after in vivo challenge with cow's milk. Predepletion level (see Fig. 3 A) is set to 1 (dotted line). The depleted and nondepleted PBMC samples were tested for proliferative activity, unstimulated and after in vitro stimulation with bovine β-LG or the mitogen PHA. Radioactive thymidine (3H-Tdr) was added 6 d after addition of antigens to the cell cultures. The cells were harvested 12–16 h later. Incorporation of 3H-Tdr was determined in a β-counter. Student's t test was used for statistical analysis. For comparison with absolute proliferative activity (cpm values) of in vitro–stimulated PBMCs collected from allergic and tolerant children before and after in vivo challenge, see Fig 3 A.
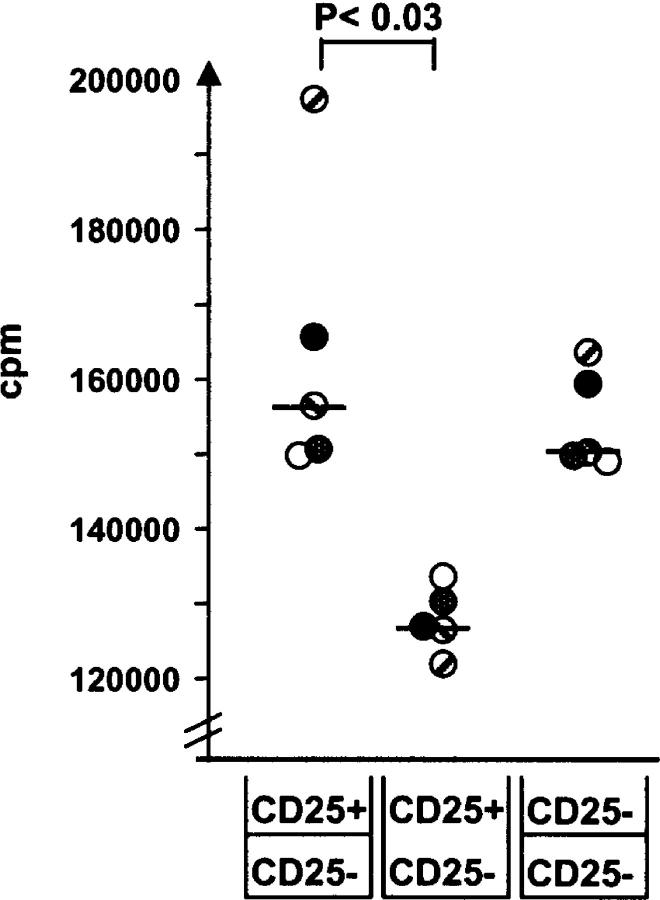
To examine whether the suppressive effect of CD25+ cells was mediated via a cell contact-dependent mechanism or by soluble factors, we examined FACS®-sorted T cell subsets in a transwell system. Due to severe ethical restraints on blood sampling in children under 2 yr of age, we were unable to purify autologous APCs for antigen-specific stimulation in these experiments. Instead, we employed anti-CD3/CD28 activation of the purified T cell subsets. When CD25+ and CD25− T cells isolated from PBMCs collected 1 wk after in vivo milk challenge were cocultured, a significantly reduced proliferative activity (P < 0.03) was observed compared with the result obtained when the two subsets were separated by the transwell membrane (Fig. 5). This suggested that at least part of the suppression was mediated via cell contact. When CD4+CD25− T cells were cultured in both the upper and the lower chamber, the proliferation was similar to that seen when the CD25− subset was cultured in the lower chamber and the CD25+ subset in the upper chamber (Fig. 5).
Figure 5.
Immunosuppression exerted by CD4+CD25+ T cells in peripheral blood from children tolerant against cow's milk is partly cell contact dependent. CD4+CD25+ T cells and CD4+CD25− T cells were FACS® sorted and cultured in transwell plates. In some wells, the CD4+CD25+ T cells were cultured in the upper chamber and the CD4+CD25− T cells in the lower chamber, whereas in other plates both subsets were cultured in the lower chambers. Additional controls consisted of CD4+CD25− T cells cultured both in the upper and the lower wells as indicated at the bottom of the figure. Stimulation was performed with anti-CD3/CD28 beads for 48 h. The proliferative activity of CD4+CD25− was tested by addition of radioactive thymidine (3H-Tdr), and the cells were harvested 12 h later. Incorporation of 3H-Tdr was determined in a β-counter. Note that individual subjects are indicated by separate symbols, and that the y axis is broken. Student's t test was used for statistical analysis.
In addition to the cell contact-mediated effect of Treg cells, we examined production of the immunosuppressive cytokines TGF-β (Fig. 6 A) and IL-10 (Fig. 6 B) in β-LG– or PHA-stimulated PBMC cultures. We could not detect any striking differences in cytokine concentrations for samples obtained either before or after the in vivo milk challenge. However, it was notable that PBMCs from the tolerant group tended to show increased TGF-β production after β-LG stimulation when we compared the levels after and before the in vivo milk challenge (P = 0.27), and a similarly increased trend associated with tolerance was observed for IL-10 before the challenge when the two groups were compared (P = 0.14). Interestingly, the TGF-β response was significantly decreased after challenge in the allergic group (P < 0.03), although it tended to be higher than in the tolerant group before challenge (P = 0.08). Such trends for cytokine changes were not apparent when PHA was used to stimulate PBMCs (Fig. 6).
Figure 6.
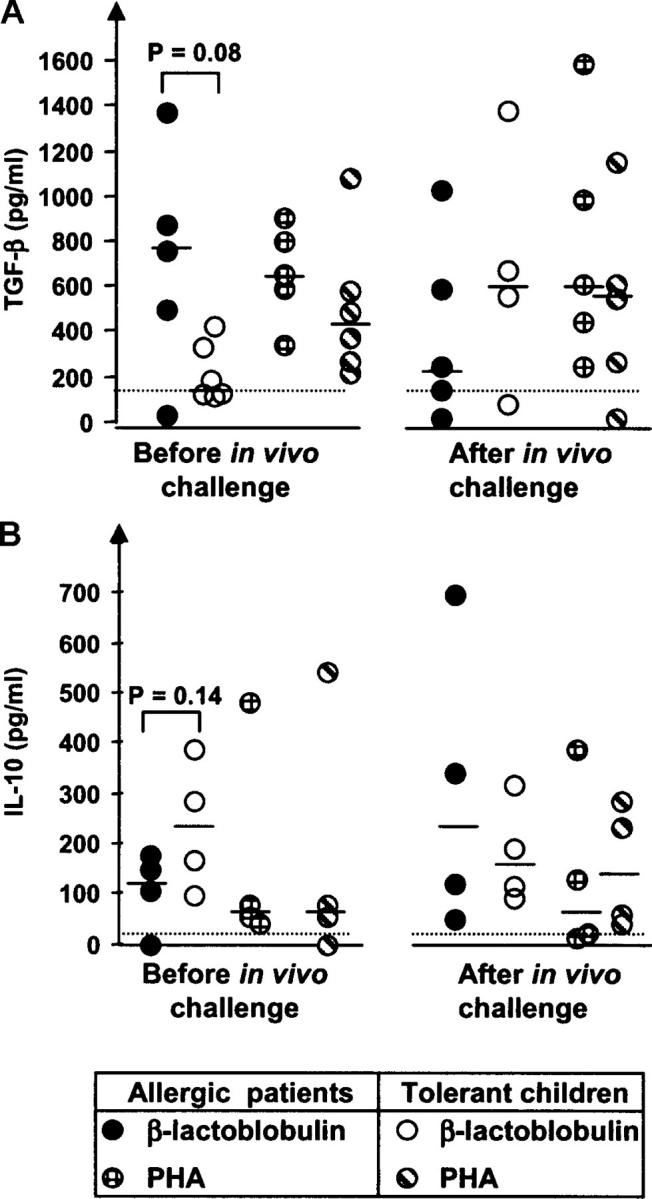
Generation of the immunosuppressive cytokines TGF-β1-LAP and IL-10 by PBMCs purified from blood of allergic or tolerant children before and 1 wk after in vivo challenge with cow's milk. (A) Production of TGF-β1-LAP determined in supernatants from PBMCs stimulated for 96 h with β-LG (1 mg/ml) or PHA (50 μg/ml) by subtracting the TGF-β1-LAP concentration in unstimulated wells. (B) Production of IL-10 under the same culture conditions as in A. Student's t test was used for statistical analysis.
Discussion
This study showed that an in vivo milk challenge induced Treg cells preferentially in previously cow's milk–allergic children who had become tolerant, that is, those who clinically had outgrown their allergy. It is intriguing how the intestinal immune system can discern between pathogens and innocuous antigens such as dietary proteins and respond by eliciting productive immunity or oral tolerance, respectively. The understanding of the latter phenomenon has been hampered by intricate mechanistic complexity involving T cell anergy, clonal deletion, skewing of the cytokine profile (immune deviation), and the action of different Treg cells. We established here for the first time that human CD4+CD25+ Treg cells induced via the gut are involved in the control of food allergy, and that this T cell subset after activation exerts its suppressive function at least partially in a cell contact-dependent manner.
Our study compared phenotypic and functional characteristics of PBMCs from children with clinically active or outgrown cow's milk allergy collected before and 1wk after an in vivo challenge with cow's milk. PBMCs from the tolerant group showed reduced proliferation induced in vitro by the major milk allergen β-LG. Furthermore, the same children had a CD25+ T cell subset within their PBMCs that was able to suppress such proliferation. In this group, 30% of CD4+CD25+ T cells expressed the maturation marker CD45RO both before and after challenge with cow's milk, whereas the corresponding figure was only some 5% in the group with active allergy. All children included in our study had negative skin prick test against cow's milk proteins and no elevated specific IgE antibody levels in serum. This suggested that the observed symptoms were most probably produced via cell-mediated immunity. Approximately 50% of children suffering from food allergy appear by standard diagnostic tests to have a non-IgE–mediated allergic reaction (43, 44), presumably delayed-type hypersensitivity, although the pathological process in the gut may be indistinguishable from an atopic disorder (45). We selected our patients to be homogeneously nonatopic, but it would certainly be of interest in the future to also study children with elevated IgE antibody levels against food allergens in a similar experimental set-up.
Such studies in young children (with repeated sampling) have profound ethical restrictions. Therefore, we had to design our in vitro experiments on the basis of the limited blood volumes available. We also had to take into account that with the large age range of the patients (6–56 mo), they had a highly variable immunological history in terms of exposure to antigens from vaccines and the environment. This related not only to their systemic but also to their mucosal immune system. Importantly, we investigated tolerance induction via the gut, and recent animal experiments have demonstrated that the systemic and local immunization routes provide different results when it comes to dampening of mucosal allergy (46). In view of this complex scenario, we decided that PHA stimulation, rather than an arbitrarily selected T cell–dependent antigen, would best ensure a consistent base-line response as a reference for the mucosal induction of Treg cells by cow's milk proteins.
The in vitro proliferative activity of PBMCs against β-LG was presumably a T cell recall response. Notably, however, there was no significant difference when we compared PBMCs collected from the two groups of children before the in vivo cow's milk challenge with regard to proliferation elicited by β-LG, frequency of CD4+CD25+ T cells, and a suppressive effect of CD25+ cells revealed by their depletion. This result probably reflected that the patients with active allergy at this time point did not have a substantially larger number of circulating memory T cells specific for β-LG than tolerant children. The avoidance of cow's milk proteins for at least 2 mo before the challenge might have reduced this number, and specific T cells activated in GALT and mesenteric LNs would presumably start homing back to the intestinal lamina propria during milk challenge. Thus, there was a reduced relative frequency of circulating CD4+CD25+ T cells in most allergic children after the challenge (Fig. 1 B).
It is of note that the β-LG preparations contained LPS comparable to an endotoxin activity of 5000 EU/ml at 5 mg/ml (Limulus Amebocyte Lysate test); but the polyclonal immunostimulation exerted by this contamination did not mask the antigen-specific T cell response elicited by β-LG in vitro (unpublished data). Interestingly, the proliferative response of PBMCs against β-LG was significantly decreased in milk-tolerant compared with allergic children after the in vivo milk challenge. Our findings therefore suggested that in the tolerant children this challenge had resulted in GALT activation and expansion not only of specific effector T cells but also of CD4+CD25+ Treg cells, which suppressed the in vitro proliferative response elicited by β-LG. The observed relative increase of this circulating subset in half of the tolerant children 1 wk after challenge (Fig. 1 B) was probably an underestimate due to homing of allergen-activated T cells to the milk protein–exposed gut lamina propria as alluded to above.
Although it was originally believed that CD4+CD25+ Treg cells are anergic, at least two independent reports recently showed that mouse CD4+CD25+ T cells initially multiply in vivo after appropriate antigen stimulation (26, 47). We cannot exclude that the increased frequency of circulating CD4+CD25+ T cells after challenge in tolerant children partly reflected expansion of ordinary memory–effector T cells, but the striking increase (fivefold) of proliferative activity against β-LG exhibited by PBMCs from these children after depletion of CD25+ cells strongly suggested that their CD4+CD25+ subset contained a substantial fraction of Treg cells. This homeostatic mechanism most likely explained that they had outgrown their cow's milk allergy. For ethical reasons we could not obtain blood at a later time point after the milk challenge, so we have no information with regard to the persistence of the elevated level of CD4+CD25+ T cells in the circulation of the tolerant children.
Expression of integrin α4β7 and CCR9 on T cells determines their homing into the small intestinal lamina propria (48). We analyzed the frequency of T cells with these markers, but there were no apparent differences between the two groups of children. It has been reported that the α4β7 subset is increased in peripheral blood from children with cow's milk allergy, but that finding was obtained with T cells kept in culture for 1 wk (49). Also, it might be unrealistic to expect that we should be able to identify a phenotypic change induced by milk proteins in the total circulating pool of T cells with gut-homing properties.
Most functional studies of Treg cells have been performed in animal models for autoimmunity, inflammatory bowel disease, and transplantation. The first human studies of CD4+CD25+ Treg cells were in fact published quite recently (39, 50–52). Evidence suggests that Treg cells may be antigen nonspecific, at least in the effector phase, and bystander suppression is a well-known phenomenon in oral tolerance (53, 54). A nonspecific effect of Treg cells could explain why we obtained a moderate enhancement of proliferation against PHA in PBMCs from tolerant children after depletion of CD25+ cells (Fig. 4). Altogether, our results implied that mucosally induced Treg cells responding to milk antigens were involved in the observed tolerance induction or, alternatively, that centrally generated Treg cells had become activated and expanded in the children with outgrown cow's milk allergy.
Expression of CTLA-4 has been associated with human CD4+CD25+ Treg cells (55). This molecule is constitutively expressed in the cytoplasm (27, 39), but once the cell is activated via the TcR complex it appears on the surface. However, the mechanistic role of surface CTLA-4 in cell contact–dependent suppression in humans is debated. Several studies show that blocking CTLA-4 with antibodies does not abolish the suppressive activity of Treg cells (39, 51, 52). We did not find any difference in the expression level of this surface marker, either between the two groups of children or the two time points for sampling of PBMCs. Nevertheless, transwell experiments demonstrated that CD4+CD25+ Treg cells from milk-tolerant children exert their antiproliferative effect via a contact-dependent mechanism. Regrettably, we had no possibility to study the involvement of other inhibitory molecules such as PD-1 (39, 56).
The role in oral tolerance of the immunosuppressive cytokine TGF-β derived from so-called regulatory Th3 cells induced in experimental animals is extensively documented (57). The reduced production of TGF-β by β-LG–stimulated PBMCs after the in vivo milk challenge in the allergic group (Fig. 6 A) is in line with a recent study by Perez-Machado et al. (58) which shows that T cells obtained from duodenal mucosa of food-allergic children had a decreased potential for TGF-β production compared with healthy controls. Conversely, we found that PBMCs stimulated by β-LG 1 wk after the in vivo challenge tended to show increased production of TGF-β in children with outgrown allergy, which could have contributed to the observed suppressive effect on proliferation. The major source for IL-10 production in β-LG–stimulated PBMC cultures in our experiments remains unknown, and the results obtained were inconclusive.
It has been shown in mouse models that the thymic epithelium is important in the generation of naturally occurring CD4+CD25+ Treg cells involved in the maintenance of tolerance (59). Because our study strongly suggests that CD4+CD25+ Treg cells reactive to dietary antigens can be induced via the gut, it will be of considerable interest to investigate whether exosomes released from the intestinal epithelium play a role in the development of such cells. We have previously obtained results indicative of a tolerance-inductive function of epithelial exosomes produced in the gut of experimental animals and therefore called these structures “tolerosomes” (60).
In conclusion, the observations presented here provide new insight into the possible relationship between mucosal induction of CD4+CD25+ Treg cells and protection against food allergy in humans. These results may aid the development of better diagnostic tools for such disorders and perhaps contribute to promote a future goal of generating Treg cells in vitro for immunotherapy of allergic diseases.
Acknowledgments
We thank Helena Kahu for her outstanding technical assistance and for creating a stimulating working atmosphere. Drs. P. Ponath (LeukoSite, Inc., Cambridge, MA) and A.I. Lazarovits are acknowledged for providing anti-CCR9 and anti-α4β7 mAb, respectively.
This work was supported by grants from the Norwegian Foundation for Health and Rehabilitation, the Norwegian Asthma and Allergy Association, the University of Oslo, the Research Council of Norway, and the Norwegian Cancer Society.
Abbreviations used in this paper: β-lactoglobulin; β-LG; PHA, phytohemagglutinin; Treg cell, regulatory T cell.
References
- 1.Brandtzaeg, P.E. 2002. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann. NY Acad. Sci. 964:13–45. [DOI] [PubMed] [Google Scholar]
- 2.Waldo, F.B., A.W. van den Wall Bake, J. Mestecky, and S. Husby. 1994. Suppression of the immune response by nasal immunization. Clin. Immunol. Immunopathol. 72:30–34. [DOI] [PubMed] [Google Scholar]
- 3.Husby, S., J. Mestecky, Z. Moldoveanu, S. Holland, and C.O. Elson. 1994. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J. Immunol. 152:4663–4670. [PubMed] [Google Scholar]
- 4.Yazdanbakhsh, M., P.G. Kremsner, and R. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science. 296:490–494. [DOI] [PubMed] [Google Scholar]
- 5.Wildin, R.S., S. Smyk-Pearson, and A.H. Filipovich. 2002. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 39:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatila, T.A., F. Blaeser, N. Ho, H.M. Lederman, C. Voulgaropoulos, C. Helms, and A.M. Bowcock. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorstenson, K.M., and A. Khoruts. 2001. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188–195. [DOI] [PubMed] [Google Scholar]
- 8.Zhang, X., L. Izikson, L. Liu, and H.L. Weiner. 2001. Activation of CD25+CD4+ regulatory T cells by oral antigen administration. J. Immunol. 167:4245–4253. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson, M.R., H. Kahu, L.A. Hanson, E. Telemo, and U.I. Dahlgren. 2000. Tolerance and bystander suppression, with involvement of CD25-positive cells, is induced in rats receiving serum from ovalbumin-fed donors. Immunology. 100:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson, M.R., H. Kahu, L.A. Hanson, E. Telemo, and U.I. Dahlgren. 2002. An established immune response against ovalbumin is suppressed by a transferable serum factor produced after ovalbumin feeding: a role of CD25+ regulatory cells. Scand. J. Immunol. 55:470–477. [DOI] [PubMed] [Google Scholar]
- 11.Hauet-Broere, F., W.W. Unger, J. Garssen, M.A. Hoijer, G. Kraal, and J.N. Samsom. 2003. Functional CD25− and CD25+ mucosal regulatory T cells are induced in gut-draining lymphoid tissue within 48 h after oral antigen application. Eur. J. Immunol. 33:2801–2810. [DOI] [PubMed] [Google Scholar]
- 12.Weiner, H.L. 2001. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 182:207–214. [DOI] [PubMed] [Google Scholar]
- 13.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 14.Trop, S., D. Samsonov, I. Gotsman, R. Alper, J. Diment, and Y. Ilan. 1999. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 29:746–755. [DOI] [PubMed] [Google Scholar]
- 15.Baecher-Allan, C., V. Viglietta, and D.A. Hafler. 2004. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 16:89–97. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 18.van Maurik, A., M. Herber, K.J. Wood, and N.D. Jones. 2002. Cutting edge: CD4+CD25+ alloantigen-specific immunoregulatory cells that can prevent CD8+ T cell-mediated graft rejection: implications for anti-CD154 immunotherapy. J. Immunol. 169:5401–5404. [DOI] [PubMed] [Google Scholar]
- 19.Edinger, M., P. Hoffmann, J. Ermann, K. Drago, C.G. Fathman, S. Strober, and R.S. Negrin. 2003. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 9:1144–1150. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, P., J. Ermann, M. Edinger, C.G. Fathman, and S. Strober. 2002. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishikawa, H., T. Kato, K. Tanida, A. Hiasa, I. Tawara, H. Ikeda, Y. Ikarashi, H. Wakasugi, M. Kronenberg, T. Nakayama, et al. 2003. CD4+CD25+ T cells responding to serologically defined autoantigens suppress antitumor immune responses. Proc. Natl. Acad. Sci. USA. 100:10902–10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azuma, T., T. Takahashi, A. Kunisato, T. Kitamura, and H. Hirai. 2003. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 63:4516–4520. [PubMed] [Google Scholar]
- 23.Sasada, T., M. Kimura, Y. Yoshida, M. Kanai, and A. Takabayashi. 2003. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 98:1089–1099. [DOI] [PubMed] [Google Scholar]
- 24.Ichihara, F., K. Kono, A. Takahashi, H. Kawaida, H. Sugai, and H. Fujii. 2003. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. 9:4404–4408. [PubMed] [Google Scholar]
- 25.Taams, L.S., J. Smith, M.H. Rustin, M. Salmon, L.W. Poulter, and A.N. Akbar. 2001. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122–1131. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki, S., T. Iyoda, K. Tarbell, K. Olson, K. Velinzon, K. Inaba, and R.M. Steinman. 2003. Direct expansion of functional CD25+CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142. [DOI] [PubMed] [Google Scholar]
- 29.McHugh, R.S., M.J. Whitters, C.A. Piccirillo, D.A. Young, E.M. Shevach, M. Collins, and M.C. Byrne. 2002. CD4+ CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323. [DOI] [PubMed] [Google Scholar]
- 30.Wing, K., A. Ekmark, H. Karlsson, A. Rudin, and E. Suri-Payer. 2002. Characterization of human CD25+CD4+ T cells in thymus, cord and adult blood. Immunology. 106:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 33.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 34.Feunou, P., L. Poulin, C. Habran, A. Le Moine, M. Goldman, and M.Y. Braun. 2003. CD4+CD25+ and CD4+ CD25− T cells act respectively as inducer and effector T suppressor cells in superantigen-induced tolerance. J. Immunol. 171:3475–3484. [DOI] [PubMed] [Google Scholar]
- 35.Ramsdell, F. 2003. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 19:165–168. [DOI] [PubMed] [Google Scholar]
- 36.Denning, T.L., H. Qi, R. Konig, K.G. Scott, M. Naganuma, and P.B. Ernst. 2003. CD4+ Th cells resembling regulatory T cells that inhibit chronic colitis differentiate in the absence of interactions between CD4 and class II MHC. J. Immunol. 171:2279–2286. [DOI] [PubMed] [Google Scholar]
- 37.Cosmi, L., F. Liotta, E. Lazzeri, M. Francalanci, R. Angeli, B. Mazzinghi, V. Santarlasci, R. Manetti, V. Vanini, P. Romagnani, et al. 2003. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 102:4107–4114. [DOI] [PubMed] [Google Scholar]
- 38.Walker, M.R., D.J. Kasprowicz, V.H. Gersuk, A. Benard, M. Van Landeghen, J.H. Buckner, and S.F. Ziegler. 2003. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J. Clin. Invest. 112:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 40.Fowler, S., and F. Powrie. 1999. Control of immune pathology by IL-10-secreting regulatory T cells. Springer Semin. Immunopathol. 21:287–294. [DOI] [PubMed] [Google Scholar]
- 41.Powrie, F., J. Carlino, M.W. Leach, S. Mauze, and R.L. Coffman. 1996. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wills-Karp, M., J. Santeliz, and C.L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69–75. [DOI] [PubMed] [Google Scholar]
- 43.Johansson, S.G., J.O. Hourihane, J. Bousquet, C. Bruijnzeel-Koomen, S. Dreborg, T. Haahtela, M.L. Kowalski, N. Mygind, J. Ring, P. van Cauwenberge, et al. 2001. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 56:813–824. [DOI] [PubMed] [Google Scholar]
- 44.Majamaa, H., P. Moisio, K. Holm, H. Kautiainen, and K. Turjanmaa. 1999. Cow's milk allergy: diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy. 54:346–351. [DOI] [PubMed] [Google Scholar]
- 45.Bischoff, S.C., J.H. Mayer, and M.P. Manns. 2000. Allergy and the gut. Int. Arch. Allergy Immunol. 121:270–283. [DOI] [PubMed] [Google Scholar]
- 46.Takabayashi, K., L. Libet, D. Chisholm, J. Zubeldia, and A.A. Horner. 2003. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in Th2-sensitized mice. J. Immunol. 170:3898–3905. [DOI] [PubMed] [Google Scholar]
- 47.Walker, L.S., A. Chodos, M. Eggena, H. Dooms, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunkel, E.J., D.J. Campbell, and E.C. Butcher. 2003. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 10:313–323. [DOI] [PubMed] [Google Scholar]
- 49.Eigenmann, P.A., L. Tropia, and C. Hauser. 1999. The mucosal adhesion receptor alpha4beta7 integrin is selectively increased in lymphocytes stimulated with beta-lactoglobulin in children allergic to cow's milk. J. Allergy Clin. Immunol. 103:931–936. [DOI] [PubMed] [Google Scholar]
- 50.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annacker, O., and F. Powrie. 2002. Homeostasis of intestinal immune regulation. Microbes Infect. 4:567–574. [DOI] [PubMed] [Google Scholar]
- 54.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. [DOI] [PubMed] [Google Scholar]
- 55.Ng, W.F., P.J. Duggan, F. Ponchel, G. Matarese, G. Lombardi, A.D. Edwards, J.D. Isaacs, and R.I. Lechler. 2001. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 98:2736–2744. [DOI] [PubMed] [Google Scholar]
- 56.Blazar, B.R., B.M. Carreno, A. Panoskaltsis-Mortari, L. Carter, Y. Iwai, H. Yagita, H. Nishimura, and P.A. Taylor. 2003. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 171:1272–1277. [DOI] [PubMed] [Google Scholar]
- 57.Mowat, A.M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Machado, M.A., P. Ashwood, M.A. Thomson, F. Latcham, R. Sim, J.A. Walker-Smith, and S.H. Murch. 2003. Reduced transforming growth factor-β1-producing T cells in the duodenal mucosa of children with food allergy. Eur. J. Immunol. 33:2307–2315. [DOI] [PubMed] [Google Scholar]
- 59.Bensinger, S.J., A. Bandeira, M.S. Jordan, A.J. Caton, and T.M. Laufer. 2001. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 194:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karlsson, M., S. Lundin, U. Dahlgren, H. Kahu, I. Pettersson, and E. Telemo. 2001. “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 31:2892–2900. [DOI] [PubMed] [Google Scholar]