Abstract
Viral infection and stimulation with lipopolysaccharide (LPS) or double stranded RNA (dsRNA) induce phosphorylation of interferon (IFN) regulatory factor (IRF)-3 and its translocation to the nucleus, thereby leading to the IFN-β gene induction. Recently, two IκB kinase (IKK)–related kinases, inducible IκB kinase (IKK-i) and TANK-binding kinase 1 (TBK1), were suggested to act as IRF-3 kinases and be involved in IFN-β production in Toll-like receptor (TLR) signaling and viral infection. In this work, we investigated the physiological roles of these kinases by gene targeting. TBK1-deficient embryonic fibroblasts (EFs) showed dramatic decrease in induction of IFN-β and IFN-inducible genes in response to LPS or dsRNA as well as after viral infection. However, dsRNA-induced expression of these genes was residually detected in TBK1-deficient cells and intact in IKK-i–deficient cells, but completely abolished in IKK-i/TBK1 doubly deficient cells. IRF-3 activation, in response not only to dsRNA but also to viral infection, was impaired in TBK1-deficient cells. Together, these results demonstrate that TBK1 as well as, albeit to a lesser extent, IKK-i play a crucial role in the induction of IFN-β and IFN-inducible genes in both TLR-stimulated and virus-infected EFs.
Keywords: Toll-like receptor, interferon regulatory factor 3, NF-κB, embryonic fibroblasts, IFN-β
Introduction
Toll-like receptors (TLRs) are essential for the recognition of invading pathogens and serve as an important link between innate and adaptive immunity (1, 2). TLRs can discriminate various microbial components, such as triacylated lipopeptides (recognized by TLR1/TLR2 heterodimer), diacylated lipopeptides (TLR2/TLR6 heterodimer), LPS (recognized by TLR4), double stranded RNA (dsRNA; recognized by TLR3), flagellin from bacterial flagella (TLR5), single stranded RNA (recognized by TLR7/8), and bacterial DNA containing the unmethylated CpG motif (TLR9; references 3–12). Recently, it has been also reported that TLR11 potentially functions to prevent infection of uropathogenic bacteria in mice (13). Intracellular signaling pathways of TLRs are now being studied extensively (1, 2). The cytoplasmic region of TLRs contains a Toll/IL-1 receptor (TIR) domain that is common in both TLR and IL-1 receptor families. The TIR domain triggers TLR signaling by recruiting a cytoplasmic molecule, MyD88, through the homophilic interaction of the TIR domains (1, 2). MyD88 also has the death domain that can associate with other death domain–containing proteins such as IL-1 receptor associated kinases 1 and 4 (14). This pathway leads to NF-κB activation through TNF receptor–associated factor (TRAF) 6 and is essential for production of proinflammatory cytokines in response to almost all TLR ligands (15–18).
TLR3 and TLR4 signaling also induce the IFN-β gene, which is distinctly regulated from the proinflammatory cytokine genes. Molecular mechanisms for type I IFN (IFN-α/β) induction have been well studied in viral infection (19–21). Induction of IFN-β is primarily regulated at transcriptional levels. Previous papers have shown that the IFN-β gene expression is regulated by several transcription factors, such as NF-κB, ATF-2/c-Jun, IRF-3, and IRF-7. Gene disruption studies have demonstrated the critical role of IRF-3 and IRF-7 in the transcription of type I IFN genes (22, 23). During viral infection, IRF-3 is mainly responsible for initial activation of the IFN-β gene (24), whereas IRF-7 expression depends on IFN-β, thereby resulting in robust induction of type I IFNs in an autocrine positive feedback manner (21, 25). IRF-3 is located in the cytoplasm in uninfected cells. After viral infection, IRF-3 is phosphorylated at multiple serine/threonine residues located in the COOH-terminal portion. Phosphorylated IRF-3 forms a homodimer that translocates into the nucleus and activates promoters containing the IRF-3 binding site, termed IFN-stimulated response element (ISRE)/IRF-binding element.
IRF-3 activation is also critical for TLR3- and TLR4-induced up-regulation of the IFN-β gene. These events are independent of MyD88, but dependent on another TIR domain–containing adaptor, TIR domain-containing adaptor inducing IFN-β (TRIF)/TIR-containing adaptor molecule 1 (26–30). The TRIF-related adaptor molecule is involved in TLR4- but not TLR3-mediated IFN-β expression (31, 32). Although the molecular mechanism to induce IRF-3 activation is poorly understood, recent papers suggested that IRF-3 is phosphorylated by two IκB kinase (IKK)-related kinases, inducible IKK (IKK-i) and TANK-binding kinase 1 (TBK1; references 33, 34). IKK-i, also called IKK-ɛ, was originally identified as an LPS-inducible kinase (35, 36). TBK1 is also known as NF-κB activating kinase or TRAF2 associated kinase (37–39). These two kinases share homology with IKK-α and IKK-β. Both kinases have been reported to interact with TRAF2 and the TRAF-binding protein TANK/I-TRAF, and function upstream of IKK-α and IKK-β (37, 40). Deficiency of TBK1 in mice resulted in the embryonic lethality caused by massive liver degeneration, and TBK1-deficient (TBK1−/−) embryonic fibroblasts (EFs) showed reduced expression of certain genes regulated by NF-κB (39). Thus, these two IKK-related kinases have been implicated in NF-κB activation. Recently, two groups have shown that IKK-i and TBK1 phosphorylate and activate IRF-3 and IRF-7, leading to the transcriptional activation of genes for IFN-β, RANTES, and ISG54 in viral infection as well as in a TRIF-dependent signaling pathway (32, 33). These in vitro studies suggest that IKK-i and TBK1 have similar functions; however, it remains unknown how the two kinases act under physiological conditions.
In this work, we have investigated the physiological roles of IKK-i and TBK1 by gene targeting. LPS-induced expression of IFN-β, but not proinflammatory cytokines, was markedly reduced in TBK1−/− cells. Similar defects were also observed in poly(I:C)-stimulated and virus-infected TBK1−/− cells. Although IKK-i −/− cells showed normal IFN-β gene induction, analysis of IKK-i/TBK1 doubly deficient (IKK-i −/−TBK1−/−) cells clearly showed that IKK-i is also critically involved in poly(I:C)-induced up-regulation of IFN-β and IFN-inducible genes. Poly(I:C)-induced activation of IRF-3, but not NF-κB, was also impaired in IKK-i −/−TBK1−/− cells, indicating that IKK-i and TBK1 act through IRF-3 to activate IFN-β. Thus, IKK-i and TBK1 play important roles in IFN-β gene induction by LPS, dsRNA, and during viral infection.
Materials and Methods
Cells, Virus, and Reagents.
Thioglycollate-elicited peritoneal cells were collected 3 d after intraperitoneal injection of 2 ml of 4% thioglycollate. EFs were prepared from day 11.5–14.5 embryos as described previously (16). Recombinant vesicular stomatitis virus (VSV) was a gift from T. Abe and Y. Matsuura (Osaka University, Osaka, Japan). Sendai virus (SeV) Z strain was provided by T. Shioda (Osaka University, Osaka, Japan). EFs were infected with 109 RNA copies/ml of VSV or multiplicity of infection 10 of SeV. LPS from Salmonella minnesota Re-595 was purchased from Sigma-Aldrich. Synthetic Pam3CSK4 (bacterial lipopeptide) was obtained from Boehringer. Poly(I:C) was purchased from Amersham Biosciences. Poly(I:C) was complexed with cationic lipids, Lipofectamin 2000 reagents (Invitrogen), and added to EFs. TNF-α and IL-1β were purchased from Genzyme.
Plasmids.
To construct the expression vectors for wild-type IKK-i and mutant IKK-i derived from the IKK-i–targeted allele, IKK-i cDNA from wild-type and IKK-i −/− EFs was sequenced, cloned, and inserted into pFLAG-CMV2 vector (Sigma-Aldrich). The human IRF-3 expression vector was constructed by ligation of human IRF-3 cDNA into pFLAG-CMV2 vector. The expression vector for human TBK1 was constructed as described previously (30). The mouse IFN-β promoter luciferase reporter was generated by PCR as described previously (30).
Generation of IKK-i−/− and TBK1−/− Mice.
Genomic DNA fragments encoding IKK-i and TBK1 were screened from the 129/Sv mice genomic library and characterized by restriction enzyme mapping and sequencing analysis. The targeting vectors were designed by replacing both a 1.0-kbp fragment of the IKK-i gene and a 1.5-kbp fragment of the TBK1 gene with a neomycin-resistant gene cassette. A herpes simplex virus thymidine kinase gene driven by MC1 promoter was used for negative selection. The targeting vectors were transfected into E14.1 ES cells. Targeted ES cells were identified among G418 and ganciclovir doubly resistant clones by PCR and Southern blotting and subsequently injected into C57BL/6 blastocysts. Male chimeric mice obtained were mated with C57BL/6 female mice. The F1 progenies were intercrossed to generate homozygotes for each mutated allele.
Measurement of IL-6 Production.
EFs were stimulated with the indicated stimulants for 18 h. Concentration of IL-6 in the culture supernatants were measured by ELISA. The ELISA kit for IL-6 was purchased from R&D Systems.
Northern Blot Analysis.
Total RNA was isolated using Sepazol-RNA I (Nacalai Tesque), electrophoresed, and transferred to nylon membranes. Hybridization was performed with the indicated cDNA probes as described previously (16). All cDNA probes were obtained from subtractive screenings as describe previously (16).
Electrophoretic Mobility Shift Assay (EMSA).
EFs (106 cells) were stimulated with 1.0 μg/ml or 10 μg/ml LPS or 10 μg/ml poly(I:C) for the indicated periods. Nuclear extracts were prepared and incubated with a radio-labeled oligonucleotide probe containing NF-κB binding sites or ISRE, and visualized by autoradiography as described previously (16).
Western Blot Analysis.
To prepare whole cell extracts, LPS- or poly(I:C)-stimulated cells were lysed in lysis buffer containing 1% Triton X-100, 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 5 mM EDTA, 10% glycerol, and protease inhibitor cocktail (Roche Diagnostics). Nuclear extracts were prepared from virus-infected cells as described previously (16). Whole cell or nuclear extracts were separated on SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membrane was blotted with specific antibodies. The membrane-bound Abs were visualized with horseradish peroxidase–conjugated Ab to rabbit IgG (Amersham Biosciences) using the ECL system (PerkinElmer). Anti–c-Jun NH2-terminal kinase (JNK) Ab, anti-extracellular signal-regulated kinase (ERK) Ab, and anti–NF-κB p65 Ab were obtained from Santa Cruz Biotechnology, Inc. Antibodies against phospho-JNK and phospho-ERK were purchased from Cell Signaling Technology. Polyclonal anti-IRF-3 antibody was raised as described previously (28).
Native PAGE Assay.
Native PAGE was performed as described previously (41). In brief, EFs (106 cells) were stimulated with 10 μg/ml poly(I:C) for the indicated periods, and whole cell lysates were prepared. Cell lysates in native PAGE buffer (62.5 mM Tris-HCl, pH 6.8, 15% glycerol) were separated on a 7.5% gel and immunoblotted with anti–IRF-3 Ab.
Reporter Assay.
Human embryonic kidney (HEK) 293 cells were seeded onto 24-well plates and transiently transfected with the indicated expression vector together with a reporter plasmid using Lipofectoamin 2000 reagents. EFs seeded onto six-well plates were transiently transfected with empty pEF-BOS vector (MOCK) or pEF-BOS-human TBK1 together with the IFN-β promoter reporter plasmid using FuGENE 6 transfection reagent (Roche Diagnostics). 24 h after transfection, the cells were stimulated with 10 μg/ml LPS for an additional 12 h. Luciferase activity of whole cell lysates was measured using a dual-luciferase reporter assay system (Promega). The Renilla luciferase reporter gene (Promega) was used as an internal control.
Microarray Analysis.
Total RNA was extracted from EFs stimulated with or without LPS for 2 or 4 h and subjected to synthesis of cRNA probe. Preparation of cRNA, hybridization, and scanning of the microarray were performed according to the manufacturer's instructions. A microarray (MG U74A version 2; Affymetrix, Inc.) was used for the analysis. Data analysis was performed with Microarray Suite software (version 5.0; Affymetrix, Inc.) and GeneSpring software (Silicon Genetics).
Results
Generation of IKK-i−/− and TBK1−/− Mice.
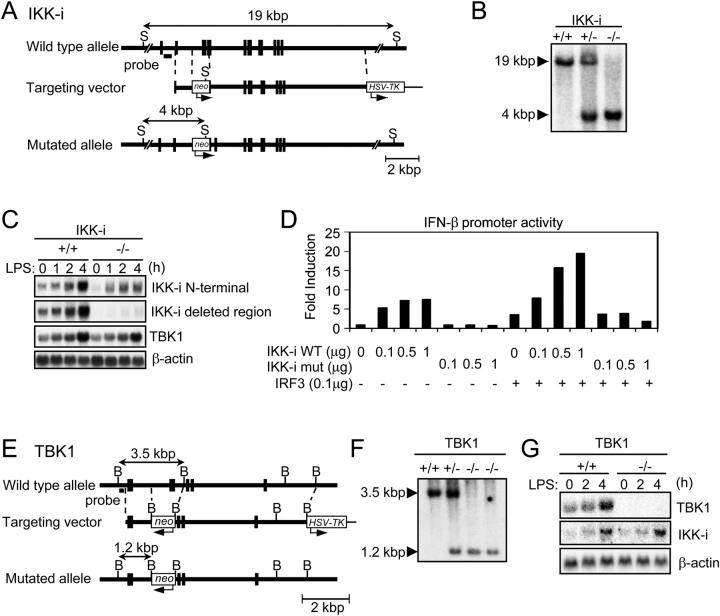
To investigate the physiological roles of IKK-i and TBK1, we generated IKK-i −/− and TBK1−/− mice by gene targeting. The targeting vector used to generate IKK-i −/− mice was constructed to replace exons 7 and 8 of the murine IKK-i gene encoding a part of the kinase domain with a neomycin resistant gene cassette (Fig. 1 A). IKK-i −/− mice were born at the expected Mendelian frequency, were fertile, and appeared to be healthy (Fig. 1 B). Using an NH2-terminal fragment of the IKK-i gene as a probe, we detected IKK-i transcripts in thioglycollate-elicited peritoneal cells from homozygous mutant mice (Fig. 1 C). However, sequencing analysis revealed that the IKK-i mRNA from the mutant allele lacked the targeted exons and contained a premature stop codon, generating a truncated protein that contained only a part of kinase domains (unpublished data). Transient transfection assay was used to assess biological activity of wild-type and the mutant IKK-i protein (Fig. 1 D). Wild-type IKK-i, but not the mutant, protein induced activation of the IFN-β promoter in a dose-dependent manner. In addition, the activity of IRF-3 was enhanced by coexpression of wild-type, but not mutant IKK-i. Therefore, we concluded that the mutant IKK-i protein does not have an activity that can lead to IFN-β induction.
Figure 1.
Generation of IKK-i −/− and TBK1−/− mice. (A) The structure of the IKK-i gene, the targeting vector, and the predicted mutated allele are shown. Closed boxes denote the coding exon. S, SphI. (B) Southern blot analysis of offspring from the heterozygote intercrosses. Genomic DNA was extracted from mouse tails, digested with SphI, electrophoresed, and hybridized with the radio-labeled probe indicated in A. Southern blotting gave a single 19-kbp band for wild type (+/+), a 4-kbp band for homozygous mutants (−/−), and both bands for heterozygous mice (+/−). (C) Northern blot analysis of thioglycollate-elicited peritoneal cells. Thioglycollate-elicited peritoneal cells from wild type and IKK-i −/− mice were stimulated with 100 ng/ml LPS for the indicated periods. Total RNA (10 μg) was electrophoresed, transferred to a nylon membrane, and hybridized using the IKK-i NH2-terminal fragment, the deleted region of IKK-i gene in targeting construct, TBK1, or β-actin cDNA fragment as a probe. (D) IFN-β promoter reporter assay. HEK293 cells were transiently cotransfected with the indicated expression vectors and the IFN-β luciferase reporter vector (0.1 μg). 36 h after transfection, luciferase activity in whole cell lysates was measured. (E) The structure of the TBK1 gene, the targeting vector, and the predicted mutated allele are shown. Closed boxes denote the coding exon. B, BamHI. (F) Southern blot analysis of EFs from the embryos of the heterozygous intercrosses. Genomic DNA was extracted from EFs, digested with BamHI, electrophoresed, and hybridized with the radiolabeled probe indicated in E. Southern blotting gave a single 3.5-kbp band for wild-type (+/+), a 1.2-kbp band for homozygous mutants (−/−), and both bands for heterozygous mice (+/−). (G) Northern blot analysis of EFs. Total RNA was extracted from wild-type and TBK1−/− EFs after stimulation with 1.0 μg/ml LPS for the indicated periods, electrophoresed, transferred to a nylon membrane, and hybridized using the IKK-i, TBK1, or β-actin cDNA fragment as a probe.
The targeting vector used to generate TBK1−/− mice was constructed to replace exon 9 of the murine TBK1 gene with a neomycin-resistant gene cassette (Fig. 1 E). Mice heterozygous for TBK1 were born and appeared healthy. However, TBK1−/− mice died at approximately embryonic day 14.5 (E14.5) as reported previously (39 and unpublished data). We obtained fibroblasts from embryos (EFs) at E12.5 (Fig. 1 F) and examined TBK1 mRNA expression. In wild-type EFs, TBK1 mRNA was detected and increased after LPS stimulation. However, in LPS-stimulated TBK1−/− EFs, TBK1 mRNA was not detected, whereas IKK-i gene induction was normal (Fig. 1 G).
IL-6 Production Was Normal in IKK-i−/− and TBK1−/− Cells.
IKK-i and TBK1 have been implicated in NF-κB activation (35–40). Because IL-6 production is dependent on NF-κB, we first measured IL-6 production by EFs in response to various stimuli known to activate NF-κB. EFs were stimulated with TNFα, IL-1β, the TLR2 ligand Pam3CSK4 (triacylated lipopeptides), and the TLR4 ligand LPS. As shown in Fig. 2 A, IKK-i −/− and TBK1−/− EFs produced similar amounts of IL-6 compared with wild-type cells in response to all stimulants tested. Moreover, EMSA analysis showed that NF-κB DNA binding activity was not affected in response to TNF-α, IL-1β, or LPS in both mutant EFs (unpublished data).
Figure 2.

Impaired induction of IFN-β and IFN-inducible genes in LPS-stimulated TBK1−/−, but not IKK-i −/− EFs. (A) IL-6 production by EFs. Control (IKK-i +/− or TBK1+/+), IKK-i −/− (top), or TBK1−/− (bottom) EFs were stimulated with 10 ng/ml TNF-α, 10 ng/ml IL-1β, 100 ng/ml Pam3CSK4, or 1.0 or 10 μg/ml LPS for 24 h. The concentration of IL-6 in the culture supernatants was measured by ELISA. Data are shown as mean ± SD of triplicate samples of one representative experiment from three independent experiments. (B) Gene induction in LPS-stimulated EFs. Control (IKK-i +/+ or TBK1+/−), IKK-i −/− (left), or TBK1−/− (right) EFs were stimulated with 1.0 μg/ml LPS for the indicated periods. Total RNA was extracted and subjected to Northern blot analysis for the indicated genes. (C) Microarray analysis of LPS-stimulated EFs. Wild-type and TBK1−/− EFs were stimulated with 1.0 μg/ml LPS, and RNA was collected at the indicated time points and used to conduct microarray analysis. Absolute expression was displayed using Genespring software. The color code for absolute signal strength is indicated on the left.
Induction of a Set of IFN-inducible Genes in Response to LPS Was Impaired in TBK1−/− EFs.
LPS stimulates IRF-3 activation, which leads to expression of IFN-β and a set of IFN-inducible genes. Therefore, we first performed Northern blot analysis to evaluate the expression of LPS-inducible genes regulated by IRF-3 (Fig. 2 B). Expression of IFN-β and IFN-inducible genes such as ISG54, IP-10, IRG1, and RANTES were up-regulated in LPS-stimulated IKK-i −/− EFs. These responses were also intact in bone marrow–derived dendritic cells, and thioglycollate-elicited peritoneal macrophages of IKK-i −/− mice (unpublished data). In contrast, although mRNA induction of RANTES and IL-6 was similar to that of wild-type EFs, mRNA induction of IFN-β, ISG54, IP-10, and IRG1 was severely impaired in TBK1−/− EFs.
Next, we performed DNA microarray analysis of LPS-stimulated EFs. Wild-type and TBK1−/− EFs were stimulated with LPS for 2 and 4 h. Genes induced in wild-type EFs are shown in Fig. 2 C. LPS-stimulated expression of IFN-inducible genes such as Mx2, IRF-7, IFN-inducible GTPase, and ISG15 were not observed in TBK1−/− EFs. In contrast, TBK1−/− EFs showed normal induction of MyD88-dependent genes such as IL-6, ICAM-1, and IκB-β (Fig. 2 C).
Next, we examined activation of intracellular signaling events in LPS-stimulated EFs. EMSA analysis revealed augmentation of NF-κB binding activity in both IKK-i −/− and TBK1−/− EFs, comparable to that observed in wild-type EFs (Fig. 3 A). Alternately, ISRE binding activity was not augmented in TBK1−/− EFs, but was observed in both wild-type and IKK-i −/− EFs. Next, we examined the activation of mitogen-activated protein (MAP) kinases. Wild-type, IKK-i −/−, and TBK1−/− cells showed comparable levels of phosphorylation of JNK and ERK (Fig. 3 B).
Figure 3.
Requirement of TBK1 in LPS-induced ISRE-binding and activation of the IFN-β promoter. (A) Impaired ISRE-binding in TBK1−/− EFs. Control (IKK-i +/+ or TBK1+/−), IKK-i −/−, or TBK1−/− EFs were stimulated with 1.0 μg/ml LPS for the indicated periods, and ISRE binding (left) and NF-κB binding activity (right) were determined by EMSA. (B) Activation of MAP kinases in LPS-stimulated EFs. Control (IKK-i +/− or TBK1+/−), IKK-i −/−, or TBK1−/− EFs were stimulated with 10 μg/ml LPS for the times indicated. Whole cell lysates were prepared and blotted with antiphospho-JNK1/2 Ab (phospho-JNK1/2) or antiphospho-ERK1/2 Ab (phospho-ERK1/2). The total amounts of JNK1/2 and ERK1/2 were also determined. One representative experiment is shown. (C) The expression of TBK1 restored activation of IFN-β promoter in TBK1−/− EFs. TBK1+/+ (WT) or TBK1−/− EFs were cotransfected with human TBK1 (hTBK1) and the IFN-β promoter luciferase reporter. 24 h after transfection, the cells were stimulated with or without 10 μg/ml LPS for an additional 12 h before luciferase activity was measured. Similar results were obtained from two independent experiments.
To confirm the direct involvement of TBK1 in induction of IFN-β, we investigated whether reconstitution of TBK1 in TBK1−/− EFs could restore activation of the IFN-β promoter upon LPS stimulation (Fig. 3 C). LPS activated the IFN-β promoter in mock-transfected wild-type EFs but not in TBK1−/− EFs. In contrast, transient expression of human TBK1 conferred the ability to activate the IFN-β promoter in LPS-stimulated TBK1−/− EFs. Thus, these results demonstrate that TBK1 is essential for induction of the TLR4-mediated IFN-β expression.
The Response to Poly(I:C) Is Impaired in TBK1−/− EFs.
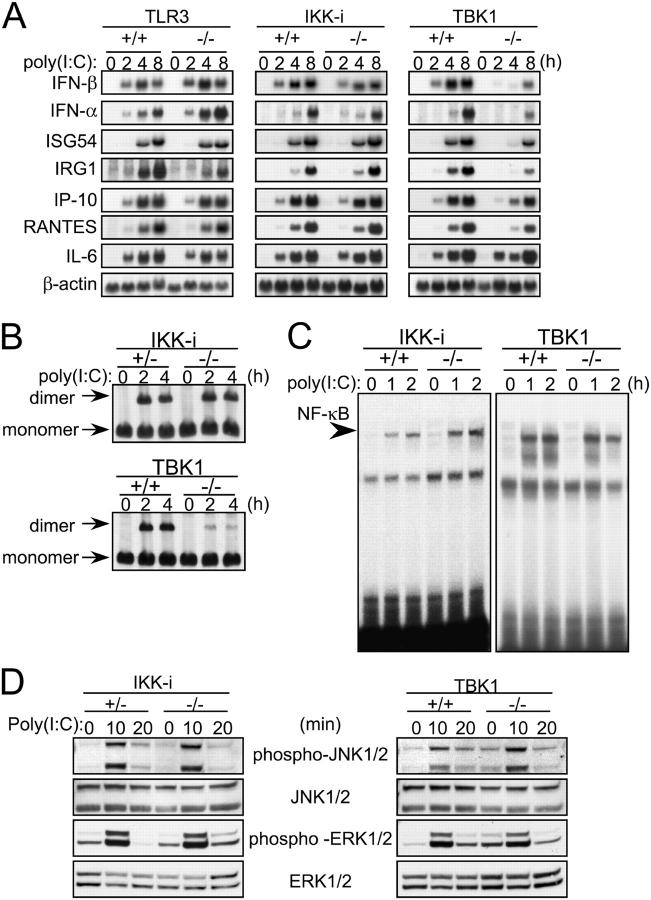
Next, we examined the response to the TLR3 ligand, poly(I:C). Because simple addition of poly(I:C) alone to the culture did not activate EFs, a poly(I:C) complex with cationic lipid was first formed and added to the culture to stimulate the cells, and the expression of IFN-β and IFN-inducible genes was monitored by Northern blot analysis (Fig. 4 A). In wild-type EFs, gene induction of IFN-β, IFN-α, ISG54, RANTES, IP-10, and IL-6 was observed after 2 or 4 h of stimulation. Induction of these genes was also observed in TLR3-deficient EFs, suggesting that transfection of poly(I:C) to EFs activates these genes in a TLR3-independent manner. Induction of these genes in IKK-i −/− EFs was comparable to that observed in wild-type EFs. However, in TBK1−/− cells, induction of the IFN-β, IFN-α, ISGF54, and IRG1 genes was severely reduced. Alternately, mRNA induction of RANTES, IP-10, and IL-6 was not impaired in these cells.
Figure 4.
Impaired induction of IFN-β and IFN-inducible genes in poly(I:C)-stimulated TBK1−/−, but not IKK-i −/− EFs. (A) mRNA induction in poly(I:C)-stimulated EFs. Wild-type, TLR3−/−, IKK-i −/−, or TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) for the indicated period. Total RNA was isolated and subjected to Northern blot analysis for the indicated genes. (B) Formation of IRF-3 dimer upon poly(I:C) stimulation. Control (IKK-i +/− or TBK1+/+), IKK-i −/−, or TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) and incubated for the indicated periods. Whole cell extracts were prepared and subjected to native PAGE. Monomeric and dimeric IRF-3 were detected by Western blotting. (C) NF-κB DNA binding activity. Wild-type, IKK-i −/−, or TBK1−/− EFs were stimulated with 10 μg/ml poly(I:C) for the times indicated, and NF-κB binding was determined by EMSA. (D) MAP kinase activation in poly(I:C)-stimulated EFs. Control (IKK-i +/− or TBK1+/+), IKK-i −/−, or TBK1−/− EFs were stimulated with 10 μg/ml poly(I:C) for the indicated periods. Whole cell lysates were prepared and blotted with antiphospho-JNK1/2 (phospho-JNK1/2) or antiphospho-ERK1/2 Ab (phospho-ERK1/2). The total amounts of JNK1/2 and ERK1/2 were also determined. One representative experiment from two independent experiments is shown.
Next, we evaluated the activation status of the transcription factors IRF-3 and NF-κB. IRF-3 dimerization was normally induced in IKK-i −/− EFs, but dramatically decreased in TBK1−/− EFs (Fig. 4 B). However, both IKK-i −/− and TBK1−/− EFs showed similar NF-κB DNA binding activity to that observed in wild-type cells. (Fig. 4 C). Moreover, in both IKK-i −/− and TBK1−/− EFs, the activation of MAP kinases including JNK and ERK were normally induced as compared with wild-type cells (Fig. 4 D). Thus, the deficiency of TBK1 leads to the impairment of poly(I:C)-induced IRF-3 activation.
TBK1 Is Essential for Induction of Type I IFNs and IFN-inducible Genes in Virus-infected EFs.
Viral infection also induces phosphorylation and dimerization of IRF-3, leading to the induction of IFN-β and other antiviral molecules (42–44). Therefore, next we examined whether virus-induced gene expression is mediated by IKK-i or TBK1 (Fig. 5 A). EFs were infected with VSV or SeV, and mRNA expression was examined by Northern blot analysis. In IKK-i −/− EFs, induction of mRNA for IFN-β, RANTES, and IP-10 was similar to that of wild-type cells in VSV or SeV infection. Alternately, in TBK1−/− cells, the expression of IFN-β and ISG54 mRNA was not observed, and induction of IP-10 was markedly diminished compared with wild-type cells. Although RANTES mRNA induction was almost normal in VSV infection, the induction was diminished in SeV infection. To evaluate IRF-3 activation, the nuclear proteins prepared from VSV-infected wild-type and TBK1−/− EFs were immunoblotted to detect IRF-3. As shown in Fig. 5 B, accumulation of IRF-3 and NF-κB p65 in the nucleus was observed in wild-type cells. The accumulation of IRF-3 was impaired in TBK1−/− cells, whereas NF-κB p65 translocation was not affected. These results indicate that TBK1 is an essential molecule for nuclear translocation of IRF-3 that leads to mRNA induction of IFN-β and other antiviral molecules in viral infection.
Figure 5.

Requirement of TBK1 for IFN-inducible gene expression in virus-infected EFs. (A) Control (IKK-i +/− or TBK1+/−), IKK-i −/−, or TBK1−/− EFs were infected with recombinant VSV or SeV for the indicated periods. Total RNA was extracted and subjected to Northern blot analysis with probes for the indicated genes. Similar results were obtained from three independent experiments. (B) Nuclear translocation of IRF-3 and NF-κB p65 in response to VSV infection. EFs were infected with recombinant VSV for the indicated periods, and nuclear proteins were extracted, separated by SDS-PAGE, and blotted with anti-IRF-3 and NF-κB p65 Abs.
IKK-i/TBK1 Doubly Deficient EFs Show That IKK-i Is Also Involved in Induction of IRF3-regulated Genes.
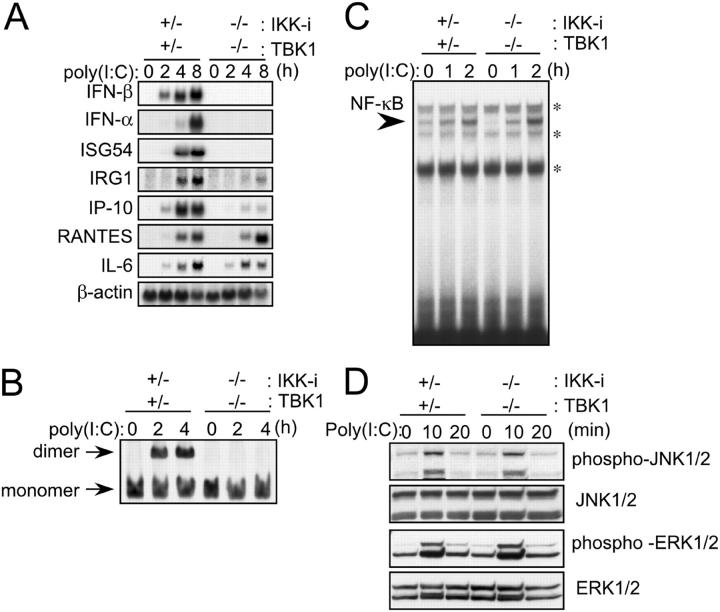
It has been reported that both IKK-i and TBK1 phosphorylate IRF-3 and IRF-7 in viral infection. Furthermore, as shown in Fig. 4 A, the induction of IFN-β mRNA, although severely reduced, still remained in poly(I:C)-stimulated TBK1−/− EFs. These results led us to investigate a possibility that this residual induction of IFN-β mRNA is mediated by IKK-i. For this purpose, we established immortalized IKK-i −/−TBK1−/− cells and analyzed gene inductions upon poly(I:C) stimulation. In control cells, the induction of the IFN-β, IFN-α, ISGF54, IRG1, IP-10, RANTES, and IL-6 genes was observed with the same kinetics as that observed in primary cells (Figs. 4 A and 6 A). In IKK-i −/−TBK1−/− cells, mRNA induction of IFN-β, IFN-α, and ISG54 was completely abolished, and the induction of IRG1 and IP-10 genes was severely impaired (Fig. 6 A). Moreover, IRF-3 dimerization was abolished (Fig. 6 B). Meanwhile, RANTES and IL-6 gene expression was augmented, and activation of NF-κB and induction of JNK and ERK phosphorylation were normally induced in doubly deficient cells (Fig. 6 D). Thus, IKK-i is also involved in poly(I:C)-induced IFN-β induction and IRF-3 activation.
Figure 6.
Involvement of IKK-i in IFN-β gene expression in poly(I:C) signaling. (A) Impaired mRNA induction in poly(I:C)-stimulated IKK-i −/−TBK1−/− cells. Immortalized control and IKK-i −/−TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) and incubated for the indicated period. Total RNA was isolated and subjected to Northern blot analysis for the indicated genes. The similar results were obtained from two immortalized EFs. (B) Formation of IRF-3 dimer. Immortalized control (IKK-i +/−TBK1+/−) and IKK-i −/−TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) and incubated for the indicated periods. Whole cell extracts were prepared and subjected to native PAGE. Monomeric and dimeric IRF-3 proteins were detected by Western blotting. The similar results were obtained from two independently established immortalized EFs. (C) NF-κB DNA binding activity. Immortalized IKK-i +/−TBK1+/− and IKK-i −/−TBK1−/− EFs were transfected with 10 μg/ml poly(I:C) for the indicated periods and NF-κB binding was determined by EMSA. Similar results were obtained from two lines of immortalized EFs. *, nonspecific bands. (D) MAP kinase activation. Immortalized IKK-i +/−TBK1+/− and IKK-i −/−TBK1−/− EFs were stimulated with 10 μg/ml poly(I:C) for the times indicated. Whole cell lysates were prepared and blotted with antiphospho-JNK1/2 Ab (phospho-JNK1/2) or antiphospho-ERK1/2 Ab (phospho-ERK1/2). The total amounts of JNK1/2 and ERK1/2 were also determined. One representative experiment from two independent experiments is shown.
Discussion
Activation of TLR3 by dsRNA and of TLR4 by LPS results in the induction of a set of IFN-induced genes including IFN-β. These gene inductions are regulated in a MyD88-independent but TRIF-dependent manner and are activated by the transcription factor IRF-3 (16, 17, 23, 28, 29). Recent in vitro findings have shown that IKK-i and TBK1 interact with TRIF and phosphorylate IRF-3 (30, 33, 34). Here, we have examined the physiological role of these kinases through the generation of knockout mice. TBK1−/−, but not IKK-i −/−, EFs showed a marked decrease in the induction of the IFN-β and IFN-inducible genes in response to LPS. In addition, TBK1−/− fibroblasts showed impaired response to poly(I:C) as demonstrated by delayed induction of IFN-β and IFN-inducible genes, and diminished IRF-3 dimerization. These responses were completely abolished in IKK-i/TBK1 doubly deficient fibroblasts, demonstrating that IKK-i is also involved in poly(I:C)-induced IRF-3 activation. However, mRNA induction of IFN-β and ISG54 was almost completely abolished in LPS-stimulated or virus-infected TBK1−/− EFs. Alternately, IKK-i −/− fibroblasts showed normal levels of gene induction after viral infection. These results indicate that the involvement of IKK-i in IRF-3 activation is varied, depending on stimulant. Further studies should be conducted to clarify the mechanisms of how IKK-i can compensate poly(I:C) stimulation, but neither LPS stimulation nor virus infection. In addition, studies using cells other than fibroblasts (e.g., by generating IKK-i −/−TBK1−/− mice under TNFα−/− background) are required to precisely determine the relative contribution of IKK-i and TBK1 in the responses in vivo.
IKK-i and TBK1 were originally identified as molecules that are structurally related to IKK-α and IKK-β. Overexpression of IKK-α, IKK-β, IKK-i, or TBK1 leads to NF-κB activation. However, IKK-i and TBK1 differ from IKK-α and IKK-β in the way they activate NF-κB. IKK-α and IKK-β phosphorylate both serines 32 and 36 of I-κBα, whereas IKK-i and TBK1 phosphorylate only serine 36 (35–38). Furthermore, previous papers have shown that IKK-i and TBK1 interact with and phosphorylate TANK/I-TRAF (37, 40), and the association of TANK with NEMO–IKK-γ and IKK–α/β complexes is dramatically increased in the presence of IKK-i or TBK1 (45), suggesting that IKK-i and TBK1 are involved in NF-κB activation upstream of IKK–α/β. However, a previous gene paper revealed that NF-κB DNA binding activity is up-regulated upon either TNF-α or IL-1 stimulation in TBK1−/− EFs (39). We have also found that up-regulation of NF-κB DNA binding activity in TBK1−/− EFs was comparable to that observed in wild-type cells in response to LPS as well as TNF-α and IL-1β (unpublished data). A previous work suggested that TLR4-induced signaling events leading to activation of NF-κB and IRF-3 are diverged at TRIF, and depend on TRAF6 and TBK1, respectively (30). Thus, IKK-i and TBK1 are dispensable for NF-κB activation, at least, in response to these stimuli.
TLR3 or TLR4 stimulation of macrophages leads to the immediate up-regulation of IFN-β, IP-10, and RANTES genes in an IRF-3–dependent manner (46). The present work shows that induction of IFN-β and IP-10 transcription was dramatically decreased in TBK1−/− EFs infected with VSV or SeV. However, RANTES mRNA was induced in TBK1−/− EFs in response to LPS and VSV infection. Although RANTES mRNA induction appeared to be slightly diminished in TBK1−/− EFs compared with wild-type EFs, the reduction may be due to the lack of secondary up-regulation by autocrine production of IFN-β. The RANTES gene might be activated by a MyD88-dependent and IRF3-independent manner as well. These results indicate that RANTES mRNA expression is regulated differently from that of IFN-β, depending on the cell type or the stimulus applied.
When poly(I:C) is directly introduced to the cytoplasm of EFs via lipofectamin, IFN-β and IFN-inducible genes are induced in a TLR3-independent fashion (Fig. 4 A). This poly(I:C)-induced TLR3-independent cellular activation is also reported in dendritic cells (47). Interestingly, poly(I:C)-induced gene expression and activation of IRF-3 were diminished in TBK1−/− EFs, suggesting that TBK1 also plays an important role in IRF-3 activation in the TLR3–TRIF-independent signaling pathway. Furthermore, the analysis of IKK-i −/−TBK1−/− EFs revealed that IKK-i can in part compensate the deficiency of TBK1.
In conclusion, our present studies demonstrate that TBK1 as well as, albeit to a lesser extent, IKK-i are essential for the activation of IFN-β and IFN-inducible genes in EFs. Control of these kinases will modulate expression of IFN-β and IFN-inducible genes without affecting induction of proinflammatory cytokines, which should enable us to obtain new therapeutic treatment for septic shock and viral infection.
Acknowledgments
The authors would like to thank Drs. Y. Matsuura and T. Abe for recombinant VSV; Dr. T. Shioda for SeV virus; Drs. H. Kuwata and S. Uematsu for helpful discussions; M. Hashimoto for secretarial assistance; N. Okita, N. Iwami, and Y. Fukuda for technical assistance; and Mr. P. Lee for critical reading of the paper.
This work was supported by grants from Special Coordination Funds; the Ministry of Education, Culture, Sports, Science and Technology; the Naito Foundation; the Virtual Research Institute of Aging of Nippon Boehringer Ingelheim; and by research fellowships from the Japan Society for the Promotion of Science for Young Scientists. H. Hemmi is a research fellow of the Japan Society for the promotion of Science. M. Yamamoto is supported by the Junior Research Associate from the Institute of Physical and Chemical Research.
Abbreviations used in this paper: dsRNA, double stranded RNA; EF, embryonic fibroblast; EMSA, electrophoretic mobility shift assay; ERK, extracellular signal-regulated kinase; IKK, IκB kinase; IKK-i, inducible IKK; IRF, IFN regulatory factor; ISRE, IFN-stimulated response element; JNK, c-Jun NH2-terminal kinase; MAP, mitogen-activated protein; poly(I:C), polyinosine-polycytidylic acid; SeV, Sendai virus; TBK1, TANK-binding kinase 1; TIR, Toll/IL-1 receptor; TLR, Toll-like receptor; TRAF, TNF receptor–associated factor; TRIF, TIR domain-containing adaptor inducing IFN-β; VSV, vesicular stomatitis virus.
References
- 1.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. First published on October 4, 2001; 10.1146/annurev.immunol. 20.083001.084359. [DOI] [PubMed]
- 2.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. First published on December 19, 2002; 10.1146/annurev.immunol.21.120601. 141126. [DOI] [PubMed]
- 3.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R.L. Modlin, and S. Akira. 2002. Role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10–14. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi, O., T. Kawai, P.F. Muhlradt, M. Morr, J.D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933–940. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 7.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752. [PubMed] [Google Scholar]
- 9.Hayashi, F., K.D. Smith, A. Ozinsky, T.R. Hawn, E.C. Yi, D.R. Goodlett, J.K. Eng, S. Akira, D.M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410:1099–1103. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 11.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529. First published on February 19, 2004; 10.1126/science.1093620. [DOI] [PubMed]
- 12.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, C. Reis, and E. Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–15231. First published on February 19, 2004; 10.1126/science.1093616. [DOI] [PubMed]
- 13.Zhang, D., G. Zhang, M.S. Hayden, M.B. Greenblatt, C. Bussey, R.A. Flavell, and S. Ghosh. 2004. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 303:1522–1526. [DOI] [PubMed] [Google Scholar]
- 14.Janssens, S., and R. Beyaert. 2003. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell. 11:293–302. [DOI] [PubMed] [Google Scholar]
- 15.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P. F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino, K., T. Kaisho, T. Iwabe, O. Takeuchi, and S. Akira. 2002. Differential involvement of IFN-β in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14:1225–1231. [DOI] [PubMed] [Google Scholar]
- 18.Toshchakov, V., B.W. Jones, P.Y. Perera, K. Thomas, M.J. Cody, S. Zhang, B.R. Williams, J. Major, T.A. Hamilton, M.J. Fenton, and S.N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3:392–398. First published on March 18, 2002; 10.1038/ni774. [DOI] [PubMed]
- 19.Sen, G.C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255–281. [DOI] [PubMed] [Google Scholar]
- 20.Grandvaux, N., B.R. tenOever, M.J. Servant, and J. Hiscott. 2002. The interferon antiviral response: from viral invasion to evasion. Curr. Opin. Infect. Dis. 15:259–267. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi, T., and A. Takaoka. 2002. The interferon-α/β system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111–116. [DOI] [PubMed] [Google Scholar]
- 22.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity. 13:539–548. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi, S., H. Negishi, M. Asagiri, C. Nakajima, T. Mizutani, A. Takaoka, K. Honda, and T. Taniguchi. 2003. Essential role of IRF-3 in lipopolysaccharide-induced interferon-β gene expression and endotoxin shock. Biochem. Biophys. Res. Commun. 306:860–866. [DOI] [PubMed] [Google Scholar]
- 24.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy, D.E., I. Marie, and A. Prakash. 2003. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr. Opin. Immunol. 15:52–58. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. A novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169:6668–6672. [DOI] [PubMed] [Google Scholar]
- 27.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nat. Immunol. 4:161–167. First published on January 21, 2003; 10.1038/ni886. [DOI] [PubMed]
- 28.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 301:640–643. First published on July 10, 2003; 10.1126/science.1087262. [DOI] [PubMed]
- 29.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S.O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, et al. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 424:743–748. First published on July 20, 2003; 10.1038/nature01889. [DOI] [PubMed]
- 30.Sato, S., M. Sugiyama, M. Yamamoto, Y. Watanabe, T. Kawai, K. Takeda, and S. Akira. 2003. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171:4304–4310. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto, M., S. Sato, H. Hemmi, S. Uematsu, K. Hoshino, T. Kaisho, O. Takeuchi, K. Takeda, and S. Akira. 2003. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4:1144–1150. First published on October 12, 2003;10.1038/ni986. [DOI] [PubMed]
- 32.Fitzgerald, K.A., D.C. Rowe, B.J. Barnes, D.R. Caffrey, A. Visintin, E. Latz, B. Monks, P.M. Pitha, and D.T. Golenbock. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J. Exp. Med. 198:1043–1055. First published on September 29, 2003; 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed]
- 33.Sharma, S., B.R. tenOever, N. Grandvaux, G.P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science. 300:1148–1151. First published on April 17, 2003; 10.1126/science. 1081315. [DOI] [PubMed]
- 34.Fitzgerald, K.A., S.M. McWhirter, K.L. Faia, D.C. Rowe, E. Latz, D.T. Golenbock, A.J. Coyle, S.M. Liao, and T. Maniatis. 2003. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491–496. First published on April 14, 2003; 10.1038/ni921. [DOI] [PubMed]
- 35.Shimada, T., T. Kawai, K. Takeda, M. Matsumoto, J. Inoue, Y. Tatsumi, A. Kanamaru, and S. Akira. 1999. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IκB kinases. Int. Immunol. 11:1357–1362. [DOI] [PubMed] [Google Scholar]
- 36.Peters, R.T., S.M. Liao, and T. Maniatis. 2000. IKKɛ is part of a novel PMA-inducible IκB kinase complex. Mol. Cell. 5:513–522. [DOI] [PubMed] [Google Scholar]
- 37.Pomerantz, J.L., and D. Baltimore. 1999. NF-κB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 18:6694–6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tojima, Y., A. Fujimoto, M. Delhase, Y. Chen, S. Hatakeyama, K. Nakayama, Y. Kaneko, Y. Nimura, N. Motoyama, K. Ikeda, et al. 2000. NAK is an IκB kinase-activating kinase. Nature. 404:778–782. [DOI] [PubMed] [Google Scholar]
- 39.Bonnard, M., C. Mirtsos, S. Suzuki, K. Graham, J. Huang, M. Ng, A. Itie, A. Wakeham, A. Shahinian, W.J. Henzel, et al. 2000. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 19:4976–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura, F., T. Kawai, K. Nakanishi, and S. Akira. 2000. NF-κB activation through IKK-i-dependent I-TRAF/TANK phosphorylation. Genes Cells. 5:191–202. [DOI] [PubMed] [Google Scholar]
- 41.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-κB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells. 6:375–388. [DOI] [PubMed] [Google Scholar]
- 42.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. Gene induction pathways mediated by distinct IRFs during viral infection. 2001. Biochem Biophys. Res. Commun. 283:1150–1156. [DOI] [PubMed] [Google Scholar]
- 43.Lin, R., C. Heylbroeck, P. Genin, P.M. Pitha, and J. Hiscott. 1999. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell. Biol. 19:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genin, P., M. Algarte, P. Roof, R. Lin, and J. Hiscott. 2000. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription factors. J. Immunol. 164:5352–5361. [DOI] [PubMed] [Google Scholar]
- 45.Chariot, A., A. Leonardi, J. Muller, M. Bonif, K. Brown, and U. Siebenlist. 2002. Association of the adaptor TANK with the IκB kinase (IKK) regulator NEMO connects IKK complexes with IKKɛ and TBK1 kinases. J. Biol. Chem. 277:37029–37036. First published on July 19, 2002; 10.1074/jbc.M205069200. [DOI] [PubMed]
- 46.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 17:251–263. [DOI] [PubMed] [Google Scholar]
- 47.Diebold, S.S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L.E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 424:324–328. First published on June 22, 2003; 10.1038/nature01783. [DOI] [PubMed]