Abstract
CD4+CD25+ regulatory T (T reg) cells play a pivotal role in control of the immune response. Transforming growth factor-β (TGF-β) has been shown to be required for T reg cell activity; however, precisely how it is involved in the mechanism of suppression is poorly understood. Using the T cell transfer model of colitis, we show here that CD4+CD45RBhigh T cells that express a dominant negative TGF-β receptor type II (dnTβRII) and therefore cannot respond to TGF-β, escape control by T reg cells in vivo. CD4+CD25+ T reg cells from the thymus of dnTβRII mice retain the ability to inhibit colitis, suggesting that T cell responsiveness to TGF-β is not required for the development or peripheral function of thymic-derived T reg cells. In contrast, T reg cell activity among the peripheral dnTβRII CD4+CD25+ population is masked by the presence of colitogenic effector cells that cannot be suppressed. Finally, we show that CD4+CD25+ T reg cells develop normally in the absence of TGF-β1 and retain the ability to suppress colitis in vivo. Importantly, the function of TGF-β1−/− T reg cells was abrogated by anti–TGF-β monoclonal antibody, indicating that functional TGF-β can be provided by a non–T reg cell source.
Naturally occurring regulatory T (T reg) cells mediate a nonredundant role in control of the immune response. Among these populations, the most well-characterized are those contained within the CD4+CD25+ subset (1–3). Although initially identified for their ability to prevent autoimmune disease (4), CD4+CD25+ T reg cells are now known to mediate a more general suppressive role, acting to limit immune pathology in the face of chronic immune stimulation (5–8).
Even though the list of immune responses affected by CD4+CD25+ T reg cells continues to expand, their mechanism of action remains obscure. Attention has focused on the role of cell contact–dependent mechanisms and the actions of immunoregulatory cytokines such as IL-10 and TGF-β (1). The relative contribution of these different mechanisms to suppressor function is highly controversial and may vary depending on the nature of the immune response being regulated. For example, TGF-β appears to play a nonredundant role in control of intestinal inflammation and diabetes (6, 9, 10), but not gastritis (11). A proportion of CD4+CD25+ cells express TGF-β1 on their surface (12) and this has been implicated in their suppressor function in vitro. However, TGF-β1−/− CD4+CD25+ cells retain T reg cell activity in vitro, indicating that TGF-β1 synthesis by CD4+CD25+ cells is not essential for cell contact–dependent suppression (11).
The TGF-βs, encompassing TGF-β1, TGF-β2, and TGF-β3, are highly pleiotropic cytokines with diverse effects on many developmental and physiological processes. TGF-β1 is the most abundant form in lymphoid organs and has a number of effects on cells of the immune system, including inhibition of T cell proliferation and differentiation and negative effects on macrophage activation and DC maturation (for review see reference 13). It plays a pivotal role in immune regulation as TGF-β1−/− mice develop a multi-organ inflammatory disease (14, 15). Recently, TGF-β1 has been shown to act as a costimulatory factor for expression of FoxP3 (16), leading to the differentiation of CD4+CD25+ T reg cells from peripheral CD4+CD25− progeny (17, 18). These results raise the possibility that, in addition to playing a role in the effector function of T reg cells, TGF-β also stimulates their differentiation. The broad immunoregulatory properties of TGF-β1 fit well with its involvement in the function of T reg cells; however, its precise role is unknown. It remains to be established whether T reg cells themselves are the crucial cellular source of TGF-β1 and, furthermore, which are the important cellular targets. Answers to these questions are complicated by the fact that both TGF-β1 and its receptor are expressed by many different cell types.
TGF-β1 binds to the TGF-βRI and RII heterodimeric receptor and induces signaling via activation of the Smad pathway. Recently, mice with T cell–restricted expression of a dominant negative form of the TGF-βRII have been generated (dominant negative TGF-β receptor II [dnTβRII]; references 19, 20). In these mice, the transgenic (Tg) TGF-βRII has a truncated intracellular kinase domain and therefore retains cytokine binding activity, but fails to trigger downstream signaling events. Unlike TGF-β1−/− mice that develop an inflammatory disease within the first weeks of life, dnTβRII mice survive into adulthood. However, these mice do show an accumulation of activated T cells and succumb to an inflammatory disease at ∼12 wk of age, indicating the physiological significance of TGF-β signaling in T cell homeostasis.
In this paper, we have used cells from dnTβRII and TGF-β1–deficient mice in the T cell adoptive transfer model of colitis (21, 22) to further dissect the role of TGF-β1 in the function of CD4+CD25+ T reg cells. Our results show that the function of T reg cells requires the direct action of TGF-β on pathogenic T cells and that in the absence of this interaction, colitogenic T cells escape T reg cell–mediated control. In addition, we provide evidence that the CD4+CD25+ T reg cell pool in the thymus is fully functional in the absence of TGF-β signaling, but that peripheral dnTβRII CD4+CD25+ cells induce rather than prevent colitis. Finally, we demonstrate that CD4+CD25+ T reg cells from TGF-β1–deficient mice retain the ability to prevent the development of colitis, even though T reg cell activity remains TGF-β dependent.
Results
Pathogenic T cells must respond to TGF-β for suppression of colitis
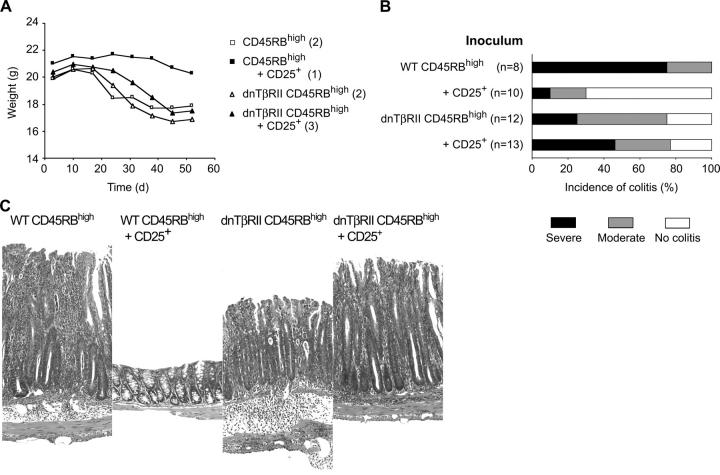
To determine whether TGF-β has direct effects on the colitogenic T cell population in vivo, we compared the ability of CD4+CD25+ cells to inhibit colitis induced by CD4+CD45RBhigh cells isolated from WT or dnTβRII mice. T cell populations were isolated from 6–8-wk-old dnTβRII mice before the development of inflammatory disease. At this time, the frequency of CD4+ cells expressing high levels of CD45RB was similar in dnTβRII and WT controls (unpublished data). To probe the relative ability of these populations to induce colitis, CD4+CD45RBhigh cells isolated from either WT or dnTβRII mice were transferred to syngeneic RAG-1−/− recipients. As shown in Fig. 1 A, the kinetics of the wasting disease was similar regardless of the genotype of the donor cells and the majority of mice in both groups developed colitis, although the incidence of severe disease was reduced in mice transfused with dnTβRII cells (Fig. 1, B and C). As dnTβRII mice develop colitis later in life (19), this latter finding may be the consequence of a reduction in potentially colitogenic cells in the naive (CD45RBhigh) pool and their transition into an antigen-experienced (CD45RBlow) phenotype (23).
Figure 1.
CD4+CD25+ T cells are unable to prevent colitis induced by transfer of dnTβRII CD4+CD45RBhigh cells. RAG-1−/− mice received 2 × 105 CD4+CD45RBhigh cells isolated from either WT or dnTβRII mice. In addition, some mice also received an equivalent number of WT CD4+CD25+ T cells. (A) Data show the mean weight of six animals per group and are representative of three independent experiments. Numbers in parentheses indicate number of animals with clinical signs of disease killed at day 45 and weights for these animals are included in the mean weights. CD4+CD25+ cells confer significant protection from wasting induced by WT CD4+CD45RBhigh cells (P < 0.03) but fail to prevent progressive weight loss induced by dnTβRII CD4+CD45RBhigh cells (NS). (B) Incidence and severity of colitis at the time of sacrifice. (n) Number of mice in each group. Data are pooled from three independent experiments. CD4+CD25+ cells confer significant protection from colitis induced by WT CD4+CD45RBhigh cells (P < 0.001), but fail to prevent colitis induced by dnTβRII CD4+CD45RBhigh cells (NS). (C) Representative photomicrographs of distal colon of RAG-1−/− mice after transfer of CD4+ T cells. Original magnification, 250 (hematoxylin and eosin).
The aforementioned data indicate a somewhat reduced colitogenic potential among the dnTβRII CD4+ CD45RBhigh T cell population compared with WT controls. Despite this, although CD4+CD25+ T reg cells were sufficient to inhibit the development of wasting disease and colitis induced by WT CD4+CD45RBhigh cells, they failed to prevent pathology induced by transfer of dnTβRII Tg cells (Fig. 1). Indeed, mice that received a mixture of WT CD4+CD25+ T cells and dnTβRII CD4+CD45RBhigh T cells exhibited a similar incidence and severity of colitis to those that received dnTβRII CD4+CD45RBhigh T cells alone (Fig. 1). These results are consistent with a requirement for TGF-β for prevention of colitis by T reg cells and, in addition, show that TGF-β signaling on the potentially colitogenic T cell population is a crucial part of the mechanism of immune suppression.
TGF-β inhibits Th1 cell accumulation and effector function
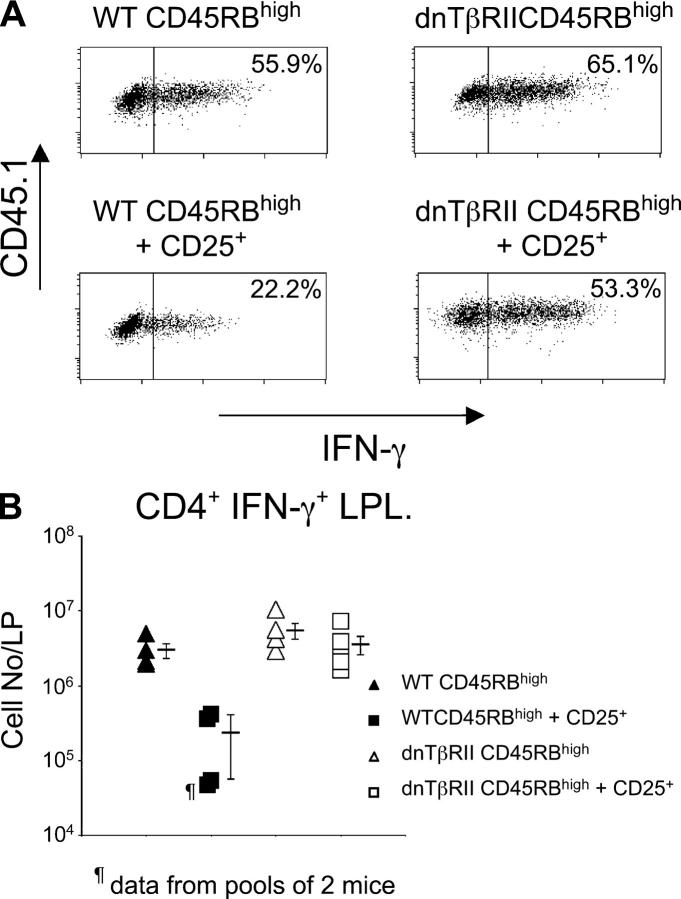
TGF-β1 has potent negative regulatory effects on CD4+ T cells in vitro, by inhibiting their proliferation, differentiation, and effector function (13). To further investigate how TGF-β acting on CD4+CD45RBhigh cells in vivo is able to prevent colitis, regulatory and pathogenic populations were isolated from congenic mice expressing different alleles of CD45. As described previously (24), transfer of WT CD4+CD45RBhigh cells to RAG-1−/− mice led to accumulation of IFN-γ–secreting Th1 cells in the colon (Fig. 2). A similar accumulation of IFN-γ+ cells was also observed after transfer of dnTβRII CD4+CD45RBhigh cells. Cotransfer of CD4+CD25+ T cells inhibited the accumulation of WT CD4+CD45RBhigh cells in the colon and also reduced the frequency of those cells capable of secreting IFN-γ (Fig. 2). In contrast, T reg cell transfer had no effect on the ability of dnTβRII CD4+CD45RBhigh progeny to differentiate into Th1 cells or accumulate in the intestine. Together, these data suggest that an essential part of CD4+CD25+ T reg cell activity in vivo requires the ability of TGF-β to act directly on pathogenic T cells to suppress their accumulation and effector function.
Figure 2.
Cytokine production by the progeny of transferred CD4+CD45RBhigh cells isolated from the LP. RAG-1−/− mice received CD4+ T cells as described in Fig. 1. 6–8 wk after transfer, LP cells were isolated and stimulated for 18 h with plate-bound anti-CD3 antibody. (A) Levels of cytokine expression were determined by flow cytometry. Scatter plots are gated on CD4+CD45.1+ lymphocytes to identify progeny of transferred CD4+CD45RBhigh cells and are representative of four to five mice per group. (B) Absolute numbers of cytokine-producing cells were determined by multiplying the frequency of cytokine producing cells after stimulation in vitro by the total number of LP leukocytes. Data are pooled from two independent experiments.
Peripheral CD4+CD25+ cells that cannot respond to TGF-β fail to suppress colitis
As TGF-β has been shown to induce the differentiation of CD4+CD25+ T reg cells from naive precursors (17), it is possible that immune pathology in dnTβRII mice also reflects deficiencies in the development, differentiation, or function of CD4+CD25+ T reg cells. To assess this, a phenotypic and functional analysis of the CD4+CD25+ T cells from the spleen and thymus of young 4–6-wk-old dnTβRII mice was performed. CD4+CD25+ cells appeared to develop normally in the absence of TGF-β signaling and were present at a similar frequency in the thymus of WT and dnTβRII mice. There was a small increase in the proportion of CD4+CD25+ cells in the spleen of dnTβRII mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20040685/DC1); however, there was no significant change in the activation status of these cells as expression of CD62L, CD45RB, and CD69 was similar to WT CD4+CD25+ cells (unpublished data). T reg cell activity was clearly present within the splenic dnTβRII CD4+ CD25+ pool as this population was able to inhibit T cell proliferation in vitro with similar efficiency to WT CD4+ CD25+ cells (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20040685/DC1).
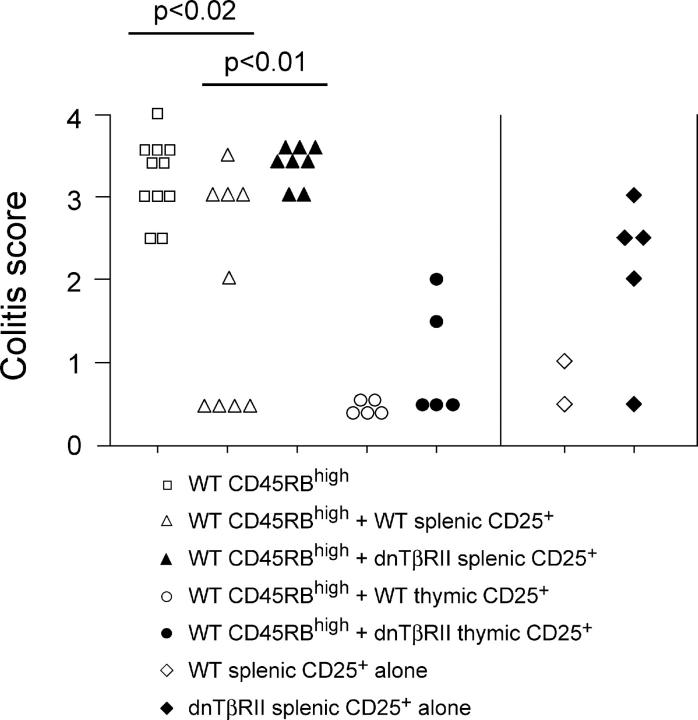
Despite the presence of demonstrable T reg cell activity within the splenic dnTβRII CD4+CD25+ population, these cells behaved very differently in vivo when compared with WT CD4+CD25+ cells. First, dnTβRII CD4+CD25+ splenocytes failed to provide statistically significant protection from CD4+CD45RBhigh–induced colitis even when isolated from young (3–4 wk old) mice and, second, they induced colitis themselves when transferred alone to RAG-1−/− mice (Fig. 3). The lack of apparent T reg cell activity in vivo amongst splenic dnTβRII CD4+CD25+ cells was not attributable to impaired thymic development of CD4+CD25+ T reg cells as dnTβRII CD4+CD25+ thymocytes, like their WT counterparts, were able to suppress colitis (Fig. 3). Thymic-derived dnTβRII CD4+CD25+ cells accumulated in the spleen and MLN of CD4+CD45RBhigh–restored RAG-1−/− mice (unpublished data), indicating that responsiveness to TGF-β is not required for the peripheral accumulation or function of CD4+CD25+ cells.
Figure 3.
Suppression of colitis by thymic but not splenic dnTβRII CD4+CD25+ cells. RAG-1−/− mice received 2–4 × 105 CD4+CD45RBhigh cells alone or in combination with 1.5–2 × 105 CD4+CD25+ cells isolated from the spleen or thymus of either WT or dnTβRII Tg mice. In addition, some mice received splenic CD4+CD25+ cells alone. Mice were killed 6–8 wk after transfer, and colons were taken for histological analysis. Data show colitis scores for individual mice taken from three independent experiments.
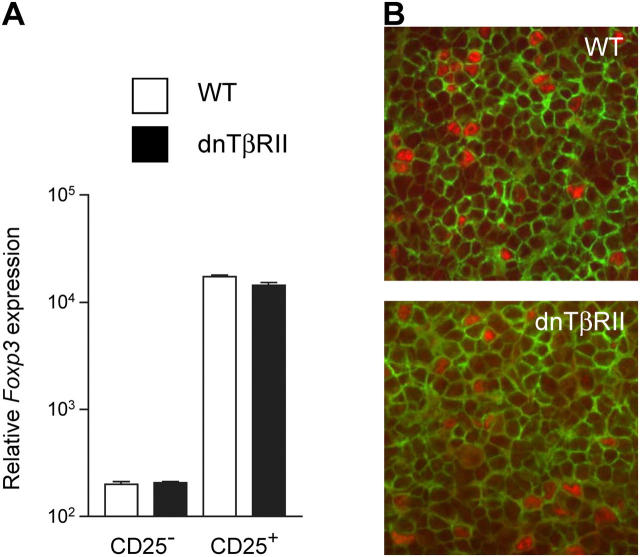
The ability of splenic dnTβRII CD4+CD25+ cells to induce rather than inhibit colitis is consistent with the finding that potentially colitogenic CD4+CD45RBhigh cells from dnTβRII are not controlled by T reg cells and most likely reflects an accumulation of activated colitogenic effector cells that are refractory to suppression. However, as TGF-β1 can induce FoxP3 expression amongst peripheral CD4+ T cells (17, 18), it is also possible that the lack of T reg cell activity is due to a deficiency of FoxP3+ cells amongst the splenic dnTβRII CD4+CD25+ pool. Quantification of FoxP3 mRNA expression revealed no significant differences between splenic CD4+CD25+ cells isolated from dnTβRII and WT mice (Fig. 4 A). This was confirmed at a single cell level, as the frequency of CD4+ cells in the spleen expressing FoxP3 protein was similar in dnTβRII and WT mice (Fig. 4 B). FoxP3+ cells were also abundant in the spleen and MLN of colitic RAG-1−/− mice that had received dnTβRII CD4+CD25+ cells 8 wk earlier, indicating that the development of colitis was not a consequence of the inability of dnTβRII FoxP3+ cells to accumulate in vivo (unpublished data).
Figure 4.
CD4+CD25+ cells from dnTβRII mice express normal levels of FoxP3. (A) CD4+CD25+ and CD4+CD25− cells were isolated from spleens of WT (white bars) and dnTβRII (black bars) mice. mRNA was recovered and analyzed for expression of FoxP3 and CD3γ by quantitative real-time PCR. Data show FoxP3 mRNA expression normalized to CD3γ levels as mean ± SEM for triplicate wells. A second experiment gave similar results. (B) Tissue sections from spleens of WT and dnTβRII mice were stained for CD4 (green) and FoxP3 (red). FoxP3+CD4+ cells show red nuclear staining and green surface staining. Frequency of FoxP3+ cells among CD4+ cells: WT 6.4 ± 1.3% and dnTβRII 6.6 ± 1.1%. Numbers represent mean and SEM from three individual mice per group. Original magnification, 200.
Together, the data demonstrate that responsiveness to TGF-β is not essential for the development or function of naturally arising CD4+CD25+ T reg cells. These cells are present in the thymus of dnTβRII mice and retain the ability to function in the periphery and suppress colitis in the T cell transfer model. FoxP3+ cells are present in normal frequency amongst splenic dnTβRII CD4+ cells; however, their activity is masked by the presence of colitogenic effector cells that express CD25 and are refractory to T reg cell–mediated control.
TGF-β1−/− CD4+CD25+ T reg cells are fully functional in vivo
There is conflicting data concerning the role of TGF-β1 in the suppressive activity of CD4+CD25+ T reg cells in vitro. Some investigators report high levels of TGF-β1 produced by and bound to the surface of activated CD4+CD25+ cells and that blockade of TGF-β is sufficient to abrogate CD4+CD25+ T reg cell–mediated suppression (12). In contrast, others have failed to find any requirement for T reg cell–derived TGF-β1 in vitro (11). As TGF-β is required for suppression of CD4+CD45RBhigh cell–induced colitis (6, 9), we examined whether CD4+CD25+ cells themselves are the critical source of TGF-β1 in this model. This approach was confounded by the fact that TGF-β1−/− mice develop a fatal lymphoproliferative inflammatory disease early after birth and fail to survive beyond 3–4 wk of age (14, 15). As a result, the CD25+ pool in these mice is likely to be highly distorted, containing an elevated frequency of activated effector cells. To circumvent this, CD4+CD25+ cells were isolated from BALB/c TGF-β1−/− mice that had been crossed onto the BALB/c DO11.10 TCR Tg line (DO11.10.TGF-β1−/−). In these mice, the reduced T cell receptor diversity prevents the early onset of the multi-organ inflammatory disease and mice survive beyond 12 wk (unpublished data).
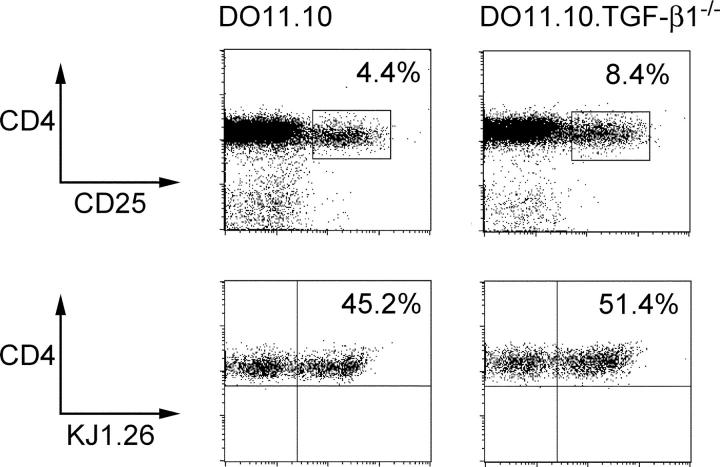
In concordance with a previous paper (25), functional CD4+CD25+ cells were present in DO11.10 mice with approximately half expressing the clonotypic T cell receptor, identified by KJ-1.26 (Fig. 5). A somewhat higher frequency of CD4+CD25+ cells was present in DO11.10.TGF-β1−/− mice, but a similar proportion of these expressed the Tg TCR (Fig. 5). There was no statistically significant difference in the frequency of activated CD62Llow cells present amongst the CD4+CD25+ population from DO11.10.TGF-β1−/− mice (47.5% ± 2.3; n = 6) compared with the equivalent population from DO11.10 TGF-β1+/− mice (36.4% ± 3.9; n = 6). Importantly, expression of FoxP3 mRNA was also identical between groups, suggesting no significant change in the development of naturally arising FoxP3+CD4+CD25+ T reg cells in the absence of TGF-β1 (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20040685/DC1). Consistent with this, DO11.10.TGF-β1−/−CD4+CD25+ cells were able to suppress antigen-induced T cell activation in vitro with similar potency to DO11.10.TGF-β1+/− CD4+ CD25+ cells (unpublished data). To analyze functional activity in vivo, CD4+CD25+ cells from DO11.10 or DO11.10.TGF-β1−/− mice were transferred either alone or together with BALB/c CD4+CD45RBhigh cells into C.B-17 SCID mice. Strikingly and contrary to expectation, DO11.10.TGF-β1−/− CD4+CD25+ cells were able to prevent colitis similarly to the equivalent population taken from DO11.10 mice (Fig. 6, A and B). The accumulation of DO11.10.TGF-β1−/− CD4+CD25+ progeny in vivo after transfer was indistinguishable from CD4+CD25+ cells isolated from DO11.10 TGF-β1+/− mice (unpublished data). These results provide unequivocal evidence that CD4+ CD25+ cells can develop in the complete absence of TGF-β1 and, despite being unable to synthesize this cytokine, retain suppressor function in vivo.
Figure 5.
Frequency of KJ-1.26+ cells is similar among CD4+CD25+ cells from either DO11.10 or DO11.10 TGF-β1−/− mice. Mice analyzed at 6–8 wk of age. CD4 enriched splenocytes from either DO11.10 or DO11.10 TGF-β1−/− mice were analyzed by flow cytometry for the expression of CD4 and CD25. Numbers indicate percentage of CD25+ cells among CD4+ cells. CD4+CD25+ cells were further analyzed for the expression of the clonotypic TCR by mAb KJ-1.26. Numbers indicate percentage KJ-1.26+ cells among CD4+CD25+ cells. Data are representative of six mice per group.
Figure 6.
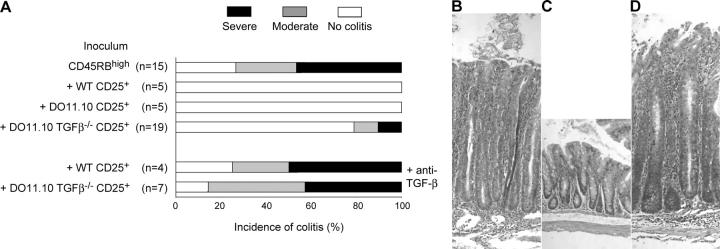
CD4+CD25+ T cells from DO11.10.TGF-β1−/− mice retain the ability to suppress colitis. C.B-17 SCID mice received 4 × 105 CD4+CD45RBhigh cells alone or in combination with 105 CD4+CD25+ cells isolated from 6–8-wk-old WT, DO11.10, or DO11.10.TGF-β1−/− mice. Some mice received anti–TGF-β mAb. (A) Incidence and severity of colitis at time of sacrifice. (n) Number of mice in each group. Data are pooled from five independent experiments. CD4+CD25+ cells from WT, DO11.10, and DO11.10 TGF-β1−/− mice confer significant protection from colitis (P < 0.02, P < 0.01, and P < 0.002, respectively). Mice receiving CD4+CD45RBhigh cells together with CD4+CD25+ cells from DO11.10 TGF-β1−/− mice are significantly different from mice that in addition received anti–TGF-β mAb once a week (P < 0.007). (B–D) Representative photomicrographs of colon from C.B-17 SCID mice after transfer of (B) CD4+CD45RBhigh cells, (C and D) CD4+CD45RBhigh, and DO11.10 TGF-β1−/− CD4+CD25+ cells. (D) Mice treated with anti–TGF-β mAb once a week. Original magnification, 200 (hematoxylin and eosin).
Suppression by TGF-β1−/− T reg cells remains TGF-β dependent
It is possible that TGF-β1−/− CD4+CD25+ T reg cells, having developed in a TGF-β1–deficient environment, are able to use other factors to control the development of colitis. To test this, C.B-17 SCID mice received WT CD4+ CD45RBhigh cells, together with CD4+CD25+ cells from either WT, DO11.10, or DO11.10 TGF-β1−/− mice. Some mice also received an anti–TGF-β mAb. Significantly, administration of anti–TGF-β mAb abrogated suppression of colitis mediated not only by WT CD4+CD25+ cells (Fig. 6 and reference 6) and DO11.10 CD4+CD25+ cells (unpublished data), but also by DO11.10 TGF-β1−/− CD4+CD25+ T reg cells (Fig. 6). Together, these data show that TGF-β plays a crucial role in CD4+CD25+ T reg cell–mediated suppression of colitis, even when the TGF-β1 is not produced by the CD4+CD25+ T reg cell.
Discussion
There is now accumulating evidence that TGF-β is an important mediator of T reg activity in vivo (1). However, it remains to be established precisely how TGF-β is involved in T reg function: specifically, which cells are the targets of TGF-β signaling and whether the CD4+CD25+ T reg cell population is an important source of this cytokine. Combining the T cell transfer model of colitis with the use of dnTβRII mice, we provide evidence that suppression of colitis by CD4+CD25+ T reg cells requires the direct action of TGF-β on the colitogenic T cell population, where it serves to prevent the accumulation of Th1 effector cells in the colon. This conclusion is further supported by the finding that colitogenic T cells accumulate in the peripheral CD4+ CD25+ pool of dnTβRII mice. Significantly, responsiveness to TGF-β is not required for the development or function of thymic CD4+CD25+ T reg cells. However, although FoxP3+ cells are present in the peripheral CD4+CD25+ pool from dnTβRII mice, it was not possible to measure the T reg cell activity in vivo due to the presence of pathogenic T cells that are refractory to T reg cell–mediated control. In addition, CD4+CD25+ T reg cells from TGF-β1−/− mice retain the ability to prevent colitis. Under these circumstances, suppression is still abrogated by administration of anti–TGF-β mAb indicating the following: (a) TGF-β remains central to the function of CD4+CD25+ T reg cells even when they cannot synthesize this cytokine themselves and (b) sufficient TGF-β1 can be provided by cellular sources other than the T reg cell population.
In the absence of TGF-β signaling in T cells, dnTβRII mice develop inflammatory infiltrates in the lung and colon with circulating autoantibodies, indicating that T cell responsiveness to TGF-β is required for normal immune homeostasis (19). The ability of TGF-β to directly suppress T cell responses may be a consequence of its ability to inhibit effector T cell differentiation (26, 27), to stimulate T reg cell function and expansion (17, 28–30), or a combination of both of these activities. In our work, transfer of CD4+ CD45RBhigh cells from dnTβRII mice into RAG-1−/− mice led to the development of wasting disease and colitis even in the presence of a dose of CD4+CD25+ T reg cells capable of preventing colitis induced by WT CD4+CD45RBhigh cells. These results provide the first evidence in vivo that the absence of a functional TGF-β receptor on pathogenic CD4+ T cells renders them refractory to control by CD4+CD25+ T reg cells. Similar to these results, in a model of diabetes, CD8+ T cells from dnTβRII mice showed enhanced diabetogenic potential and escaped control by CD4+CD25+ T reg cells (31). Together, these results demonstrate that the function of CD25+ T reg cells is critically dependent on the direct inhibitory effects of TGF-β on potentially pathogenic T cells.
The inability of CD4+CD25+ T reg cells to suppress dnTβRII CD4+CD45RBhigh cells in vivo does not seem to be attributable to a general hyperreactivity of the colitogenic population, but rather to their refractoriness to suppression. Several lines of evidence support this conclusion. First, WT and dnTβRII CD4+CD45RBhigh cells induced wasting disease in RAG-1−/− recipients with similar kinetics. Second, the incidence and severity of colitis was somewhat reduced in mice restored with dnTβRII CD45RBhigh cells, arguing against enhanced pathogenicity of these cells. Finally, dnTβRII CD4+TCR Tg T cells do not show hyperreactivity and exhibit a similar dose response curve upon antigen-induced activation (unpublished data).
TGF-β1 has been shown to inhibit T cell responses in vitro at several levels. It acts on naive T cells to inhibit expression of T-bet, a transcription factor critical for the development of a Th1 phenotype (27, 32) and has also been shown to inhibit IFN-γ secretion by Th1 effector cells (26, 33). TGF-β–dependent suppression by CD4+CD25+ T reg cells is associated with a reduction not only in the number of T cells in the colon but also in the frequency of those that can produce IFN-γ at this site. As such, TGF-β may also act at several levels in vivo, preventing accumulation of T cells in the intestine as well as inhibiting the acquisition of effector function by these cells.
TGF-β1 has been shown to induce FoxP3 expression and acquisition of T reg function amongst peripheral CD25−CD4+ cells in vitro (16, 17). However, it is unknown whether this pathway is operational in vivo or whether TGF-β1 is also involved in the generation of CD4+CD25+ T reg cells in the thymus. To address this, we analyzed the thymic and peripheral CD4+CD25+ T cell pool in dnTβRII mice. Our results show that dnTβRII CD4+CD25+ thymocytes express similarly high levels of FoxP3 mRNA as WT CD4+CD25+ cells (unpublished data). These cells were also fully functional based on their ability to accumulate in vivo and prevent colitis in the T cell transfer model, indicating that T cell responsiveness to TGF-β is not required for the development or peripheral function of thymic-derived CD4+CD25+ T reg cells. In contrast, peripheral dnTβRII CD4+CD25+ cells failed to inhibit CD4+CD45RBhigh-induced colitis and were pathogenic when transferred alone to RAG-1−/− recipients. The lack of demonstrable T reg cell activity in vivo amongst peripheral dnTβRII CD4+CD25+ cells was not the consequence of a complete absence of T reg cells as dnTβRII CD4+CD25+ cells expressed similarly high levels of FoxP3 mRNA as WT CD4+CD25+ cells and also retained the ability to suppress T cell activation in vitro. In addition, the frequency of CD4+ cells expressing FoxP3 protein was similar in dnTβRII and WT CD4+ cells. Together, the data suggest that FoxP3+ T reg cells are present among splenic dnTβRII CD4+CD25+ cells, but their activity cannot be measured due to the presence of colitogenic T cells that accumulate in the CD4+CD25+ pool postthymically and cannot be controlled by T reg cells. As colitogenic T cells in the naive T cell pool in dnTβRII mice are resistant to T reg cell–mediated control, these cells may be driven into the activated CD4+ CD25+ pool by exposure to intestinal antigens in the periphery (23). The CD4+CD25+ pool, even in WT mice, contains ∼70% FoxP3+ cells, making it likely that the effector cells reside within the FoxP3− fraction of dnTβRII CD4+CD25+ cells. These findings illustrate that the resistance to suppression of pathogenic T cells is an important factor in determining T reg cell activity and that the mere presence of FoxP3+CD4+CD25+ in an inflammatory lesion is not necessarily indicative of functional suppression.
CD4+CD25+ T reg cells from DO11.10.TGF-β1−/− mice retained the ability to prevent colitis, demonstrating that production of TGF-β1 by T reg cells is not essential for their function in vivo. These results are consistent with a recent paper that showed that TGF-β1−/− CD4+CD25+ cells could ameliorate immune pathology induced by transfer of TGF-β1−/− CD4+CD25− cells to immune-deficient recipients (34). Recently, Nakamura et al. found that TGF-β1−/− CD4+CD25+ cells failed to prevent colitis in the T cell transfer model. That work was performed on the C57BL/6 background, making it possible that the conflicting results are attributable to differences in genetic background. Alternatively, as Nakamura et al. purified CD4+CD25+ from TGF-β1−/− mice that develop severe lymphoproliferative disease early after birth; it cannot be excluded that the function of T reg cells in that study was obscured by the presence of colitogenic effector cells in the activated CD4+CD25+ T cell pool. Suppression of colitis by DO11.10.TGF-β1−/− CD4+CD25+ cells was inhibited by anti–TGF-β mAb, indicating that TGF-β remains central to the function of CD4+CD25+ T reg cells even when they do not synthesize it themselves. Production and regulation of TGF-β is very complex. In this instance, it seems likely that TGF-β1−/− CD4+CD25+ T reg cells act to induce TGF-β production by other hematopoietic or stromal cells. A wide variety of cells are capable of producing TGF-β and identifying the relevant ones in this model, which would require cell type–specific deletion of TGF-β among key candidates, such as DCs. It is also worth noting that TGF-β is produced in a latent form that can be found bound to the surface of CD4+CD25+ T reg cells (12). Activation of TGF-β is controlled by the presence of additional factors such as thrombospondin-1 (35–37), making it possible that CD4+CD25+ T reg cells contribute to this activation process. Although CD4+CD25+ T reg cells do not have to synthesize TGF-β to function in vivo, they may be required to express it on their surface, focusing it for presentation to effector T cells as has been described previously (12, 38). Indeed, expression of membrane-bound latency–associated peptide was found to identify a population of CD4+CD25− T reg cells capable of inhibiting colitis (38). An alternative possibility is that TGF-β1−/− CD4+CD25+ T reg cells function via TGF-β2 secretion. However, the fact that TGF-β1−/− mice develop severe immune pathology suggests that TGF-β2 cannot compensate for the role of TGF-β1 in immune regulation.
In summary, this paper identifies T cell responsiveness to TGF-β as an important factor that determines susceptibility to control by CD4+CD25+ T reg cells. Impaired T cell responsiveness to TGF-β may be involved in the pathogenesis of inflammatory bowel disease as intestinal T cells from inflammatory bowel disease patients expressed high levels of the inhibitor of TGF-β signaling Smad 7, allowing them to escape the inhibitory effects of TGF-β (39).
MATERIALS AND METHODS
Mice and antibodies
C57BL/6, C57BL/6 RAG-1–deficient (RAG-1−/−), C57BL/6.SJL.CD45 congenic mice, and C57BL/6.CD4-restricted dnTβRII (19) as well as BALB/c, DO11.10, DO11.10 TGF-β1–deficient (TGF-β1−/−), and C.B-17 SCID mice were bred under specific pathogen-free conditions and maintained in microisolator cages with filtered air at the School of Pathology in Oxford and used in experiments between 3 and 8 wk of age. All procedures were conducted in accordance with the Animals (Scientific Procedures) Act of 1986.
The following mAbs were used for CD4 enrichment: YTS169 (40), anti-CD8; TIB120, anti–MHC class II (American Type Culture Collection); M1/70, anti–Mac-1 (TIB128; American Type Culture Collection); and RA3-6B2 (41), anti-B220. FITC-conjugated anti-CD45RB (16A); Cy-Chrome–conjugated anti-CD4 (RM4-5); biotinylated anti-CD25 (7D4); allophycocyanin-conjugated anti-CD8α (53–6.7); and PE-conjugated streptavidin (BD Biosciences) were used for FACS sorting. PerCP-conjugated anti-CD4 (RM4-5), FITC-conjugated anti-CD4 (RMA4-4), biotinylated anti-CD45.1 (A20), FITC-conjugated anti-CD45.2 (104), PE-conjugated anti–TCR-β chain (H57-597), FITC-conjugated anti–IFN-γ (XMG1-2), PE-conjugated anti-CD25 (PC61), allophycocyanin-conjugated streptavidin (BD Biosciences), and biotinylated anticlonotypic TCR (KJ-1.26; reference 42) were used for analysis of T cell populations. Anti–mouse CD3ɛ (145-2C11) was purified from hybridoma supernatants and used in proliferation assays in vitro. For in vivo experiments, 1D11.16.8 (anti–mouse TGF-β1/2; reference 43) was purified from hybridoma supernatant by affinity chromatography and shown to contain <0.1 EU endotoxin per milligram of protein.
Purification of T cell subsets
CD4+ T cell subsets were isolated from spleen or thymus as described previously (44). In brief, single cell suspensions were depleted of CD8+, MHC class II+, Mac-1+, and B220+ cells by negative selection using sheep anti–rat–coated Dynabeads (Dynal). The resulting CD4+ enriched population was stained with Cy-Chrome–conjugated anti-CD4, FITC-conjugated anti-CD45RB, allophycocyanin-conjugated anti-CD8α, biotinylated anti-CD25 mAb, and streptavidin-PE. Subpopulations of CD4+ cells were isolated by cell sorting on a FACSVantage (BD Biosciences) or MoFlow High performance Cell Sorter (DakoCytomation).
T cell transfer model of colitis.
RAG-1−/− or C.B-17 SCID mice were injected i.p. with sorted CD4+ T cell populations as described previously (44). Mice received purified CD4+CD45RBhigh T cells alone or in combination with CD4+CD25+ T cells as specified. Some mice received CD4+CD25+ T cells alone. In addition, some mice also received 2.0 mg of anti–TGF-β mAb (clone 1D11.16.8) injected i.p. in PBS 1 d after T cell reconstitution and then once a week for the duration of the experiment. After T cell reconstitution, mice were weighed weekly and inspected for clinical signs of disease. Mice presenting clinically severe disease were killed according to the UK Animals Scientific Procedures Act of 1986.
Histological examination.
Colons were removed and paraffin embedded sections were cut and stained with hematoxylin and eosin. Inflammation was scored in a blinded fashion, on a scale of 0–4 (6): grade 0–1, no colitis; grade 2, moderate colitis; and grades 3–4: severe colitis.
Cell preparation and flow cytometry.
For analysis of surface marker expression, cells from the spleen and thymus were stained with fluorochrome-conjugated antibodies. Single cell suspensions were depleted of erythrocytes by hypotonic lysis and labeled with the required antibodies. For analysis of colitogenic effector populations, lamina propria (LP) lymphocytes were purified as described previously (45). In brief, colons were cut into 0.5–1.0 cm pieces and incubated in PBS/10% FCS/5.0 mM EDTA to remove the epithelial layer. The remaining tissue was further digested with collagenase/dispase (Sigma-Aldrich), and the released lymphocytes were recovered by centrifugation on a Percoll gradient (Amhersam Biosciences). This resulting population was stained for expression of CD4, CD45.2, and TCR-β chain, and the proportion of CD4+ T cells were determined by flow cytometric analysis on a FACSort using CELLQuest software (BD Biosciences). For detection of cytokines, LP lymphocytes were cultured overnight in complete medium in wells coated with 10 μg/ml anti–mouse CD3ɛ. 10 μg/ml brefeldin A (Sigma-Aldrich) was added for the final 2 h of incubation, and surface and cytoplasmic staining was performed as described previously (46). Labeled cells were analyzed on a FACSort or FACSCalibur using CELLQuest software.
Quantitative PCR
RNA was purified from sorted cells using Tri Reagent (Sigma-Aldrich) and reverse transcribed into cDNA using oligo-dT and Superscript First Strand synthesis system (Invitrogen) according to the manufacturer's instructions. Quantitative real-time PCR was performed using the following primers and probes: FoxP3, 5′-TTCTCACAACCAGGCCACTTG-3′, 5′-CCCAGGAAAGACAGCAACCTT-3′, and 5′-FAM-ATCCTACCCACTGCTGGCAAATGGAGTC-TAMRA-3′; CD3γ, 5′-CACCAAGAGCAAGGAAGAAGATG-3′, 5′-TTACAGAATGTGTGAAAACTGCATTG-3′, and 5′-VIC-ACATAGGCACCATATCCGGCTTTATCTTCG-TAMRA-3′, using a Chromo4 Detector (MJ Research). The reference cDNA sample used to make standard curves was obtained from CD4-enriched splenocytes. Samples were analyzed in triplicate and normalized levels of FoxP3 were calculated as the relative quantity of FoxP3 divided by the relative quantity of CD3γ in each sample, multiplied by 104.
Immunohistology
Tissue samples were snap frozen, cryocut, and acetone fixed. Slides were blocked with donkey serum (Sigma-Aldrich). Tissue sections were stained for CD4 (clone RMA4-5 FITC conjugated; BD Biosciences) and FoxP3. FoxP3 staining was performed using rabbit polyclonal anti–mouse FoxP3 antibodies (provided by F. Ramsdell, Zymogenetics, Seattle, WA; reference 47) and donkey anti–rabbit IgG Cy5 (Jackson ImmunoResearch Laboratories). The specificity of FoxP3 staining has been confirmed by the absence of nuclear staining in organs from FoxP3−/− mice (unpublished data).
Statistical analysis.
The Mann-Whitney U test was used for comparison of weight, clinical scores, cell numbers, and frequencies of cell surface markers. Differences were considered statistically significant when P < 0.05.
Online supplemental material
Fig. S1 shows the flow cytometric analysis of T cell subsets from spleen and thymus of WT and dnTβRII mice. Fig. S2 depicts the T cell proliferation assay. FACS-sorted CD4+ T cell subsets were cultured in 96-well plates in RPMI 1640 containing 10% FCS, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, and 100 U of penicillin and streptomycin. 2.5 × 104 WT CD4+CD45RBhigh cells were cultured with increasing numbers of CD4+CD25+ cells from either WT or dnTβRII Tg mice together with γ-irradiated T cell–depleted splenocytes as APCs and anti-CD3ɛ antibody. After 72 h, cells were pulsed with 0.5 μCi [3H]thymidine (Amersham Biosciences) and harvested onto glass fiber filters 18 h later. Proliferation was measured using liquid scintillation counting. Fig. S3 shows relative FoxP3 mRNA levels from splenic CD4+CD25+ cells from DO11.10.TGF-β1+/− or DO11.10.TGF-β1−/− mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20040685/DC1.
Acknowledgments
The authors wish to thank N. Rust for excellent technical assistance with cell sorting, S. Biddolph and L. Darley for processing of histological samples, and Dr. S. Clark and staff for care of experimental animals. We gratefully acknowledge Dr. F. Ramsdell for the provision of polyclonal anti-FoxP3 antibody and Dr. H. Uhlig for his immunohistology expertise. We also wish to thank Drs. O. Annacker, K. Maloy, and M. Letarte for critical review of this text.
S. Read, L. Fahlén, and F. Powrie were funded by the Wellcome Trust. R.A. Flavell is an investigator of the Howard Hughes Medical Institute. Work from the R.A. Flavell lab was supported by grants from the National Institutes of Health (no. R01 DK51665) and the American Diabetes Association.
The authors have no conflicting financial interests.
Abbreviations used: dnTβRII, dominant negative TGF-β receptor II; LP, lamina propria; Tg, transgenic; T reg, regulatory T.
L. Fahlén and S. Read contributed equally to this work.
References
- 1.Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. [DOI] [PubMed] [Google Scholar]
- 2.Shevach, E.M. 2002. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi, T., and S. Sakaguchi. 2003. Naturally arising CD25+CD4+ regulatory T cells in maintaining immunologic self-tolerance and preventing autoimmune disease. Curr. Mol. Med. 3:693–706. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 5.Shimizu, J., S. Yamazaki, and S. Sakaguchi. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218. [PubMed] [Google Scholar]
- 6.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 8.Hori, S., T.L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291. [DOI] [PubMed] [Google Scholar]
- 9.Powrie, F., J. Carlino, M.W. Leach, S. Mauze, and R.L. Coffman. 1996. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RBlow CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belghith, M., J.A. Bluestone, S. Barriot, J. Megret, J.F. Bach, and L. Chatenoud. 2003. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 9:1202–1208. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo, C.A., J.J. Letterio, A.M. Thornton, R.S. McHugh, M. Mamura, H. Mizuhara, and E.M. Shevach. 2002. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 196:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelik, L., and R.A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 2:46–53. [DOI] [PubMed] [Google Scholar]
- 14.Shull, M.M., I. Ormsby, A.B. Kier, S. Pawlowski, R.J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni, A.B., C.G. Huh, D. Becker, A. Geiser, M. Lyght, K.C. Flanders, A.B. Roberts, M.B. Sporn, J.M. Ward, and S. Karlsson. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA. 90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobbold, S.P., R. Castejon, E. Adams, D. Zelenika, L. Graca, S. Humm, and H. Waldmann. 2004. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 172:6003–6010. [DOI] [PubMed] [Google Scholar]
- 17.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+ CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantini, M.C., C. Becker, G. Monteleone, F. Pallone, P.R. Galle, and M.F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 19.Gorelik, L., and R.A. Flavell. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181. [DOI] [PubMed] [Google Scholar]
- 20.Lucas, P.J., S.J. Kim, S.J. Melby, and R.E. Gress. 2000. Disruption of T cell homeostasis in mice expressing a T cell–specific dominant negative transforming growth factor β II receptor. J. Exp. Med. 191:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powrie, F. 1995. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 3:171–174. [DOI] [PubMed] [Google Scholar]
- 22.Leach, M.W., A.G. Bean, S. Mauze, R.L. Coffman, and F. Powrie. 1996. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am. J. Pathol. 148:1503–1515. [PMC free article] [PubMed] [Google Scholar]
- 23.Asseman, C., S. Read, and F. Powrie. 2003. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J. Immunol. 171:971–978. [DOI] [PubMed] [Google Scholar]
- 24.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou, P., R. Borojevic, C. Streutker, D. Snider, H. Liang, and K. Croitoru. 2004. Expression of dual TCR on DO11.10 T cells allows for ovalbumin-induced oral tolerance to prevent T cell-mediated colitis directed against unrelated enteric bacterial antigens. J. Immunol. 172:1515–1523. [DOI] [PubMed] [Google Scholar]
- 26.Ludviksson, B.R., D. Seegers, A.S. Resnick, and W. Strober. 2000. The effect of TGF-beta1 on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur. J. Immunol. 30:2101–2111. [DOI] [PubMed] [Google Scholar]
- 27.Gorelik, L., S. Constant, and R.A. Flavell. 2002. Mechanism of transforming growth factor β–induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagiwa, S., J.D. Gray, S. Hashimoto, and D.A. Horwitz. 2001. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166:7282–7289. [DOI] [PubMed] [Google Scholar]
- 29.Peng, Y., Y. Laouar, M.O. Li, E.A. Green, and R.A. Flavell. 2004. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+ CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. USA. 101:4572–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schramm, C., S. Huber, M. Protschka, P. Czochra, J. Burg, E. Schmitt, A.W. Lohse, P.R. Galle, and M. Blessing. 2004. TGFbeta regulates the CD4+CD25+ T-cell pool and the expression of Foxp3 in vivo. Int. Immunol. 16:1241–1249. [DOI] [PubMed] [Google Scholar]
- 31.Green, E.A., L. Gorelik, C.M. McGregor, E.H. Tran, and R.A. Flavell. 2003. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. USA. 100:10878–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, C.H., C. Seguin-Devaux, N.A. Burke, T.B. Oriss, S.C. Watkins, N. Clipstone, and A. Ray. 2003. Transforming growth factor β blocks Tec kinase phosphorylation, Ca++ influx, and NFATc translocation causing inhibition of T cell differentiation. J. Exp. Med. 197:1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitani, A., I.J. Fuss, K. Nakamura, O.M. Schwartz, T. Usui, and W. Strober. 2000. Treatment of experimental (trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-β1 plasmid: TGF-β1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor β2 chain downregulation. J. Exp. Med. 192:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mamura, M., W. Lee, T.J. Sullivan, A. Felici, A.L. Sowers, J.P. Allison, and J.J. Letterio. 2004. CD28 disruption exacerbates inflammation in TGF-beta1−/− mice: in vivo suppression by CD4+CD25+ regulatory T cells independent of autocrine TGF-beta1. Blood. 103:4594–4601. [DOI] [PubMed] [Google Scholar]
- 35.Crawford, S.E., V. Stellmach, J.E. Murphy-Ullrich, S.M. Ribeiro, J. Lawler, R.O. Hynes, G.P. Boivin, and N. Bouck. 1998. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 93:1159–1170. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro, S.M., M. Poczatek, S. Schultz-Cherry, M. Villain, and J.E. Murphy-Ullrich. 1999. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J. Biol. Chem. 274:13586–13593. [DOI] [PubMed] [Google Scholar]
- 37.Schultz-Cherry, S., S. Ribeiro, L. Gentry, and J.E. Murphy-Ullrich. 1994. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J. Biol. Chem. 269:26775–26782. [PubMed] [Google Scholar]
- 38.Oida, T., X. Zhang, M. Goto, S. Hachimura, M. Totsuka, S. Kaminogawa, and H.L. Weiner. 2003. CD4+CD25− T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J. Immunol. 170:2516–2522. [DOI] [PubMed] [Google Scholar]
- 39.Monteleone, G., A. Kumberova, N.M. Croft, C. McKenzie, H.W. Steer, and T.T. MacDonald. 2001. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J. Clin. Invest. 108:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobbold, S.P., A. Jayasuriya, A. Nash, T.D. Prospero, and H. Waldmann. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 312:548–551. [DOI] [PubMed] [Google Scholar]
- 41.Coffman, R.L. 1982. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol. Rev. 69:5–23. [DOI] [PubMed] [Google Scholar]
- 42.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 157:1149–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasch, J.R., D.R. Pace, W. Waegell, D. Inenaga, and L. Ellingsworth. 1989. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J. Immunol. 142:1536–1541. [PubMed] [Google Scholar]
- 44.Powrie, F., M.W. Leach, S. Mauze, L.B. Caddle, and R.L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 45.Powrie, F., M.W. Leach, S. Mauze, S. Menon, L.B. Caddle, and R.L. Coffman. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhigh CD4+ T cells. Immunity. 1:553–562. [DOI] [PubMed] [Google Scholar]
- 46.Openshaw, P., E.E. Murphy, N.A. Hosken, V. Maino, K. Davis, K. Murphy, and A. O'Garra. 1995. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 182:1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schubert, L.A., E. Jeffery, Y. Zhang, F. Ramsdell, and S.F. Ziegler. 2001. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 276:37672–37679. [DOI] [PubMed] [Google Scholar]