Abstract
The cytokine thymic stromal lymphopoietin (TSLP) has recently been implicated in the pathogenesis of atopic dermatitis (AD) and other allergic diseases in humans. To further characterize its role in this disease process, transgenic mice were generated that express a keratinocyte-specific, tetracycline-inducible TSLP transgene. Skin-specific overexpression of TSLP resulted in an AD-like phenotype, with the development of eczematous lesions containing inflammatory dermal cellular infiltrates, a dramatic increase in Th2 CD4+ T cells expressing cutaneous homing receptors, and elevated serum levels of IgE. These transgenic mice demonstrate that TSLP can initiate a cascade of allergic inflammation in the skin and provide a valuable animal model for future study of this common disease.
Atopic dermatitis (AD) is a common allergic inflammatory disease of the skin that is characterized by intense pruritus and chronic eczematous plaques and is often associated with a personal or family history of allergic disease (1, 2). In addition, it is the most common chronic skin disease in childhood, affecting an estimated 7–12% of school-aged children (3). Although most cases are mild and usually clear up, some patients experience severe or widespread disease that can be physically, socially, and/or emotionally debilitating (4). Although the underlying cause of AD remains unclear, its pathogenesis is thought to involve a Th2 cell–mediated allergic inflammatory cascade (5, 6). This type of inflammation is characterized by the production of proallergic cytokines (IL-4, IL-5, IL-13, and TNF-α) by CD4+ T cells, leading to elevated levels of IgE and leukocytic infiltration of the dermis (6, 7). Local DCs are thought to play a crucial role in this process by polarizing naive CD4+ T cells to differentiate into Th2 lymphocytes. However, the initiating factors that influence these important antigen-presenting cells to instruct T helper cell polarization toward this proallergic phenotype remain unknown.
One candidate factor for the local activation of skin DCs during allergic inflammation is the cytokine thymic stromal lymphopoietin (TSLP). TSLP is expressed primarily by epithelial cells, including keratinocytes, whereas the TSLP receptor is expressed by hematopoietic cells, including monocytes and CD11c+ DCs (8–10). In vitro, human TSLP can activate CD11c+ DCs and induce their production of the Th2 cell–attracting chemokines CCL17 and CCL22 (9). Moreover, naive CD4+ T cells, primed by TSLP-treated DCs, underwent extensive proliferation and differentiation into Th2 lymphocytes on restimulation, with the production of IL-4, IL-5, IL-13, and TNF-α (11). Additionally, TSLP is highly expressed by keratinocytes in acute and chronic lesions of AD, where it is associated with the activation and migration of DCs within the dermis (11). These data support a role for TSLP as one of the initiating factors in AD, responsible for activating cutaneous DCs and endowing them with the ability to polarize CD4+ T cells toward a Th2 cell allergic response.
Based on these data, we hypothesized that targeted expression of TSLP to the skin of transgenic mice would result in an AD-like phenotype. We report that mice expressing an inducible, epidermal-specific TSLP transgene demonstrate clinical, histologic, cellular, and biochemical characteristics that closely resemble those of human AD. These include dermal infiltrates containing lymphocytes, mast cells, and eosinophils, an increase in Th2 cytokines in the effected skin, and elevated circulating levels of IgE. Disease is associated with a dramatic increase in IL-4–producing CD4+ T cells, which also express CCR4 and other skin homing receptors, in both the skin-draining LNs and the spleen. Consistent with the hypothesis that TSLP can act directly on myeloid cells, transgenic mice lacking T cells still develop disease. Collectively, these mice directly demonstrate that TSLP can initiate an AD-like allergic inflammation on transgenic expression in the skin.
Results
Skin-specific expression of TSLP results in moderate to severe dermatitis
Mice containing a tetracycline-inducible, skin-specific TSLP transgene were generated by crossing tetO-TSLP mice with mice expressing the reverse tetracycline transactivator in the skin under control of the keratin 5 promoter (K5-rtTA mice; reference 12). Animals carrying both transgenes (referred to as K5-TSLP mice), along with nontransgenic littermates, were started on dietary doxycycline (dox) at approximately 5 wk of age. Skin-targeted transgene expression in K5-TSLP mice was confirmed by RT-PCR (Fig. 1 A). In addition, expression of TSLP directly in the skin from K5-TSLP and control mice was examined. As shown in Fig. 1 B, TSLP expression was evident in the epidermis of K5-TSLP mice, whereas only scattered cells expressed TSLP in the skin from control mice. dox-treated mice carrying single transgenes, as well as untreated K5-TSLP mice, were also examined and were indistinguishable from nontransgenic littermates with respect to all parameters studied (unpublished data).
Figure 1.
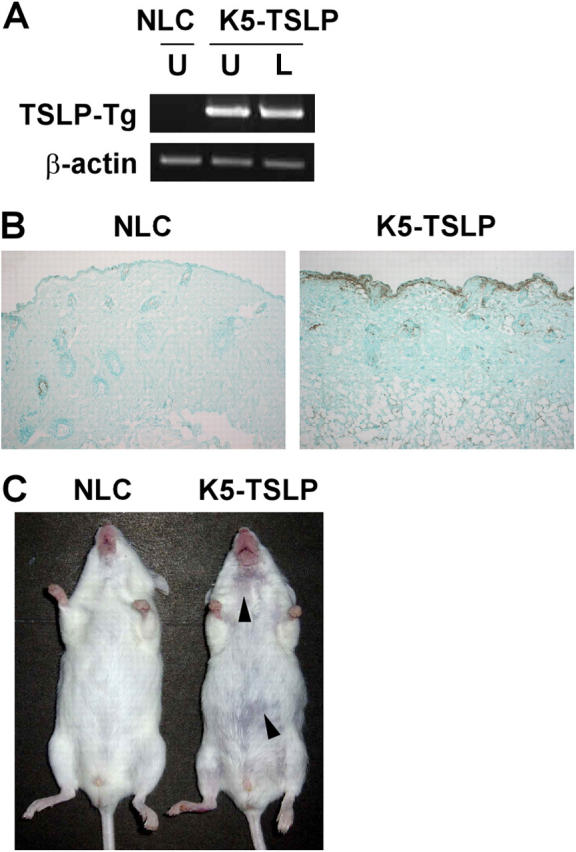
Skin-targeted transgenic expression of TSLP causes eczematous-like skin lesions accompanied by pronounced lymphadenopathy and splenomegaly. (A) RT-PCR analysis of TSLP transgene expression in the skin of a K5-TSLP mouse and a normal littermate control (NLC) after 3 wk of dox treatment. From the K5-TSLP mouse, total RNA isolated from affected lesional skin (L) and unaffected skin (U) were analyzed separately. (B) An immunohistochemical localization of TSLP in the skin of a K5-TSLP mouse as compared with NLC. TSLP in a K5-TSLP mouse was expressed in the epidermis and its basement membrane (brown). (C) Images showing a K5-TSLP mouse and NLC after 3 wk of dox treatment. Arrowheads mark eczematous lesions on the snout and trunk. Images are representative of >10 K5-TSLP mice analyzed.
K5-TSLP mice developed skin disease beginning at ∼2–3 wk of dox treatment. Their lesions consisted of mild erythema at 2 wk with progression to obvious eczematous-like changes, including erythema, crusting, and erosions, by 3–4 wk (Fig. 1 C). Lesions were most common on the snout, postauricular region, nape of the neck, and upper back. Less commonly, lesions were found on the anterior neck and hind legs. The majority of mice exhibited a mild xerosis that was generalized, and conjunctivitis was observed in a few cases (not depicted). Together, these clinical features closely resemble those observed during human AD. In addition to the exterior manifestations of disease, these mice also displayed pronounced splenomegaly and lymphadenopathy (unpublished data).
Eczematous skin samples from K5-TSLP mice were subjected to histological examination and compared with skin from nontransgenic littermates (Fig. 2). By 5 wk of dox treatment, lesional skin from K5-TSLP mice demonstrated all of the characteristic changes of eczematous inflammation observed in human AD, including acanthosis (epidermal hypertrophy), spongiosis (interepidermal edema), hyperkeratosis (thickening of the stratum corneum), and a dermal mononuclear cell infiltrate. These features were not present in either clinically normal K5-TSLP skin or nontransgenic skin. A more detailed examination of the inflammatory infiltrate revealed, in addition to a predominance of lymphocytes and macrophages, an abundance of mast cells and eosinophils. In contrast to the other, nonspecific changes observed, the presence of these latter cell types is highly suggestive of an allergic inflammatory process.
Figure 2.
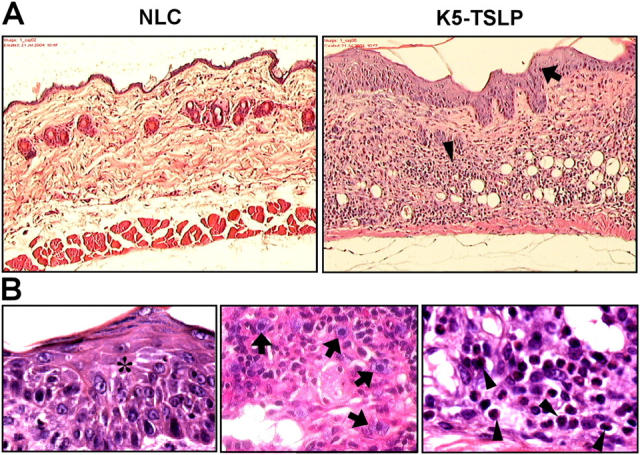
Histological observations of the skin in K5-TSLP mice. Paraffin-embedded sections of lesional skin from a K5-TSLP mouse and an NLC were stained with H&E. (A) Skin sections showing pronounced acanthosis (arrow) and dermal infiltration (arrowhead) at 10×. (B) Images of lesional skin from a K5-TSLP mouse showing epidermal spongiosis (asterisk) and dermal infiltration by mast cells (arrows) and eosinophils (arrowheads) at 100×.
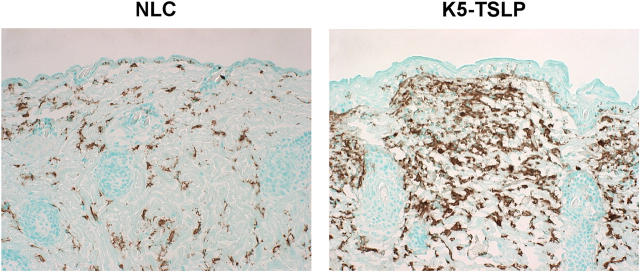
To further investigate the nature of the inflammation seen in the skin of K5-TSLP mice, skin sections were stained with BM8, an antibody that recognizes activated macrophages and Langerhans cells in the skin. Skin sections from K5-TSLP mice showed extensive accumulation of BM8+ cells, with only scattered cells staining in control skin. Interestingly, the staining was restricted to the dermis, with little or no staining of cells in the epidermis (Fig. 3). These data are consistent with the presence of an inflammatory infiltrate, containing activated macrophages, mast cells, and eosinophils, in the dermis of K5-TSLP mice. We also observed increased expression of CD31 on endothelial cells in the dermis from affected K5-TSLP skin, which was consistent with an inflammatory response (unpublished data).
Figure 3.
Immunohistochemical localizations of infiltrated macrophages and Langerhans cells in the skin of a K5-TSLP mouse. As compared with the NLC, a larger number of macrophages and Langerhans cells (BM8+) are infiltrated into affected skin areas of a K5-TSLP mouse.
Cellular and hematological analyses of K5-TSLP mice reveal a Th2 inflammatory response targeted to the skin
AD and the other allergic diseases are generally characterized by dysregulated Th2 lymphocyte activation, IgE overproduction, activation of mast cells, and eosinophilia. K5-TSLP mice displayed pronounced splenomegaly and lymphadenopathy in both cutaneous and noncutaneous LNs (unpublished data). Moreover, consistent with the proposed function of TSLP in the differentiation of Th2 cells, intracellular cytokine analysis revealed a dramatic increase in the frequency of IL-4–producing CD4+ T cells in both the spleens and LNs of K5-TSLP mice (Fig. 4 A and Table I). Many of these IL-4–producing cells coexpressed IL-10 or TNF-α; however, there was little or no increase in the frequency of IFN-γ–producing cells. RT-PCR from erythematous skin of K5-TSLP mice confirmed this Th2 cell profile, with an increase in the proallergic cytokines IL-4, IL-5, and TNF-α and undetectable IFN-γ (Fig. 4 B). Consistent with this Th2 response, analysis of serum immunoglobulins showed increases in both IgG1 and IgE, along with a decrease in IgG2a in K5-TSLP mice (Table II).
Figure 4.
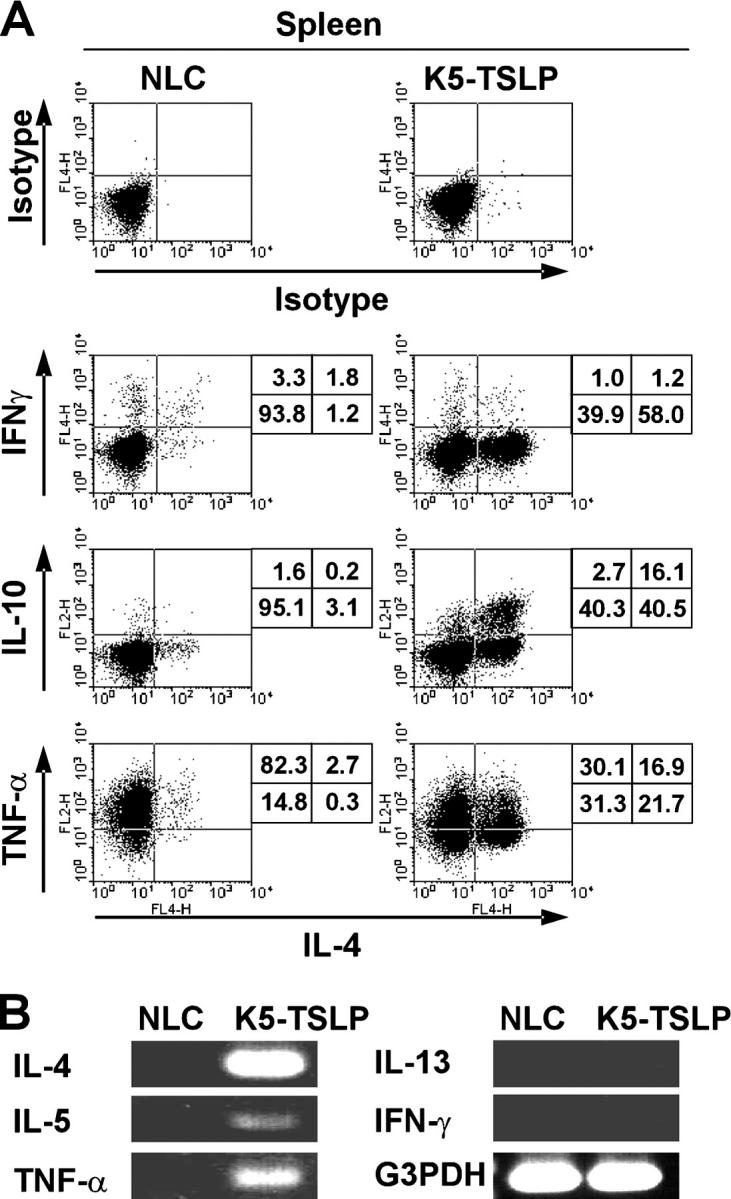
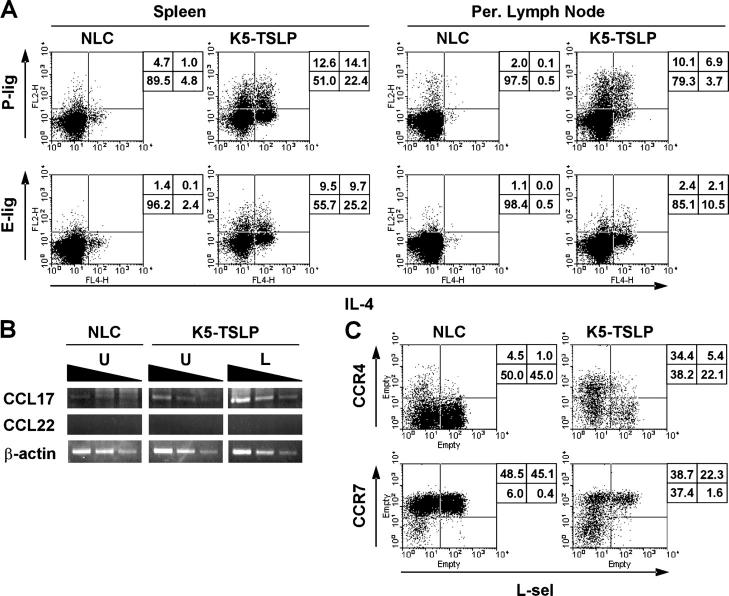
The CD4+ T cell response in K5-TSLP mice is Th2 cell polarized. (A) Intracellular proallergic cytokine staining of splenic CD4+ T cells isolated from a K5-TSLP mouse and an NLC after 5 wk of dox treatment. The percentages of cells in each quadrant are shown. Data are representative of more than five animals analyzed in this manner. (B) RT-PCR analysis of cytokine gene expression in the lesional skin of a K5-TSLP mouse and skin from an NLC.
Table I.
Cytokine production by CD4+ T cells in K5-TSLP and control mice
| Spleen
|
PLNa
|
MLN
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | γ+/4− | γ−/4+ | γ+/4+ | γ+/4− | γ−/4+ | γ+/4+ | γ+/4− | γ−/4+ | γ+/4+ |
| NLC | 2.09 | 3.18 | 1.70 | 0.15 | 0.82 | 0.07 | 0.24 | 3.46 | 0.02 |
| NLC | 3.44 | 1.23 | 1.51 | 0.68 | 0.40 | 0.05 | 0.62 | 1.35 | 0.09 |
| K5-TSLP | 1.43 | 40.94 | 0.64 | 1.45 | 6.14 | 0.20 | 1.75 | 10.17 | 0.41 |
| K5-TSLP | 0.94 | 60.26 | 0.78 | 2.60 | 15.17 | 0.58 | 1.89 | 11.87 | 0.41 |
| K5-TSLP | 1.34 | 46.96 | 0.90 | 3.41 | 4.08 | 0.31 | 0.78 | 7.26 | 0.07 |
After 5 wk of dox treatment, IFN-γ and IL-4 production by CD4+ T cells from the spleen, cutaneous peripheral LNs (PLN), and mesenteric LNs (MLN) of K5-TSLP and NLC mice were assessed by intracellular cytokine staining/flow cytometry after in vitro stimulation with PMA/ionomycin. Values shown are the percentage of CD4+ T cells producing the indicated combinations of IFN-γ and IL-4.
Inguinal LNs in K5-TSLP mice; pooled inguinal, axillary, brachial, and cervical LNs in NLC.
Table II.
Serum immunoglobulin levels in K5-TSLP and control mice
| Genotype | Doxa | IgG1 b | IgG2a b | IgEb |
|---|---|---|---|---|
| mg/ml | mg/ml | mg/ml | ||
| K5-TSLP | 3 | 339.8 | 41.6 | 50.5 |
| NLC | 3 | 191.2 | 176.6 | n.d. |
| K5-TSLP | 4 | 379.0 | 14.7 | 82.9 |
| K5-TSLP | 4 | 369.0 | 7.1 | 72.1 |
| NLC | 4 | 283.5 | 75.6 | n.d. |
| K5-TSLP | 5 | 143.8 | 14.6 | 54.3 |
| NLC | 5 | 71.5 | 148.0 | n.d. |
n.d., none detected.
Number of weeks of dietary dox treatment.
Measured by ELISA.
Compared with controls, there was a 14-fold increase in IL-4–producing cells in the skin-draining LNs and an 18-fold increase in the spleen, but just a 4-fold increase in the intestinal mesenteric LNs (Table I). In addition, histological analysis of hematoxylin and eosin (H&E)–stained sections of both the small intestine and colon from K5-TSLP mice showed no inflammation in these tissues (unpublished data). These results highlight the organ specificity of the disease phenotype, which may in part be mediated by selective migration of the responding Th2 CD4+ T cells to the skin. Homing to the inflamed skin requires expression of ligands for either P- or E-selectin (referred to as P- or E-ligand) and is associated with expression of CCR4 and CCR10, which are receptors for the cutaneous chemokines CCL17 and CCL27 (13–15). CCR4 is also expressed by nearly all of the IL-4–producing Th2 cells (16, 17). Based on these expression patterns, we hypothesized that these molecules may play important roles in the tissue- and Th2 cell–specific inflammation observed in K5-TSLP mice.
Using P- and E-selectin fusion proteins, CD4+ T cells from the spleen and subcutaneous-draining LNs of K5-TSLP mice were examined for P- and E-ligand expression. In both tissues, there was a striking increase in the expression of both P- and E-ligand among the total CD4+ T cell population (Fig. 5 A). Moreover, these P- and E-ligand–expressing cells were dramatically enriched in IL-4 producers, demonstrating a direct correlation between Th2 effector cell function and expression of cutaneous homing receptors in the K5-TSLP animals.
Figure 5.
CD4+ T cells in the K5-TSLP mice express cutaneous homing receptors. Flow cytometry analysis of homing receptor expression by CD4+ T cells isolated from a K5-TSLP mouse and an NLC after 5 wk of dox treatment. Data are representative of more than five animals analyzed in this manner. (A) P-ligand (P-lig) and E-ligand (E-lig) expression was assessed by binding to P- and E-selectin–IgM fusion proteins in conjunction with intracellular cytokine staining to detect expression of IL-4. The percentages of cells in each quadrant are shown. Per., peripheral. (B) RT-PCR analysis of chemokine expression in the skin of a K5-TSLP mouse and NLC after 3 wk of dox treatment. From the K5-TSLP mouse, total RNA isolated from affected lesional skin (L) and unaffected skin (U) were analyzed separately. The levels of mRNA of CCL17, CCL22, and β-actin were determined by semiquantitative RT-PCR analysis with threefold serial dilution of the template cDNA. (C) CCR4 and CCR7 expression assessed by binding to CCL22- and CCL19-IgG3 fusion proteins in conjunction with staining for L-selectin (L-sel). The percentages of cells in each quadrant are shown.
RT-PCR was performed to assess expression of cutaneous chemokines in the skin of K5-TSLP and control mice (Fig. 5 B). Levels of CCL17 mRNA were dramatically increased in the affected skin from K5-TSLP mice, whereas unaffected skin also showed elevated levels and skin from control mice had low but detectable levels (Fig. 5 B). We were unable to detect expression of CCL22, the only other known CCR4 ligand, in any of the skin samples. Consistent with the expression of CCL17 in the skin and the Th2 phenotype of the cells, CD4+ T cells in the spleens of K5-TSLP mice showed a substantial increase in expression of CCR4, as assessed by staining with a CCL22-Fc fusion protein (Fig. 5 C). CCR4 expression was correlated with a loss of the lymphoid tissue homing receptors L-selectin and CCR7, indicating a shift in the tropism of these pathogenic cells away from the secondary lymphoid tissues and into the cutaneous lesions.
Skin inflammation in K5-TSLP mice is T cell independent
The data described in Figs. 4 and 5 demonstrate that CD4+ T cells in the skin-draining LNs of K5-TSLP mice have a pronounced Th2 phenotype. These data suggest a role for CD4+ T cells in the development and/or progression of skin inflammation in these mice. However, TSLP is thought to directly activate DCs and other myeloid cells, and the accumulation of CD4+ Th2 cells may be a secondary effect of TSLP overexpression that serves to amplify and sustain the disease process. Therefore, to directly determine if Th2 cells were required for disease in the K5-TSLP mice, these animals were crossed with mice lacking T cells (TCRβ−/− mice), resulting in K5-TSLP/T cell−/− mice. K5-TSLP/T cell−/− mice, as well as controls, were given dietary dox for 3 wk, and skin inflammation was assessed histologically. Epidermal thickening and dermal infiltration was observed in the skin of both K5-TSLP and K5-TSLP/T cell−/− mice (Fig. 6, A–D). An examination of the cells contained in the dermal infiltrates revealed mostly mast cells and eosinophils, which was similar to what was seen in the TCRβ sufficient, K5-TSLP mice (Fig. 6 E). In addition, the K5-TSLP/T cell−/− mice displayed little or no serum IgE, demonstrating that T cells are necessary for the production of this isotype and that B cells do not contribute greatly to the overall phenotype seen in these mice (unpublished data). These data demonstrate that T cells are not required for the skin inflammation induced by TSLP, suggesting instead that this cytokine acts directly on myeloid lineage cells to promote atopic inflammation.
Figure 6.
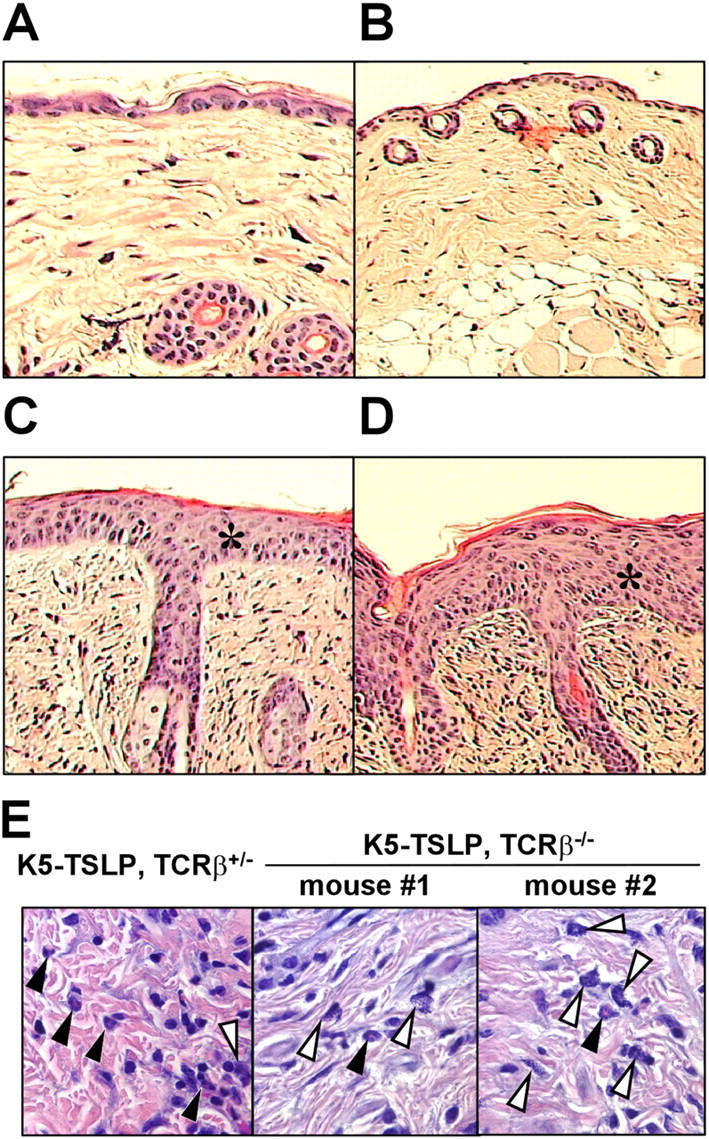
Histological observations of the skin in different genotypes of mice that were crossed between K5-TSLP and TCRβ KO mice. (A) A wild-type mouse without the transgene for K5-TSLP and KO alleles for TCRβ. (B) A mouse without the transgene for K5-TSLP but with KO alleles for TCRβ. (C) A mouse with the transgene for K5-TSLP but without KO alleles for TCRβ. (D) A mouse with the transgene for K5-TSLP and KO alleles for TCRβ. Skin sections show pronounced acanthosis (asterisks) and infiltration in K5-TSLP mice with and without KO alleles for TCRβ at 10× (C and D), suggesting that skin symptoms of AD occur in K5-TSLP mice, regardless of whether αβ T cells are present. All paraffin-embedded sections were stained with H&E. (E) H&E-stained skin sections from K5-TSLP, TCRβ+/− and K5-TSLP, TCRβ−/− mice at 100×, showing the presence of eosinophils (closed arrowheads) and mast cells (open arrowheads) in skin from both sets of mice.
Discussion
The current model for the pathogenesis of allergic diseases holds that, after entry of allergen into the body, local DCs process antigen and migrate to the draining LNs. There, they prime naive allergen-specific CD4+ T cells, leading to their differentiation into Th2 effector lymphocytes that secrete proallergic inflammatory cytokines, including IL-4, IL-5, IL-13, and TNF-α. IL-4, in conjunction with cell surface interactions, promotes class switch of allergen-specific B cells to IgE, resulting in mast cell activation, whereas IL-5 promotes the differentiation and activation of eosinophils. The degranulated products of mast cells and eosinophils then contribute directly to the clinical phenotype seen in atopic disease (5–7).
An essential step in this pathway is the activation of DCs and their subsequent maturation into cells capable of priming a Th2 effector T cell response. However, the factors that promoted this maturation remained obscure until recently. TSLP can activate CD11c+ DCs in vitro, and these DCs subsequently promote differentiation of Th2 effector cells (9, 11), suggesting that this cytokine may function as one of the initiating factors in allergic inflammation in vivo. Consistent with this hypothesis, TSLP is expressed in the affected skin of AD patients, where it is associated with DC activation and migration (11). We show that transgenic overexpression of this cytokine in murine skin results in allergic inflammation with the clinical, histologic, and immunologic characteristics of human AD. This AD-like phenotype is accompanied by massive lymphadenopathy and splenomegaly and a dramatic increase in both the frequency and absolute number of IL-4–producing Th2 CD4+ T cells. In a similar fashion, mice that express a lung-specific TSLP transgene develop a spontaneous airway inflammatory disease resembling human asthma (unpublished data). These data provide the first direct demonstration that overexpression of this cytokine in vivo does indeed result in tissue-specific allergic inflammation.
The precise function of TSLP in promoting allergic inflammation remains to be defined. Its unique ability to enable DCs to prime Th2 responses strongly suggests that this is one mechanism by which TSLP causes the AD-like syndrome in the K5-TSLP mice. However, although most strongly expressed by myeloid lineage cells and CD11c+ DCs in humans (9), the specific γ chain–like TSLP receptor (TSLPR) is also expressed by a variety of T and B cell lines (unpublished data; Rawlings, D.J, personal communication). Indeed, TSLP was first identified as a factor produced by thymic epithelium that could promote B cell differentiation (18). In this regard, it is similar to the related cytokine IL-7, and the functional TSLP receptor complex consists of a heterodimer of TSLPR and the IL-7 receptor α chain (10, 19). It is therefore possible that a portion of the phenotype observed in the K5-TSLP mice is a result of this cytokine acting on CD4+ T cells, as recently shown by Al-Shami et al. (20). However, the finding that K5-TSLP, TCRβ−/− mice still develop skin inflammation suggests that CD4+ T cells are not required for disease development and progression and that TSLP may directly activate macrophages, eosinophils, mast cells, and other myeloid effector cells that mediate allergic inflammation.
Epithelial overexpression of TSLP likely represents one of the early events in allergic inflammation. The fact that TSLP is not universally overexpressed in the skin of AD patients, but rather is limited to those areas manifesting disease, implies that other local factors must play a role in triggering TSLP overexpression on contact with allergen in affected individuals. Identifying these triggers is therefore essential for understanding the etiology of allergic diseases such as AD. It has long been recognized that genetic factors play a role in susceptibility to AD and other allergic diseases. Therefore, it will be of great interest to determine whether polymorphisms in the TSLP gene correlate with susceptibility to allergic inflammation. However, as many other factors, such as skin barrier disruption, Staphylococcus aureus colonization, and various other immunologic abnormalities, have been linked to AD, it seems likely that a genetic polymorphism in TSLP would be one of many predisposing factors that act in conjunction to induce a disease phenotype.
The adhesion molecules and chemoattractant receptors that mediate leukocyte homing are responsible for recruiting the necessary and appropriate cell types to sites of infection and inflammation (21). Such molecules are differentially associated with specific cell types, tissues, and/or inflammatory settings and are thus necessary for precisely correlating inflammatory cell function and tissue localization in any given disease setting. As such, they also serve as important targets for disease intervention. P- and E-selectin mediate homing of CD4+ T cells into inflamed skin, and their expression is rapidly up-regulated on vascular endothelial cells during cutaneous inflammation (22). Reciprocally, CD4+ T cells up-regulate selectin ligands on activation in cutaneous lymphoid tissues (23). In the dox-treated K5-TSLP mice, there is a dramatic increase in the frequency of Th2 cells that coexpress E- and/or P-ligand, which is consistent with the skin-specific expression of TSLP in this model. Lymphocyte migration to the skin is also mediated by cutaneous chemokines and their corresponding receptors. The K5-TSLP mice had an elevated frequency of CD4+ T cells expressing the chemokine receptor CCR4. CCR4 is associated with homing to sites of cutaneous inflammation (24, 25), and its ligand CCL17 is expressed in the affected skin of AD patients and in the K5-TSLP mice (26). In addition to its role in T cell homing to the skin, CCR4 expression is strongly associated with Th2 phenotype cells (16). Interestingly, human DCs activated by TSLP produce the CCR4 ligands CCL17 and CCL22 (11). In the secondary lymphoid tissues, this may set up a positive feedback loop by attracting IL-4–producing Th2 cells to TSLP-stimulated DCs, further promoting their selective activation and expansion.
Th2 cytokine–mediated allergic reactions are orchestrated in both innate and adaptive immune cells. Interestingly, K5-TSLP, TCRβ−/− mice show AD symptoms such as thickening of the skin and cellular infiltrates, similar to those seen in TCR+ K5-TSLP mice (Figs. 2 and 6). In addition, rag2-deficient mice expressing a lung-specific TSLP transgene still developed asthma-like symptoms (unpublished data). Our data are consistent with a two-stage model for TSLP-mediated inflammation. Initially, local production of TSLP by epithelial cells leads to the stimulation and recruitment of macrophages and eosinophils, resulting in the cellular infiltrate seen in the K5-TSLP, TCRβ−/− mice. In addition, skin-resident DCs, matured through exposure to TSLP, migrate to the draining LNs and attract and activate Th2 CD4+ T cells. Thus, the initial targets of TSLP expression at sites of inflammation are likely myeloid-derived cells, which are then capable of initiating the disease process. Lymphoid cells are then involved in the progression and amplification of the disease. Several lines of evidence support this model. First, K5-TSLP mice lacking T cells still develop skin-specific inflammatory disease, and rag2-deficient mice expressing a lung-specific TSLP transgene develop airway inflammation (Fig. 6 and not depicted). Second, TSLP treatment of CD11c+ DCs and bone marrow–derived macrophages leads directly to Stat5 activation and CCL17 production (unpublished data; Roach, S.K., personal communication), indicating that TSLP can directly activate myeloid lineage cells. In addition, Osborn et al. (27) showed that systemic TSLP transgene expression affected myelopoiesis. Finally, eosinophils were capable of migrating to the lung and producing IL-4 in rag2-deficient mice infected with Nippostrongylus brasiliensis (28). Addition of antigen-activated CD4+ T cells led to eosinophil degranulation at the site of inflammation, which was consistent with a secondary, amplifying role for T cells in the disease observed in K5-TSLP mice.
The K5-TSLP mice present a novel model for AD, based on the tissue-specific expression of a cytokine shown to be at elevated levels in human patients. As TSLP is likely involved in the initiating events of the inflammatory cascade, these mice will allow for the dissection of downstream cells and mediators that are critical for disease development and progression. The data presented here demonstrate that T cells are not required for disease development. Future work will focus on the role of inflammatory mediators, such as IL-4, that have been implicated in the pathology of AD and other allergic diseases (29). This inducible animal model of AD allows for the identification and characterization of the various aspects of AD pathogenesis from subclinical onset to final disease phenotype and provides a preclinical model for the analysis of new therapeutic modalities for the treatment of this disease.
Materials and mETHODS
Generation of inducible skin-specific TSLP transgenic mice (K5-rtTA/tetO-TSLP).
A cDNA encoding mouse TSLP was cloned into a vector containing a heptameric tetracycline operator, CMV minimal promoter, and rabbit β-globin intron (provided by D. Mathis, Harvard Medical School, Boston, MA). The tetO-TSLP insert was isolated and microinjected into [(C3H × C57BL/6F1) × C57BL/6] F2 oocytes. The offspring were assessed for vector incorporation by PCR amplification of genomic DNA processed from tail clippings using the following primers for the TSLP cDNA: 5′-GACAGCATGGTTCTTCTCAG-3′ and 5′-CTGGAGATTGCATGAAGG-3′ (30 cycles of 94°C for 60 s denaturation, 55°C for 60 s annealing, and 72°C for 60 s extension). To generate tetracycline-inducible skin-specific TSLP transgenic mice, tetO-TSLP mice were crossed with K5-rtTA mice (provided by A. Glick, National Institutes of Health, Bethesda, MD; reference 12). These latter mice contain the mouse reverse tetracycline transactivator (rtTA) under the control of the keratin 5 (K5) promoter. Pups were screened for the presence of K5-rtTA using the following primers: 5′-AGCTGCTTAATGAGGTCGGA-3′ and 5′-GCTTGTCGTAATAATGGCGG-3′ (30 cycles of 94°C for 60 s denaturation, 55°C for 60 s annealing, and 72°C for 60 s extension for both sets). After weaning, mice containing both transgenes (referred to as K5-TSLP) were treated with the tetracycline analogue dox at a concentration of 1 mg/ml in the drinking water to induce rtTA transactivation of the TSLP transgene. Nontransgenic and single-positive tetO-TSLP littermates were included as controls. The TCRβ KO mice were purchased from the Jackson Laboratory and crossed with K5-TSLP mice. The pups were screened using the primers and conditions recommended by the vendor. All mice were maintained under specific pathogen-free conditions without manipulation and were killed for analysis after 2.5–5 wk of dox treatment. All animal work was conducted under conditions approved by the Benaroya Research Institute Animal Care and Use Committee.
Detection of TSLP expression.
Total RNA was isolated from skin samples using the RNeasy Mini Kit (QIAGEN). First strand cDNA was synthesized from 1–2 μg of total RNA using oligo(dT) priming and RT (SuperScript II; Invitrogen). TSLP cDNA was PCR amplified from the resulting first strand cDNA using the transgene-specific primers 5′-GACAGCATGGTTCTTCTCAG-3′ and 5′-AGCCACCACCTTCTGATAGG-3′ (to distinguish from endogenous TSLP). The PCR reaction mixture was run through 32 amplification cycles (94°C for 60 s denaturation, 55°C for 60 s annealing, and 72°C for 60 s extension), and the products were run on 1.5% agarose gel electrophoresis to detect TSLP mRNA expression. Products of PCR amplification using primers for β-actin (5′-GAGAGGGAAATCGTGCGTGA-3′ and 5′-ACATCTGCTGGAAGGTGGAC-3′) were included as controls.
Histopathology and immunohistochemistry.
Skin sections were fixed in 10% buffered formalin (VWR Scientific Products), embedded in paraffin, and stained with H&E.
Antibodies and flow cytometry.
Single cell suspensions were prepared from the spleen, cutaneous peripheral LNs (cervical, axillary, and inguinal nodes), and intestinal mesenteric LNs. For surface staining, the following antibodies were used: αCD4-PerCP (clone RM4-5; BD Biosciences), αCD8α–FITC (clone 53-6.7; eBioscience), and αCD62L-APC (clone MEL-14; eBioscience). For intracellular cytokine staining, isolated splenic or LN cells were activated in complete DMEM containing 50 ng/ml PMA, 1 μg/ml ionomycin, and 10 μg/ml monensin (all chemicals were obtained from Sigma-Aldrich) for 4 h at 37°C and subsequently washed, surface stained for expression of CD4 and CD8, fixed with 4% paraformaldehyde in PBS, and permeabilized in Perm/Wash buffer (BD Biosciences). Cells were then incubated for 30 min at 4°C with different combinations of the following cytokine-specific mAbs (all were obtained from eBioscience) diluted in Perm/Wash buffer: αIL-4–APC (clone 11B11), αIL-4–PE (clone 11B11), αIFN-γ–FITC (clone XMG1.2), αIFN-γ–APC (clone XMG1.2), αIL-10–PE (clone JES5-16E3), and αTNF-α–PE (clone MP6-XT22). Labeled cells were analyzed by flow cytometry. To assess binding of CD4+ T cells to P- and E-selectin (provided by J. Lowe, University of Michigan, Ann Arbor, MI), cells were incubated sequentially with either a P- or E-selectin–human IgM fusion protein (produced in COS-7 cells as previously described; reference 30), followed by biotinylated goat anti–human IgM (Jackson ImmunoResearch Laboratories) and streptavidin–PE (eBioscience). After selectin–ligand staining, cells were activated with PMA/ionomycin and stained for expression of IL-4 as described above. CCL19– and CCL22–human IgG3 fusion proteins were used to examine expression of the chemokine receptors CCR7 and CCR4, respectively. cDNAs encoding amino acids 26–108 of CCL19 and 25–92 of CCL22 were amplified from RNA isolated from murine spleen and cloned in frame downstream of the human CD5 signal sequence and upstream of the Fc portion of human IgG3 in the PEAK13 expression vector (provided by B. Seed, Harvard Medical School, Boston, MA). These constructs were transiently transfected into COS-7 cells, and the supernatants of these transfected cells were used directly to stain lymphocytes from the indicated mice. PE-labeled goat–anti–human IgG (Jackson ImmunoResearch Laboratories) was used to detect binding of the fusion protein. As a specificity control, supernatants were preincubated for 5 min with blocking antibodies directed against either CCL19 (clone 87102; R&D Systems) or CCL22 (clone 158132; R&D Systems), which completely abrogated staining.
Cutaneous cytokine and chemokine expression.
Cytokine and chemokine expression in the skin was analyzed using RT-PCR. For IL-4, IL-5, IL-13, TNF-α, and IFN-γ, we used previously described primers and conditions (31). For chemokine expression, the following primer sets were used: CCL17, 5′-AGGTCACTTCAGATGCTGCTCC-3′ and 5′-TCATGGCCTTGGGTTTTTCACC-3′; CCL22, 5′-ATGGCTACCCTGCGTGTCCCACTC-3′ and 5′-CTAGGACAGTTTATGGAGTAGC-3′; and CCL27, 5′-TGTTACTGTTGCTTCTGAGCCCG-3′ and 5′-GTTTTGCTGTTGGGGGTGTGAG-3′. PCR reactions were run through 32–35 amplification cycles (94°C for 60 s denaturation, 55°C for 60 s annealing, and 72°C for 60 s extension), and products were separated on 1.5% agarose gels to detect cytokine and chemokine mRNA expression. Products of PCR amplification using primers for the housekeeping gene glycerol-3-phosphate dehydrogenase (5′-GGTCATCCATGACAACTTTGG-3′ and 5′-CATACCAGGAAATGAGCTTGAC-3′) or β-actin (as described in Detection of TSLP expression section) were included as controls.
Measurement of serum immunoglobulin levels.
Sera were analyzed by ELISA for total IgG1, IgG2a, and IgE. For total serum IgG1 and IgG2a, plates were coated overnight with 2 μg/ml goat anti–mouse Ig (H+L; Southern Biotechnology Associates, Inc.), blocked with PBS/1% BSA, and incubated sequentially with serial dilutions of mouse sera, 2 μg/ml alkaline phosphatase (AP)–conjugated rat anti–mouse IgG1/IgG2a (1:1,000; Southern Biotechnology Associates, Inc.), and 1 mg/ml of the AP substrate DNP phosphate (DNPP; Sigma-Aldrich). For total serum IgE, plates were coated with 2 μg rat anti–mouse IgE (BD Biosciences), blocked with PBS/1% BSA, and incubated sequentially with serial dilutions of mouse sera, biotinylated rat anti–mouse IgE (1:1,000; BD Biosciences), SAV-AP (1:1,000; BD Biosciences), and 1 mg/ml DNPP. All samples were analyzed in duplicate or triplicate. Plates were read on a microplate autoreader (EL311S; Bio-Tek Instruments, Inc.) at 405 nm, and concentrations were calculated by plotting against standard curves generated from purified IgG1, IgG2a (Southern Biotechnology Associates, Inc.), and IgE (BD Biosciences).
Online supplemental material.
Fig. S1 shows the measurement of serum IgE levels. Total IgE levels in serum were determined using ELISA, as described in the previous section. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20041503/DC1.
Acknowledgments
The authors would like to thank Dr. John Lowe for the P- and E-selectin constructs; Dr. Daniel Branstatter, Robert Underwood, and Marcia Usui for assistance with histopathology; and Dr. H. Denny Liggitt for critical comments.
This work was supported in part by National Institutes of Health grant AI44259 (to S.F. Ziegler).
A. Brewer and M.R. Comeau are employees of Amgen Corporation. The authors have no other conflicting financial interests.
Abbreviations used: AD, atopic dermatitis; dox, doxycycline; H&E, hematoxylin and eosin; NLC, normal littermate control; TSLP, thymic stromal lymphopoietin.
J. Yoo and M. Omori contributed equally to this work.
References
- 1.Oranje, A.P., and F.B. de Waard-van der Spek. 2002. Atopic dermatitis: review 2000 to January 2001. Curr. Opin. Pediatr. 14:410–413. [DOI] [PubMed] [Google Scholar]
- 2.Kang, K., and S.R. Stevens. 2003. Pathophysiology of atopic dermatitis. Clin. Dermatol. 21:116–121. [DOI] [PubMed] [Google Scholar]
- 3.Eichenfield, L.F., J.M. Hanifin, T.A. Luger, S.R. Stevens, and H.B. Pride. 2003. Consensus conference of pediatric atopic dermatitis. J. Am. Acad. Dermatol. 49:1088–1095. [DOI] [PubMed] [Google Scholar]
- 4.Gupta, M.A., and A.K. Gupta. 2003. Psychiatric and psychological co-morbidity in patients with dermatologic disorders: epidemiology and management. Am. J. Clin. Dermatol. 4:833–842. [DOI] [PubMed] [Google Scholar]
- 5.Leung, D.Y.M., M. Boguniewicz, M.D. Howell, and Q. Hamid. 2004. New insights into atopic dermatitis. J. Clin. Invest. 113:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novak, N., T. Bieber, and D.Y.M. Leung. 2003. Immune mechanisms leading to atopic dermatitis. J. Allergy Clin. Immunol. 112:S128–S138. [DOI] [PubMed] [Google Scholar]
- 7.Sinke, J.D., V.P.M.G. Rutten, and T. Willemse. 2002. Immune dysregulation in atopic dermatitis. Vet. Immunol. Immunopathol. 87:351–356. [DOI] [PubMed] [Google Scholar]
- 8.Sims, J.E., D.E. Williams, P.J. Morrissey, K. Garka, D. Foxworthe, V. Price, S.L. Friend, A. Farr, M.A. Bedell, N.A. Jenkins, et al. 2000. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 192:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reche, P.A., V. Soumelis, D.M. Gorman, T. Clifford, M. Liu, M. Travis, S.M. Zurawski, J. Johnston, Y.J. Liu, H. Spits, et al. 2001. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167:336–343. [DOI] [PubMed] [Google Scholar]
- 10.Park, L.S., U. Martin, K. Garka, B.C. Gliniak, J.P. DiSanto, W. Muller, D.A. Largaespada, N.G. Copeland, N.A. Jenkins, A.G. Farr, et al. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soumelis, V., P.A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673–680. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, I., T. Owolabi, M. Marco, C. Lam, and A. Glick. 2000. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J. Invest. Dermatol. 115:788–794. [DOI] [PubMed] [Google Scholar]
- 13.Tietz, W., Y. Allemand, E. Borges, D. von Laer, R. Hallmann, D. Vestweber, and A. Hamann. 1998. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 161:963–970. [PubMed] [Google Scholar]
- 14.Reiss, Y., A.E. Proudfoot, C.A. Power, J.J. Campbell, and E.C. Butcher. 2001. CC chemokine receptor (CCR) 4 and the CCR10 ligand cutaneous T cell–attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales, J., B. Homey, A.P. Vicari, S. Hudak, E. Oldham, J. Hedrick, R. Orozco, N.G. Copeland, N.A. Jenkins, L.M. McEvoy, and A. Zlotnik. 1999. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl. Acad. Sci. USA. 96:14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, C.H., L. Rott, E.J. Kunkel, M.C. Genovese, D.P. Andrew, L. Wu, and E.C. Butcher. 2001. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonecchi, R., G. Bianchi, P.P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P.A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin, S.D., R.M. Koelling, S.L. Friend, D.E. Isaksen, S.F. Ziegler, R.M. Perlmutter, and A.G. Farr. 1999. Thymic stromal lymphopoietin (TSLP): a cytokine that promotes the development of IgM+ cells in vitro and signals via a novel mechanism. J. Immunol. 162:677–683. [PubMed] [Google Scholar]
- 19.Pandey, A., K. Ozaki, H. Baumann, S.D. Levin, A. Puel, A.G. Farr, S.F. Ziegler, W.J. Leonard, and H.F. Lodish. 2000. Cloning of a novel receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 1:59–64. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shami, A., R. Spolski, J. Kelly, T. Fry, P.L. Schwartzberg, A. Pandey, C.L. Mackall, and W.J. Leonard. 2004. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 200:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell, D.J., G.F. Debes, B. Johnston, E. Wilson, and E.C. Butcher. 2003. Targeting T cell responses by selective chemokine receptor expression. Semin. Immunol. 15:277–286. [DOI] [PubMed] [Google Scholar]
- 22.Kansas, G.S. 1996. Selectins and their ligands: current concepts and controversies. Blood. 88:3259–3287. [PubMed] [Google Scholar]
- 23.Campbell, D.J., and E.C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel, E.J., J. Boisvert, K. Murphy, M.A. Vierra, M.C. Genovese, A.J. Wardlaw, H.B. Greenberg, M. Hodge, L. Wu, E.C. Butcher, and J.J. Campbell. 2002. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am. J. Pathol. 160:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soler, D., T.L. Humphreys, S.M. Spinola, and J.J. Campbell. 2003. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 101:1677–1682. [DOI] [PubMed] [Google Scholar]
- 26.Vestergaard, C., K. Bang, B. Gesser, H. Yoneyama, K. Matsushima, and C.G. Larsen. 2000. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J. Invest. Dermatol. 115:640–646. [DOI] [PubMed] [Google Scholar]
- 27.Osborn, M.J., P.L. Ryan, N. Kirchhof, A. Panoskaltsis-Mortari, F. Mortari, and K.-S.R.S. Tudor. 2004. Overexpression of murine TSLP impairs lymphopoiesis and myelopoiesis. Blood. 103:843–851. [DOI] [PubMed] [Google Scholar]
- 28.Shinkai, K., M. Mohrs, and R.M. Locksley. 2002. Helper T cells regulate type-2 innate immunity in vivo. Nature. 420:825–829. [DOI] [PubMed] [Google Scholar]
- 29.Chan, L.S., N. Robinson, and L. Xu. 2001. Expression of IL-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J. Invest. Dermatol. 117:977–983. [DOI] [PubMed] [Google Scholar]
- 30.Knibbs, R.N., R.A. Craig, P. Maly, P.L. Smith, F.M. Wolber, N.E. Faulkner, J.B. Lowe, and L.M. Stoolman. 1998. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J. Immunol. 161:6305–6315. [PubMed] [Google Scholar]
- 31.Horikawa, T., T. Nakayama, I. Hikita, H. Yamada, R. Fujisawa, T. Bito, S. Harada, A. Fukunaga, D. Chantry, P.W. Gray, et al. 2002. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int. Immunol. 14:767–773. [DOI] [PubMed] [Google Scholar]