Abstract
The enzyme 11β–hydroxysteroid dehydrogenase (HSD) type 1 converts inactive cortisone into active cortisol in cells, thereby raising the effective glucocorticoid (GC) tone above serum levels. We report that pharmacologic inhibition of 11β-HSD1 has a therapeutic effect in mouse models of metabolic syndrome. Administration of a selective, potent 11β-HSD1 inhibitor lowered body weight, insulin, fasting glucose, triglycerides, and cholesterol in diet-induced obese mice and lowered fasting glucose, insulin, glucagon, triglycerides, and free fatty acids, as well as improved glucose tolerance, in a mouse model of type 2 diabetes. Most importantly, inhibition of 11β-HSD1 slowed plaque progression in a murine model of atherosclerosis, the key clinical sequela of metabolic syndrome. Mice with a targeted deletion of apolipoprotein E exhibited 84% less accumulation of aortic total cholesterol, as well as lower serum cholesterol and triglycerides, when treated with an 11β-HSD1 inhibitor. These data provide the first evidence that pharmacologic inhibition of intracellular GC activation can effectively treat atherosclerosis, the key clinical consequence of metabolic syndrome, in addition to its salutary effect on multiple aspects of the metabolic syndrome itself.
Glucocorticoids (GCs) influence a wide variety of physiologic functions, including immune and inflammatory responses, stress responses, aspects of development, and metabolism. Thus, it is not surprising that the levels of these multipotent hormones are tightly regulated. Secretion of GCs from the adrenal cortex is controlled by negative feedback via the hypothalamic-pituitary-adrenal (HPA) axis. The main regulators of intracellular GC levels are 11β–hydroxysteroid dehydrogenase (HSD) enzymes. Two isoforms of 11β-HSD have been cloned and characterized (1, 2). 11β-HSD type 1 is an NADP(H)-dependent enzyme that acts primarily as a reductase in intact cells, converting the inactive 11-keto metabolites cortisone (in humans) or 11-dehydrocorticosterone (in rodents) into the active GCs cortisol or corticosterone, respectively. 11β-HSD1 is expressed in most tissue types and potentiates the action of endogenous GCs by increasing their local concentration. 11β-HSD type 2 is an NAD(H)-dependent enzyme that catalyzes the reverse reaction, oxidizing active GCs to their inactive 11-keto forms. Although 11β-HSD1 is widely expressed, 11β-HSD2 expression is limited to tissues that express the mineralocorticoid receptor, such as the kidney and gut, as well as to the placenta. By inactivating cortisol, 11β-HSD2 prevents it from binding to the mineralocorticoid receptor, thus conferring aldosterone specificity on the receptor. In the placenta, the enzyme prevents maternal GCs from reaching the fetal circulation.
The metabolic syndrome is a cluster of cardiovascular risk factors, including visceral obesity, insulin resistance, dyslipidemia, and hypertension. It has been noted that the features of metabolic syndrome are also seen in patients with increased circulating GCs, or Cushing's syndrome. However, patients with metabolic syndrome do not exhibit increased circulating GC levels. Thus, it has been suggested that metabolic syndrome may result from increased intracellular GC tone, as may occur with elevated 11β-HSD1 activity, and that pharmacologic inhibition of 11β-HSD1 may alter intracellular GC levels and be therapeutic for metabolic syndrome (3–5).
Several recent experiments in mice support this hypothesis. Overexpression of 11β-HSD1 in murine adipose leads to a metabolic syndrome–like phenotype, including increased central obesity, hypertension, impaired glucose tolerance, and hypertriglyceridemia (6, 7). These transgenic mice have elevated intraadipose corticosterone levels but normal circulating levels. Conversely, mice that fail to express 11β-HSD1 are resistant to the development of metabolic syndrome (8, 9). 11β-HSD1 KO mice resist hyperglycemia provoked by obesity or stress, resist weight gain on high-fat (HF) feeding, and have a cardioprotective lipid phenotype, including elevated high density lipoprotein (HDL) cholesterol and low triglyceride levels. This beneficial phenotype occurs despite activation of the HPA axis in these mice, which underscores that changes in intracellular, rather than circulating, GC levels determine the metabolic phenotype in mice.
Though the predominant source of morbidity and mortality in metabolic syndrome is from atherosclerotic cardiovascular disease, the effect of 11β-HSD1 inhibition on atherogenesis has not been studied. A potent and selective nonsteroidal inhibitor of murine and human 11β-HSD1 was dosed in murine models of diet-induced obesity (DIO) and type 2 diabetes and a mouse model of atherosclerosis, the apolipoprotein E (apoE) KO mouse. We report that pharmacologic inhibition of 11β-HSD1 can ameliorate multiple facets of metabolic syndrome as well as prevent atherosclerotic lesion progression in these disease models.
Results
Potency and pharmacodynamic (PD) activity of 11β-HSD1 inhibitor
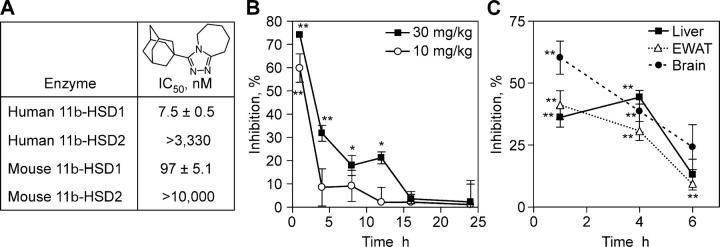
To examine the role of 11β-HSD1 in the etiology of metabolic syndrome and to explore the role of 11β-HSD1 in atherosclerotic plaque formation, we tested the effect of pharmacologic inhibition of 11β-HSD1 using a novel potent and selective nonsteroidal inhibitor in murine models of diabetes, obesity, and atherosclerosis. Compound 544 (3-(1-adamantyl)-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[4,3-a]azepine; Fig. 1 A) is a potent inhibitor of both mouse and human 11β-HSD1 with >100- or >450-fold selectivity over mouse or human 11β-HSD2, respectively (Fig. 1 A). The compound is a competitive inhibitor for cortisone, but not NADPH, suggesting that it interacts with the steroid binding site (unpublished data). The specificity of the inhibitor was assessed in >170 receptor binding and enzyme assays, and no activity at concentrations <10 μM was detected, including GC receptor binding.
Figure 1.
Characteristics of the 11β-HSD1 inhibitor compound 544. (A) Structure and in vitro potency of compound 544. Potency of compound 544 against human and murine 11β-HSD1 and -HSD2 was determined as described in Materials and methods. The IC50 was determined from >25 determinations for each enzyme. (B) PD activity of 11β-HSD1 inhibitor in serum. Compound 544 was orally administered to mice and the percent inhibition of [3H]cortisone conversion to cortisol was measured relative to controls dosed with vehicle only. Data are a representative study of at least two experiments. Statistical significance is calculated between the treatment groups and vehicle controls (*, P < 0.05; **, P < 0.005). Values represent the mean ± SEM. (C) PD activity of 11β-HSD1 inhibitor in key organs. Data are the mean of two experiments. Statistical significance is calculated between the treatment groups and vehicle controls (*, P < 0.05; **, P < 0.005). Values represent the mean ± SEM.
A PD mouse model was used to evaluate the efficacy of compound 544 in inhibiting 11β-HSD1 reductase activity in the entire animal. Evidence from 11β-HSD1 KO mice suggests that 11β-HSD1 is the only 11β reductase in mice capable of converting inactive to active GC (8). 2 min of exposure after i.v. administration of [3H]cortisone resulted in 58% conversion to [3H]cortisol in the vehicle-treated ICR mice (n = 3 per time point). Oral administration of compound 544 at 10 or 30 mg/kg inhibited 11β reductase activity at 1 h by 60 or 75%, respectively. For the higher dose, this effect diminished to ∼30% by 4 h with essentially no inhibition by 16 h (Fig. 1 B). Inhibitor administered at 10 mg/kg lowered enzyme activity by <10% by 4 h and was ineffective by 12 h.
Serum measurements of cortisol elaboration in the PD mouse model reflect the sum of inhibition occurring in the various 11β-HSD1–expressing tissues. Cortisone administered i.v. enters cells from the circulation, is converted by 11β-HSD1 to cortisol, and the cortisol, specifically labeled with tritium, reenters the circulation, where it is detected in serum by HPLC. To understand the degree of 11β-HSD1 activity inhibition in specific organs of interest, the liver, epididymal fat, and brains from mice dosed with compound 544 at 30 mg/kg were excised, minced, and exposed to [3H]cortisone ex vivo for 10 min (liver) or 2 h (epididymal white adipose tissue [EWAT] and brain). The elaboration of [3H]cortisol in the media can be dose-dependently inhibited by compound 544 administration before death (Fig. 1 C). Liver inhibition averaged 35% over the first 4 h after inhibitor administration (30 mg/kg) and nearly disappeared by 6 h. EWAT showed a similar time course of inhibition, whereas inhibition in the brain was shorter lived and diminished to low levels by 4 h.
11β-HSD1 inhibition decreases body weight gain, food intake, and fat pad weight in DIO mice
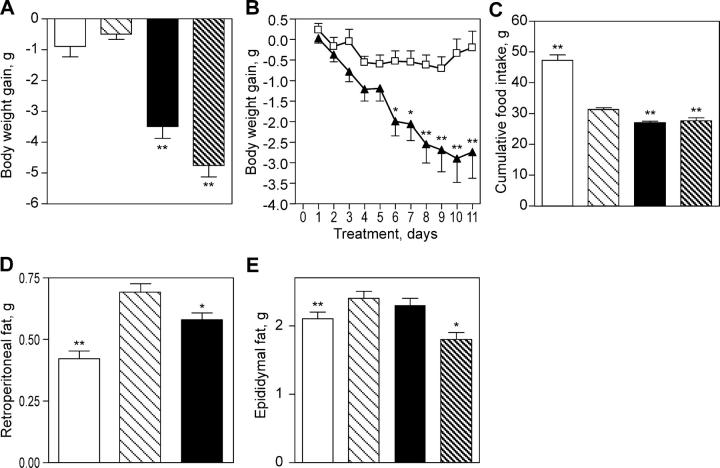
Inhibition of 11β-HSD1 lowers body weight and appetite in DIO mice. The DIO mice (n = 42) had an average body weight of 45.1 ± 0.5 g before dosing with compound 544 (vehicle control group: n = 41, 48.0 ± 0.6 g). After 11 d of dosing with 11β-HSD1 inhibitor at 20 mg/kg twice daily, their body weights were reduced by 7% (P < 0.001), whereas the body weight of the vehicle group was essentially unchanged (Fig. 2, A and B). Dosing with compound 544 also affected the animals' feeding behavior, resulting in a 12.1% reduction in cumulative food intake compared with the vehicle-treated animals (P < 0.001; Fig. 2 C). Furthermore, compound 544 caused a preferential loss of central fat pad weight, as there was a reduction of retroperitoneal fat weight in DIO mice (n = 24) compared with vehicle-treated control animals (Fig. 2 D), but it essentially had no effect on epididymal fat weight (Fig. 2 E). We did not observe changes of 11β-HSD1 mRNA expression in the liver or adipose after treatment of the DIO mice with the inhibitor (unpublished data). Unlike the DIO mice, the HF/streptozotocin (STZ) animals in our studies had only slightly greater average body weights than controls (41 ± 1.3 g vs. 39 ± 1.9 g, respectively), and 11β-HSD1 inhibition had little effect (39 ± 4 g for compound 544). Interestingly, there was a statistically nonsignificant lowering of serum leptin levels in the HF/STZ mice (5.88 ± 1.1 ng/ml for compound 544 vs. 9.6 ± 2.3 ng/ml for vehicle [P = 0.17] and 3.1 ± 0.5 ng/ml for chow/saline controls), despite no decreases in average body weight (39 ± 4 g for compound 544 vs. 41 ± 1.3 g for vehicle and 39 ± 1.9 g for chow/saline controls).
Figure 2.
11β-HSD1 inhibition decreases body weight gain, food intake, and fat pad weight in DIO mice. Compound 544–treated DIO mice (closed bars) were compared with obese animals dosed with vehicle (light hatched bars) and with lean controls (open bars). Animals treated with RU38486 served as positive controls (dark hatched bars). Compound 544 lowers body weight gain (A and B), food intake (C), and retroperitoneal fat pad weight (D), but not epididymal fat pad weight (E). Data shown are representative (B) or the mean (A and C–E) of at least two studies. Body weight gain was calculated relative to the weight of each individual animal before initiation of compound treatment. Statistical significance is calculated between the treatment groups and vehicle controls (*, P < 0.05; **, P < 0.005). Values represent the mean ± SEM.
11β-HSD1 inhibition lowers fasting glucose and improves insulin resistance in murine models of type 2 diabetes
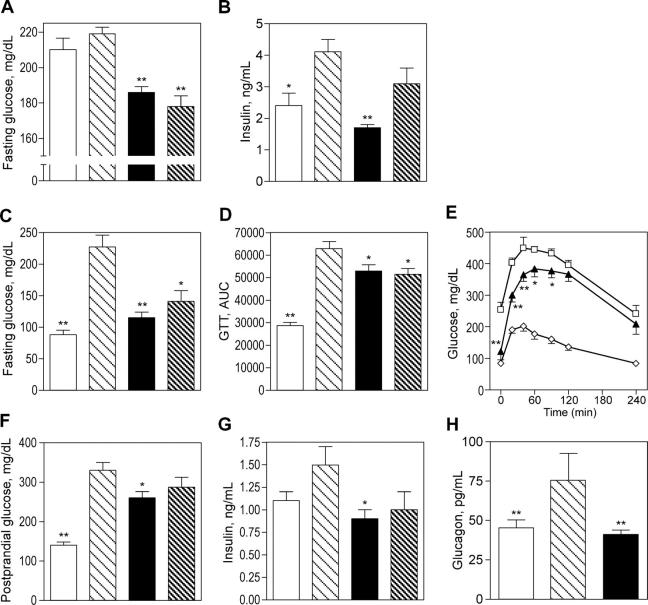
Inhibition of 11β-HSD1 improved insulin sensitivity, hyperglycemia, and other glycemic parameters in murine DIO and HF/STZ models. 7–12-mo-old DIO C57BL/6J mice on an HF diet from weaning were hyperglycemic and hyperinsulinemic (Fig. 3, A and B). 11β-HSD1 inhibition with compound 544 for 11 d in DIO mice at 20 mg/kg per os [PO], twice daily, lowered fasting serum glucose levels by 15% compared with vehicle-treated animals (P < 0.001; Fig. 3 A). Insulin levels were lowered below those in the lean controls (P < 0.001; Fig. 3 B).
Figure 3.
11β-HSD1 inhibition lowers fasting glucose and improves insulin resistance in murine models of type 2 diabetes. Compound 544 (closed bars and closed triangles) was administered twice daily by oral gavage to DIO obese mice (A and B) or HF/STZ mice (C–H). The effects were compared with affected animals that were dosed with vehicle (light hatched bars and open squares) and with unaffected lean or chow/saline controls (open bars and open diamonds). Animals treated with RU38486 served as a positive control (dark hatched bars). Compound 544 lowers fasting glucose (A, DIO and C, HF/STZ), serum insulin (B, DIO and G, HF/STZ), glucagon (H, HF/STZ), postprandial glucose (F, HF/STZ), and glucose excursions after oral glucose challenge (D and E). Data shown are the mean of at least two studies. Statistical significance is calculated between the treatment groups and vehicle controls for DIO and between the treated groups and the difference between vehicle and chow/saline controls for HF/STZ (*, P < 0.05; **, P < 0.005). Values represent the mean ± SEM.
The HF/STZ model manifests hyperglycemia, glucose intolerance, insulin resistance, and a loss of insulin-secreting β cells, all of which are characteristics of type 2 diabetes in human patients (10). ICR mice are induced to develop diabetes through combined insulin resistance, induced by HF feeding, and insulin deficiency, induced by a low dose of STZ. These diabetic HF/STZ animals (n = 17) were orally dosed with the 11β-HSD1 inhibitor at 30 mg/kg twice daily. Treated animals were compared with vehicle controls (n = 16) as well as to normoglycemic, normoinsulinemic mice (chow/saline control; n = 18) kept on a chow diet and sham injected with saline instead of STZ. Compound 544 lowered fasting and postprandial glucose levels and improved insulin sensitivity in HF/STZ animals after 9 d of oral dosing. An oral or i.p. glucose tolerance test performed after an overnight fast on day 9 of dosing revealed that the 11β-HSD1 inhibitor lowered fasting glucose nearly to chow/saline control levels (80% correction, P < 0.001; Fig. 3 C) and decreased the glucose serum excursions after glucose challenge by 29% (area under the curve correction calculated relative to the difference between the HF/STZ vehicle and chow/saline control; Fig. 3 D). The mean of two studies dosed at 30 mg/kg bis in die ([BID]; P = 0.02) is shown as a representative experiment in Fig. 3 E. Postprandial glucose levels for HF/STZ animals were significantly higher than chow/saline control levels (P < 0.001; Fig. 3 F) and were lowered 37% relative to controls by inhibition of 11β-HSD1 (P < 0.01). Insulin levels were also elevated in HF/STZ animals (Fig. 3 G), though they were not as high as in the HF-fed animals before partial β cell destruction by STZ treatment (2.7 ± 0.4 ng/ml). Treatment with the 11β-HSD1 inhibitor normalized these levels to the chow/saline control values (P < 0.01). Similarly, glucagon is elevated significantly in the serum of HF/STZ animals, and compound 544 treatment reduced these levels to those of the control (P < 0.005; Fig. 3 H).
11β-HSD1 inhibition lowers serum lipids in murine models of metabolic syndrome or atherosclerosis
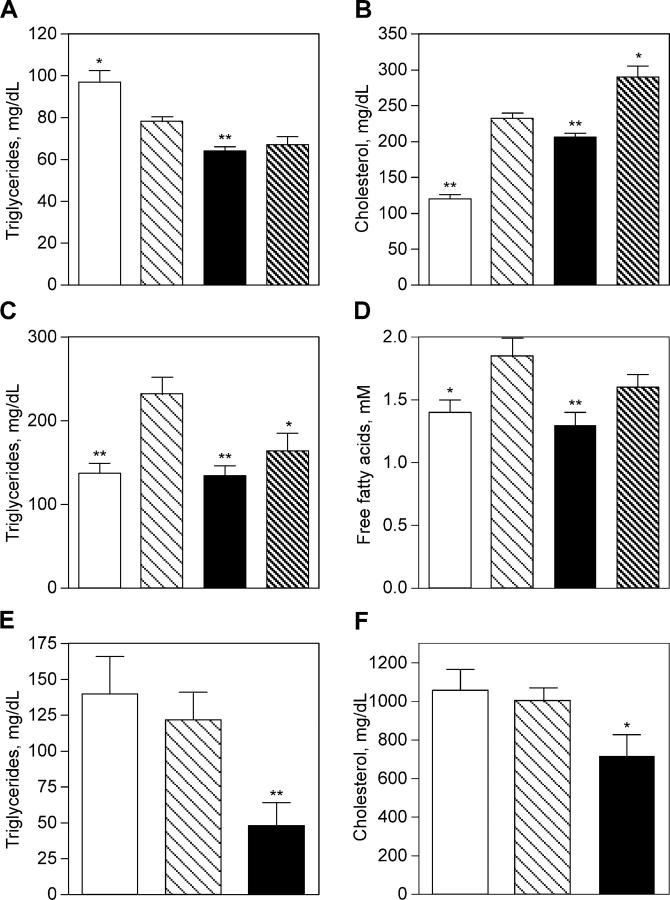
Inhibition of 11β-HSD1 activity improved lipid profiles in our murine models. Compound 544 lowered serum triglycerides in the HF/STZ, DIO, and apoE KO mice. It lowered free fatty acids in the HF/STZ model (the endpoint was not examined in the other models) and cholesterol in the DIO and apoE KO mice (not tested in HF/STZ). Triglyceride levels were reduced by 18% in DIO mice compared with vehicle-treated animals (P = 0.002; Fig. 4 A). Serum cholesterol levels were nearly doubled in DIO mice versus lean controls, and 11β-HSD1 inhibition lowered serum cholesterol by 24% (P = 0.012; Fig. 4 B). Serum triglycerides were increased by 1.75-fold in HF/STZ animals as compared with chow/saline control levels, and 11β-HSD1 inhibitor treatment abrogated this increase (96% correction, P < 0.005; Fig. 4 C). Serum-free fatty acids were increased in HF/STZ animals, though the difference compared with chow/saline controls was modest (P < 0.05; Fig. 4 D). Nevertheless, 11β-HSD1 inhibition significantly lowered free fatty acids (122% correction, P = 0.003). In apoE KO mice, inhibition of 11β-HSD1 (10 mg/kg of compound 544 in feed for 8 wk beginning at 16 wk of age; n = 8) lowered circulating levels of cholesterol by 28% (P = 0.03; Fig. 4 F) and serum triglycerides by 61% relative to vehicle controls (P < 0.005; Fig. 4 E).
Figure 4.
11β-HSD1 inhibition lowers serum lipids in murine models of metabolic syndrome or atherosclerosis. Compound 544 (closed bars) was administered twice daily by oral gavage to DIO mice (A and B), HF/STZ mice (C and D), or apoE KO mice (E and F). The effects were compared with affected animals that were dosed with vehicle (light hatched bars) and with control animals (open bars). Controls included baseline 16-wk-old animals for apoE KO mice, lean animals for DIO mice, or chow/saline-treated animals for HF/STZ mice. Animals treated with RU38486 served as a positive control for DIO and HF/STZ models (A–D, dark hatched bars). Compound 544 lowers triglycerides (A, DIO; C, HF/STZ; and E, apoE KO), serum cholesterol (B, DIO and F, apoE KO), and free fatty acids (D, HF/STZ). Data shown are the mean of at least two studies. Statistical significance is calculated between the treatment groups and vehicle controls for DIO, the treated groups and the difference between vehicle and chow /saline controls for HF/STZ, or the difference between vehicle and baseline controls for apoE KO (*, P < 0.05; **, P < 0.005). Values represent the mean ± SEM.
The GC (progesterone) receptor antagonist RU38486 (mifepristone) showed comparable effects to the 11β-HSD1 inhibitor in the HF/STZ and DIO mouse models (10 mg/kg PO, twice daily; Figs. 2–4, dark hatched bars), further suggesting that the effects of 11β-HSD1 inhibition are indeed obtained through a lessening of signaling through the GC receptor. RU38486 lowered fasting glucose in both models and improved insulin sensitivity and glucagon levels in the HF/STZ mice. RU38486 lowered body weight, food intake, and fat pad weights in the DIO mice and lowered triglycerides in the HF/STZ animals. In vitro assays confirmed that RU38486 lacked inhibitory activity against either mouse or human 11β-HSD1 at concentrations ≤10 μM (unpublished data).
11β-HSD1 inhibition decreases aortic lesion area in a mouse model of atherosclerosis
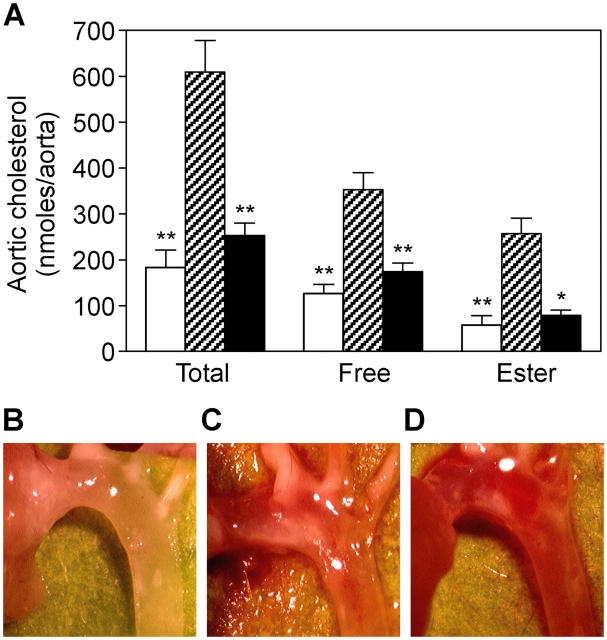
11β-HSD1 inhibition not only improves cardiovascular disease risk factors such as insulin resistance, obesity, and dyslipidemia, but we show that it also dramatically slows plaque progression in atherosclerotic lesions. Targeted deletion of the apoE gene diminishes hepatic uptake of circulating cholesterol and causes the development of aortic atherosclerotic plaques in C57BL/6 mice on an atherogenic HF diet (11, 12). Such animals received compound 544 (10 mg/kg/day; n = 8) in the feed for 8 wk, beginning at 16 wk of age (baseline). In the period from 16–24 wk of age, the untreated animals tripled the amount of cholesterol deposited in aortic lesions (n = 8; Fig. 5 A). Compound 544 almost completely prevented this plaque progression (84% less total aortic cholesterol; P < 0.001; Fig. 5 A,). Aortic cholesterol ester, the species of cholesterol that is found in atherosclerotic aortas, but not normal aortas, showed a similar pattern of accumulation (89% inhibition of accumulation with 11β-HSD1 inhibition; P < 0.05) as did free aortic cholesterol. There was no significant effect of 11β-HSD1 inhibition on body weight in the vehicle and compound 544–treated (10 mg/kg) groups (36.5 ± 2 g compared with 35 ± 1.6 g, respectively), whereas postprandial glucose levels were modestly decreased in these two groups (302 ± 20 mg/dL compared with t 166.8 ± 23 mg/dL, respectively; P < 0.001). Representative images of aortic arch specimens from apoE KO mice show the diminution of lesion area with compound 544 treatment (Fig. 5 D compared with C [24-wk-old vehicle controls] and B [16-wk-old baseline controls]). Although the effect of 11β-HSD1 inhibition on serum lipids and glucose may contribute to decreased atherosclerotic lesion progression, the extent of the effect appears disproportionate to these metabolic improvements and suggests an additional direct effect of 11β-HSD1 inhibition on the artery wall. Indeed, preliminary data show that serum levels of the proinflammatory cytokine monocyte chemoattractant protein (MCP)–1 are lowered by compound 544 in these apoE KO mice (vehicle group, 231 ± 44 pg/ml vs. compound 544 group, 107 ± 15 pg/ml; P = 0.02). Further studies are needed to completely elucidate the mechanism of the antiatherogenic effect of HSD1 inhibition.
Figure 5.
11β-HSD1 inhibition decreases aortic lesion area in a mouse model of atherosclerosis. Compound 544 (A, closed bars) lowered aortic total or free cholesterol and cholesterol ester content in apoE-deficient mice to nearly baseline levels when compared with untreated controls (A, hatched bars). apoE KO animals killed at 16 wk represent the baseline lesion area at initiation of the study (A and B, open bars). Aortic arch lesions, visible as milky opacities in a normally translucent aortic wall, are visibly diminished in size on compound 544 treatment (D) when compared with untreated controls (C). Statistical significance is calculated between the treated animals and the difference between baseline animals and vehicle controls (*, P < 0.05; **P, < 0.005). Values represent the mean ± SEM.
Discussion
Using recently defined diagnostic criteria (13), it has been estimated that >20% of the adult population of the United States has metabolic syndrome and that the incidence rises to >40% of the population ≥60 yr of age (14). Metabolic syndrome exacts an enormous cost on Western societies, primarily through a strong increase in the risk of atherosclerosis and coronary heart disease. Given the seriousness of the cardiovascular health threat posed by metabolic syndrome, the third report of the National Cholesterol Education Program recommended that physicians treat the disease aggressively, even though the committee acknowledged that weapons at the disposal of physicians are few and that, in the case of lifestyle interventions such as weight loss and exercise, compliance is poor. Given the difficulty in achieving lasting lifestyle changes, an urgent need for pharmacologic intervention clearly exists. However, the mechanisms by which the metabolic syndrome affects heart disease are a long-standing enigma. The experiments described here demonstrate a novel pharmacologic means of controlling metabolic syndrome and curtailing the risk of atherosclerosis. They further suggest a critical role for elevated intracellular cortisol levels in the acceleration of atherosclerosis associated with metabolic syndrome.
A recent prospective study in 714 nondiabetic adults suggests that visceral obesity precedes the development of other metabolic syndrome components, including insulin resistance (15). Although patients with metabolic syndrome have normal circulating levels of GCs, it is still possible that local dysregulation of intracellular GC tone occurs in these patients (16). An aberrantly high intracellular activation of GCs by 11β-HSD1 has indeed been noted in the adipose and skeletal muscle of obese insulin-resistant humans (17–20). Experimental manipulation of 11β-HSD1 activity in animal models also points to the importance of intracellular GC tone in the metabolic syndrome phenotype. Transgenic overexpression of 11β-HSD1 in murine visceral fat or selective disruption of the gene leads to a metabolic syndrome–like phenotype (6, 7), or confers protection from development of the metabolic syndrome phenotype (8), respectively. Treatment of Zucker rats with carbenoxolone, a nonspecific inhibitor of 11β-HSD1 and -HSD2, led to inhibition of 11β-HSD1 in the liver and concomitantly improved the lipid profile of the animals (21). Finally, recent studies with an arylsulfonamidothiazole class of an 11β-HSD1 inhibitor with >30-fold selectivity over 11β-HSD2 show improvement of insulin sensitivity in diabetic mouse models (22, 23).
GCs regulate several inflammatory and metabolic functions. Patients with Cushing's syndrome develop central obesity and a redistribution of adipose tissue away from subcutaneous depots and into the visceral compartment. Furthermore, GCs are orexigenic. Therefore, it is not surprising that the lowering of GC tone via inhibition of 11β-HSD1 activity would lower body weight and food intake, as shown here for the DIO mouse model. It is interesting to note that treated animals lost more weight in the visceral, or retroperitoneal, depot than in the epididymal fat pad. Although the mechanism for the fat redistribution effect of GCs has not been completely elucidated, it is clear that different depots vary in their degree of metabolic activity (24) and that, therefore, a hormone that regulates metabolic function of adipose tissue would have varying effects in these different depots. Various groups have noted increased 11β-HSD1 activity or expression in adipose tissue from obese human subjects (17–19, 25, 26), but another has failed to see any difference (27). Given the effects of GCs on adipogenesis, one can expect a therapeutic benefit for lower prereceptor generation of activated GCs in adipose tissue even in the absence of an increase in that activity in obesity.
As part of the stress response, GCs mobilize fuel sources to enable the organism to respond to the stressor. As such, they increase hepatic gluconeogenesis and antagonize the effects of insulin, which decreases glucose utilization by cells and leads to elevated circulating levels of glucose and insulin resistance. 11β-HSD1 is expressed in the islets of Langerhans, and activation of 11-dehydrocholesterol by 11β-HSD1 in isolated islets inhibits insulin release, an effect that can be inhibited by carbenoxolone (28). GCs have been shown to destroy β cells in the islets of HF-fed rats (29), and 11β-HSD1 expression and activity are increased in the islets of Zucker diabetic fatty rats with β cell destruction (30). Lowering GC levels by adrenalectomy or GC receptor antagonism can improve hyperglycemia and insulin resistance (31–33). In the diabetic models presented here, 11β-HSD1 inhibition had a profound effect on fasting glucose. This is consistent with a lowering of GC tone in the liver and a lessening of hepatic gluconeogenesis. Compound 544 increased insulin sensitivity, as evidenced by the decreased serum glucose excursions on glucose tolerance testing in the HF/STZ model, which is also consistent with decreased GC tone, antagonizing the effects of insulin on glucose uptake in adipose tissue and skeletal muscle. With improvements in hyperglycemia, the animals also had lower circulating insulin and glucagon levels. PPARγ agonists reduce the expression and activity of 11β-HSD1 in adipose tissue (30, 34), and suggest that the beneficial effect of PPARγ agonists on metabolic syndrome may, at least in part, be caused by their ability to diminish GC production by 11β-HSD1. RU38486 showed comparable effects to the 11β-HSD1 inhibitor, further suggesting that the effects of 11β-HSD1 inhibition are indeed obtained through a lessening of signaling through the GC receptor.
GCs are known to have multiple effects on lipid metabolism. These hormones increase lipolysis in adipocytes and regulate hepatic lipoprotein production such that patients with Cushing's syndrome have elevated serum-free fatty acids, triglycerides, and cholesterol. Inhibition of 11β-HSD1 caused the lowering of serum triglycerides in the HF/STZ, DIO, and apoE KO mice. Free fatty acids were lowered in the HF/STZ model and cholesterol was lowered in the DIO and apoE KO mice. These data are also consistent with the observation that 11β-HSD1 KO mice have a cardioprotective lipid phenotype (35). Finally, PPARα agonists such as fibrates are very effective agents for treating dyslipidemia and have been shown to reduce 11β-HSD1 expression and activity in murine liver (36).
The metabolic syndrome is associated with a dramatic increase in the risk of coronary heart disease, the principal source of morbidity and mortality in this syndrome (37, 38). The data presented here thus suggest that inhibition of 11β-HSD1 may be an effective means for lowering cardiovascular risk in metabolic syndrome. The addition of compound 544 to the diet of apoE KO mice almost completely blocked plaque progression. The degree of improvement in aortic lipid content with 11β-HSD1 inhibition seems greater than can be accounted for by the relatively modest lowering of serum triglycerides, cholesterol, and postprandial glucose in this model. The precise mechanism for this beneficial effect of lowered GC tone on the artery wall is unclear. We know that the cells of the artery wall, specifically macrophages, smooth muscle, and endothelial cells, which are also present in atherosclerotic plaques, express 11β-HSD1 activity (39–41). Treatment of aortic rings with antisense oligonucleotides against 11β-HSD1 attenuated the contractile response to phenylephrine (42). Lowering vessel wall and intraplaque production of an antiinflammatory hormone such as cortisol by inhibiting 11β-HSD1 might be expected to exacerbate rather than slow the progression of atherosclerotic disease. However, our investigations into the inflammatory state of the apoE mice show that circulating levels of the proinflammatory cytokine MCP-1 are decreased with 11β-HSD1 inhibition. Furthermore, preliminary efforts to profile gene expression in the aortic plaques of 11β-HSD1 inhibitor–treated apoE KO mice reveal that aortic MCP-1 expression is lowered by inhibitor treatment. Gene signatures from primary human aortic smooth muscle cells treated with 11β-HSD1 inhibitor in culture also reveal decreases in proinflammatory gene expression (Luo, M.-J., personal communication). We intend to follow up on these preliminary observations that 11β-HSD1 inhibitors act to slow plaque progression through a direct antiinflammatory effect on vascular cells in order to understand the relative contribution of lowering serum lipids versus an antiinflammatory effect at the vessel wall. Although this antiinflammatory and atheroprotective effect of lowering GC production may seem surprising, it is not without precedent. Studies on Cushing's disease patients one year after remission and normalization of GC levels show considerably decreased intimal medial thickness of the carotid wall by ultrasound as well as notable increases in carotid systolic lumen diameter and distensibility coefficient, with only relatively modest improvements in body weight, hyperglycemia, and dyslipidemia (43). Experiments are underway to more completely characterize the effects of 11β-HSD1 inhibition on the inflammatory status of the apoE KO mice and on the expression profile of the key cells of the atherosclerotic lesion, using in situ approaches, such as laser capture microdissection of plaques from animals treated with inhibitor, as well as in vitro approaches, such as exposure of vascular cells to 11β-HSD1 inhibitors in culture.
There is ample data in the literature to suggest that the categorization of GCs as purely antiinflammatory hormones may be an oversimplification. Physiologists studying adrenalectomized animals in the early 20th century determined that GCs were a necessary part of a coordinated response to stress or injury, and it was only after these hormones were purified and administered at high doses that their potent antiinflammatory actions were discovered. Studies performed at GC concentrations closer to the basal, unstimulated state reveal GC-mediated enhancement of such diverse inflammatory processes as hepatic acute phase response, delayed-type hypersensitivity response, inflammatory cell cytokine secretion, expression of cytokine/chemokine receptors, and release of the proinflammatory mediator macrophage migration inhibitory factor (44, 45). For example, GCs have been shown to increase the expression of proinflammatory receptors in macrophages (TLR4, IL1R, TNFR, etc.) and to enhance immune responses such as the delayed type hypersensitivity response (46, 47). Gene profiling of human peripheral blood mononuclear cells has shown that GCs can regulate some genes in opposite directions, depending on the activation state of the target cells (47). These data suggest a highly complex regulatory role of GC in human immune functions.
It has been difficult to quantify a relationship between the metabolic syndrome risk factors and atherosclerosis as a means of assessing risk because the extent of risk appears greater than the sum of the individual parts For instance, the 4-yr risk of incident myocardial infarction among men from age 40 to 65 in the Prospective Cardiovascular Münster study was increased 2.5 times in the presence of either type 2 diabetes or hypertension, 8 times in the presence of both factors, and 19 times in the presence of both factors plus an abnormal lipid profile (48). Our observations suggest an explanation for these findings. We propose that the mechanistic contributors to atherosclerotic disease are not drawn only from the short list of known risk factors (hyperlipidemia, insulin resistance, and hypertension) but from other processes as well. We have shown that lowering intracellular GC production by HSD1 inhibition can be antiinflammatory, lowering circulating levels of the proinflammatory cytokine MCP-1. Our results suggest that excess GCs are a candidate for the underlying dysregulation that generates a host of conditions that in turn contribute synergistically to atherogenesis. In keeping with this notion, Kumari et al. (49) have shown that chronic mild stress can induce or accelerate the development of atherosclerosis in the apoE-deficient mouse. We propose that excess intracellular GCs cause atherogenesis through a broad network of physiologic responses to this protean hormone.
Because 11β-HSD1 is expressed in the hypothalamus and pituitary and adrenal glands, 11β-HSD1 inhibition would be expected to affect the function of the HPA axis. Indeed, 11β-HSD1 KO mice have evidence of altered HPA feedback, including elevated corticosterone at nadir and adrenal hyperplasia (8). Although circulating GCs are slightly elevated, mice lacking 11β-HSD1 have no signs of Cushing's disease–like symptoms such as fat redistribution or insulin resistance, and they reproduce normally and have apparently normal behavioral reactions to fasting, acute restraint stress, and forced swimming (8, 35, 50). Pharmacologic inhibition of 11β-HSD1 did not affect the terminal circulating corticosterone levels in serum for any of the models used here (DIO model: vehicle, 101.4 ± 12.1 ng/ml vs. compound 544, 80.9 ± 12.2 ng/ml; HF/STZ model: vehicle, 96 ± 24 ng/ml vs. compound 544, 95 ± 26 ng/ml; and apoE−/− model: vehicle, 82 ± 11 ng/ml vs. compound 544, 71 ± 9 ng/ml). This lack of effect, although not examined rigorously in our experiments, suggests that partial pharmacologic inhibition of 11β-HSD1 is sufficient to have beneficial effects and may not be enough to measurably alter HPA function. In additional studies, DIO mice chronically dosed with 11β-HSD1 inhibitor were subjected to restraint stress. These mice showed considerable reductions in body weight and improvement in insulin sensitivity with no changes in adrenal or thymus weights and no changes in basal or restraint-induced elevations in corticosterone or adrenocorticotropic hormone (unpublished data). These data suggest that inhibition of 11β-HSD1 at levels sufficient to give beneficial metabolic effects does not have deleterious effects on the capacity of the HPA axis to respond to a standard stressor.
In summary, our data support the notion that the inhibition of 11β-HSD1 can be beneficial in metabolic syndrome and atherosclerotic disease. These studies used a potent and specific adamantyl-triazole inhibitor of 11β-HSD1 with good in vitro and in vivo efficacy. This 11β-HSD1 inhibitor, compound 544, improved insulin resistance and glucose, insulin, and glucagon levels in mouse models of type 2 diabetes and obesity. 11β-HSD1 inhibition also lowered body weight and central fat pad mass and improved dyslipidemia in mouse models of obesity, diabetes, or atherosclerosis. Most strikingly, the 11β-HSD1 inhibitor almost completely prevented atherosclerotic plaque progression in the apoE KO, a mouse model of atherosclerosis. Metabolic features such as hyperglycemia (51, 52) and vessel wall abnormalities (43) are reversible in treated Cushing's disease patients, suggesting that humans, like mice, will benefit from lowering 11β-HSD1 activity and, thus, active GC generation in key metabolic organs.
Materials and Methods
Reagents
All reagents were obtained from Sigma-Aldrich unless otherwise stated.
11β-HSD1 inhibitor
Compound 544 (3-(1-adamantyl)-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[4,3-a]azepine) was dosed by oral gavage in either 5% hydroxypropyl-β-cyclodextrin (for activity measurements) or in 0.5% methylcellulose containing 5% Tween 80 (in disease models). A direct comparison of the two vehicles shows that compound 544 administered in either vehicle yields comparable results on enzyme activity inhibition. For the apoE KO mouse model, compound 544 was administered in the feed.
Animal husbandry
All experimental animal procedures were approved by the Institutional Animal Care and Use Committee of Merck Research Laboratories. Unless otherwise stated, all animals were maintained under controlled conditions of 25°C and 12-h light/dark cycles (7:00 a.m./7:00 p.m.), with food and water available ad libitum. Twice daily dosing occurred at ∼9:00 a.m. and 5:00 p.m.
Measurement of 11β-HSD1 and -HSD2 activity in vitro
In vitro enzyme assays were performed with a 100,000-g membrane fraction of a postnuclear supernatant from Chinese hamster ovary cells (CHO-K1; American Type Culture Collection) stably transfected with an expression vector (pcDNA3.1; Invitrogen) encoding human or murine 11β-HSD1 or -HSD2 and 20 nM [3H]cortisone (11β-HSD1 reductase assay; American Radiolabeled Chemical) or [3H]cortisol (11β-HSD2 dehydrogenase assay; GE Healthcare) with 1 mM cofactor (NADPH or NAD, respectively). Anticortisol-coated (East Coast Biologics) scintillation proximity assay beads (protein A–coated yttrium silicate; GE Healthcare) were used to capture [3H]cortisol generated in or remaining from the enzyme reaction. CHO-K1 cells transfected with the control vector lack both enzyme activities (unpublished data). Percent inhibition was calculated relative to a noninhibited control.
Measurement of 11β-HSD1 activity in vivo
Compound 544 was dosed orally in three 13-wk-old ICR male mice (Taconic Farms) per group on a standard chow diet (7012; Harlan Teklad) using 5% hydroxypropyl-β-cyclodextrin as vehicle. After compound administration, animals were either challenged with an i.v. bolus of tritiated cortisone, whose generation by 11β-HSD1 was measured by detection of circulating levels (serum PD assay), or tissues were isolated and exposed to tritiated cortisone ex vivo (tissue PD assay).
For the serum PD assay, 0.2 ml [3H]cortisone in saline was injected i.v. by tail vein at 3.3 μM for a 20 nM final dose in a 35-g mouse, assuming a distribution over total body water of 25 ml. The animal was killed 2 min after administration of substrate, and blood was drawn by cardiac puncture into clotting tubes. The time of administration was previously determined to be within linearity of the in vivo 11β reductase reaction and resulted in recovery of 75% of radioactivity (unpublished data). Steroids were extracted from 200 μl of serum with 5 vol of ethyl acetate. Samples were air dried and resuspended in 200 μl of 16 μg/ml of unlabeled cortisone/cortisone (wt/wt = 1:1) and subsequently analyzed by HPLC using a C18 column (Inertsil; Phenomenex). The relative levels of [3H]cortisone and its reduced product, [3H]cortisol, were determined for the compound- and vehicle-dosed control groups by radiochromatography. The percent conversions of [3H]cortisone to [3H]cortisol, as well as the percentage of inhibition, were calculated as the ratio of the peak area for cortisol over the combined peak areas for cortisone and cortisol. Other peaks constituted <15% of the total.
For tissue PD assay, EWAT, liver, and brain were removed and minced into 2–3-mm pieces in RPMI 1640 with 5% FCS (Sigma-Aldrich) and 15 mM Hepes (GIBCO), and 50 U/ml penicillin–streptomycin (GIBCO BRL) containing 20 nM [3H]cortisone. The total volume (in milliliters) added was equivalent to approximately five times the mass of tissue (in milligrams). Tissue was then incubated at 37°C in a 5% CO2 atmosphere for 10 min (liver) to 2 h (brain and EWAT). Cortisol content of the supernatant was determined by scintillation proximity assay.
DIO model
6–10-mo-old C57BL/6J male mice (The Jackson Laboratory) were fed on an HF diet (34% fat by weight with 60% kcal from fat; 97070; Harlan Teklad) from week 4, and water was given ad libitum. The animals were housed individually in cages and adjusted to the dosing regimen by vehicle administration (10% Tween 80 in 0.5% Methocel) 7 d before the initiation of treatment. 20 mg/kg 11β-HSD1 inhibitor, 25 mg/kg of RU38486, or vehicle was administered by oral gavage (PO) twice daily (BID), with one dose given in the morning and the other dose given in the afternoon. The food intake and weight of the animals were recorded daily. The treatments were resumed for 11 d, and the last dose was given in the afternoon before termination of the study. Food was withdrawn from the mice 4 h before death.
HF/STZ model
4-wk-old male ICR mice (Taconic Farms) were fed an HF diet (36% fat by weight with 58.4% kcal from fat; D00031501; Research Diets Inc.) for 3 wk to generate peripheral insulin resistance, followed by a single injection of 90 mg/kg of body weight of freshly prepared STZ to induce partial β cell dysfunction. 4 wk after administration of STZ, animals (n = 8) on an HF diet ad libitum and housed 3–4 per cage were dosed for 9 d with 11β-HSD1 inhibitor (30 mg/kg BID in 0.5% methylcellulose and 5% Tween 80), RU38486 (10 mg/kg BID), or vehicle alone, with one dose given in the morning and the other dose given in the afternoon. A group of mice fed a chow diet and injected with saline instead of STZ served as controls (chow/saline controls). Two other groups consisting either of animals fed a chow diet and injected with STZ or of HF-diet animals injected with vehicle were used to monitor the contribution of STZ or an HF diet to the development of diabetes (unpublished data).
apoE KO mouse model
C57BL/6 male apoE KO mice (The Jackson Laboratory) were weaned at 4 wk of age onto an atherogenic Western diet containing 21% fat and 0.15% cholesterol (88137; Harlan Teklad). At 16 wk of age, mice were separated into the following treatment groups: Western diet only (vehicle) or Western diet containing 0.01% 11β-HSD1 inhibitor (equivalent to ∼10 mg/kg/day). After 8 wk of treatment, mice were killed, serum was collected, and aortas were dissected from the aortic root to the right renal artery for cholesterol extraction. Aortic lesions were quantitated by measuring total cholesterol, free cholesterol, and cholesterol ester incorporated into the vessel, as previously described (53). Samples were collected from 16-wk-old mice on an atherogenic diet to establish baseline control levels of aortic and serum lipid. It should be noted, however, that murine cholesterol profiles are quite distinct from those seen in humans. Circulating cholesterol is found almost exclusively as HDL particles, whereas humans have more low density lipoprotein than HDL. apoE deficiency renders yet another, distinct lipoprotein profile in these mice, where chylomicrons and very low density lipoprotein comprise the dominant population of particles.
Serum measurements
Overnight fasting and postprandial blood glucose levels were detected by a glucometer (One Touch; LifeScan, Inc.) for the HF/STZ model. Serum glucose levels were determined spectrophotometrically by the hexokinase method (Hitachi 911; Roche Molecular Biochemicals). Serum levels of insulin and leptin were measured by ELISA (Alpco). Serum cholesterol levels were measured by cholesterol and triglyceride assay kits (439-17501 and 997-37492; Wako Chemicals). Serum levels of triglycerides and free fatty acids levels were quantified by a colorimetric diagnostic kit (Roche Molecular Biochemicals).
Statistics
A two-factor linear model with analysis of variance was used to account for interstudy variability for endpoints. All comparisons used P = 0.05 to claim statistically significant differences. The logarithmic scale was used to better satisfy assumptions of normality and constant variance and to naturally express comparisons as percent differences. All calculations were performed in the R software environment (http://www.r-project.org).
Acknowledgments
We would like to thank Ms. Michele Mariano for help with serum chemistry; Ms. Easter Frazier for animal handling; and Ms. Llnon Slossberg, Mr. Greg Rouen, and Dr. Gerard Kieczykowski for preparation of compound 544.
The authors have no conflicting financial interests.
Abbreviations used: apoE, apolipoprotein E; BID, bis in die; DIO, diet-induced obesity; EWAT, epididymal white adipose tissue; GC, glucocorticoid; HDL, high density lipoprotein; HF, high fat; HPA, hypothalamic-pituitary-adrenal; HSD, hydroxysteroid dehydrogenase; MCP, monocyte chemoattractant protein; PD, pharmacodynamic; PO, per os; STZ, streptozotocin.
M. Strowski's present address is Medizinische Klinik mit Schwerpunkt Hepatologie und Gastroenterologie, Campus Virchow-Klinikum, Charité–Universitätsmedizin Berlin, 13353 Berlin, Germany.
References
- 1.Agarwal, A.K., C. Monder, B. Eckstein, and P.C. White. 1989. Cloning and expression of rat cDNA encoding corticosteroid 11 beta-dehydrogenase. J. Biol. Chem. 264:18939–18943. [PubMed] [Google Scholar]
- 2.Albiston, A.L., V.R. Obeyesekere, R.E. Smith, and Z.S. Krozowski. 1994. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol. Cell. Endocrinol. 105:R11–R17. [DOI] [PubMed] [Google Scholar]
- 3.Walker, B.R., and J.R. Seckl. 2003. 11beta-hydroxysteroid dehydrogenase type 1 as a novel therapeutic target in metabolic and neurodegenerative disease. Expert Opin. Ther. Targets. 7:771–783. [DOI] [PubMed] [Google Scholar]
- 4.Masuzaki, H., and J.S. Flier. 2003. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)–a promising drug target for the treatment of metabolic syndrome. Curr. Drug Targets Immune Endocr. Metabol. Disord. 3:255–262. [DOI] [PubMed] [Google Scholar]
- 5.Stewart, P.M. 2003. Tissue-specific Cushing's syndrome, 11beta-hydroxysteroid dehydrogenases and the redefinition of corticosteroid hormone action. Eur. J. Endocrinol. 149:163–168. [DOI] [PubMed] [Google Scholar]
- 6.Masuzaki, H., J. Paterson, H. Shinyama, N.M. Morton, J.J. Mullins, J.R. Seckl, and J.S. Flier. 2001. A transgenic model of visceral obesity and the metabolic syndrome. Science. 294:2166–2170. [DOI] [PubMed] [Google Scholar]
- 7.Masuzaki, H., H. Yamamoto, C.J. Kenyon, J.K. Elmquist, N.M. Morton, J.M. Paterson, H. Shinyama, M.G. Sharp, S. Fleming, J.J. Mullins, et al. 2003. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J. Clin. Invest. 112:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotelevtsev, Y., M.C. Holmes, A. Burchell, P.M. Houston, D. Schmoll, P. Jamieson, R. Best, R. Brown, C.R. Edwards, J.R. Seckl, and J.J. Mullins. 1997. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. USA. 94:14924–14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotelevtsev, Y., R.W. Brown, S. Fleming, C. Kenyon, C.R. Edwards, J.R. Seckl, and J.J. Mullins. 1999. Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2. J. Clin. Invest. 103:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo, J., J. Quan, J. Tsai, C.K. Hobensack, C. Sullivan, R. Hector, and G.M. Reaven. 1998. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism. 47:663–668. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, S.H., R.L. Reddick, J.A. Piedrahita, and N. Maeda. 1992. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 258:468–471. [DOI] [PubMed] [Google Scholar]
- 12.Plump, A.S., J.D. Smith, T. Hayek, K. Aalto-Setala, A. Walsh, J.G. Verstuyft, E.M. Rubin, and J.L. Breslow. 1992. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71:343–353. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults. 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 14.Ford, E.S., W.H. Giles, and W.H. Dietz. 2002. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 287:356–359. [DOI] [PubMed] [Google Scholar]
- 15.Palaniappan, L., M.R. Carnethon, Y. Wang, A.J. Hanley, S.P. Fortmann, S.M. Haffner, and L. Wagenknecht. 2004. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 27:788–793. [DOI] [PubMed] [Google Scholar]
- 16.Bujalska, I.J., S. Kumar, and P.M. Stewart. 1997. Does central obesity reflect “Cushing's disease of the omentum”? Lancet. 349:1210–1213. [DOI] [PubMed] [Google Scholar]
- 17.Rask, E., T. Olsson, S. Soderberg, R. Andrew, D.E. Livingstone, O. Johnson, and B.R. Walker. 2001. Tissue-specific dysregulation of cortisol metabolism in human obesity. J. Clin. Endocrinol. Metab. 86:1418–1421. [DOI] [PubMed] [Google Scholar]
- 18.Rask, E., B.R. Walker, S. Soderberg, D.E. Livingstone, M. Eliasson, O. Johnson, R. Andrew, and T. Olsson. 2002. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J. Clin. Endocrinol. Metab. 87:3330–3336. [DOI] [PubMed] [Google Scholar]
- 19.Paulmyer-Lacroix, O., S. Boullu, C. Oliver, M.C. Alessi, and M. Grino. 2002. Expression of the mRNA coding for 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J. Clin. Endocrinol. Metab. 87:2701–2705. [DOI] [PubMed] [Google Scholar]
- 20.Whorwood, C.B., S.J. Donovan, D. Flanagan, D.I. Phillips, and C.D. Byrne. 2002. Increased glucocorticoid receptor expression in human skeletal muscle cells may contribute to the pathogenesis of the metabolic syndrome. Diabetes. 51:1066–1075. [DOI] [PubMed] [Google Scholar]
- 21.Livingstone, D.E., and B.R. Walker. 2003. Is 11beta-hydroxysteroid dehydrogenase type 1 a therapeutic target? Effects of carbenoxolone in lean and obese Zucker rats. J. Pharmacol. Exp. Ther. 305:167–172. [DOI] [PubMed] [Google Scholar]
- 22.Alberts, P., L. Engblom, N. Edling, M. Forsgren, G. Klingstrom, C. Larsson, Y. Ronquist-Nii, B. Ohman, and L. Abrahmsen. 2002. Selective inhibition of 11beta-hydroxysteroid dehydrogenase type 1 decreases blood glucose concentrations in hyperglycaemic mice. Diabetologia. 45:1528–1532. [DOI] [PubMed] [Google Scholar]
- 23.Alberts, P., C. Nilsson, G. Selen, L.O. Engblom, N.H. Edling, S. Norling, G. Klingstrom, C. Larsson, M. Forsgren, M. Ashkzari, et al. 2003. Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology. 144:4755–4762. [DOI] [PubMed] [Google Scholar]
- 24.Abate, N., and A. Garg. 1995. Heterogeneity in adipose tissue metabolism: causes, implications and management of regional adiposity. Prog. Lipid Res. 34:53–70. [DOI] [PubMed] [Google Scholar]
- 25.Engeli, S., J. Bohnke, M. Feldpausch, K. Gorzelniak, U. Heintze, J. Janke, F.C. Luft, and A.M. Sharma. 2004. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes. Res. 12:9–17. [DOI] [PubMed] [Google Scholar]
- 26.Westerbacka, J., H. Yki-Jarvinen, S. Vehkavaara, A.M. Hakkinen, R. Andrew, D.J. Wake, J.R. Seckl, and B.R. Walker. 2003. Body fat distribution and cortisol metabolism in healthy men: enhanced 5beta-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J. Clin. Endocrinol. Metab. 88:4924–4931. [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson, J.W., B. Sinha, I. Bujalska, M. Hewison, and P.M. Stewart. 2002. Expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue is not increased in human obesity. J. Clin. Endocrinol. Metab. 87:5630–5635. [DOI] [PubMed] [Google Scholar]
- 28.Davani, B., A. Khan, M. Hult, E. Martensson, S. Okret, S. Efendic, H. Jornvall, and U.C. Oppermann. 2000. Type 1 11beta-hydroxysteroid dehydrogenase mediates glucocorticoid activation and insulin release in pancreatic islets. J. Biol. Chem. 275:34841–34844. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa, A., J.H. Johnson, M. Ohneda, C.T. McAllister, L. Inman, T. Alam, and R.H. Unger. 1992. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J. Clin. Invest. 90:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duplomb, L., Y. Lee, M.Y. Wang, B.H. Park, K. Takaishi, A.K. Agarwal, and R.H. Unger. 2004. Increased expression and activity of 11beta-HSD-1 in diabetic islets and prevention with troglitazone. Biochem. Biophys. Res. Commun. 313:594–599. [DOI] [PubMed] [Google Scholar]
- 31.Shimomura, Y., G.A. Bray, and M. Lee. 1987. Adrenalectomy and steroid treatment in obese (ob/ob) and diabetic (db/db) mice. Horm. Metab. Res. 19:295–299. [DOI] [PubMed] [Google Scholar]
- 32.Spear, G.S., K. Ohshima, G.A. Bray, and M.V. Caple. 1986. Effect of adrenalectomy on the pancreas of db/db mice. Horm. Metab. Res. 18:743–745. [DOI] [PubMed] [Google Scholar]
- 33.Friedman, J.E., Y. Sun, T. Ishizuka, C.J. Farrell, S.E. McCormack, L.M. Herron, P. Hakimi, P. Lechner, and J.S. Yun. 1997. Phosphoenolpyruvate carboxykinase (GTP) gene transcription and hyperglycemia are regulated by glucocorticoids in genetically obese db/db transgenic mice. J. Biol. Chem. 272:31475–31481. [DOI] [PubMed] [Google Scholar]
- 34.Berger, J., M. Tanen, A. Elbrecht, A. Hermanowski-Vosatka, D.E. Moller, S.D. Wright, and R. Thieringer. 2001. Peroxisome proliferator-activated receptor-gamma ligands inhibit adipocyte 11beta-hydroxysteroid dehydrogenase type 1 expression and activity. J. Biol. Chem. 276:12629–12635. [DOI] [PubMed] [Google Scholar]
- 35.Morton, N.M., M.C. Holmes, C. Fievet, B. Staels, A. Tailleux, J.J. Mullins, and J.R. Seckl. 2001. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11beta-hydroxysteroid dehydrogenase type 1 null mice. J. Biol. Chem. 276:41293–41300. [DOI] [PubMed] [Google Scholar]
- 36.Hermanowski-Vosatka, A., D. Gerhold, S.S. Mundt, V.A. Loving, M. Lu, Y. Chen, A. Elbrecht, M. Wu, T. Doebber, L. Kelly, et al. 2000. PPARalpha agonists reduce 11beta-hydroxysteroid dehydrogenase type 1 in the liver. Biochem. Biophys. Res. Commun. 279:330–336. [DOI] [PubMed] [Google Scholar]
- 37.Trevisan, M., J. Liu, F.B. Bahsas, and A. Menotti. 1998. Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am. J. Epidemiol. 148:958–966. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, P.W., W.B. Kannel, H. Silbershatz, and R.B. D'Agostino. 1999. Clustering of metabolic factors and coronary heart disease. Arch. Intern. Med. 159:1104–1109. [DOI] [PubMed] [Google Scholar]
- 39.Thieringer, R., C.B. Le Grand, L. Carbin, T.Q. Cai, B. Wong, S.D. Wright, and A. Hermanowski-Vosatka. 2001. 11 Beta-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J. Immunol. 167:30-35. [DOI] [PubMed] [Google Scholar]
- 40.Cai, T.Q., B. Wong, S.S. Mundt, R. Thieringer, S.D. Wright, and A. Hermanowski-Vosatka. 2001. Induction of 11beta-hydroxysteroid dehydrogenase type 1 but not 2 in human aortic smooth muscle cells by inflammatory stimuli. J. Steroid Biochem. Mol. Biol. 77:117–122. [DOI] [PubMed] [Google Scholar]
- 41.Brem, A.S., R.B. Bina, T.C. King, and D.J. Morris. 1998. Localization of 2 11beta-OH steroid dehydrogenase isoforms in aortic endothelial cells. Hypertension. 31:459–462. [DOI] [PubMed] [Google Scholar]
- 42.Souness, G.W., A.S. Brem, and D.J. Morris. 2002. 11 beta-hydroxysteroid dehydrogenase antisense affects vascular contractile response and glucocorticoid metabolism. Steroids. 67:195–201. [DOI] [PubMed] [Google Scholar]
- 43.Faggiano, A., R. Pivonello, S. Spiezia, M.C. De Martino, M. Filippella, C. Di Somma, G. Lombardi, and A. Colao. 2003. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing's disease during active disease and 1 year after disease remission. J. Clin. Endocrinol. Metab. 88:2527–2533. [DOI] [PubMed] [Google Scholar]
- 44.Sapolsky, R.M., L.M. Romero, and A.U. Munck. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21:55–89. [DOI] [PubMed] [Google Scholar]
- 45.Yeager, M.P., P.M. Guyre, and A.U. Munck. 2004. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol. Scand. 48:799–813. [DOI] [PubMed] [Google Scholar]
- 46.Dhabhar, F.S., and B.S. McEwen. 1999. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc. Natl. Acad. Sci. USA. 96:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galon, J., D. Franchimont, N. Hiroi, G. Frey, A. Boettner, M. Ehrhart-Bornstein, J.J. O'Shea, G.P. Chrousos, and S.R. Bornstein. 2002. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 16:61–71. [DOI] [PubMed] [Google Scholar]
- 48.Assmann, G., and H. Schulte. 1988. The Prospective Cardiovascular Munster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am. Heart J. 116:1713–1724. [DOI] [PubMed] [Google Scholar]
- 49.Kumari, M., C. Grahame-Clarke, N. Shanks, M. Marmot, S. Lightman, and P. Vallance. 2003. Chronic stress accelerates atherosclerosis in the apolipoprotein E deficient mouse. Stress. 6:297–299. [DOI] [PubMed] [Google Scholar]
- 50.Yau, J.L., J. Noble, C.J. Kenyon, C. Hibberd, Y. Kotelevtsev, J.J. Mullins, and J.R. Seckl. 2001. Lack of tissue glucocorticoid reactivation in 11beta-hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proc. Natl. Acad. Sci. USA. 98:4716–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nosadini, R., S. Del Prato, A. Tiengo, A. Valerio, M. Muggeo, G. Opocher, F. Mantero, E. Duner, C. Marescotti, F. Mollo, and F. Belloni. 1983. Insulin resistance in Cushing's syndrome. J. Clin. Endocrinol. Metab. 57:529–536. [DOI] [PubMed] [Google Scholar]
- 52.Page, R., M. Boolell, A. Kalfas, S. Sawyer, R. Pestell, G. Ward, and F. Alford. 1991. Insulin secretion, insulin sensitivity and glucose-mediated glucose disposal in Cushing's disease: a minimal model analysis. Clin. Endocrinol. (Oxf.). 35:509–517. [DOI] [PubMed] [Google Scholar]
- 53.Sparrow, C.P., C.A. Burton, M. Hernandez, S. Mundt, H. Hassing, S. Patel, R. Rosa, A. Hermanowski-Vosatka, P.R. Wang, D. Zhang, et al. 2001. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 21:115–121. [DOI] [PubMed] [Google Scholar]