Abstract
The fast-moving field of dendritic cell (DC) biology is hard to keep pace with. Here we report on advances from the recent Keystone Symposium, “Dendritic Cells at the Center of Innate and Adaptive Immunity,” organized in Vancouver, BC on Feb. 1–7, 2005 by Anne O'Garra, Jacques Banchereau, and Alan Sher. New insights into the molecular mechanisms of DC function and their influence on immune regulation, their role in infectious and autoimmune disease, and new clinical applications are highlighted.
DC maturation
DCs are “immunological sensors” that reside throughout the body in an immature form. Immature DCs can capture invading microbes, which provide a source of antigens and ligands for Toll-like receptors (TLRs) that trigger a process of maturation and/or differentiation. The mature DCs migrate to draining secondary lymphoid tissues, present the captured antigen, and up-regulate costimulatory molecules and cytokines. Mature DCs both initiate and direct T and B cell responses. During the meeting, it was reported that a combination of TLR agonists that mobilize both MyD88 and TRIF adaptors for TLR signal transduction—such as the TLR4 ligand lipopolysaccharide (LPS) with the synthetic viral RNA mimic and TLR7 ligand R848, or alternatively the TLR3 ligand poly IC with R848—synergize to induce elevated and sustained interleukin (IL)-12 production by human DCs, enhancing T helper (Th) type 1 responses in vitro (Federica Sallusto, Bellinzona, Switzerland) (1). TLR signals were also reported to alter the expression of Notch family ligands on DCs, up-regulating Delta-4 and down-regulating Jagged1, a finding that confirms earlier mouse data (2). Delta-4–expressing DCs promoted Th1 responses, whereas Jagged promoted Th2 responses. Thus, modulation of Notch ligands on DCs may be an important mechanism by which TLR maturational signals push Th responses in the Th1 or Th2 direction.
Another type of microbial recognition occurs via C-type lectin receptors (CLRs), which primarily mediate the uptake of microbial antigens. Carl Figdor (Nijmegen, Netherlands) demonstrated that DC-SIGN binds numerous pathogens and is organized in well-defined membrane microdomains (3). The CLR Dectin-1, which binds β-glycans and zymosan from fungi, induces TNF and IL-12 production (Siamon Gordon, Oxford, UK). These fungal products are recognized by DCs through both TLR2 and Dectin-1, and TNF production has been shown to be dependent on the TLR signaling adaptor MyD88. Caetano Reis e Sousa (London, UK) showed that zymosan binding to Dectin-1 on DCs additionally induced high levels of IL-10 and IL-2, which required Syk kinase (4). In contrast, IL-12 production was dependent on MyD88 and independent of Syk. A fine balance between these signaling pathways in response to fungal products such as zymosan will undoubtedly determine the outcome of an immune response to fungi.
Mechanisms of type I IFN production in DCs
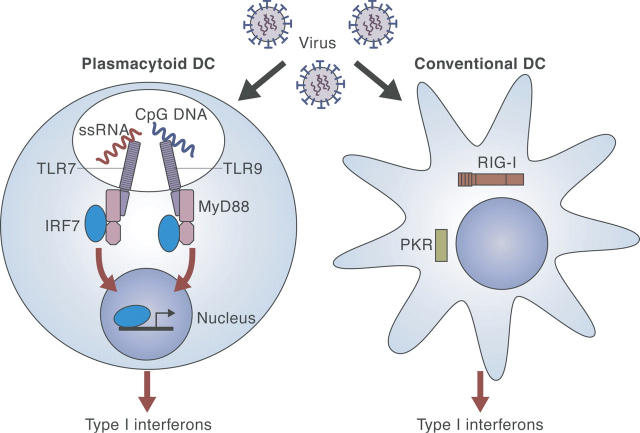
Type I interferon (IFN) is especially important for antiviral immunity. Although various kinds of cells including macrophages, fibroblasts, or conventional DCs can also produce type I IFN, a unique DC subset, the plasmacytoid dendritic cells (pDCs), is the most potent type I IFN producer. New insights into the mechanisms controlling type I IFN production were presented during the meeting. Giorgio Trinchieri (Bethesda, MD) reported that pDC-depleted mice or mutant mice lacking pDCs showed severely decreased production of type I IFN in certain viral infections, which depended mainly on TLR7 and TLR9, which recognize ssRNA and unmethylated CpG DNA, respectively. MyD88, an adaptor molecule for both TLR7 and TLR9 signaling, was shown by Tadatsugu Taniguchi (Tokyo, Japan) to associate directly with the transcription factor, IRF-7, which is essential for IFN-α induction (5). However, type I IFN can also be induced in non-pDCs by viruses in a TLR-independent manner (Christine Biron, Providence, RI). It was also reported that apoptotic cells can trigger type I IFN production in a TLR-independent manner, although the full mechanism for this was unclear (Kasper Hoebe, La Jolla, CA). Insight into intracellular components of TLR-independent pathways for induction of type I IFN was provided by Hiroki Kato (Osaka, Japan), who found that double-stranded RNA stimulated an RNA helicase (RIG-I) to induce type I IFN in fibroblasts and myeloid DCs (mDCs). RIG-1 induced type I IFN by activating IFN regulatory factor (IRF)-3 via IκB kinase–related kinases. An overview of type I IFN production in DCs based on these new findings is shown in Fig. 1.
Figure 1.
Recognition of viral infection for induction of type I IFNs in conventional and plasmacytoid DCs. In pDCs, viral infection is recognized by TLRs, leading to type I IFN induction. Non-pDCs are suggested to utilize a TLR-independent system.
Th1/Th2 and regulatory responses
DCs exposed to certain microenvironmental stimuli are known to promote the differentiation of CD4+ T cells into Th1, Th2, or T regulatory (T reg) cell subsets. IL-10 production by DCs is known to favor Th2 and T reg cell development, and IL-12 production is required for Th1 cell development. However, little is known about the intracellular signaling pathways within DCs that underlie differential cytokine secretion. Although most TLR signaling in DCs is known to prime Th1 responses, Bali Pulendran (Atlanta, GA) has previously shown that signaling through TLR2 induced DCs to secrete IL-10, by a mechanism dependent on the MEK kinase ERK, without secretion of IL-12p70, and thus favored Th2 responses (6). Similarly, work from Anne O'Garra's laboratory (National Institute for Medical Research, London, UK) showed that a delicate balance in signaling through ERK determines IL-10 versus IL-12 production by DCs. Pulendran also showed that zymosan (a ligand for TLR2, TLR6, and Dectin-1) stimulates DCs to robustly secrete IL-10 (which appeared to be ERK dependent) but not inflammatory cytokines, both in vitro and in vivo.
A potential new mode of DC self-regulation to control Th1 responses was proposed by Marika Sarfati (Montreal, Canada). Ligation of CD47 on DCs by thrombospondin-1 (TSP-1; also produced by DCs) altered DC maturation and negatively regulated Th1 responses (7). TSP-1 production by immature DCs was enhanced upon activation and may thereby act as an autocrine “deactivator.” Accordingly, CD47 −/− DCs displayed an activated phenotype, produced more IL-12, and thereby induced enhanced Th1 responses. Future work will examine if CD47 −/− mice are prone to autoimmunity, as expected.
DC cell biology
The efficiency of antigen presentation on MHC class II by DCs correlates with their low levels of lysosomal proteases, which allows antigen persistence (8) (Ira Mellman, New Haven, CT). It remains to be defined how DCs are able to cross-present exogenous nonreplicating antigens for presentation on MHC class I. The loading compartment may be either the endoplasmic reticulum (ER) or a mixed phagosome-ER compartment, a concept which had drawn much attention recently (9, 10) and was strongly challenged by Mellman who has failed to find evidence for phagosome-ER fusion by several approaches. In a friendly discussion, Sebastian Amigorena (Paris, France) suggested that Mellman's inability to find fusion of GFP-tagged ER resident proteins with phagosomes might be explained by a low sensitivity of his detection method. Amigorena focused on the role of Rab27a in cross-presentation. Rab27a, a GTP-binding protein, which interacts with myosins and mediates actin binding, is expressed in DC phagosomes and MHC class II endocytic compartments. Rab27a deficiency leads to enhanced protein degradation, high phagosome acidification, and a delay in phagosome fusion to the lysosome, all of which hamper cross-presentation.
DC subsets and plasticity
DCs can develop along two pathways, CD11c+ mDCs (also called conventional DCs) and pDCs. mDCs have long been known to include distinct subsets such as Langerhans cells and interstitial (dermal) DCs in humans and CD8+ and CD8− DCs in mice. Jacques Banchereau (Dallas, TX) extended mDC diversity into a wider rainbow of mDC subsets. He showed that in vitro different cytokines, such as IFNα/β, IL-15, IL-4, and TNF, skew the differentiation of monocytes, which are potential precursors of mDCs, into different DC subtypes. These subtypes have both common and unique properties and may ultimately shape the type of T cells that respond in vivo upon microbial invasion. The cytokines produced by microbe-activated cells (pDCs, keratinocytes, mast cells) will determine the type of DCs that the monocyte differentiates into. Yong-Jun Liu (Houston, TX) showed that the cytokine thymic stromal lymphopoietin (TSLP) activated mDCs causing them to polarize T cells, leading to an inflammatory Th2 response characterized by TNF expression. However, in the thymus, TSLP produced by Hassal's corpuscles activated DCs to polarize CD4+CD8− thymocytes into CD4+ CD25+CD8− regulatory T cells.
Elina Zuniga (La Jolla, CA) showed that infection with LCMV induces a proportion of immature bone marrow pDCs to differentiate into mDCs (11). This claim was based on down-regulation of all known pDC markers studied (B220, Ly6C, and 120G8), up-regulation of CD11c and CD11b (a prototype mDC marker), enhanced antigen-presenting capacity, and the up-regulation of TLR3 and TLR4 expression, which enabled responsiveness to LPS (a property of mDCs not shared by pDCs). Nonetheless, this finding was met with skepticism by Jacques Banchereau who argued that morphological changes and receptor modulation—well-known characteristics of DCs—might not equate to lineage infidelity.
DC–T cell engagement in vivo
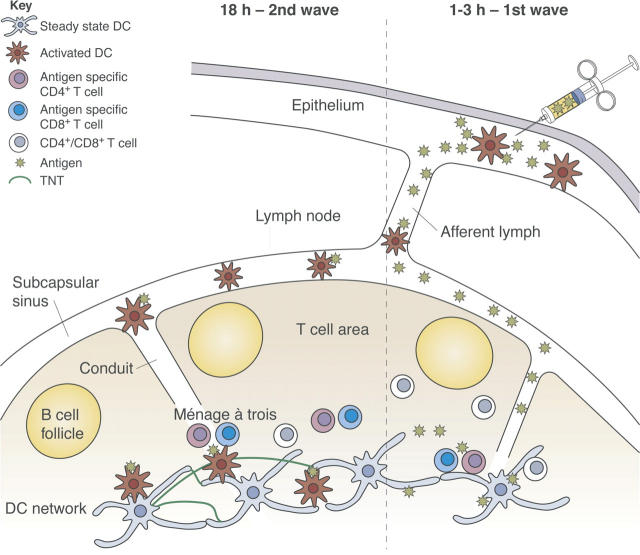
With the advent of new imaging technology, it is becoming possible to witness how DC and T cells interact in vivo, which will undoubtedly provide new insights on these interactions during an immune response. Michel Nussenzweig (New York, NY) and Ronald Germain (Bethesda, MD), each presented movies and images of DC–T cell interactions in the lymph nodes of living mice. Germain demonstrated by intravital two-photon microscopy the ménage à trois between DCs, CD4+, and CD8+ T cells. In the course of productive immune responses, the antigen-specific T cells establish interactions with DCs that can last for several hours. Amigorena, also using two-photon microscopy, failed to find stable interactions between DCs and CD8+ T cells during the induction of tolerance. Nussenzweig contested this finding, as he observed that antigen-specific T cells stopped for prolonged interaction with the DC whether in the course of tolerance or immunity. He found that in the steady-state most lymph node DCs are entangled in an extensive sessile network. The DC network surrounds the B cell follicles, extends into the T cell zones, and is particularly dense in the border between the T and B cell follicle, where T cell–dependent immune responses are initiated. Mature DCs, which migrate into lymph nodes from tissues, eventually integrate into the network (12). Russell Salter (Pittsburgh, PA) showed that myeloid-lineage DCs and monocytes can be triggered to flux calcium by mechanical contact with a microprobe, and that the signal can be propagated within seconds to other cells at distances up to a hundred microns away. This communication occurred via membranous connections called tunneling nanotubules (TNTs), indicating that within the DC network TNTs might permit transmission of signals between cells. These findings regarding DC–T cell interactions are illustrated in Fig. 2.
Figure 2.
Two waves of antigen delivery. Antigen administered subcutaneously is delivered in two successive waves to the draining lymph node, and presented by different DCs which initiate different effector functions.
Marc Jenkins (Minneapolis, MA) showed that antigen injected intradermally is delivered to lymph nodes in two waves. A recombinant fluorescing antigen first diffused through lymphatic vessels into the subcapsular sinus and conduit network, reaching the resident DC network within 30 minutes, which presents the processed antigen within 3 hours. In the second wave, DCs that had acquired antigen at the injection site delivered antigen into the T cell areas by 18 hours (Fig. 2). It is thought that resident and migratory DCs educate T cells to acquire different functions.
DCs in autoimmunity and allergy
Since DCs are critical in initiating and perpetuating the class of the immune response and cytokine production in response to antigens, one might expect that alterations of DC homeostasis might determine autoimmune and allergic pathologies. Fiona Powrie (Oxford, UK) showed that in Rag −/− mice CD11c+CD11b+ DCs activated with CD40-specific antibody induced colitis and systemic wasting disease. Systemic wasting was triggered by production of IL-12 and could be inhibited by IL-12 p40-specific antibody, but local inflammation was induced by IL-23 production, since treatment with anti-p19 abrogated local disease. This implies that cytokine antagonists might be useful interventions in these diseases.
Overproduction of type I IFNs has been associated with some autoimmune disease states such as systemic lupus erythematosus (SLE). Virginia Pascual (Dallas, TX) reported that certain arthritis patients develop an SLE-like syndrome during anti-TNF treatment (13). Gene expression profiles in these patients show highly up-regulated expression of IFN-inducible genes, which were similar to those up-regulated in SLE patients. Virus-induced IFN-α was suppressed by TNF in vitro and augmented by anti-TNF antibody. Based on these results, Pascual proposed that an imbalance between TNF and IFN-α production might cause SLE in anti-TNF–treated rheumatoid arthritis patients. Cross talk between DCs and non-DCs could be important for type I IFN production both in physiologic and pathologic conditions (13). Finally, Pascual reported a spectacular improvement when children suffering from a devastating form of juvenile arthritis, who failed to respond to anti-TNF treatment, were treated with an IL-1α/β antagonist (14).
A pathogenic role for pDCs and IFNα (15) in human psoriasis was suggested by Michel Gilliet (Houston, TX). This skin disease is characterized by chronic relapses and is triggered by factors such as infection, stress and drugs. Gilliet showed that precursors of pDCs infiltrated the skin of psoriasis patients and were activated to produce IFNα at an early stage of the disease (16). Blocking IFNα signaling and/or production prevented induction of psoriasis in a model in which human skin from psoriatic patients was transplanted into mice.
In a mouse model of allergic asthma, capture and presentation of an inhaled protein by airway DCs was shown to lead to T cell tolerance (Bart Lambrecht, Rotterdam, Netherlands) (17). pDCs maintained this tolerance by suppressing the development of Th2 cytokine responses. It was not clear whether pDCs mediated this effect by inducing Th1 cytokines, type I IFNs, or IL-10 expression, all of which could result in down-regulation of allergic responses. However, mDC are essential for inducing effector Th2 responses (17). Exposure of human respiratory tract DCs to granulocyte-macrophage colony-stimulating factor, previously reported to be released by respiratory cells in response to allergens, was shown to activate DCs to support Th2 cell differentiation (Alexander Faith, London, UK).
From DC and NK cells to DCs in infection
A new appreciation of DCs interactions with NK cells was one of the highlights of this meeting. Early IL-2 responses from bacteria-stimulated DCs contribute to activation of NK cells (18). In addition, DCs activated with TLR ligands have been shown to induce an early influx and activation of NK cells in the lymph node and to be important for the development of Th1 responses (19). Nicolas Glaichenhaus (Valbonne, France) extended our understanding of NK–DC interactions in vivo with beautiful two-photon images of NK cells in the lymph nodes: 80% of these cells were located in the outer paracortex near the lymphatics and close to DCs. In the steady-state, NK cells moved slowly but were seen to crawl over the DCs making long lasting (>20 minutes) contact. During infection with Leishmania major, antigen-specific T cells were relocated to the outer paracortex of the lymph node. However, NK cell distribution, colocalization with DCs, and the speed at which they moved were not changed.
Alan Sher (Bethesda, MD) has been searching for single TLR ligands responsible for the IL-12 production by CD8+ DC stimulated with soluble Toxoplasma gondii antigen (STag). In addition to cyclophilin, which had been previously identified as a ligand of CCR5 (20, 21), Sher and colleagues identified another STag protein, profilin, which was shown to signal through TLR11 and induce large amounts of IL-12 in CD8α1 DCs. Tlr11 −/− mice showed decreased IL-12 production in response to STag. These results implicate TLR11 as a receptor for apicomplexan protozoan parasites and for IL-12 production by DCs (22).
A new population of DC-like cells, whose function remains a mystery, was identified in other infections in mice. Malin Sundquist (Goteborg, Sweden) described large numbers of CD11c intermediate CD11b+ DCs in mesenteric lymph nodes of mice infected orally with Salmonella. Jean Langhorne (London, UK) reported a large increase of DCs with a similar phenotype in the spleen of mice infected with malaria.
Human papilloma virus infection (HPV) is thought to be the causative agent for most cases of cervical cancer. W. Martin Kast (Los Angeles, CA) has found a possible mechanism for evasion of T cell immunity to HPV (23). Langerhans cells at the site of infection did not elicit virus-specific immune responses, and Langerhans cells incubated with HPV virus-like particles in vitro up-regulated PI3K, which were previously shown to affect cytokine gene regulation. However, when PI3K pathways were blocked, Langerhans cells induced virus-specific T cell responses in vitro. Thus, HPV may prevent the priming of antiviral T cell responses by inhibiting Langerhans cell activation and/or migration.
In addition to DCs, innate viral recognition by radioresistant cells, possibly epithelial cells, is also involved in establishing Th1 immunity against viral infection (Akiko Iwasaki, New Haven, CT) (24).
TLR agonists and antagonists in vaccines and therapy
Small synthetic agonists of some TLRs can be easily manufactured. Robert Coffman (Dynavax, Berkeley, CA) found that short oligonucleotide TLR9 agonists coupled to antigen activate DCs, thereby increasing antigen uptake and providing a strong adjuvant effect. Coffman reported that pulmonary administration of this TLR9 agonist in a mouse model of allergic asthma led to specific inhibition of the Th2 response to subsequent allergen challenge. Coffman also described a novel antagonist that inhibited TLR7, 8, and 9 stimulation and IFN-α production by pDCs, which suggested its use as a potential therapy for SLE.
DC therapy and immunomonitoring
DCs generated in vitro by culturing either monocytes or CD34+ hematopoietic progenitor cells are considered promising therapeutic vaccines in cancer. Gerold Schuler (Erlangen, Germany) presented the results of the first phase III randomized clinical trial of DC vaccination in stage IV metastatic melanoma patients. He found that of the patients who received the DC vaccine, those who enjoyed a better health status or expressed a particular HLA haplotype (A2+/B44−) survived significantly longer. Thus, overall health status and HLA type may influence the success of active immunotherapy, and these parameters should be considered in the design of future vaccines. Hideki Ueno (Dallas, TX) presented EPIMAX, a novel high throughput strategy for predicting and monitoring the immune response of vaccinated patients. By simultaneously analyzing T cell proliferation and production of multiple cytokines in response to pools of peptides from overlapping libraries, it was possible to accurately define the specificity and the type of T cell responses in patients, before or after DC vaccination. This allowed for the monitoring of multiple types of tumor antigen-specific T cells which may affect the outcome of the vaccine in cancer.
In vivo targeting of antigen to DCs might eventually replace vaccination with ex vivo–generated, antigen-loaded DCs. A single dose of an antibody specific for the DC-specific lectin DEC-205 fused to HIV-gag induced efficient CD4+ T cell immunity against HIV-gag protein that protected mice against a challenge with recombinant vaccinia-gag virus (Ralph Steinman, New York, NY). They anticipate that this will form the basis of future vaccines in cancer and infectious disease therapies.
About the authors
Eynav Klechevsky is a PhD student working with Yoram Reiter at the Technion Israel Institute of Technology in Haifa, Israel, and with Jacques Banchereau at the Baylor Institute for Immunology Research in Dallas, Texas, on class I antigen presentation in various DC subsets and its influence on immune responses.
Hiroki Kato is a student working in Shizuo Akira's laboratory in the Research Institute for Microbial Diseases of Osaka University in Japan. His main research topic is the mechanism of type I IFN induction in response to RNA viruses, focusing on which molecules recognize RNA viral infection and trigger IFN responses.
Anne-Marit Sponaas works in Jean Langhorne's laboratory in the Parasitology Division at the National Institute for Medical Research in London. The lab studies the regulation of immune responses to malaria parasites, and Sponaas is interested in the role of DCs in this process.
Acknowledgments
The authors thank Anne O'Garra, Jacques Banchereau, Ralph Steinman, Tsuneyasu Kaisho, and Jean Langhorne for their help and support in writing this review.
References
- 1.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Nat. Immunol. 10.1038/ni1223. [DOI] [PMC free article] [PubMed]
- 2.Amsen, D., J.M. Blander, G.R. Lee, K. Tanigaki, T. Honjo, and R.A. Flavell. 2004. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 117:515–526. [DOI] [PubMed] [Google Scholar]
- 3.Cambi, A., F. de Lange, N.M. van Maarseveen, M. Nijhuis, B. Joosten, E.M. van Dijk, B.I. de Bakker, J.A. Fransen, P.H. Bovee-Geurts, F.N. van Leeuwen, et al. 2004. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J. Cell Biol. 164:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers, N.C., E.C. Slack, A.D. Edwards, M.A. Nolte, O. Schulz, E. Schweighoffer, D.L. Williams, S. Gordon, V.L. Tybulewicz, G.D. Brown, and C. Reis E Sousa. 2005. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 22:507–517. [DOI] [PubMed] [Google Scholar]
- 5.Honda. K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-1 interferon-dependent immune responses. Nature. 434:772–777. [DOI] [PubMed] [Google Scholar]
- 6.Dillon, S., A. Agrawal, T. Van Dyke, G. Landreth, L. McCauley, A. Koh, C. Maliszewski, S. Akira, and B. Pulendran. 2004. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172:4733–4743. [DOI] [PubMed] [Google Scholar]
- 7.Doyen, V., M. Rubio, D. Braun, T. Nakajima, J. Abe, H. Saito, G. Delespesse, and M. Sarfati. 2003. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 198:1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delamarre, L., M. Pack, H. Chang, I. Mellman, and E.S. Trombetta. 2005. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 307:1630–1634. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins, M. 2003. ER-mediated phagocytosis: a new membrane for new functions. Nat. Rev. Immunol. 3:280–291. [DOI] [PubMed] [Google Scholar]
- 10.Guermonprez, P., L. Saveanu, M. Kleijmeer, J. Davoust, P. Van Endert, and S. Amigorena. 2003. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 425:397–402. [DOI] [PubMed] [Google Scholar]
- 11.Zuniga, E.I., D.B. McGavern, J.L. Pruneda-Paz, C. Teng, and M.B. Oldstone. 2004. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat. Immunol. 5:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindquist, R.L., G. Shakhar, D. Dudziak, H. Wardemann, T. Eisenreich, M.L. Dustin, and M.C. Nussenzweig. 2004. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5:1243–1250. [DOI] [PubMed] [Google Scholar]
- 13.Palucka, A.K., J.P. Blanck, L. Bennett, V. Pascual, and J. Banchereau. 2005. Cross-regulation of TNF and IFN-α in autoimmune diseases. Proc. Natl. Acad. Sci. USA. 102:3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual, V., F. Allantaz, E. Arce, M. Punaro, and J. Banchereau. 2005. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilliet, M., C. Conrad, M. Geiges, A. Cozzio, W. Thurlimann, G. Burg, F.O. Nestle, and R. Dummer. 2004. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 140:1490–1495. [DOI] [PubMed] [Google Scholar]
- 16.Nestle, F.O., C. Conrad, A. Tun-Kyi, B. Homey, M. Gombert, O. Boyman, G. Burg, Y.-J. Liu, and M. Gilliet. 2005. Plasmacytoid predendritic cells initiate psoriasis through IFN-α production. J. Exp. Med. 202:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rijt, L.S., S. Jung, A. Kleinjan, N. Vos, M. Willart, C. Duez, H.C. Hoogsteden, and B.N. Lambrecht. 2005. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granucci, F., I. Zanoni, N. Pavelka, S.L. Van Dommelen, C.E. Andoniou, F. Belardelli, M.A. Degli Esposti, and P. Ricciardi-Castagnoli. 2004. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J. Exp. Med. 200:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Fontecha, A., L.L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260–1265. [DOI] [PubMed] [Google Scholar]
- 20.Yarovinsky, F., J.F. Andersen, L.R. King, P. Caspar, J. Aliberti, H. Golding, and A. Sher. 2004. Structural determinants of the anti-HIV activity of a CCR5 antagonist derived from Toxoplasma gondii. J. Biol. Chem. 279:53635–53642. [DOI] [PubMed] [Google Scholar]
- 21.Aliberti, J., J.G. Valenzuela, V.B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J.M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485–490. [DOI] [PubMed] [Google Scholar]
- 22.Yarovinsky, F., D. Zhang, J.F. Andersen, G.L. Bannenberg, C.N. Serhan, M.S. Hayden, S. Hieny, F.S. Sutterwala, R.A. Flavell, S. Ghosh, and A. Sher. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 308:1626–1629. [DOI] [PubMed] [Google Scholar]
- 23.Fausch, S.C., L.M. Fahey, D.M. Da Silva, and W.M. Kast. 2005. Human papillomavirus can escape immune recognition through langerhans cell phosphoinositide 3-kinase activation. J. Immunol. 174:7171–7178. [DOI] [PubMed] [Google Scholar]
- 24.Sato, A., and A. Iwasaki. 2004. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl. Acad. Sci. USA. 101:16274–16279. [DOI] [PMC free article] [PubMed] [Google Scholar]