Abstract
B7-DC, one of the recently described B7 family members, has the capacity to inhibit T cell responses via engagement of the immunoreceptor tyrosine-based inhibitory motif–containing inhibitory PD-1 receptor as well as enhance responses via an as yet unidentified costimulatory receptor. B7-DC is highly homologous to a coinhibitory B7 family member, B7-H1, which also binds PD-1. It is currently unclear which B7-DC function—costimulation or inhibition—predominates in vivo. To study in vivo functions of B7-DC, we evaluated immune responses in B7-DC knockout (KO) mice. Although not eliminated, interferon-γ (IFN-γ) production by CD4 T cells and IFN-γ–dependent humoral responses were reduced in B7-DC KO mice relative to wild type mice. Antigen-specific CD8 T cell responses and cytotoxic T lymphocyte (CTL) activity were also diminished in B7-DC KO mice. Hepatic tumors grew more quickly in B7-DC KO mice, associated with a decrease in intrahepatic tumor-specific CD8 T cells. These results highlight the contrasting in vivo roles of B7-DC and B7-H1 and indicate that B7-DC functions as a tuning molecule, selectively augmenting T helper 1 and CTL responses.
The B7 family of costimulatory and coinhibitory molecules critically regulates qualitative and quantitative aspects of T cell responses to antigen (1, 2). The complexity of this regulation depends upon a number of factors including differential kinetics and patterns of expression of the B7 family molecules by different APC types; differential kinetics, patterns of expression; and signaling outcomes of their multiple receptors on T cells; and effects of backward signaling by the B7 family members themselves. The well-characterized prototype B7 family molecules B7-1 (CD80) and B7-2 (CD86) costimulate T cells via interaction with CD28 expressed on both naive and activated T cells and down-regulate T cell responses via interaction with the activation-dependent counter-regulatory receptor CTLA-4 (3, 4). Although B7-1 and B7-2 are considered largely redundant, recent evidence suggests partially distinct roles resulting from different capacities for homodimerization and resultant binding kinetics in CD28 and CTLA-4.
B7-H1 (PDL1) and B7-DC (PDL2) represent a second molecular pair within the B7 family (5–8). They are the most homologous of any pair of B7 family molecules, and both bind PD-1, an immunoreceptor tyrosine-based inhibitory motif–containing receptor expressed on activated T cells (and B cells) that seems to down-modulate T cell responses via multiple mechanisms including cell-cycle inhibition and apoptosis induction (9). In accordance with their PD-1 specificity, both B7-H1 and B7-DC were shown to inhibit T cell responses under certain conditions of in vitro T cell stimulation (5, 10–12). Paradoxically, both molecules also were shown to costimulate T cells in vitro under some conditions (6, 7, 13, 14). A partial explanation for these apparently conflicting results came from two lines of investigation that provided evidence that T cell costimulation by B7-H1 and B7-DC occurs via a second receptor. First, a structure-based mutational analysis of B7-H1 and B7-DC revealed PD-1 binding residues that, when mutated, result in molecules that have lost PD-1 binding capacity but retained costimulatory activity (13). Another set of studies demonstrated that naive T cells from PD-1 KO mice are costimulated by B7-DC in a similar fashion to naive T cells from WT mice (13, 14). B7-H1 can also stimulate PD-1 KO T cells (Housseau F, unpublished data). Taken together, these results suggest a model similar to the B7-1/2 pair in which naive T cells express a costimulatory receptor, and activated T cells turn on an inhibitory receptor.
Despite these analogies with the B7-1/2 pair, the radically different expression patterns of B7-H1 and B7-DC suggest that they could have distinct in vivo biologic functions. B7-H1 is quite ubiquitously expressed on the surface of many cell types, including stromal cells within many organs. In contrast, although B7-DC mRNA has been reported in many tissue types, surface expression is much more restricted, in particular to DCs and a subset of macrophages (5–7, 15, 16). Many tumors express B7-H1, but relatively few express B7-DC. The in vivo role of B7-H1 has been studied much more extensively than that of B7-DC. B7-H1 expression on tumors inhibits the activity of tumor-specific CTLs (17). Apoptosis of CD8 T cells in the liver is dramatically reduced in B7-H1 KO mice, and hepatitis is increased (18). These results point to an important inhibitory role for B7-H1 in vivo, protecting both tissues and tumors from immune attack. Collectively, these results support a predominantly negative regulatory role for B7-H1.
At present, analysis of the in vivo role of B7-DC has been limited. The effects of in vivo anti-B7-DC mAb injection have been variable in different model systems. In a model of Th2-dependent asthma, anti-B7-DC antibodies exacerbated disease in association with increased IL-4 production in the lung (19). In an experimental autoimmune encephalitis model, anti-B7-DC antibody administration was reported to diminish disease, whereas this treatment exacerbated autoimmune diabetes in nonobese diabetic (NOD) mice (20, 21). Interpretation of these results is complicated by partial and variable antagonistic activity of different antibodies, variable tissue access, variable effects in inducing elimination of B7-DC–expressing cells, and potential effects of backward signaling into DCs through cross-linking of B7-DCs (22, 23). To evaluate the in vivo role of B7-DC more definitively, we have analyzed immune responses in B7-DC KO mice. Our results demonstrate diminished but not absent Th1 and CTL responses in B7-DC KO mice, indicating that, in contrast to B7-H1, B7-DC serves as a costimulator of Th1 responses in vivo and augments CTL responses. Further, B7-DC seems to serve more as a rheostat rather than as an on–off switch for these immune functions.
Results
Leukocyte populations are normal in B7-DC knockout mice
To knock out the B7-DC gene and simultaneously introduce a reporter for B7-DC expression, we used a knock-out/knock-in construct that replaced B7-DC with the EGFP gene (Fig. 1a). Animals derived from recombinant embryonic stem cells were backcrossed nine generations onto the BALB/c background. Initially, myeloid and lymphoid populations from the thymus, secondary lymphoid tissues, and liver were analyzed. Overall cell numbers and proportions of standard subsets of T, B, NK, NK T cells, and DC were fairly similar in the thymus and spleen of WT and B7-DC KO mice (Fig. S1 available at http://www.jem.org/cgi/content/full/jem.20050072/DC1). This similarity contrasts with the recently reported B7-H1 KO mice, in which the total number of CD8 T cells in the liver is significantly increased (18). Importantly, the expression pattern of MHC class II as well as other B7 family members, including B7-1 (CD80), B7-2 (CD86), and B7-H1, on DCs was not appreciably affected by the absence of B7-DC (Fig. 1b). Likewise, the cell yield from bone marrow–derived DC (BMDC) cultures was essentially identical in WT and B7-DC KO mice (Fig. 1c).
Figure 1.
Generation and characterization of B7-DC gene KO mice. (a) The targeting map of B7-DC genomic locus. The signal peptide with the ATG initiation codon of B7-DC was replaced with EGFP and NEO cassette. After mating with CMV-Cre mouse, the NEO cassette was deleted. The 5′ probe indicated was used for Southern blot analysis of mouse genomic DNA. (b) Flow cytometry analysis of B7-DC, B7-H1, B7-1, B7-2, and MHC class II (H-2Kd) expression on the surface of BMDCs from WT and B7-DC KO mice on the BALB/c background. Mouse BM cells were cultured with GM-CSF for 6 d and with GM-CSF and IL-4 for additional 2 d. Data are representative of six experiments. (c) Absolute numbers of BMDCs from WT or B7-DC KO mice (n = 13/group).
In vitro T cell responses in the absence of B7-DC
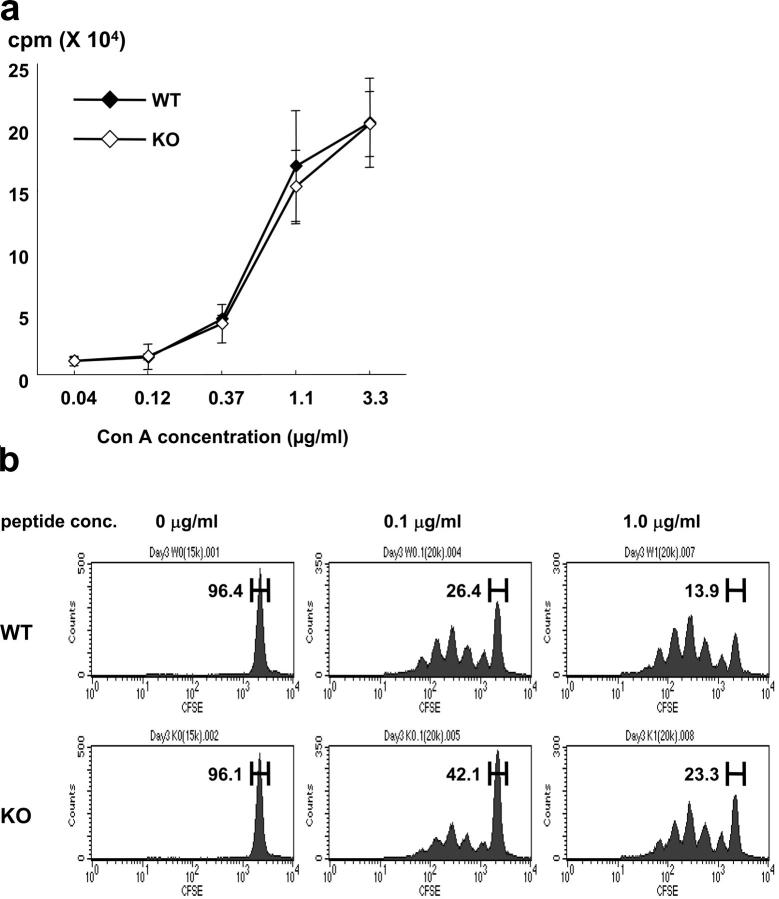
As described in earlier publications, surface expression of B7-DC is largely restricted to DCs and a subset of macrophages, with essentially no detectable expression on B cells or T cells in any activation state (16). Indeed, purified T cells from B7-DC KO mice proliferate in response to the T cell mitogen, Concanavalin A (ConA), equivalently to WT T cells (Fig. 2a). In contrast, naive OVA-specific CD4 T cells (from the DO.11.10 TCR transgenic line) are stimulated to proliferate somewhat less efficiently than WT DCs in vitro by OVA-pulsed B7-DC KO DCs (Fig. 2b). Given that MHC II, B7-1, B7-2, and B7-H1 are not altered on B7-DC KO DCs (Fig. 1b), these data are compatible with a costimulatory role for B7-DC. However, they could also reflect effects on other costimulatory molecules or backward signaling into DCs by B7-DC.
Figure 2.
In vitro T cell proliferation. (a) Purified T cells from WT and B7-DC KO mice were cultured with the indicated concentration of ConA for 3 d. 16 h before the end of culture, 3H-thymidine was added, and its uptake was measured. (b) Purified DO11.10 CD4 T cells were labeled with CFSE and cultured for 3 d with BMDCs from WT and B7-DC KO mice in the presence of various concentrations of OVA323-339 peptide. CFSE dilution as a measure of cell division of CD4+KJ1-26+ T cells was analyzed by FACS. Data are representative of three independent experiments.
Earlier studies have shown that when plate-bound B7-DC-Fc plays a costimulatory role, particularly when used in conjunction with B7-1-Fc or B7-2-Fc, there is a pronounced effect on IL-2 and IFN-γ, (14). We therefore further evaluated the role of B7-DC in Th1 versus Th2 differentiation upon culturing purified DO11.10 CD4 T cells with BMDCs from WT or B7-DC KO mice under Th1- or Th2-generating conditions in the presence of OVA323-339 peptide. Fig. 3 shows that T cells cultured with BMDCs from B7-DC KO mice generated roughly 40% fewer IFN-γ+ cells than did T cells cultured with BMDCs from WT mice (59.7% vs. 35.0%, respectively). The same reduction in IFN-γ+ CD4 T cells was observed in the presence of additional anti-mouse CD3 antibody during restimulation (81.7% vs. 50.6%, respectively). In contrast, culture under Th2-generating conditions failed to demonstrate a significant difference in IL-4+ T cells whether WT or B7-DC KO BMDCs were used.
Figure 3.
In vitro Th1 and Th2 cell generation by dendritic cells. 1 × 104 purified DO11.10 CD4 T cells were cultured with 3 ×104 BMDCs from WT or B7-DC KO mice with OVA323-339 peptide for 5 d in the presence of recombinant IL-12 and anti-IL-4 mAb for Th1 generation or recombinant IL-4 and anti-IFN-γ mAb for Th2 generation. 6 h before end of culture, additional OVA323-339 peptide with or without anti-CD3 mAb was added for restimulation. After surface CD4 was stained, cells were fixed and permeabilized, and intracellular IFN-γ and IL-4 were stained. The data shown are gated on CD4+ cells. Data are representative of four independent experiments.
These results support the notion that B7-DC selectively costimulates Th1 responses. The effect is certainly not all-or-none, suggesting the this particular B7 family member tunes IFN-γ responses rather than being absolutely necessary for their generation.
Effect of B7-DC on in vivo antigen-specific T cell proliferative and cytokine responses
To analyze the role of B7-DC in the priming of in vivo immune responses, we adoptively transferred naive OVA-specific DO11.10 cells and measured responses to immunization with peptide loaded onto DCs. 5 million purified carboxyfluorescein succinimidyl (CFSE)-labeled DO11.10 CD4 T cells were transferred into WT or B7-DC KO mice on d −1. Because it remains unresolved whether priming by ex vivo–loaded DCs involves direct presentation or transfer of antigen from injected DCs to endogenous DCs, the genotype of the BMDCs was matched to the immunized host; that is, WT mice were immunized with WT DCs, and B7-DC KO mice were immunized with B7-DC KO BMDCs. This design assures that all potential DCs presenting antigen in vivo are uniformly WT or B7-DC KO. When these mice were immunized by OVA323-339 peptide–pulsed BMDCs, a significant effect of B7-DC on T cell expansion was observed. Whereas OVA-specific KJ1-26+ T cells expanded to 11.6%, 1.8%, 4.1%, and 7.6% of total CD4 T cells in spleen, peripheral lymph nodes (PLN), liver, and hepatic lymph node (HLN), respectively, expansion in B7-DC KO mice was 7.7% (34% reduction), 0.9% (50% reduction), 2.4% (42% reduction), and 3.0% (61% reduction) in the respective compartments by d 3 after immunization (Fig. 4a). By d 5, B7-DC KO mice showed similar reductions relative to those in WT mice in all compartments except PLN, in which there was no significant reduction (Fig. 4b). A more pronounced effect of B7-DC deletion was observed in the analysis of CFSE dilution in DO11.10 CD4 T cells (Figs. 4, c and d, and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20050072/DC1). For example, the ratios of undivided cells (no CFSE dilution) to undivided cells were significantly different, particularly in the liver and HLN, on d 3 and d 5. On d 3, there were 8.7 times more undivided cells in B7-DC KO mouse liver than in WT mouse liver and 2.7 times more undivided cells in B7-DC KO mouse HLN than in WT mouse HLN. On d 5 there were 2.9 times more undivided cells in B7-DC KO mouse liver than in WT mouse liver and 2.4 times more undivided cells in B7-DC KO mouse HLN than in WT mouse HLN). Analysis of CD4+ KJ1-26+ T cell expansion and CFSE dilution showed similar trends in differences between B7-DC KO and WT mice among the various compartments; namely, liver > HLN > spleen > PLN.
Figure 4.
In vivo CD4 T cell stimulation after DC immunization. 5 ×106 purified CFSE-labeled DO11.10 CD4 T cells were transferred into WT or B7-DC KO mice on d −1. Mice were then immunized with 1 ×106 OVA323-339 peptide–pulsed BMDCs on d 0. Peptide-pulsed BMDCs from WT mice were injected into WT mice, and peptide-pulsed BMDCs from B7-DC KO mice were injected into B7-DC KO mice, respectively. (a and b) Proportion and (c and d) cell division (CFSE dilution) of CD4+KJ1-26+ T cells in spleen, PLN, liver, and HLN were analyzed by FACS on d 3 (a and c) and d 5 (b and d). The CFSE-labeled CD4+KJ1-26+ T cells in liver, immunized with BMDCs without OVA323-339 peptide, are also shown in Fig. S2 as negative controls. (e) The absolute number of DO11.10 CD4 T cells in spleen and liver was calculated on d 3 and 5 by the formula: total cell number × percent CD4+ cells × percent CD4+ cells staining with KJ1-26. *, Significant difference (P < 0.001) observed in liver of six pairs of B7-DC KO mice and WT mice on d 3 and 5. Data are representative of three independent experiments.
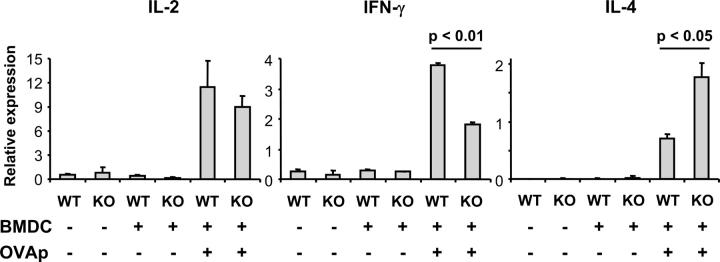
To follow up on the in vitro results suggesting that B7-DC selectively contributes to Th1 responses, we analyzed in vivo cytokine responses to immunization with OVA peptide-pulsed DCs in animals receiving DO11.10 CD4 T cells 1 d before immunization, as described previously. To avoid effects on cytokine patterns caused by in vitro stimulation, we analyzed cytokine mRNA by quantitative RT-PCR from OVA-specific T cells purified directly from immunized animals. 5 d after immunization, CD4+KJ1-26+ T cells were sorted out, and the RNA was prepared for quantitative RT-PCR. Cytokine responses as assessed by mRNA were highly antigen specific—virtually none were detected in purified CD4+KJ1-26+ T cells after immunization with unpulsed DCs. Significantly less IFN-γ, slightly less IL-2, and significantly more IL-4 was consistently observed in B7-DC KO mice immunized with peptide-pulsed B7-DC KO BMDCs than in WT mice immunized with WT BMDCs (Fig. 5). These results demonstrate that, in the absence of B7-DC, cytokine production is skewed from Th1 to Th2 with decreased IFN-γ and increased IL-4 production. As in the in vitro studies, the effect is partial, compatible with a tuning function for B7-DC.
Figure 5.
In vivo Th1 response in B7-DC KO mice. 5 ×106 purified DO11.10 CD4 T cells were adoptively transferred into WT and B7-DC KO mice on d −1 as in Fig. 4. Mice were then immunized with 106 OVA323-339 peptide-pulsed BMDCs on d 0. Peptide-pulsed BMDCs from WT mice were injected into WT mice, and peptide-pulsed BMDCs from B7-DC KO mice were injected into B7-DC KO mice, respectively. On d 5, CD4+KJ1-26+ T cells were sorted out, and their mRNA was extracted. The expression level of each cytokine was detected by real-time RT-PCR, and relative transcripts were calculated by the formula given in Material and methods. Data are representative of three independent experiments.
Effect of B7-DC on antibody-class switching
The patterns of cytokine expression from both the in vitro and in vivo studies described previously suggested that B7-DC has a positive costimulatory role in enhancing Th1-type responses. It was therefore of interest to analyze the effect of B7-DC deletion on antibody isotypes in response to immunization, because isotype switching is dependent on production of specific cytokines by Th cells and, as such, partially reflects Th1/2 balance. WT and B7-DC KO mice were immunized with the T cell–dependent antigen NP17-KLH in alum, and the serum levels of NP-specific antibody isotypes were measured. Significantly, all classes of antibody were essentially identical in WT and B7-DC KO mice with the exception of the IFN-γ–dependent IgG2a class, which was dramatically reduced in B7-DC KO mice (Fig. 6). These results support a functional role for B7-DC in costimulating IFN-γ–dependent class switching in vivo.
Figure 6.
Class switching of antibodies in B7-DC KO mice. 100 μg of NP17-KLH with alum was injected twice (2-wk interval between injections) into WT mice (n = 7) or B7-DC KO mice (n = 7). Sera were collected, and the titers of isotype of NP17-specific antibody were evaluated by ELISA. Each dot indicates the titer of a single mouse. *, Significantly different (P < 0.001) from WT mice.
Effect of B7-DC on in vivo cytotoxic T lymphocyte priming
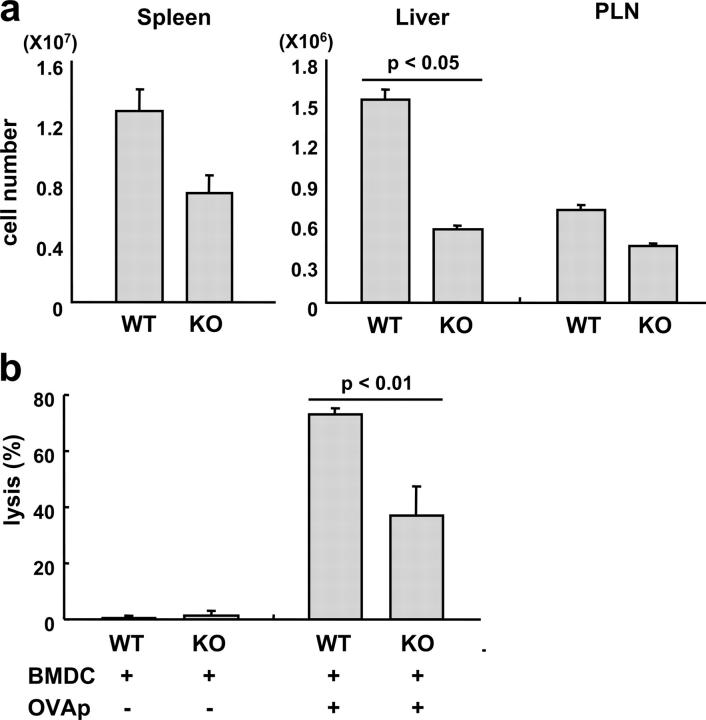
To determine whether B7-DC plays a role in MHC class I–restricted CTL generation, we transferred 2 × 106 T cells from H-2Kd+ hemagglutinin (HA)-specific TCR transgenic mice (24, 25) into BALB/c WT or B7-DC KO mice and immunized them with unloaded BMDCs as control or with BMDCs loaded with the cognate peptide (HA518-526). As in the in vivo CD4 T cell experiments, WT mice were immunized with WT BMDCs, and B7-DC KO mice were immunized with B7-DC KO BMDCs. As with antigen-specific CD4 T cells, expansion of HA-specific CD8 T cells was lower in B7-DC KO mice than in WT mice, particularly in the liver and, to a lesser extent, in the spleen (Fig. 7). As with CD4 T cells, the smallest difference in absolute numbers of HA-specific CD8 T cells in WT and B7-DC KO mice was observed in PLN. To measure CTL activity directly, an in vivo CTL assay was done in which two populations of BALB/c splenocytes differentially labeled with CFSE, one of which loaded with HA518-526, were transferred into the immunized mice. As a control, some immunized mice received splenocytes in which neither the CFSEhigh or CFSElow cells were peptide loaded. In vivo CTL activity is determined based on the selective reduction in ratio of peptide-loaded cells (CFSElow) to unloaded cells (CFSEhigh) reisolated from the spleen 10 h after injection. As seen in Fig. 7b, in vivo CTL activity in immunized B7-DC KO mice was roughly half that of WT mice. Thus, B7-DC seems to play a costimulatory role in vivo for CTL proliferation and activation, either indirectly by enhancing Th function or directly by costimulating CD8 T cells. Because only the CTL epitope of HA was used in the immunizations, a direct role in CD8 T cell costimulation independent of CD4 T cells is the most likely mechanism. This notion is supported by findings that B7-DC can strongly costimulate purified CD8 T cells in vitro (15).
Figure 7.
CD8 T cell responses and CTL activity in B7-DC KO mice. 2 × 106 T cells from H-2Kd+ HA-specific TCR transgenic mice (clone 4 mice) were transferred into BALB/c WT or B7-DC KO mice on d −1 followed by immunization with 1 ×106 peptide (HA518-526)-loaded BMDCs. As in the CD4 T cell experiments in Figs. 4 and 5, WT mice were immunized with WT BMDCs, and B7-DC KO mice were immunized with B7-DC KO BMDCs. (a) Expansion of HA-specific CD8 T cells was measured by FACS analysis of hepatic, lymph node, and splenic lymphocytes. Because there is no clonotypic antibody for this TCR, donor clone 4 T cells were marked with Thy1.1, and recipients were Thy1.2. (b) To measure CTL activity directly, an in vivo CTL assay was done in which two populations of differentially CFSE- labeled Balb/c splenocytes, one of which was loaded with HA518-526, were transferred into the immunized mice. As a control, some immunized mice received splenocytes in which neither the CFSEhi or CFSElo cells were peptide loaded. In vivo CTL activity is determined based on the selective reduction in the ratio of peptide-loaded cells (CFSElo) to unloaded cells (CFSEhi) reisolated from the spleen 10 h after injection, calculated as described in Materials and methods. Data are representative of three independent experiments.
Effect of B7-DC on tumor growth and tumor-specific CD8 T cell responses
IFN-γ responses are crucial for both natural and induced antitumor immunity. Tumors arise more frequently and grow more quickly in mice with deletions in either the IFN-γ gene or components of the IFN-γ signaling pathway. In general, strong Th1 responses tend to be linked to strong CD8 CTL responses, and the combination of strong Th1 and strong CD8 CTL responses is typically associated with successful antitumor immunity. We therefore analyzed the growth of hepatic metastases of the well-characterized CT26 colon tumor in WT and B7-DC KO mice as well as the CD8 T cells specific for the immunodominant H-2Ld-restricted CT26 antigen, AH1. CT26 is a partially immunogenic tumor, because injection of WT tumor cells into BALB/c mice elicits an AH1-specific T cell response. This CD8 T cell response slows tumor growth, but the tumor ultimately outgrows the antitumor immune response and kills the host animal. The mortality rate in CT26-bearing mice is roughly proportional to the strength of the natural antitumor response generated, because CD8-depleted animals succumb to tumor faster than WT animals. Fig. 8a demonstrates that B7-DC KO mice bearing hepatic metastases of CT26 die more quickly than WT mice. This mortality is associated with more rapid growth of CT26 tumors in the liver (Fig. 8b). Tetramer staining of AH1-specific CD8 T cells reveals a marked decrease in the number of AH1-specific CD8 T cells in the livers of tumor-bearing B7-DC KO mice relative to WT mice (Fig. 8, c and d). Thus, B7-DC seems to contribute in a positive fashion to the endogenous generation of intrahepatic CT26-specific CD8 responses in animals bearing hepatic CT26 metastases.
Figure 8.
Antitumor responses in B7-DC KO mice. Hepatic metastases of CT26 were established by intrahemisplenic injection of 1 × 105 CT26 cells into either WT or B7-DC KO mice on d 0 (n = 10). (a) Mouse survival was observed (P < 0.01). (b) Livers were excised from WT and B7-DC KO mice on d 15, and liver weight and number of tumor nodules was measured. (c) Hepatic lymphocytes were isolated on d 3 and stained with AH-1 peptide–loaded Ld tetramer or control (β-galactosidase–loaded Ld tetramer). (d) Total number of AH-1–specific CD8 T cells were analyzed by FACS staining. Data in b, c, and d are representative of three independent experiments.
Discussion
We present in vitro and in vivo experiments in B7-DC KO mice that support a predominantly costimulatory role for B7-DC in the generation of both Th1 and CTL responses. In some cases, decreased IFN-γ responses in B7-DC KO mice are associated with increased IL-4 (Th2) responses (Fig. 5), whereas in other cases, such as isotype switching, IL-4–dependent antibody isotypes (IgG1) are unaffected. In all cases, the effects are partial rather than complete. We therefore propose that, unlike a molecule such as T-bet, on which Th1 responses depend absolutely (26), the role of B7-DC is to amplify or tune Th1 responses. Fine-tuning of immune responses seems to be critical so that they do not undershoot or overshoot. The importance of not undershooting the response to an infectious organism is obvious. Conversely, the toxicities of overzealous immune responses are readily apparent in mice deficient in inhibitory checkpoint molecules and in humans treated with antibodies such as anti-CTLA-4 (27).
The most straightforward interpretation of these results is that decreased IFN-γ and CTL responses in B7-DC KO mice are caused by loss of a costimulatory signal to T cells that interacts with B7-1/2 costimulation. However, an additional possibility is that DC activation caused by backward signaling by B7-DC is diminished. The fact that B7-1, B7-2, and surface MHC II are not appreciably different in B7-DC KO mice and WT mice suggests that DC activation status is not appreciably affected by the absence of B7-DC. Nonetheless, a definitive distinction between effects on forward versus backward signaling will require further experimentation.
Taken together, our results strongly refute the notion that B7-DC is a redundant inhibitory ligand for B7-H1, even though both bind PD-1. In particular, the finding of diminished CD4 and CD8 responses in the livers of B7-DC KO mice stands in striking contrast to the increased numbers and responses of activated CD8 T cells in the livers of B7-H1 KO mice (18). Direct comparisons between these two KO models in identical systems and strain backgrounds remain to be made. However, a preliminary picture of distinct roles for these B7 family members seems to be emerging from the different expression patterns seen in B7-DC (predominantly DCs) and B7-H1 (expression on both hematopoietic and nonhematopoietic cells of peripheral organs as well as many tumors) mice and the respective KO mice. Thus, B7-DC seems to have a primarily costimulatory role in immune priming, whereas B7-H1 has a predominantly down-modulating role for activated T cells in peripheral organs and tumors. Further support for this distinct physiology comes from the findings of Liu and colleagues that B7-DC–transfected tumors enhance antitumor immunity in contrast to B7-H1–expressing tumors that are protected from elimination by activated tumor-specific T cells (17, 28). The finding that tumors grow faster and elicitation of tumor-specific CD8 T cells is impaired in B7-DC KO mice further accentuates the physiologic differences between these two molecules and suggests that B7-DC signals may significantly enhance antitumor immunity.
Many questions remain to be answered about B7-DC physiology. Foremost is the identification of its cognate costimulatory receptor. We hypothesize that the costimulatory role of B7-DC observed in vivo is largely mediated by this costimulatory receptor rather than by PD-1. Consistent with this notion, treatment of mice with anti-PD1 antibodies did not alter B7-DC costimulatory effects (unpublished data). However, it is possible that, under certain circumstances, PD-1 may transmit costimulatory rather than inhibitory signals. In addition, it will be important to determine how B7-DC signals interact with other signaling pathways that affect the Th1/Th2 balance. Along these lines, we are crossing the B7-DC KO mice onto a C57/BL6 background, which is more “Th1 prone” than the BALB/c background on which the current studies were performed. A recent study suggested that the IFN-γ receptor differentially localizes into the immune synapse in these two strains (29). Comparison between these two strains may allow us to determine whether the Th1 skewing by B7-DC is affected by interactions with other background genes.
Materials and Methods
Mice.
6–8-week-old BALB/c mice and antigen-specific TCR transgenic DO11.10 mice (H-2d) were purchased from the National Cancer Institute and Jackson ImmunoResearch Laboratories, respectively. Thy1.1 background HA (clone 4) transgenic mice were the gift from E. Fuchs (Johns Hopkins University). B7-DC knock-out/knock-in mice were generated using standard methodologies (30). A genomic fragment containing exon 2 of the B7-DC gene, which includes first methionine, was cloned from a 129/SvJ mouse genomic library. The short and long fragments, two of loxP sites and one of EGFP site (CLONTECH Laboratories, Inc.) were incorporated into pNTKV1901 (Stratagene; Fig.1 a). This targeting vector was linearized and electroporated into R1 embryonic stem cells. Genomic DNAs from 288 G418-resistant clones were digested with BamHI and screened by Southern blot using 5′ probe. Eight homologous recombinants were obtained. Germline chimeras were generated from embryonic stem #144 by aggregation with d-2.5 embryonic 8 cells. The NEO cassette was deleted by crossing with Cre transgenic mice (Jackson ImmunoResearch Laboratories), and its deletion was confirmed by Southern blot and PCR (unpublished data). Offspring were backcrossed with BALB/c more than nine generations and typed as B7-DC WT and KO by Southern blot and PCR. B7-DC expression was completely abolished on BMDCs (Fig. 1), and spleen DCs (unpublished data). All experimental protocols were reviewed and approved by Johns Hopkins University Institutional Animal Care and Use Committee.
Antibodies and reagents.
PE-conjugated anti-mouse B7-DC (TY25) and anti-mouse B7-H1 (M1H6) mAbs were purchased from eBioscience. PE-conjugated anti-mouse OVA-specific TCR (KJ1-26) mAb was purchased from Caltag Laboratories. Purified anti-mouse CD16/32 (2.4G2), IL-4 (BVD-1D11), and IFN-γ (R4-6A2) mAbs, PE-conjugated CD8 (Ly-2), CD80 (1G10), CD86 (GL1), Pan-NK cells (DX5), and I-Ad (AMS-32.1) mAbs, Cy-Chrome-conjugated CD45R/B220 (RA3-6B2) mAbs, and APC-conjugated CD3 (145-2C11), CD4 (RM4-5), and CD11c (HL3) mAbs were purchased from BD Biosciences. Recombinant mouse GM-CSF, IL-4, and IL-12 were purchased from R&D Systems.
Cell preparations and in vitro cultures.
For in vitro T cell assays, single-cell suspensions of spleen from B7-DC WT or KO mice were prepared by purification by Pan T cell isolation kit (Miltenyi Biotec) and cultured in complete medium with ConA for 72 h. The last 18 h before end of culture, 3H-thymidine was pulsed, and its incorporation was measured. For antigen-specific responses, CD4 T cells from DO11.10 mice were purified by using a CD4+ T cell isolation kit (Miltenyi Biotec) and labeled with CFSE before culture. The purity of CD4 T cells was monitored by FACS and was always >97%. BMDCs were prepared by culturing with recombinant mouse GM-CSF (1,000 unit/ml) in the presence or absence of IL-4 (1,000 unit/ml).
Flow cytometric analysis.
Cells were preincubated with unlabeled anti-CD16/32 mAb to avoid nonspecific binding of antibodies to Fc receptor and then incubated with PE- or APC-conjugated mAb. After washing twice with FACS buffer, the stained cells (live-gated on the basis of forward and side scatter profiles) were analyzed on a FACSCalibur (BD Biosciences).
Intracellular staining.
1.0 × 104 purified CD4 T cells from DO11.10 mice were cultured for 5 d with 3.0 × 104 BMDCs and 0.3 μg/ml of OVA323-339 peptide in the presence of recombinant IL-12 (1.0 unit/ml) and anti-mouse IL-4 mAb (10 μg/ml) for Th1 generation or with recombinant IL-4 (100 unit/ml) and anti-mouse IFN-γ mAb (10 μg/ml) for Th2 generation. 6 h before the end of culture, additional OVA323-339 peptide (1.0 μg/ml) and GolgiStop (BD Biosciences) were added to each well for restimulation with or without purified anti-CD3 mAb (1.0 μg/ml). After surface CD4 was stained, cells were fixed and permeabilized by Cytofix/Cytoperm kit (BD Biosciences). Then intracellular IFN-γ and IL-4 were stained and analyzed by FACS.
In vivo proliferation assay.
5 million purified CD4 T cells from DO11.10 mice or CD8 T cells from clone 4 mice were labeled with CFSE and transferred to WT or B7-DC KO mice on d −1. For immunization, OVA323-339 peptide– or HA518-526 peptide– pulsed BMDCs were injected into WT or B7-DC KO mouse on d 0. Peptides were obtained from Colorado State University. Single-cell suspensions were prepared from each organ of immunized mice, cell numbers were counted, and CFSE dilution of CD4+KJ1-26+ T cells or CD8+Thy1.1+ T cells were analyzed by FACS. In vivo cytotoxic assay was performed by transferring CFSEhi (1 μM)- and CFSElo (0.1 μM)-labeled Balb/c splenocytes. HA518-526 peptide was pulsed only on the CFSElo population. The proportions of CFSEhi and CFSElo cells were measured by FACS on d 5, and the cytotoxicity was calculated by the formula: % cytotoxicity = (% CFSEhi − %CFSEhi control) × 100 / (% CFSEhi × % CFSElo control).
T helper cell 1/2 response in vivo.
5 million purified DO11.10 CD4 T cells were transferred intravenously on d −1. Mice were immunized with 1 million OVA323-339 peptide–pulsed BMDCs on d 0. 0.5 million CD4+KJ1-26+ T cells from spleen were sorted by FACS-Vantage (Becton Dickinson) at the Johns Hopkins Medical Institutions Oncology Center Cell Sorting Facility. The purity among live cells was >99%. Total mRNA from sorted cells was extracted, and first-strand cDNA was prepared by You-Prime First-Strand Beads (Amersham Biosciences). Real-time PCR reactions were performed in ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as described (31, 32). Data were treated according to the manufacturer's instructions in User Bulletin #2. Briefly, standard curves were prepared by plotting the cycle threshold (Ct) by log ng input RNA. The inverse logs were calculated and experimental values were divided by 18s internal controls. Relative transcripts were determined by the formula: 1/2(CTtarget - CTcontrol).
Antibody class switching.
Mice were injected with 100 μg NP17-KLH (Biosearch Technologies, Inc.) with alum (Pierce Chemical Co.) on d 0 and 14. Mice sera were collected on d 28. The titers of isotype and subclass of anti-NP–specific antibody were detected by ELISA using NP25-BSA (Biosearch Technologies, Inc.) for antibody capture and SBA Clonotyping system/HRP kit (Southern Biotechnology Associates, Inc.) for detection (33).
Liver metastatic model and tumor specific cytotoxic T lymphocyte detection.
For generating liver-specific tumor metastasis, 1 × 105 cells of the Balb/c mouse–derived colon cancer cell line, CT26 were injected via the hemispleen technique on day 0 (34). Survival was monitored every day, and all dead mice were confirmed to possess liver metastases. To analyze antigen-specific CTLs after generating liver metastases of CT26, liver lymphocytes were extracted by the Percoll technique and stained with AH-1 peptide–pulsed MHC class I tetramer together with anti-mouse CD8 mAb.
Online supplemental material.
Fig. S1 shows the flow cytometric analysis of lymphocyte subsets in thymus and spleen of B7-DC KO mice. Fig. S2 shows in vivo CD4 T cell proliferation in the liver after dendritic cell immunization. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050072/DC1.
Acknowledgments
This work was supported in part by a National Institutes of Health grant and gifts from William and Betty Topercer, Dorothy Needle, and the Commonwealth Foundation. This project was partly supported by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government. This project was partly supported by Uehara Memorial Foundation. Dr Pardoll is a Januey Scholar and holds the Seraph Chair for Cancer Research.
The authors have no conflicting financial interests.
Abbreviations used: BMDC, bone marrow–derived dendritic cell; CFSE, carboxyfluorescein succinimidyl; ConA, concanavalin A; HA, hemagglutinin; NOD, nonobese diabetic; PHN, peripheral hepatic nodes; PLN, peripheral lymph nodes.
References
- 1.Sharpe, A.H., and G.J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116–126. [DOI] [PubMed] [Google Scholar]
- 2.Chen, L. 2004. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 4:336–347. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, C.A., M.S. Kuhns, J.G. Egen, and J.P. Allison. 2001. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19:565–594. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, C.A., and J.P. Allison. 1999. Costimulatory regulation of T cell function. Curr. Opin. Cell Biol. 11:203–210. [DOI] [PubMed] [Google Scholar]
- 5.Latchman, Y., C.R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A.J. Long, J.A. Brown, R. Nunes, et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268. [DOI] [PubMed] [Google Scholar]
- 6.Tseng, S.Y., M. Otsuji, K. Gorski, X. Huang, J.E. Slansky, S.I. Pai, A. Shalabi, T. Shin, D.M. Pardoll, and H. Tsuchiya. 2001. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong, H., G. Zhu, K. Tamada, and L. Chen. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 8.Freeman, G.J., A.J. Long, Y. Iwai, K. Bourque, T. Chernova, H. Nishimura, L.J. Fitz, N. Malenkovich, T. Okazaki, M.C. Byrne, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okazaki, T., Y. Iwai, and T. Honjo. 2002. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr. Opin. Immunol. 14:779–782. [DOI] [PubMed] [Google Scholar]
- 10.Carter, L., L.A. Fouser, J. Jussif, L. Fitz, B. Deng, C.R. Wood, M. Collins, T. Honjo, G.J. Freeman, and B.M. Carreno. 2002. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 32:634–643. [DOI] [PubMed] [Google Scholar]
- 11.Rodig, N., T. Ryan, J.A. Allen, H. Pang, N. Grabie, T. Chernova, E.A. Greenfield, S.C. Liang, A.H. Sharpe, A.H. Lichtman, and G.J. Freeman. 2003. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33:3117–3126. [DOI] [PubMed] [Google Scholar]
- 12.Mazanet, M.M., and C.C. Hughes. 2002. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169:3581–3588. [DOI] [PubMed] [Google Scholar]
- 13.Wang, S., J. Bajorath, D.B. Flies, H. Dong, T. Honjo, and L. Chen. 2003. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J. Exp. Med. 197:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin, T., G. Kennedy, K. Gorski, H. Tsuchiya, H. Koseki, M. Azuma, H. Yagita, L. Chen, J. Powell, D. Pardoll, and F. Housseau. 2003. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J. Exp. Med. 198:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida, M., Y. Iwai, Y. Tanaka, T. Okazaki, G.J. Freeman, N. Minato, and T. Honjo. 2002. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol. Lett. 84:57–62. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki, T., H. Akiba, H. Iwai, H. Matsuda, M. Aoki, Y. Tanno, T. Shin, H. Tsuchiya, D.M. Pardoll, K. Okumura, et al. 2002. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 169:5538–5545. [DOI] [PubMed] [Google Scholar]
- 17.Dong, H., S.E. Strome, D.R. Salomao, H. Tamura, F. Hirano, D.B. Flies, P.C. Roche, J. Lu, G. Zhu, K. Tamada, et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800. [DOI] [PubMed] [Google Scholar]
- 18.Dong, H., G. Zhu, K. Tamada, D.B. Flies, J.M. van Deursen, and L. Chen. 2004. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 20:327–336. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto, K., H. Inoue, T. Nakano, M. Tsuda, Y. Yoshiura, S. Fukuyama, F. Tsushima, T. Hoshino, H. Aizawa, H. Akiba, et al. 2004. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J. Immunol. 172:2530–2541. [DOI] [PubMed] [Google Scholar]
- 20.Salama, A.D., T. Chitnis, J. Imitola, M.J. Ansari, H. Akiba, F. Tushima, M. Azuma, H. Yagita, M.H. Sayegh, and S.J. Khoury. 2003. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 198:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari, M.J., A.D. Salama, T. Chitnis, R.N. Smith, H. Yagita, H. Akiba, T. Yamazaki, M. Azuma, H. Iwai, S.J. Khoury, et al. 2003. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen, L.T., S. Radhakrishnan, B. Ciric, K. Tamada, T. Shin, D.M. Pardoll, L. Chen, M. Rodriguez, and L.R. Pease. 2002. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J. Exp. Med. 196:1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Radhakrishnan, S., L.T. Nguyen, B. Ciric, D.R. Ure, B. Zhou, K. Tamada, H. Dong, S.Y. Tseng, T. Shin, D.M. Pardoll, et al. 2003. Naturally occurring human IgM antibody that binds B7-DC and potentiates T cell stimulation by dendritic cells. J. Immunol. 170:1830–1838. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez, J., S. Aung, W.L. Redmond, and L.A. Sherman. 2001. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J. Exp. Med. 194:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan, D.J., R. Liblau, B. Scott, S. Fleck, H.O. McDevitt, N. Sarvetnick, D. Lo, and L.A. Sherman. 1996. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J. Immunol. 157:978–983. [PubMed] [Google Scholar]
- 26.Szabo, S.J., S.T. Kim, G.L. Costa, X. Zhang, C.G. Fathman, and L.H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 100:655–669. [DOI] [PubMed] [Google Scholar]
- 27.Phan, G.Q., J.C. Yang, R.M. Sherry, P. Hwu, S.L. Topalian, D.J. Schwartzentruber, N.P. Restifo, L.R. Haworth, C.A. Seipp, L.J. Freezer, et al. 2003. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA. 100:8372–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, X., J.X. Gao, J. Wen, L. Yin, O. Li, T. Zuo, T.F. Gajewski, Y.X. Fu, P. Zheng, and Y. Liu. 2003. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J. Exp. Med. 197:1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldonado, R.A., D.J. Irvine, R. Schreiber, and L.H. Glimcher. 2004. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature. 431:527–532. [DOI] [PubMed] [Google Scholar]
- 30.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J.C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA. 90:8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura, Y., C.J. Thoburn, E.C. Bright, M.L. Phelps, T. Shin, E.C. Matsui, W.H. Matsui, S. Arai, E.J. Fuchs, G.B. Vogelsang, et al. 2004. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 104:2187–2193. [DOI] [PubMed] [Google Scholar]
- 32.Gorski, K.S., T. Shin, E. Crafton, M. Otsuji, F.M. Rattis, X. Huang, E. Kelleher, L. Francisco, D. Pardoll, and H. Tsuchiya. 2003. A set of genes selectively expressed in murine dendritic cells: utility of related cis-acting sequences for lentiviral gene transfer. Mol. Immunol. 40:35–47. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe, N., M. Gavrieli, J.R. Sedy, J. Yang, F. Fallarino, S.K. Loftin, M.A. Hurchla, N. Zimmerman, J. Sim, X. Zang, et al. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4:670–679. [DOI] [PubMed] [Google Scholar]
- 34.Jain, A., J.E. Slansky, L.C. Matey, H.E. Allen, D.M. Pardoll, and R.D. Schulick. 2003. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann. Surg. Oncol. 10:810–820. [DOI] [PubMed] [Google Scholar]