Abstract
Interleukin (IL)-23 is a heterodimeric cytokine that shares the identical p40 subunit as IL-12 but exhibits a unique p19 subunit similar to IL-12 p35. IL-12/23 p40, interferon γ (IFN-γ), and IL-17 are critical for host defense against Klebsiella pneumoniae. In vitro, K. pneumoniae–pulsed dendritic cell culture supernatants elicit T cell IL-17 production in a IL-23–dependent manner. However, the importance of IL-23 during in vivo pulmonary challenge is unknown. We show that IL-12/23 p40–deficient mice are exquisitely sensitive to intrapulmonary K. pneumoniae inoculation and that IL-23 p19−/−, IL-17R−/−, and IL-12 p35−/− mice also show increased susceptibility to infection. p40−/− mice fail to generate pulmonary IFN-γ, IL-17, or IL-17F responses to infection, whereas p35−/− mice show normal IL-17 and IL-17F induction but reduced IFN-γ. Lung IL-17 and IL-17F production in p19−/− mice was dramatically reduced, and this strain showed substantial mortality from a sublethal dose of bacteria (103 CFU), despite normal IFN-γ induction. Administration of IL-17 restored bacterial control in p19−/− mice and to a lesser degree in p40−/− mice, suggesting an additional host defense requirement for IFN-γ in this strain. Together, these data demonstrate independent requirements for IL-12 and IL-23 in pulmonary host defense against K. pneumoniae, the former of which is required for IFN-γ expression and the latter of which is required for IL-17 production.
To perform its primary function of gas exchange, the mucosal surface of the lung is continuously exposed to the environment. Given the considerable infectious risk to the host, complex mechanisms exist to prevent the development of bacterial pneumonia (1, 2). The immune system in the lung consists of both innate and adaptive components, and it is clear these two systems are highly interdependent for optimal host defense.
Upon bacterial recognition, innate immune cells release cytokines such as tumor necrosis factor α to initiate the inflammatory response (3, 4). Innate effector cells are also important in the initiation of adaptive immunity by secreting cytokines such as IL-12 (5, 6). A key role of IL-12 is the induction of IFN-γ, an important cytokine in pulmonary defense against Klebsiella pneumoniae (7, 8). The identification of IL-23 as a cytokine with characteristics similar to, but distinct from, IL-12 has created particular interest in deciphering the role of this cytokine in the immune response to infection (9). IL-23 is secreted as a heterodimer composed of a p40 subunit identical to that of IL-12 and a unique p19 subunit that shares sequence homology with IL-12 p35. IL-23 is expressed predominantly by stimulated antigen-presenting cells, including dendritic cells (DCs), peripheral blood monocytes/macrophages, and brain microglia cells (9–11). Whereas IL-12 is important in stimulating IFN-γ production by naive T cells, IL-23 is reported to elicit IFN-γ from human memory T cells (9). Recombinant IL-23 can also signal directly on peritoneal macrophages to induce tumor necrosis factor α and IL-1β, suggesting that IL-23 acts as an autocrine proinflammatory cytokine in these cells (11). The importance of IL-23 during an infectious challenge is suggested by studies showing that certain bacterial, fungal, and mycobacterial infections are less severe in animals lacking the IL-12 p35 subunit compared with those deficient in IL-12/23 p40 (12–15). Recently, the creation of IL-23 p19−/− mice has shown that IL-23 is critical for the generation of memory T cell–dependent humoral and cell-mediated immune responses to antigen (16).
In addition, IL-23 stimulates T cell production of IL-17 (IL-17A) and IL-17F (17), cytokines that promote neutrophilic inflammation through the induction of granuloctye CSF (G-CSF), granulocyte macrophage CSF, monocyte chemoattractant protein 1, IL-1β, IL-6, and the neutrophil chemokines growth-related oncogene-α, IL-8 (human), and keratinocyte chemoattractant (KC), LPS-induced C-X-C chemokine (LIX), and macrophage inflammatory protein 2 (MIP-2) (mouse) in a variety of target cells (18–22). We have shown that IL-17 signaling is critical for maintenance of a granulopoietic response and survival from pulmonary K. pneumoniae infection (21). More recent work demonstrates that bone marrow–derived myeloid dendritic cell (mDC) cultures pulsed with this bacteria secrete IL-23 only if intact Toll-like receptor 4 (TLR4) is present (23).
However, the role of IL-23 during in vivo pulmonary K. pneumoniae challenge is unknown. We show that IL-23 is a cytokine critical to host survival during infection and demonstrate that IL-23 is the dominant in vivo stimulus for pulmonary IL-17 and IL-17F production in this model. Alveolar macrophages (AMs) and pulmonary mDCs are significant sources of IL-23 during in vitro challenge with this pathogen, whereas lung plasmacytoid dendritic cells (pDCs) are not. Furthermore, defective bacterial clearance in IL-23 p19−/− mice could be abolished by administration of exogenous IL-17, and this treatment restored lung G-CSF and LIX production. A more moderate improvement in bacterial clearance was also observed in IL-12/23 p40−/− mice, which are defective in mounting an IFN-γ response, further demonstrating an IFN-γ–independent role of the IL-23/IL-17 axis in host defense.
RESULTS
Reduced survival of IL-12– and IL-23–deficient mice during pulmonary K. pneumoniae challenge
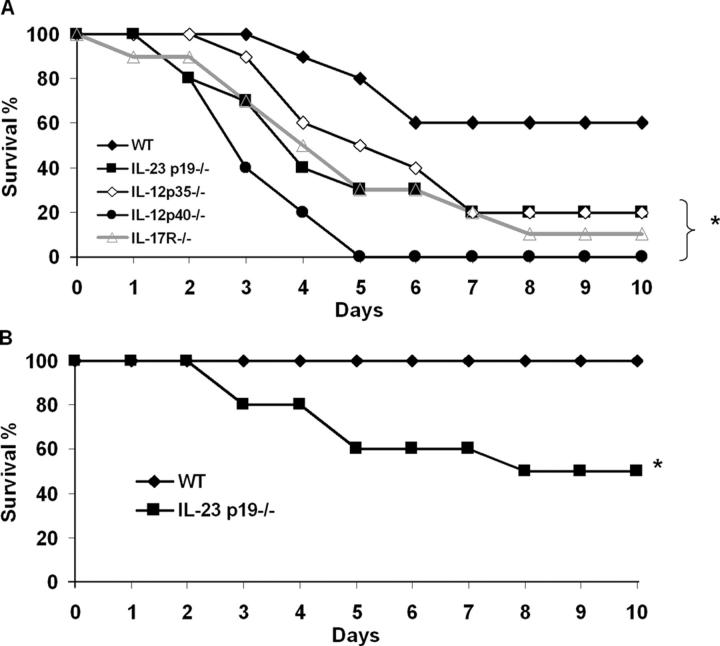
IL-12, STAT4, IFN-γ, and IL-17 signaling have each been shown to be critical for host defense against pulmonary K. pneumoniae infection (6, 21, 24). However, the precise role of IL-12 and IL-23 during infection has not been demonstrated. To determine the independent requirement for IL-12 and IL-23 expression in surviving this infection model, we challenged wild-type (WT), IL-12/23 p40−/−, IL-12p 35−/−, and IL-23 p19−/− mice with 104 CFU K. pneumoniae. IL-12/23 p40−/− mice showed a rapid decline in survival (P < 0.05 by log rank test compared with WT) (Fig. 1 A). Survival was also diminished in IL-17R−/−, IL-23 p19−/−, and IL-12 p35−/− mice compared with wild-type C57BL/6 mice. To further characterize the requirement of IL-23 in pulmonary host defense, we next challenged WT and p19−/− mice with a sublethal dose of bacteria (103 CFU). As shown in Fig. 1 B, IL-23–deficient mice display marked sensitivity to a normally well-controlled bacterial inoculum. Together, these results suggest that both IL-12 and IL-23 are critical for pulmonary host defense against K. pneumoniae.
Figure 1.
Animals with targeted gene deletion in the IL-12, IL-17, and IL-23 signaling pathways demonstrate increased mortality during intrapulmonary K. pneumoniae infection. (A) WT C57BL/6, IL-23 p19−/−, IL-12 p35−/−, IL-12 p40−/−, or IL-17R−/− mice were challenged with 104 CFU intratracheal K. pneumoniae and survival was recorded every 12 h (n = 16–20 per group). IL-12 p40 knockout mice showed the greatest susceptibility to infection (*P < 0.01 compared with C57BL/6 [log rank test]). Survival differences between IL-23 p19−/−, IL-17R−/−, and IL-12 p35−/− mice were not statistically significant. (B) WT and p19−/− mice were also challenged with a lower dose (103 CFU) of bacteria, demonstrating significant mortality in p19−/− mice to a sublethal dose of K. pnuemoniae (*P < 0.01 compared with WT mice; n = 10 per group).
Bronchoalveolar lavage (BAL) cell IL-23 p19, IL-12/23 p40, and IL-12 p35 mRNA expression following K. pneumoniae infection
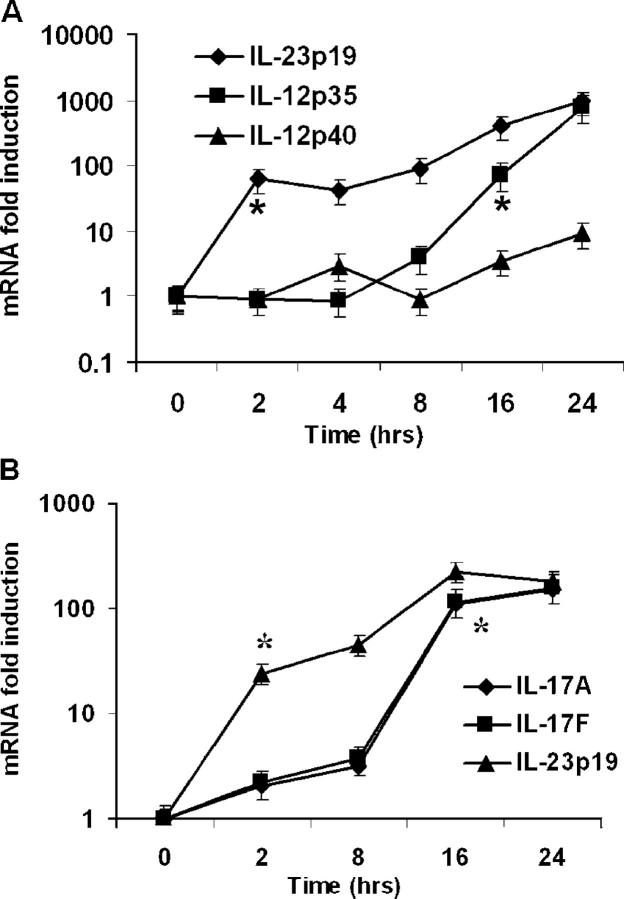
As both IL-12 and IL-23 are critical for host defense in this model, we analyzed the time course of IL-23 p19, IL-12 p40, and IL-12 p35 gene expression in bronchoalveolar lavage (BAL) cells over time following K. pneumoniae infection via real-time reverse-transcription polymerase chain reaction (RT-PCR). Prior to bacterial challenge, p40 transcripts were present in BAL cells (Ct 32 ± 2.3). In contrast, baseline IL-12 p35 and IL-23 p19 transcripts were not detected. Inoculation with K. pneumoniae increased expression of all three genes over baseline levels, though significant increases in IL-23 p19 transcripts occurred sooner than increases in p40 or p35 mRNA (Fig. 2 A). IL-12 p35 messenger RNA (mRNA) did not show significant induction until 16 h after K. pneumoniae inoculation, suggesting that because p40 transcripts are already present, BAL cell IL-23 is induced rapidly and sooner than IL-12 p70 expression. These results were confirmed at the protein level, because IL-23 was detectable via ELISA 2 h after K. pneumoniae challenge (173 ± 17 pg/mL; n = 5), whereas IL-12 p70 was undetectable at this early time point.
Figure 2.
Cytokine mRNA expression following pulmonary K. pneumoniae infection. Animals were administered 104 CFU bacteria and killed at specified time points. BAL cell pellet and lung homogenate mRNA were assayed via real-time RT-PCR. (A) Increases in BAL cell IL-23 p19, IL-12/23 p40, and IL-12 p35 mRNA expression during infection (n = 4–5 per group). (B) Whole lung tissue IL-23 p19, IL-17, and IL-17F mRNA expression following infection (n = 4–5 per group). Data are normalized for 18s ribosomal RNA content and plotted as fold change over baseline (time 0) expression. *Earliest significant (P < 0.05) increase in expression compared with time zero transcripts. Error bars represent mean ± SD.
Because IL-23 elicits T cell IL-17 production in response to in vitro K. pneumoniae challenge, we examined the time course of IL-23 p19, IL-17, and IL-17F mRNA expression in lung tissue following intrapulmonary infection using real-time RT-PCR. Transcripts for these genes were undetectable at baseline, but significant increases in their expression were seen following infection, with p19 mRNA increasing as early as 2 h and peaking at 16 h after challenge (Fig. 2 B).
IL-23 expression in lung cell subpopulations in response to K. pneumoniae
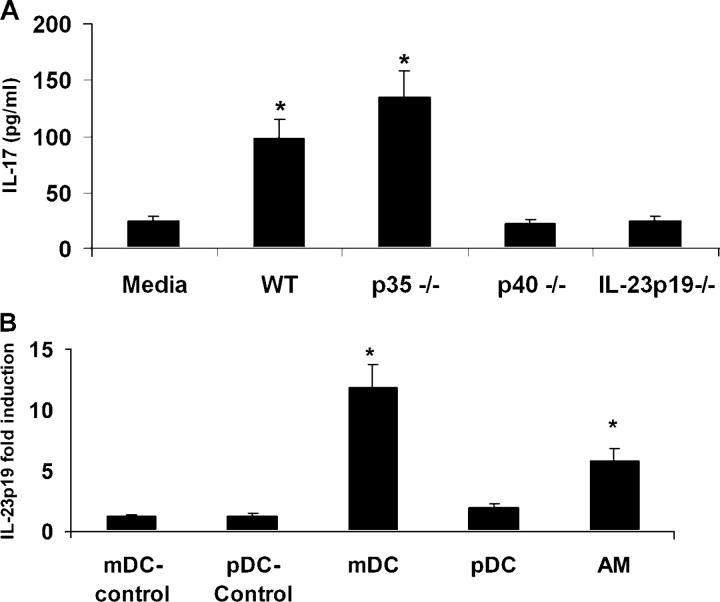
Because AMs are critical to pulmonary host defense against K. pneumoniae (25), express TLR4 (26), and are resident in the alveoli, we hypothesized that they are an early source of IL-23 in response to infection. AM production of IL-23 was assayed using IL-17 production by splenocytes as a bioassay (17, 21, 27). AMs from WT, IL-23 p19−/−, IL-12 p35−/−, and IL-12/23 p40−/− animals were recovered by BAL of naive animals and treated with pathogen in vitro as described in Materials and methods. Cell-free supernatants collected 24 h after WT AM culture with K. pneumoniae showed significant IL-17–inducing capacity when transferred onto splenocyte cultures, whereas IL-12/23 p40−/− or IL-23 p19−/− AM culture supernatants failed to stimulate splenocyte IL-17 production (Fig. 3 A). Culture supernatants from p35−/− AMs stimulated splenocyte IL-17 production to a significantly greater extent than did supernatants from WT AMs. In contrast, supernatants from bacteria-stimulated AM did not induce splenocyte IFN-γ production, and IL-12 p70 was not detectable in these AM supernatants (IL-12 p70 limit of detection 8 pg/mL; unpublished data). These data demonstrate a strict requirement for IL-23 in AM-induced IL-17 stimulation, and demonstrate that, in vitro, IL-12 is not a significant AM product in response to K. pneumoniae over this time course.
Figure 3.
AM IL-23 expression is required for induction of splenocyte IL-17 expression in response to K. pneumoniae. (A) AMs from naive WT, p35−/−, p40−/−, and p19−/− mice were recovered via BAL and exposed in vitro to K. pneumoniae. After 24 h, supernatants were harvested, centrifuged, and placed onto adherent cell-depleted WT splenocytes for 24 h to assay IL-17 induction (n = 5 per group; *P < 0.05 compared with media control). (B) IL-23 p19 mRNA expression in unexposed mDCs (mDC-control) or pDCs (pDC-control) or mDCs, AMs, and pDCs following 2-h in vitro exposure to K. pneumoniae. Data are expressed as fold increase in p19 expression compared with mDC- or pDC-control (n = 4–5 per group; *P < 0.05 compared with mDC-control). Error bars represent mean ± SD.
Bone marrow–derived cultures of mDCs and pDCs have recently been shown to differ in their ability to drive T cell activation in response to LPS, likely as a result of differential TLR4 expression by these DC subsets (28). Whether differences exist in the ability of lung resident DC populations to express IL-23 in response to K. pneumoniae is unknown. Using FACS, we isolated both mDCs and pDCs from lung digests of naive C57BL/6 mice to more than 95% purity. AMs and DCs were cultured with bacteria or media control for 2 h. mDCs exhibited an almost twofold greater IL-23 p19 mRNA expression compared with AMs, whereas pDCs expressed approximately one tenth the level seen in mDC cultures and one fourth the level of p19 transcripts seen in AM cultures (Fig. 3 B). IL-23 p19 transcripts were not detected in cells not exposed to bacteria.
Differential requirements for IL-12 and IL-23 in pulmonary IFN-γ, IL-17, and IL-17F expression
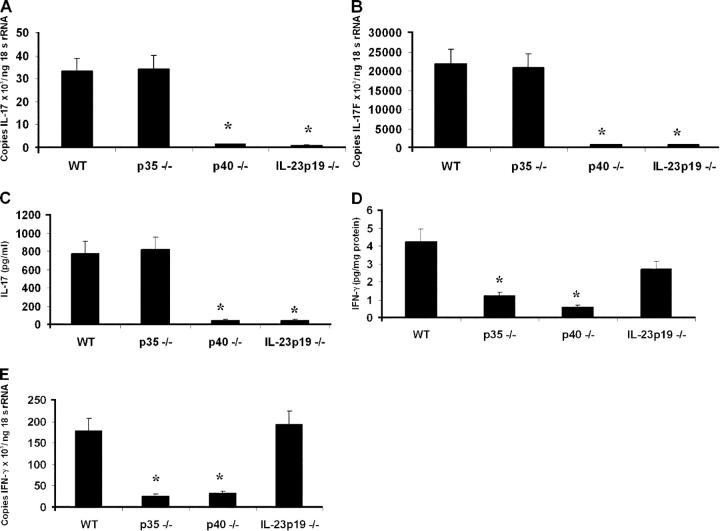
Given these survival phenotypes and the known requirements for IFN-γ and IL-17 in pulmonary host defense against this infection, we sought to determine the in vivo requirements for IL-12 and IL-23 signaling in pulmonary IL-17, IL-17F, and IFN-γ expression. WT, IL-12 p35−/−, IL-12/23 p40−/−, and IL-23 p19−/− animals were administered 104 CFU K. pneumoniae via intratracheal injection. Transcripts for IL-17 and IL-17F were undetectable in lung tissue before bacterial challenge (unpublished data). Sixteen hours after infection, WT and IL-12 p35−/− mice demonstrate significant induction of both IL-17 (Fig. 4 A) and IL-17F (Fig. 4 B), indicating IL-12 is unnecessary for the pulmonary IL-17/17F responses to this pathogen. In contrast, p40−/− and IL-23 p19−/− mice had significantly reduced levels of IL-17 and IL-17F transcripts in lung tissue after bacterial challenge. These results were confirmed at the protein level for IL-17 in lung homogenate 24 h after bacterial challenge (Fig. 4 C). IL-12 is, however, required to generate the IFN-γ response to infection, as evidenced by significantly attenuated lung IFN-γ levels in p35−/− and p40−/− mice (Fig. 4 D). IFN-γ levels were not significantly reduced in the IL-23 p19−/− mice. To confirm the dispensable nature of IL-23 in mediating IFN-γ induction, whole lung RNA was assayed for IFN-γ transcripts 24 h after infection and was found to be similar in IL-23 p19−/− and WT control mice (Fig. 4 E).
Figure 4.
IL-23 expression is required for lung IL-17 and IL-17F expression, whereas IL-12 is necessary for IFN-γ induction in response to K. pneumoniae infection. WT, p35−/−, p40−/−, and p19−/− mice were infected with 104 CFU K. pneumoniae and killed 24 h after infection. (A, B) Whole lung homogenate IL-17 (A) and IL-17F (B) mRNA expression as measured via real-time RT-PCR. (C) Whole lung homogenate IL-17 protein expression in repeat experiments. (D) Lung homogenate IFN-γ content in response to infection, indicating IL-23 is not sufficient to induce IFN-γ in the absence of IL-12. (E) Lung homogenate IFN-γ mRNA 24 h after K. pneumoniae infection confirms equivalent IFN-γ expression in WT and IL-23 p19−/− mice (n = 6 per group; *P < 0.05 compared with WT). Error bars represent mean ± SD.
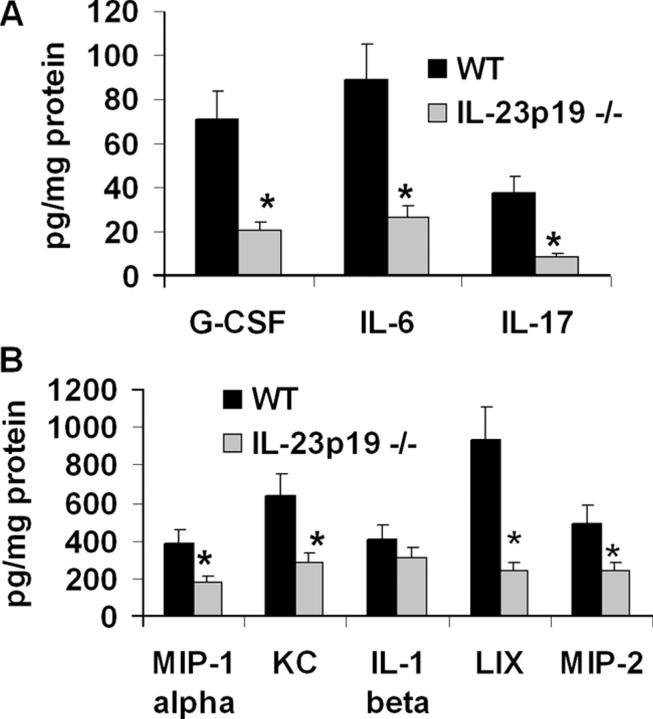
Cytokines and chemokines up-regulated by IL-17 are reduced in IL-23 p19–deficient mice during K. pneumoniae infection
Because IL-23 is critical for lung IL-17 induction after gram-negative challenge, we analyzed whether inflammatory mediators known to be up-regulated by IL-17 (such as G-CSF, IL-6, MIP-2, KC, and LIX) (21, 22) were also reduced in IL-23 p19−/− mice during infection. At 24 h postinoculation, lungs of knockout mice contained significantly attenuated levels of G-CSF, IL-6, MIP-1α, and the ELR+ CXC chemokines KC, MIP-2, and LIX compared with WT controls (Fig. 5 A and B), although differences in IL-1β were not significant. These data are similar to those observed in IL-17 receptor–deficient mice (21), further supporting a functional role for the IL-23/IL-17 axis in specific components of the inflammatory response to this pathogen.
Figure 5.
IL-23 p19−/− mice have reduced cytokine and chemokine responses to K. pneumoniae infection. Whole lung cytokine and chemokine content were measured in lung homogenates 24 h after 104 CFU K. pneumoniae delivery. Values are normalized to homogenate protein concentration (n = 6 per group; *P < 0.05 compared with WT). Error bars represent mean ± SD.
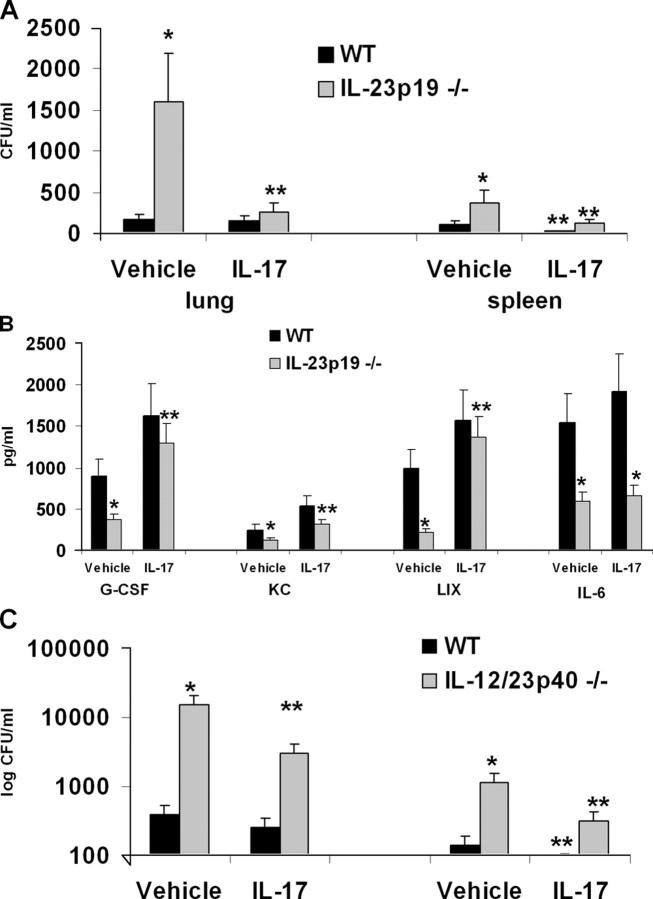
Rescue of IL-23 p19 knockout mice with IL-17 restores bacterial host defense and G-CSF and LIX levels in IL-23 p19 knockout mice
To confirm a role for IL-17 in host defense against K. pneumoniae in IL-23 p19−/− mice, we performed rescue experiments using recombinant murine IL-17 administered intratracheally 12 h after infection, the time at which IL-17 becomes detectable in BAL fluid in this model (29). Mice were killed 24 h later. For these experiments, we administered a dose of 1.5 μg of recombinant IL-17; this resulted in a mean level of IL-17 in BAL fluid 12 h after administration of 482 ± 92 pg/mL, which is a physiological concentration of IL-17 in this model (29). Vehicle treated IL-23 p19−/− mice demonstrate significantly higher burdens of K. pneumoniae in lung tissue as well as greater dissemination to the spleen 36 h postinoculation compared with WT control mice (Fig. 6 A). Administration of IL-17 significantly improved lung antibacterial host defense in IL-23 p19−/− mice and significantly reduced dissemination to the spleen in both WT and p19−/− mice. This enhanced clearance was associated with an increase in G-CSF and KC levels in BAL fluid, but not in IL-6, as measured 24 h after IL-17 treatment (Fig. 6 B). Among the CXC chemokines, IL-17 administration had the most dramatic effect on increasing LIX in IL-23 p19−/− mice (Fig. 6 B). We did not detect any differences in IFN-γ in BAL fluid at this 24-h time point (12.3 + 3.9 pg/mL in WT vs. 9.8 + 5.4 pg/mL in IL-23 p19−/− mice). However, to investigate whether restoration of IL-17 signaling in the absence of IFN-γ was at least partially protective, we performed a similar rescue experiment in IL-12/23 p40 mice. In this setting, IL-17 delivery resulted in a more modest yet significant improvement in both lung and splenic clearance of K. pneumoniae (Fig. 6 C), although the effect was more modest compared with results observed in IL-23 p19−/− mice (Fig. 6 A).
Figure 6.
Defects in pulmonary host defense in IL-23 p19−/− mice are rescued by IL-17 treatment. WT C57BL/6 or IL-23 p19−/− mice were challenged with 104 K. pneumoniae followed by intratracheal administration of 1.5 μg recombinant murine IL-17 (or phosphate-buffered saline vehicle) 12 h later. Animals were then killed 24 h after rmIL-17 (or vehicle) delivery. (A) IL-17 improves bacterial clearance in IL-23 p19−/− mice. (B) IL-17 restores pulmonary concentrations of G-CSF, KC, and LIX in IL-23 p19−/− mice. (C) IL-17 partially improves bacterial clearance in IL-12 p40−/− mice. *P < 0.05 compared with WT vehicle-treated control. **P < 0.05 compared with vehicle-treated group of same genotype (n = 4–6 per group). Error bars represent mean ± SD.
DISCUSSION
Our current studies show that IL-23 is required for the in vivo pulmonary IL-17 and IL-17F response to K. pneumoniae infection. These results are novel in the light of previous work suggesting other cytokine signals, namely IL-15, are responsible for the pulmonary IL-17 response to lipopolysaccharide (30). Although IL-15 appears to play a role in IL-17 induction in other models of inflammation (31–33), our current data demonstrate a strict requirement for IL-23 in pulmonary IL-17/17F induction in response to K. pneumoniae challenge. Our current work also identifies two potential sources of IL-23 in the lung—AMs and mDCs—and suggests IL-23 functions very early in lung response to pathogen compared with IL-12. As early as 4 h after in vitro exposure to bacteria, media from AM culture stimulates splenocyte IL-17 production, indicating a rapid induction of bioactive IL-23 (unpublished data). In contrast, IL-12 remained undetectable in AM culture supernatants even 24 h after in vitro challenge, and these supernatants did not induce splenocyte IFN-γ expression. These findings are consistent with prior work showing that AMs produce little or no IL-12 p70 in response to isolated challenge with K. pneumoniae or LPS (5, 24). Hence, AM may be more important in initiating the early “ThIL-17” response to this pathogen rather than directing T cells into Th1 polarization (34).
In vivo, up-regulation of IL-23 p19 in BAL cells is seen as early as 2 h after K. pneumoniae infection. Cells obtained by BAL at this time are still more than 95% AMs, implicating these cells as the likely source of early IL-23 expression in the alveolar compartment, because they express p40 mRNA even before infection. Hence, induction of p19 transcription likely regulates the onset of IL-23 production in air spaces. The early increase in BAL cell p19 mRNA is followed by greater expression 16–24 h after infection, a pattern observed in both BALF cells and lung homogenate. Whether this finding is due to the alveolar recruitment of additional cell types expressing IL-23 p19 or increased AM gene expression is unknown.
Interestingly, we observed greater splenocyte IL-17 induction from bacteria-pulsed IL-12 p35−/− AM conditioned media compared with WT AM. A regulatory role for IL-12 in IL-23–mediated signaling has been previously demonstrated (17), because IL-12 and IL-23 share a common p40 subunit and both require IL-12Rβ1 binding to signal. However, we were unable to measure IL-12 p70 in bacteria-stimulated AM supernatants, regardless of genotype. One hypothesis is that AM production of bioactive IL-23 is greater in p35−/− AMs, because more intracellular p40 is available to combine with p19. In support of this is, it has been shown that the elaboration of IL-12 p70 in AMs is under posttranscriptional control, and a second stimulus (such as IFN-γ) (5) is required for AM release of IL-12 p70 heterodimer in response to LPS. Of note, higher IL-17 levels in IL-12 p35−/− mice was not observed in vivo. However, deficient STAT1 signaling results in augmented IL-23 and IL-17 expression in the context of respiratory syncytial virus infection (35).
Our prior work has shown that TLR4 signaling is required for early IL-23 p19 and IL-17 mRNA expression in the lung challenged with K. pneumoniae (23). The greater up-regulation in p19 mRNA seen in mDCs compared with pDCs also supports a TLR4-dependent mechanism for IL-23 expression in this model, because granulocyte macrophage CSF–treated, bone marrow–derived mDCs are reported to express greater amounts of TLR4 and are more responsive to LPS than Flt3 ligand-generated pDCs (28). Our data lead us to speculate that mDCs play an important role in the IL-17 recall response to bacterial challenge as mDCs readily migrate to draining lymph nodes upon antigen capture, a function not readily shared by AMs (36). The subsequent T cell expansion, IL-17 expression, and augmented neutrophil recruitment as a result of the IL-23/IL-17 axis may represent a novel “cross-talk” loop between innate and adaptive pulmonary immunity, which enables the infected lung to more rapidly contain infection.
Although IL-12 p35 was not required for the pulmonary IL-17 response to K. pneumoniae, it was requisite for IFN-γ expression in this infection. This finding is consistent with the well-studied stimulatory effect of IL-12 on IFN-γ expression (37) as well as prior work that demonstrates the requirement of intact IL-12 for the pulmonary IFN-γ response to infection (38). The inability of IL-23 to induce pulmonary IFN-γ expression in the absence of IL-12 is consistent with previous work showing the failure of recombinant IL-23 to induce splenocyte IFN-γ expression, despite up-regulation of IL-17 by this cytokine (17). These observations are likely the result of differential receptor affinity and intracellular signaling events induced by IL-12 and IL-23. IL-12 binding to the IL-12Rβ1/Rβ2 complex predominantly activates STAT4. In contrast, IL-23 binds to the IL-23R/IL-12Rβ1 complex and induces STAT3, STAT1, and possibly STAT3/STAT4 heterodimer nuclear translocation, while only weakly activating STAT4 (39).
Our finding of decreased survival following pulmonary K. pneumoniae infection in both p35−/− and p40−/− mice is consistent with other reports of the importance of intact IL-12 signaling in this infection model. The early and universal mortality observed in the p40−/− group compared with other strains suggests roles for both IL-12 and IL-23 in host defense. That a normally sublethal pathogen dose imparts 60% mortality in IL-23 p19−/− animals confirms the critical requirement for this cytokine in surviving pulmonary K. pneumoniae infection. Bacterial clearance could be significantly enhanced in IL-23 p19−/− mice by administration of recombinant IL-17 at 12 h into the infection, and this treatment restored G-CSF and LIX production without correcting IL-6 expression. These data suggest that the absence of IL-17 signaling in p19−/− mice mediates the observed phenotype and that IL-17–induced IL-6 signaling is not a critical component of host defense in this infection model. Despite the absence of IL-12 and markedly diminished lung IFN-γ induction in IL-12/23 p40−/− mice, IL-17 treatment still reduced significantly the high bacterial burden observed in these mice, further suggesting IL-17 plays a significant role in host defenses in this model independent of IL-12/IFN-γ signaling.
We recognize that the current study has important limitations. Namely, we have not identified the specific effector immune functions defective in the absence of IL-23 signaling. Because IL-17 and IL-17F elicit neutrophil recruitment in the lung (40), defects in the number or function of these cells may also underlie the observed phenotype. Impaired antimicrobial peptide production may also contribute to enhanced mortality, as IL-17 signaling has also been shown to induce airway epithelial cell expression of mucin and β defensin 2 proteins, molecules important in bacterial clearance (41–43).
Our data support a critical role for IL-23 and IL-17 in early host resistance to K. pneumoniae independent of IL-12 and IFN-γ. It is possible that IL-23 and the subsequent IL-17 pathway have evolved to handle extracellular gram-negative bacterial challenges, because IL-17 is not required for host resistance against intracellular organisms such as Listeria monocytogenes or Mycobacterium tuberculosis (Kolls et al., unpublished observations), whereas IL-12 and IFN-γ have been shown to be critical for host resistance against these pathogens (44–46). Moreover, because IL-23 is critical for autoimmune diseases such as arthritis and multiple sclerosis (47), our data suggest that targeting IL-23 p19 would be less immunosuppressive than IL-12/23 p40.
MATERIALS AND METHODS
Mice.
Specific pathogen-free C57BL/6, IL-12 p35−/−, and IL-12/23 p40−/− mice were purchased at 6–8 wk of age (The Jackson Laboratory). IL-23 p19−/− mice were provided by N. Ghilardi. All mice were housed in specific pathogen-free rooms within the animal care facilities of the Louisiana State University Health Sciences Center or Children's Hospital of Pittsburgh under Institutional Animal Care and Use Committee-approved protocols. Mice were provided with water and food ad libitum and received 12-h light/dark cycles until the date of the experiment.
Infection model.
Mice were anesthetized via intraperitoneal ketamine/xylazine injection. The neck was opened in sterile fashion, and the trachea was cannulated with a 30-gauge needle. K. pneumoniae strain 43816 serotype 2 (American Type Culture Collection) was injected in a volume of 50 μL sterile phosphate-buffered saline (PBS). For IL-17 rescue experiments, 1.5 μg of recombinant murine IL-17 (R&D Systems) was delivered via the intratracheal route in a volume of 50 μL sterile PBS following light isoflurane anesthesia. At designated time points, animals were anesthetized and killed via cardiac puncture. Serial BAL was performed using 10 mL of Ca2+- and Mg2+-free PBS containing 5 mM glucose as described previously (21). BAL cell pellets were resuspended in RNeasy lysis buffer (RLT) for RNA isolation (QIAGEN). Nonlavaged whole lung samples were obtained via dissection and homogenization (Omni GLH, Omni International) in PBS containing 0.5% Triton X-100 and protease inhibitor cocktail (Roche Complete; Roche Applied Science) for protein determinations or in buffer RLT for RNA isolation.
Cell culture.
AMs were obtained for cell culture via BAL of naive mice, and these cells were more than 95% macrophages as determined by morphology on cytologic examination (unpublished data) for all experiments. DCs were obtained as follows: after lung lavage for partial depletion of AM, lungs were finely minced and incubated at 37°C with 1 mg/mL type IV collagenase (Sigma-Aldrich) for 1 h on a rotating shaker. The cell mixture was passed through a 40-μm nylon mesh filter and centrifuged at 400 g, and red cells were lysed. The cells were washed with PBS, counted, and stained with I-Ab, CD11c, CD11b, and CD45R/B220 monoclonal antibodies (BD Biosciences) after incubation with Fc block. DCs were obtained via fluorescence-activated cell sorting using the FACSAria cytometer (BD Biosciences). mDCs were defined as IAb+, CD11c+, CD11b+, and B220−; pDCs were defined as IAb+, CD11c+, CD11b−, and B220+. Isotype controls were performed to assist in sorting. Cells were resuspended in RPMI 1640 (Invitrogen) supplemented with 10% fetal calf serum. In vitro infection was performed by adding 107 CFU K. pneumoniae to 105 cells, giving a multiplicity of infection of 100:1. Splenocytes were obtained from C57BL/6 mice via organ passage through a 70-μm nylon mesh filter and centrifuged at 400 g, and red cells were lysed using NH4Cl. The cells were washed twice with PBS and plated onto 12-well plates in RPMI 1640 + 10% FCS in a 37°C 5% CO2 incubator. After 1 h, nonadherent cells were removed for use in conditioned media studies. At specified time points after in vitro infection, AMs and DCs were harvested for mRNA assay, and supernatants were centrifuged at 20,000 g for 20 min before transfer onto adherent cell-depleted splenocyte preparations for overnight culture. After 24 h of incubation at 37°C in a 5% CO2 incubator, cells and media from splenocyte cultures were harvested for mRNA and protein assay, respectively.
Real time RT-PCR.
Total RNA from lung homogenates and cultured cells was isolated using the RNeasy mini kit (QIAGEN). Ten nanograms of total RNA was subjected to one-step RT-PCR using TaqMan linear hydrolysis chemistry on the iCycler thermocycler (Bio-Rad). Gene-specific primers and dual-labeled probe sequences for IL-23 p19, IL-17, IL-17F, IL-12/23 p40, and IL-12 p35 mRNA and 18s ribosomal RNA (rRNA) were designed using Beacon Designer 2.12 (Premier Biosoft International) as follows (primer, primer, probe): IL-17, 5′-GCTCCAGAAGGCCCTCAGA-3′, 5′-CTTTCCCTCCGCATTGACA-3′, 5′-ACCTCAACCGTTCCACGTCAC-3′; IL-17F, 5′-GCAGACACTCAGGCTGCATC-3′, 5′-CCTCCGAAGGACCAGGATTT-3′, 5′-TGCTGTCTTCCTGACCCTGGGCAT-3′; IL-23 p19, 5′-TGGCTGTGCCTAGGAGTAGCA-3′, 5′-TTCATCCTCTTCTTCTCTTAGTAGATTCATA-3′, 5′-CTCTGCATGCTAGCCTGGAAC-3′; IL-12 p35, 5′-CCAAGGTCAGCGTTCCAACA-3′, 5′-AGAGGAGGTAGCGTGATTGACA-3′, 5′-CCTCACCCTCGGCATCCAGCAGC-3′; IL-12 p40, 5′-ACAGCACCAGCTTC-TTCATCAG-3′, 5′-TCTTCAAAGGCTTCATCTGCAA-3′, 5′-CATCAAACCAGACCCGCCCAAGAA-3′; 18s rRNA, 5′-ATTCGAACGTCTGCCCTATCA-3′, 5′-GTCACCCGTGGTCACCATG-3′, 5′-TCGATGGTAGTCGCCGTGCCTACC-3′. IFN-γ mRNA was assayed using SYBR Green dye and the gene-specific primers 5′-TCAGCAACAGCAAGGCGAAA-3′ and 5′-CCGCTTCCTGAGGCTGGAT-3′. A melt curve analysis was performed after all SYBR runs to ensure single PCR product formation, and expected IFN-γ amplicon size was confirmed via gel electrophoresis of the PCR product. All samples were normalized to 18s rRNA content. Data are expressed as transcript copy numbers per nanogram of 18s rRNA (when complementary RNA standards were used) or as fold induction over baseline, using time zero gene expression levels as reference (48).
Cytokine assays.
Lung homogenate and culture supernatant cytokine levels were determined via Bio-Plex cytokine bead array (Bio-Rad Laboratories), with the exception of LIX and IL-23, which were measured via sandwich ELISA (R&D Systems and eBioscience, respectively).
Statistical analysis.
All data are presented as the mean ± SEM. Statistical analysis was performed with a commercially available statistical software program (SAS OnlineDoc 9, SAS Institute). Data were tested for differences using analysis of variance for mixed and random effect models followed by the Tukey-Kramer range test. Survival analysis was performed using the log rank test. Statistical significance was set at P < 0.05.
Acknowledgments
This work was supported by Public Health Service grants nos. P60AA009803 (to S. Nelson and G.J. Bagby), K08AA015163 (to K.I. Happel), R01AI051677 (to J.E. Shellito), T32AA07577 (to G.J. Bagby), and R01HL061271, R01HL079142 (to J.K. Kolls).
The authors have no conflicting financial interests.
Abbreviations used: AM, alveolar macrophages; BAL, bronchoalveolar lavage; G-CSF, granuloctye CSF; KC, keratinocyte chemoattractant; LIX, LPS- induced C-X-C chemokine; mDC, myeloid DC; MIP-2, macrophage inflammatory protein 2; mRNA, messenger RNA; pDC, plasmacytoid DC; rRNA, ribosomal RNA.
K.I. Happel, P.J. Dubin, and M. Zheng contributed equally to this work.
References
- 1.Mehrad, B., and T.J. Standiford. 1999. Role of cytokines in pulmonary antimicrobial host defense. Immunol. Res. 20:15–27. [DOI] [PubMed] [Google Scholar]
- 2.Zhang, P., W.R. Summer, G.J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39–51. [DOI] [PubMed] [Google Scholar]
- 3.Ulich, T.R., L.R. Watson, S.M. Yin, K.Z. Guo, P. Wang, H. Thang, and J. del Castillo. 1991. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am. J. Pathol. 138:1485–1496. [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, S., R.B. Devlin, and J.S. Haskill. 1989. Differential production of tumor necrosis factor, macrophage colony stimulating factor, and interleukin 1 by human alveolar macrophages. J. Leukoc. Biol. 45:353–361. [PubMed] [Google Scholar]
- 5.Isler, P., B.G. de Rochemonteix, F. Songeon, N. Boehringer, and L.P. Nicod. 1999. Interleukin-12 production by human alveolar macrophages is controlled by the autocrine production of interleukin-10. Am. J. Respir. Cell Mol. Biol. 20:270–278. [DOI] [PubMed] [Google Scholar]
- 6.Greenberger, M.J., S.L. Kunkel, R.M. Strieter, N.W. Lukacs, J. Bramson, J. Gauldie, F.L. Graham, M. Hitt, J.M. Danforth, and T.J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 157:3006–3012. [PubMed] [Google Scholar]
- 7.Moore, T.A., M.L. Perry, A.G. Getsoian, M.W. Newstead, and T.J. Standiford. 2002. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect. Immun. 70:6310–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida, K., T. Matsumoto, K. Tateda, K. Uchida, S. Tsujimoto, Y. Iwakurai, and K. Yamaguchi. 2001. Protection against pulmonary infection with Klebsiella pneumoniae in mice by interferon-gamma through activation of phagocytic cells and stimulation of production of other cytokines. J. Med. Microbiol. 50:959–964. [DOI] [PubMed] [Google Scholar]
- 9.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 10.Verreck, F.A., T. de Boer, D.M. Langenberg, M.A. Hoeve, M. Kramer, E. Vaisberg, R. Kastelein, A. Kolk, R. Waal-Malefyt, and T.H. Ottenhoff. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA. 101:4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, A.M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I.M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 168:1322–1327. [DOI] [PubMed] [Google Scholar]
- 13.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M.K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann, J., S. Bellmann, C. Werner, R. Schroder, N. Schutze, and G. Alber. 2001. IL-12p40-dependent agonistic effects on the development of protective innate and adaptive immunity against Salmonella enteritidis. J. Immunol. 167:5304–5315. [DOI] [PubMed] [Google Scholar]
- 15.Elkins, K.L., A. Cooper, S.M. Colombini, S.C. Cowley, and T.L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghilardi, N., N. Kljavin, Q. Chen, S. Lucas, A.L. Gurney, and F.J. De Sauvage. 2004. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 172:2827–2833. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. De Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 18.Fossiez, F., J. Banchereau, R. Murray, C. Van Kooten, P. Garrone, and S. Lebecque. 1998. Interleukin-17. Int. Rev. Immunol. 16:541–551. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic, D.V., J.A. Di Battista, J. Martel-Pelletier, F.C. Jolicoeur, Y. He, M. Zhang, F. Mineau, and J.P. Pelletier. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 160:3513–3521. [PubMed] [Google Scholar]
- 20.Witowski, J., K. Pawlaczyk, A. Breborowicz, A. Scheuren, M. Kuzlan-Pawlaczyk, J. Wisniewska, A. Polubinska, H. Friess, G.M. Gahl, U. Frei, et al. 2000. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J. Immunol. 165:5814–5821. [DOI] [PubMed] [Google Scholar]
- 21.Ye, P., F.H. Rodriguez, S. Kanaly, K.L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, et al. 2001. Requirement of interleukin 17 receptor signaling for lung cxc chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruddy, M.J., F. Shen, J.B. Smith, A. Sharma, and S.L. Gaffen. 2004. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 76:135–144. [DOI] [PubMed] [Google Scholar]
- 23.Happel, K.I., M. Zheng, E. Young, L.J. Quinton, E. Lockhart, A.J. Ramsay, J.E. Shellito, J.R. Schurr, G.J. Bagby, S. Nelson, et al. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng, J.C., X. Zeng, M. Newstead, T.A. Moore, W.C. Tsai, V.J. Thannickal, and T.J. Standiford. 2004. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J. Immunol. 173:4075–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broug-Holub, E., G.B. Toews, J.F. van Iwaarden, R.M. Strieter, S.L. Kunkel, R. Paine III, and T.J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan, J., R.D. Ye, and A.B. Malik. 2001. Transcriptional mechanisms of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L1037–L1050. [DOI] [PubMed] [Google Scholar]
- 27.Stark, M.A., Y. Huo, T.L. Burcin, M.A. Morris, T.S. Olson, and K. Ley. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 22:285–294. [DOI] [PubMed] [Google Scholar]
- 28.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y.J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye, P., P.B. Garvey, P. Zhang, S. Nelson, G. Bagby, W.R. Summer, P. Schwarzenberger, J.E. Shellito, and J.K. Kolls. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 25:335–340. [DOI] [PubMed] [Google Scholar]
- 30.Ferretti, S., O. Bonneau, G.R. Dubois, C.E. Jones, and A. Trifilieff. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170:2106–2112. [DOI] [PubMed] [Google Scholar]
- 31.Kolls, J.K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity. 21:467–476. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari-Lacraz, S., E. Zanelli, M. Neuberg, E. Donskoy, Y.S. Kim, X.X. Zheng, W.W. Hancock, W. Maslinski, X.C. Li, T.B. Strom, et al. 2004. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J. Immunol. 173:5818–5826. [DOI] [PubMed] [Google Scholar]
- 33.Ziolkowska, M., A. Koc, G. Luszczykiewicz, K. Ksiezopolska-Pietrzak, E. Klimczak, H. Chwalinska-Sadowska, and W. Maslinski. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832–2838. [DOI] [PubMed] [Google Scholar]
- 34.Langrish, C.L., B.S. McKenzie, N.J. Wilson, M.R. de Waal, R.A. Kastelein, and D.J. Cua. 2004. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 202:96–105. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto, K., J. Durbin, W. Zhou, R.D. Collins, S.B. Ho, J.K. Kolls, P.J. Dubin, J.R. Sheller, K. Goleniewska, J.F. O'Neal, et al. 2005. Respiratory syncytial virus infection in the absence of STAT1 results in airway dysfunction, induction of airway mucus, and augmented IL-17 production. J. Allergy Clin. Immunol. In press. [DOI] [PubMed] [Google Scholar]
- 36.Havenith, C.E., P.P. van Miert, A.J. Breedijk, R.H. Beelen, and E.C. Hoefsmit. 1993. Migration of dendritic cells into the draining lymph nodes of the lung after intratracheal instillation. Am. J. Respir. Cell Mol. Biol. 9:484–488. [DOI] [PubMed] [Google Scholar]
- 37.Wolf, S.F., P.A. Temple, M. Kobayashi, D. Young, M. Dicig, L. Lowe, R. Dzialo, L. Fitz, C. Ferenz, and R.M. Hewick. 1991. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 146:3074–3081. [PubMed] [Google Scholar]
- 38.Geng, Y., K. Berencsi, Z. Gyulai, T. Valyi-Nagy, E. Gonczol, and G. Trinchieri. 2000. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect. Immun. 68:2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K.P. Singh, F. Vega, et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699–5708. [DOI] [PubMed] [Google Scholar]
- 40.Hurst, S.D., T. Muchamuel, D.M. Gorman, J.M. Gilbert, T. Clifford, S. Kwan, S. Menon, B. Seymour, C. Jackson, T.T. Kung, et al. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169:443–453. [DOI] [PubMed] [Google Scholar]
- 41.Chen, Y., P. Thai, Y.H. Zhao, Y.S. Ho, M.M. DeSouza, and R. Wu. 2003. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 278:17036–17043. [DOI] [PubMed] [Google Scholar]
- 42.Kao, C.Y., Y. Chen, P. Thai, S. Wachi, F. Huang, C. Kim, R.W. Harper, and R. Wu. 2004. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173:3482–3491. [DOI] [PubMed] [Google Scholar]
- 43.Cole, A.M., and A.J. Waring. 2002. The role of defensins in lung biology and therapy. Am. J. Respir. Med. 1:249–259. [DOI] [PubMed] [Google Scholar]
- 44.Cooper, A.M., J. Magram, J. Ferrante, and I.M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 186:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flynn, J.L., J. Chan, K.J. Triebold, D.K. Dalton, T.A. Stewart, and B.R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper, A.M., D.K. Dalton, T.A. Stewart, J.P. Griffin, D.G. Russell, and I.M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmittgen, T.D., B.A. Zakrajsek, A.G. Mills, V. Gorn, M.J. Singer, and M.W. Reed. 2000. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285:194–204. [DOI] [PubMed] [Google Scholar]