Abstract
The bacterial pathogens of the genus Yersinia, the causative agents of plague, septicemia, and gastrointestinal syndromes, use a type III secretion system to inject virulence factors into host target cells. One virulence factor, YopJ, is essential for the death of infected macrophages and can block host proinflammatory responses by inhibiting both the nuclear factor κB (NF-κB) and mitogen-activated protein kinase pathways, which might be important for evasion of the host immune response and aid in establishing a systemic infection. Here, we show that YopJ is a promiscuous deubiquitinating enzyme that negatively regulates signaling by removing ubiquitin moieties from critical proteins, such as TRAF2, TRAF6, and IκBα. In contrast to the cylindromatosis tumor suppressor CYLD, which attenuates NF-κB signaling by selectively removing K63-linked polyubiquitin chains that activate IκB kinase, YopJ also cleaves K48-linked chains and thereby inhibits proteasomal degradation of IκBα. YopJ, but not a catalytically inactive YopJ mutant, promoted deubiquitination of cellular proteins and cleaved both K48- and K63-linked polyubiquitin. Moreover, an in vitro assay was established to demonstrate directly the deubiquitinating activity of purified YopJ.
Pathogenic Yersinia, Yersinia pseudotuberculosis, and Yersinia enterocolitica must express the cysteine protease YopJ to kill infected macrophages and establish a systemic infection in mice (1–4). Other members of the family of cysteine proteases to which YopJ belongs include the adenovirus protease AVP, the proapoptotic protein AvrA of Salmonella typhimurium, and the Xanthomonas campestris pv. vesicatoria protein AvrBsT, which induces localized cell death in infected plant leaves (4). Mutation of the conserved catalytic cysteine in YopJ abolishes its ability to inhibit the proinflammatory mitogen-activated protein kinase (MAPK) and NF-κB pathways that are activated within infected cells, and it has been suggested that YopJ proteolytic activity is required to remove the ubiquitin-like protein SUMO-1 from posttranslationally modified proteins (4). However, the proteins that must be de-sumoylated to shut down NF-κB or MAPK signaling remain ill defined. In this study, we identify YopJ as a promiscuous deubiquitinating protease that negatively regulates the host cell response by cleaving ubiquitin moieties from critical proteins, such as TRAF2, TRAF6, and IκBα.
Results and Discussion
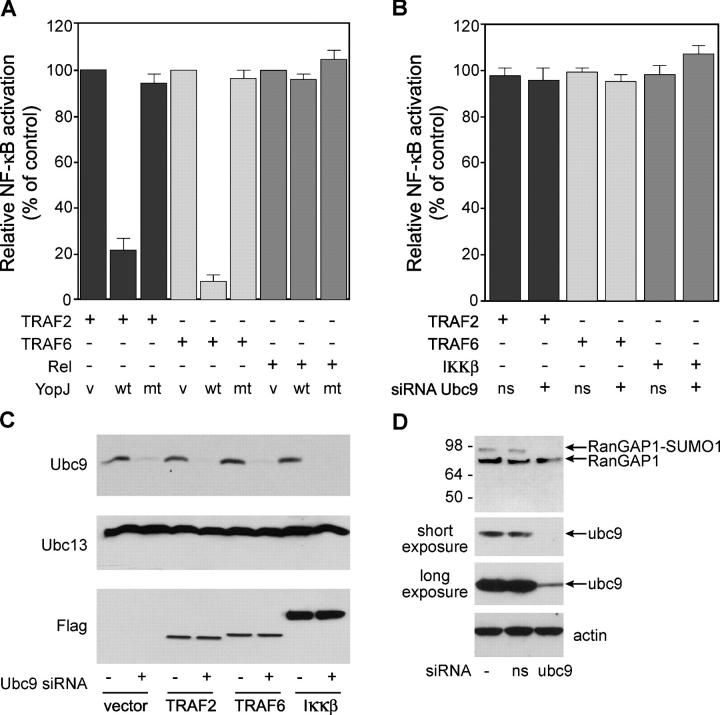
To identify the YopJ substrates that are critical to NF-κB signaling, we began by comparing the impact of YopJ on NF-κB activation in response to a variety of different stimuli. HEK293T cells were cotransfected with YopJ, a NF-κB–dependent reporter gene, and one of the following activators of NF-κB transcription: TRAF6, which is essential for NF-κB signaling when LPS engages Toll-like receptor 4; TRAF2 or TRAF5, which signal NF-κB activation downstream of TNF receptor 1; NIK, which signals NF-κB activation downstream of the lymphotoxin β receptor and the BAFF/BLyS receptor 3; IKKα or IKKβ, components of the IκB kinase (IKK) complex that is activated by diverse stimuli to phosphorylate IκB proteins and thereby targets them for ubiquitin-mediated degradation; and c-Rel, a member of the NF-κB family of transcription factors (Fig. 1 A and see Fig. 3 B; references 5–7). NF-κB transcriptional activity in response to overexpression of TRAF6, TRAF2, NIK, IKKα, or IKKβ was markedly reduced by WT YopJ, but not by the catalytic cysteine YopJ mutant C172A. These results indicate that YopJ can inhibit NF-κB signaling in response to diverse stimuli and that inhibition is dependent on its protease activity. WT YopJ did not, however, block NF-κB activation after c-Rel overexpression, indicating that YopJ does not have a direct effect on the transcriptional activity of NF-κB (Fig. 1 A).
Figure 1.
YopJ inhibits NF-κB activation by diverse stimuli. (A) HEK 293T cells were transfected with an NF-κB–dependent reporter gene, the indicated activator of NF-κB signaling, and either control empty vector (v), WT YopJ (wt), or YopJ mutant C172A (mt). NF-κB activation was measured by dual-luciferase reporter assay after 36 h and is presented as a percentage of the activity induced by TRAF2 (lane 1), TRAF6 (lane 4), or Rel (lane 7) alone. Expression of YopJ, TRAF2, TRAF6, and Rel was confirmed by Western blotting (not depicted). (B) 293T cells that were transfected with siRNAs targeting Ubc9 or a nonspecific siRNA (ns) were cotransfected with Flag-tagged TRAF2, TRAF6, or IKKβ, and NF-κB activation was measured after 36 h. NF-κB activity is presented as a percentage of the activity induced by either TRAF2, TRAF6, or IKKβ in the absence of any siRNA oligos. (C) Cell lysates from B were Western blotted for Ubc9 (top), Ubc13 (middle), and TRAF2, TRAF6, or IKKβ (bottom). (D) 293T cells that were transfected with or without siRNAs targeting Ubc9 were cotransfected with RanGAP1. Modification of RanGAP1 by SUMO-1 was measured after 36 h by immunoblotting with RanGAP1 antibody (top). The actin blot shows equal protein loading (bottom).
Figure 3.
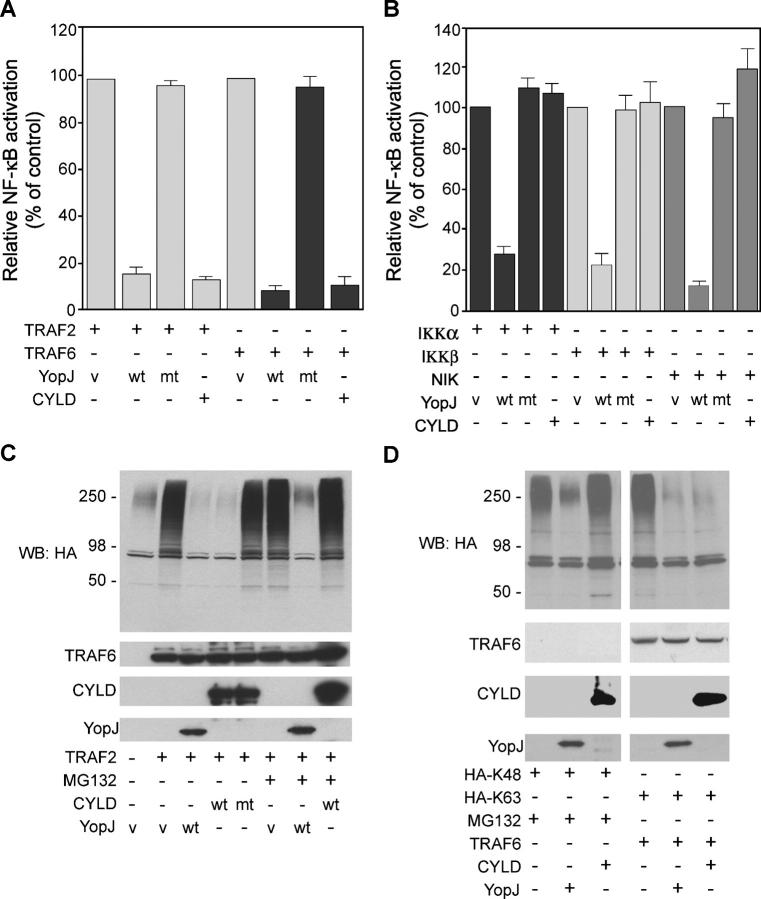
Comparison of YopJ and CYLD activities. (A and B) 293T cells were transfected with an NF-κB–dependent reporter gene, the indicated activator of NF-κB signaling, and either control empty vector (v), WT YopJ (wt), YopJ mutant C172A (mt), or CYLD. NF-κB activation was measured by dual-luciferase reporter assay after 36 h. (C) 293T cells were transfected with HA-ubiquitin and either empty vector (v), Flag-tagged WT YopJ (wt), Flag-tagged YopJ mutant C172A (mt), or Flag-tagged CYLD. Cells were then treated with 50 μM MG132 for 1 h before harvesting. Total cellular ubiquitinated proteins were detected by Western blotting with HA antibodies. (D) 293T cells were transfected with HA-tagged “K48-only” ubiquitin or “K63-only” ubiquitin together with TRAF6, WT YopJ, or CYLD. Cells were treated with 50 μM MG132 for 1 h before harvesting and analyzed as in C.
Given that SUMO-1–conjugated proteins have been reported as targets for YopJ (4), we next examined whether sumoylation of signaling components is a requirement for NF-κB activation, such that removal of SUMO-1 by YopJ might lead to inhibition of the NF-κB pathway. To test this hypothesis, we transfected 293T cells with siRNA against Ubc9, the E2 enzyme essential for protein sumoylation (8–9), and determined whether TRAF2, TRAF6, or IKKβ still activated an NF-κB–dependent reporter gene (Fig. 1 B). Significant knockdown of Ubc9 expression was confirmed by Western blotting (Fig. 1 C), but there was no inhibition of TRAF2-, TRAF6-, or IKKβ-induced NF-κB activity (Fig. 1 B). We verified that Ubc9 knockdown inhibited sumoylation by Western blotting of RanGAP1, a known target for sumoylation (10–11). The slower migrating form of RanGAP1 conjugated to SUMO-1 was abolished in cells transfected with Ubc9 siRNA (Fig. 1 D). These data suggest that sumoylation may not be required for NF-κB activation, at least in response to expression of TRAF2, TRAF6, or IKKβ, which brings into question the notion that YopJ inhibits NF-κB signaling by functioning as a SUMO-1 isopeptidase.
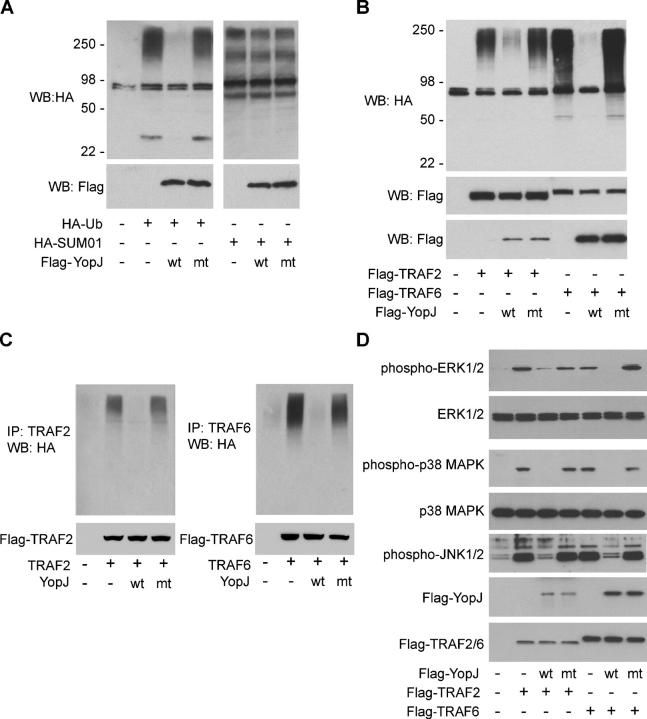
Recent studies have identified a critical role for ubiquitination in NF-κB signaling. For example, TRAF2 and TRAF6 undergo autoubiquitination and acquire polyubiquitin chains in which lysine 63 (K63) of one ubiquitin is linked to the COOH terminus of an adjacent ubiquitin. Modification of TRAF2 or TRAF6 in this manner is proposed to promote the assembly of a multiprotein complex that activates the IKK complex and hence, NF-κB signaling (12–14). The cylindromatosis tumor suppressor protein CYLD recently was defined as a deubiquitinating protease that inhibits TRAF2- and TRAF6-induced NF-κB activation by cleaving K63-linked ubiquitin chains (15–17). Therefore, we explored whether YopJ might also inhibit the NF-κB pathway by targeting ubiquitinated proteins rather than proteins conjugated to SUMO-1. To facilitate the detection of ubiquitin and SUMO-1 conjugates, 293T cells expressing YopJ were also transfected with SUMO-1 or ubiquitin having NH2-terminal hemagglutinin (HA) epitope tags. Immunoblotting of whole cell lysates with HA antibodies revealed that cells expressing YopJ contained far fewer ubiquitinated proteins than cells not expressing YopJ (Fig. 2 A, left). By contrast, YopJ did not decrease total cellular sumoylated protein levels (Fig. 2 A, right). The decrease in cellular ubiquitinated proteins mediated by YopJ was dependent on YopJ protease activity because the catalytic cysteine YopJ mutant C172A did not reduce ubiquitinated protein levels (Fig. 2 A). Consistent with the work of others (10–12), basal levels of total cellular ubiquitinated proteins in 293T cells, but not sumoylated proteins, were elevated further by overexpression of TRAF2 or TRAF6 (Fig. 2 B and not depicted). WT YopJ, but not YopJ mutant C172A, prevented this increased level of ubiquitinated proteins, further supporting the possibility that YopJ inhibits NF-κB signaling by acting as a deubiquitinating protease rather than a SUMO-1 isopeptidase.
Figure 2.
YopJ decreases cellular proteins modified by polyubiquitin chains but not proteins conjugated to SUMO-1. (A) 293T cells were transfected with HA-SUMO or HA-ubiquitin together with control empty vector (v), Flag-tagged WT YopJ (wt), or Flag-tagged YopJ mutant C172A (mt). Total sumoylated and ubiquitinated cellular proteins were detected by Western blotting with HA antibodies. (B) Western blot analysis of 293T cells transfected with Flag-tagged WT YopJ (wt) or YopJ mutant C172A (mt), HA-ubiquitin, and Flag-tagged TRAF2 or TRAF6. (C) TRAF2 or TRAF6 autoubiquitination was analyzed by immunoprecipitation of the proteins from denatured 293T cell lysates followed by immunoblotting with HA antibody (top) or anti-FLAG antibody (bottom). (D) Lysates from 293T cells transfected with the constructs indicated were Western blotted with phospho-specific antibodies recognizing activated ERK1/2, p38 MAPK, or Jun NH2-terminal kinases 1/2 (left). Western blots for all forms of ERK1/2 and p38 MAPK are also shown.
To test whether ubiquitinated TRAF2 and TRAF6 are substrates for YopJ, TRAF2 or TRAF6 was overexpressed with HA ubiquitin and YopJ and then immunoprecipitated from denatured cell lysates and immunoblotted with HA antibody. YopJ, but not YopJ mutant C172A, abolished TRAF2 and TRAF6 autoubiquitination (Fig. 2 C). This inhibition of TRAF2 and TRAF6 autoubiquitination by YopJ correlated not only with inhibition of NF-κB signaling (Fig. 1 A), but also with impaired activation of extracellular-regulated kinases (ERKs) 1 and 2, Jun NH2-terminal kinases 1 and 2, and p38 MAPK (Fig. 2 D; references 18–20). These results suggested that YopJ, like CYLD, prevents NF-κB signaling by acting as a deubiquitinating protease. However, when we compared the activities of YopJ and CYLD in our assay systems (Fig. 3), differences between the two were apparent. Although YopJ inhibited expression of an NF-κB–dependent reporter in response to overexpression of TRAF2, TRAF6, IKKα, IKKβ, and NIK, CYLD was only effective at blocking NF-κB signaling by TRAF2 and TRAF6 (Fig. 3, A and B). We also tested whether the lysine residue in ubiquitin that is used to assemble polyubiquitin chains impacts deubiquitination by YopJ and CYLD. For example, while TRAF2 and TRAF6 undergo “activating” K63-linked autoubiquitination, K48-linked polyubiquitin chains play an important role in NF-κB signaling by targeting phosphorylated IκB proteins for degradation in the 26S proteasome (21–23). To assess whether YopJ can promote the cleavage of K48-linked polyubiquitin chains as well as K63-linked chains, which are attached to TRAF2 and TRAF6, we expressed TRAF6 in cells to activate NF-κB signaling and used the proteasome inhibitor MG132 to trap the predominantly K48-linked polyubiquitinated proteins that would otherwise be degraded and not detected. We found that WT YopJ, but not CYLD, was able to block the accumulation of ubiquitinated proteins that occurred in cells treated with MG132 (Fig. 3 C). Next, we focused directly on K48- and K63-linked polyubiquitin chains by cotransfecting cells with TRAF6 and HA-tagged ubiquitin that had every lysine residue except K48 or K63 mutated to arginine (Fig. 3 D). MG132 treatment was used again to prevent the degradation of cellular proteins modified by K48-linked polyubiquitin chains. Western blotting with anti-HA antibodies revealed that YopJ interfered with the accumulation of proteins with K48- or K63-linked polyubiquitin chains, whereas CYLD only blocked the accumulation of proteins with K63-linked polyubiquitin chains (Fig. 3 D). These data indicate that YopJ can promote the cleavage of both K48- and K63-linked polyubiquitin chains and, as such, has much broader target specificity than CYLD. This difference might account for the ability of YopJ to inhibit NF-κB signaling in more settings than CYLD. For example, YopJ can inhibit NIK-induced NF-κB activation but CYLD cannot (Fig. 3 B). Such promiscuity in a bacterial enzyme would enable efficient shutdown of the multiple signaling pathways that contribute to the host immune response. Similar substrate promiscuity is associated with another Yersinia virulence factor, the tyrosine phosphatase YopH (24–25).
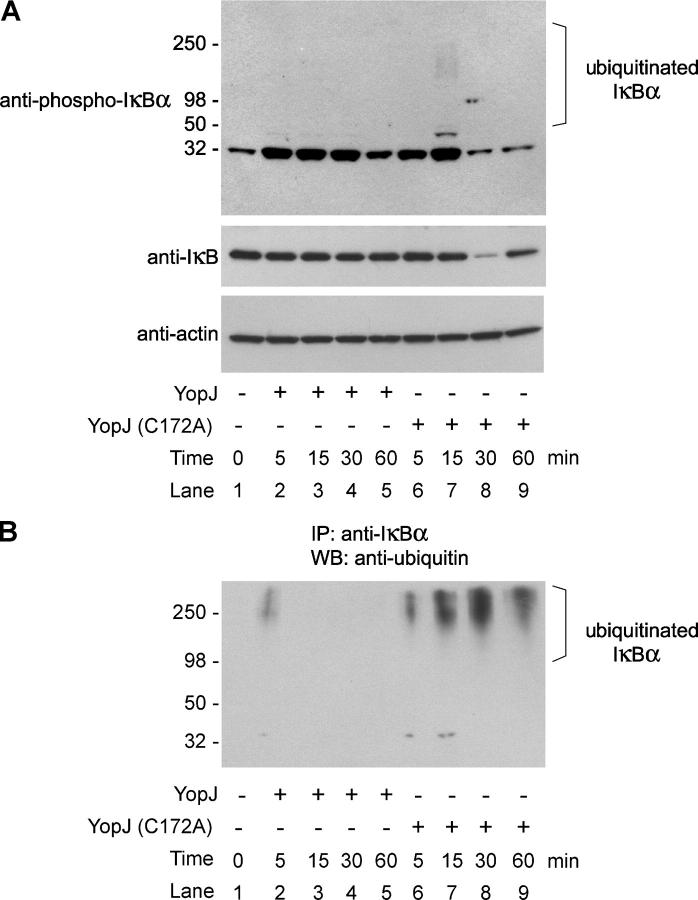
YopJ is essential for Y. pseudotuberculosis–induced macrophage apoptosis and is important for establishing a systemic infection in mice as indicated by increased clearance of a yopJ mutant 4 d after an oral infection in mice (2, 3, 26). Thus, to extend our findings in 293T cells, we tested the importance of the deubiquitinating activity associated with YopJ in the relevant setting of an actual bacterial infection. Mouse macrophages were infected with either WT Y. pseudotuberculosis or a YopJ-null strain of Y. pseudotuberculosis that had been complemented with the catalytic cysteine YopJ mutant C172A. Western blot analysis of whole cell lysates was performed at different times after infection (Fig. 4 A). At 5 min after infection, phosphorylated IκBα was observed in macrophages infected with WT YopJ Y. pseudotuberculosis as well as in macrophages infected with YopJ C172A Y. pseudotuberculosis, suggesting that both bacteria initially induced similar activation of the IKK complex, presumably through engagement of surface Toll-like receptors (Fig. 4 A, top; compare lanes 1, 2, and 6). Interestingly, the levels of phosphorylated IκBα remained elevated even at 60 min after infection with WT YopJ Y. pseudotuberculosis, whereas levels of phosphorylated IκBα in cells infected with YopJ C172A Y. pseudotuberculosis were greatly decreased by 30 min after infection (Fig. 4 A, top; compare lanes 4 and 8). Immunoblotting for total IκBα levels confirmed that IκBα was degraded in cells infected with YopJ C172A Y. pseudotuberculosis but not in cells infected with WT YopJ (Fig. 4 A, middle; compare lanes 4 and 8). Phosphorylation of IκBα normally is a prerequisite for its ubiquitination and degradation by the proteasome (5), so we investigated whether phosphorylated IκBα was not degraded in cells infected with WT YopJ Y. pseudotuberculosis because it was deubiquitinated efficiently by YopJ. To test this hypothesis, we treated macrophages with the proteasome inhibitor MG132 before infection to trap and detect the otherwise highly labile ubiquitinated IκBα. At different times after infection, IκBα was immunoprecipitated from boiled lysates and immunoblotted with ubiquitin antibodies (Fig. 4 B). High molecular weight bands, consistent with polyubiquitinated IκBα, were observed by 5 min after infection in cells infected with WT YopJ or YopJ mutant C172A bacteria. Notably, these higher molecular weight species then disappeared rapidly from cells infected with WT YopJ Y. pseudotuberculosis despite the presence of MG132 (Fig. 4 B). By contrast, the polyubiquitinated IκBα was still evident in cells infected with YopJ C172A Y. pseudotuberculosis at 60 min after infection. These results indicate that YopJ plays an essential role in reversing the ubiquitination of phosphorylated IκBα during Yersinia infection. Consequently, NF-κB transcription factors would stay sequestered in the cytoplasm by intact IκBα, thereby attenuating the antiapoptotic NF-κB pathway (2, 26, 27). We propose that the reason the IKK complex can still be activated in macrophages infected with WT YopJ Y. pseudotuberculosis (Fig. 1 A), despite the fact that YopJ can promote the deubiquitination of such upstream components as TRAF2 and TRAF6 (Fig. 2 C), is that attachment of Yersinia to the macrophage surface activates NF-κB signaling through Toll-like receptors (28) and perhaps additional cell surface receptors before the time that YopJ is injected into the macrophage by the bacteria's type III secretion system. Consistent with this notion, by immuno-gold labeling and electron microscopy, substantial levels of YopJ could not be detected in macrophages infected with Y. pseudotuberculosis until 15 min after infection (not depicted), a time when IκBα is already phosphorylated (Fig. 4 A).
Figure 4.
YopJ decreases cellular proteins modified by polyubiquitin chains during Yersinia infection of macrophages. (A) Mouse macrophages were untreated or infected with Y. pseudotuberculosis expressing WT YopJ or YopJ mutant C172A and subjected to Western blot analysis with antibodies to phosphorylated IκBα (top), total IκBα (middle), or actin (bottom). (B) Mouse macrophages were treated with 50 μM MG132 for 1 h and then infected with Y. pseudotuberculosis expressing either WT YopJ or YopJ mutant C172A. IκBα was immunoprecipitated from boiled lysates at the times indicated and immunoblotted with antibodies against ubiquitin.
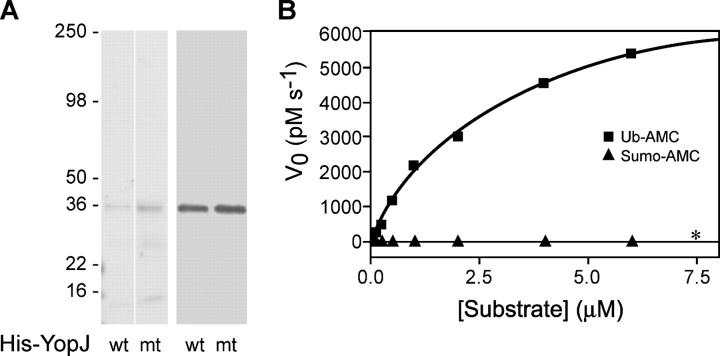
These results indicate that YopJ promotes deubiquitination, though it remains uncertain whether YopJ has intrinsic deubiquitinating activity or regulates ubiquitination indirectly via other proteins in cells. To demonstrate deubiquitinating activity for YopJ directly, we purified WT YopJ and YopJ mutant C172A from insect cells (Fig. 5 A) and incubated them with ubiquitin-7-amino-4-methylcoumarin (ubiquitin-AMC) in an in vitro deubiquitination assay (Fig. 5 B). Purified WT YopJ cleaved ubiquitin-AMC, but not SUMO-1–AMC, as judged by the release of fluorescent AMC, whereas YopJ mutant C172A was inactive against ubiquitin-AMC. Cleavage of SUMO-1–AMC under these experimental conditions was observed with recombinant SUMO protease 1 (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20051194/DC1). These results indicate that YopJ possesses intrinsic deubiquitinating protease activity that requires cysteine 172. YopJ cleaved ubiquitin-AMC with a Km of ∼3.0 uM, which is comparable with that of other known deubiquitinating proteases (29).
Figure 5.
Purified YopJ cleaves ubiquitin-AMC but not SUMO-1–AMC. (A) Coomassie blue staining (left) and anti-His immunoblotting (right) of purified YopJ protein. (B) Purified YopJ was incubated with ubiquitin-AMC (▪) or SUMO1-AMC (▴), and protease activity was determined by the release of fluorescent AMC. YopJ mutant C172A had no activity against ubiquitin-AMC (*). For WT YopJ, Km = 3.0 μM, Vmax = 7,975 pM s−1, and kcat = 0.06 s−1. kcat is likely underestimated because the calculation assumed that 100% of purified YopJ protein was active and folded correctly.
In sum, we provide the first direct evidence that a bacterial virulence factor, YopJ, is a deubiquitinating protease and have shown that this activity is responsible for YopJ-mediated inhibition of NF-κB and MAPK signaling. Given that YopJ and its related proteins function as virulence factors in bacteria that cause: (a) plague or black death (Yersinia pestis), a disease of historical significance and of recent interest due to the threat of bioterrorism, (b) gastroenteritis (Y. pseudotuberculosis, Salmonella typhimurium), and (c) black rot in plants (Xanthomonas campestris), the finding that purified YopJ is a deubiquitinase provides a facile enzymatic assay for the eventual development of a therapeutic inhibitor. Additionally, it is likely that interfering with ubiquitin signaling in the host might be a common strategy used by pathogens to evade the host immune system during the course of infection.
MATERIALS AND METHODS
Transfection experiments.
293T cells were transfected with Lipofectamine 2000 (Invitrogen). Luciferase assays were performed as described previously (30). Ubc9 siRNA duplexes with 3′ dTdT overhangs (Genentech, Inc.) or scramble II siRNA duplexes (Dharmacon) were transfected twice at 24-h intervals, with the cells being split to 80% confluency before the second transfection. Expression constructs were also included in the second transfection, and lysates were collected after an additional 36 h. Cells washed with PBS were lysed for 0.5 h at 4°C in 120 mM NaCl, 50 mM Hepes, pH 7.2, 1 mM EDTA, 0.1% NP-40, and complete protease inhibitor cocktail (Roche). Proteins were immunoprecipitated for 2–16 h at 4°C and then washed three times in lysis buffer. To assess ubiquitination, lysates were supplemented with 1% SDS (vol/vol) and heated at 90°C for 5 min. Samples were then diluted 10-fold with lysis buffer, and immunoprecipitations were performed.
Purification of YopJ.
Recombinant baculoviruses to express WT YopJ or YopJ mutant C172A with NH2-terminal 6xHIS tags from the polyhedrin promoter were generated in sf9 cells cotransfected with baculovirus gold DNA and YopJ sequences cloned into pVL1932 (BD Biosciences). Hi5 cells were infected with virus at a multiplicity of infection of 5:1 at 27°C for 3 d. Infected cells were lysed during three freeze/thaw cycles in 50 mM Tris, pH 8.0, 500 mM NaCl, 0.1% Triton X-100, and 2 mM β-mercaptoethanol. Soluble HIS-tagged proteins were recovered from a Ni-NTA agarose column (QIAGEN) in 50 mM Tris, pH 8.5, 300 mM NaCl, 2 mM β-mercaptoethanol, and 250 mM imidazole. Monomeric YopJ was obtained using a Superdex-75 gel filtration column (GE Healthcare) equilibrated with 50 mM Tris, pH 8.5, 250 mM NaCl, and 5 mM β-mercaptoethanol.
Protease assays.
WT YopJ, mutant YopJ, or SUMO protease 1 (∼140 nM; LifeSensor Inc.) was incubated with ubiquitin-AMC or Sumo-AMC (0.25–7.5 μM; Boston Biochem) in 50 mM Hepes, pH 8.0, and 1 mM DTT. Liberation of AMC at 23°C was monitored continuously in a microplate reader (excitation/emission wavelengths 380/460 nm; SpectraMax M2; Molecular Devices). Relative fluorescence units were converted to pmol AMC using a standard curve of relative fluorescence units versus AMC concentration. Kinetic constants (kcat, Km) were calculated from Michaelis-Menten plots (V0 versus [S]) with nonlinear regression analysis using GraphPad software and the assumption that 100% of purified YopJ was active.
Yersinia infections.
Yersinia strains were grown overnight with aeration in 2x YT medium at 26°C and then diluted 1:50 into 2x YT plus 20 mM sodium oxalate and 20 mM MgCl2 to be grown for 2 h at 26°C and then 2 h at 37°C. 2 × 106 RAW264.7 (ATCC TIB71) cells were seeded into six-well plates at 37°C (5% CO2) for 15–18 h and infected at a multiplicity of infection of 50:1. Centrifugation at 165 g for 5 min synchronized the infection. 50 μM MG132 was added 1 h before infection.
Online supplemental material.
Fig. S1 shows the protease activity of recombinant SUMO protease 1 or YopJ against ubiquitin-AMC or SUMO1-AMC. It is available at http://www.jem.org/cgi/content/full/jem.20051194/DC1.
Acknowledgments
We thank Kim Newton for critical reading and editorial assistance, Karen O'Rourke for excellent technical assistance, and members of the Dixit lab for helpful discussion.
This work was supported by the National Institutes of Health grants P01AI063302 and U-19AI057229 to D.M. Monack.
The authors have no conflicting financial interests.
I. Wertz's present address is Washington University School of Medicine, St. Louis, MO 63110.
References
- 1.Cornelis, G.R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA. 97:8778–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monack, D.M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA. 94:10385–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monack, D.M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orth, K., Z. Xu, M.B. Mudgett, Z.Q. Bao, L.E. Palmer, J.B. Bliska, W.F. Mangel, B. Staskawicz, and J.E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 290:1594–1597. [DOI] [PubMed] [Google Scholar]
- 5.Dixit, V., and T.W. Mak. 2002. NF-kappaB signaling. Many roads lead to madrid. Cell. 111:615–619. [DOI] [PubMed] [Google Scholar]
- 6.Rothwarf, D.M., and M. Karin. 1999. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE. 5:RE1. [DOI] [PubMed] [Google Scholar]
- 7.Hayden, M.S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195–2224. [DOI] [PubMed] [Google Scholar]
- 8.Desterro, J.M., J. Thomson, and R.T. Hay. 1997. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett. 417:297–300. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz, S.E., K. Matuschewski, D. Liakopoulos, M. Scheffner, and S. Jentsch. 1998. The ubiquitin-like proteins SMT3 and SUMO-1 are conjugated by the UBC9 E2 enzyme. Proc. Natl. Acad. Sci. USA. 95:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matunis, M.J., J. Wu, and G. Blobel. 1998. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J. Cell Biol. 140:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahajan, R., L. Gerace, and F. Melchior. 1998. Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J. Cell Biol. 140:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi, C.S., and J.H. Kehrl. 2003. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 278:15429–15434. [DOI] [PubMed] [Google Scholar]
- 13.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z.J. Chen. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 103:351–361. [DOI] [PubMed] [Google Scholar]
- 14.Wang, C., L. Deng, M. Hong, G.R. Akkaraju, J. Inoue, and Z.J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 412:346–351. [DOI] [PubMed] [Google Scholar]
- 15.Trompouki, E., E. Hatzivassiliou, T. Tsichritzis, H. Farmer, A. Ashworth, and G.E. Mosialos. 2003. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 424:793–796. [DOI] [PubMed] [Google Scholar]
- 16.Brummelkamp, T.R., S.M. Nijman, A.M. Dirac, and R. Bernards. 2003. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 424:797–801. [DOI] [PubMed] [Google Scholar]
- 17.Kovalenko, A., C. Chable-Bessia, G. Cantarella, A. Israel, D. Wallach, and G. Courtois. 2003. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 424:801–805. [DOI] [PubMed] [Google Scholar]
- 18.Orth, K., L.E. Palmer, Z.Q. Bao, S. Stewart, A.E. Rudolph, J.B. Bliska, and J.E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 285:1920–1923. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, L.E., A.R. Pancetti, S. Greenberg, and J.B. Bliska. 1999. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun. 67:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer, L.E., S. Hobbie, J.E. Galan, and J.B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-alpha production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953–965. [DOI] [PubMed] [Google Scholar]
- 21.Chen, Z., J. Hagler, V.J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586–1597. [DOI] [PubMed] [Google Scholar]
- 22.Alkalay, I., A. Yaron, A. Hatzubai, A. Orian, A. Ciechanover, and Y. Ben-Neriah. 1995. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA. 92:10599–10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaron, A., A. Hatzubai, M. Davis, I. Lavon, S. Amit, A.M. Manning, J.S. Andersen, M. Mann, F. Mercurio, and Y. Ben-Neriah. 1998. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 396:590–594. [DOI] [PubMed] [Google Scholar]
- 24.Guan, K.L., and J.E. Dixon. 1990. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 249:553–556. [DOI] [PubMed] [Google Scholar]
- 25.Bliska, J.B., K.L. Guan, J.E. Dixon, and S. Falkow. 1991. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. USA. 88:1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills, S.D., A. Boland, M.P. Sory, P. van der Smissen, C. Kerbourch, B.B. Finlay, and G.R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA. 94:12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denecker, G., W. Declercq, C.A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M.P. Sory, P. Vandenabeele, and G.R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706–19714. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, Y., and J.B. Bliska. 2003. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect. Immun. 71:1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dang, L.C., F.D. Melandri, and R.L. Stein. 1998. Kinetic and mechanistic studies on the hydrolysis of ubiquitin C-terminal 7-amido-4-methylcoumarin by deubiquitinating enzymes. Biochemistry. 37:1868–1879. [DOI] [PubMed] [Google Scholar]
- 30.Zhou, H., I. Wertz, K. O'Rourke, M. Ultsch, S. Seshagiri, M. Eby, W. Xiao, and V.M. Dixit. 2004. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 427:167–171. [DOI] [PubMed] [Google Scholar]