Abstract
The brain’s sensory processing systems are modified during perceptual learning. To learn more about the spatial organization of learning-related modifications, we trained rats to utilize the sensory signal from a single intact whisker to carry out a behavioral task. Once a rat had mastered the task, we clipped its “trained” whisker and attached a “prosthetic” one to a different whisker stub. We then tested the rat to determine how quickly it could relearn the task by using the new whisker. We observed that rats were immediately able to use the prosthetic whisker if it were attached to the stub of the trained whisker but not if it were attached to a different stub. Indeed, the greater the distance between the trained and prosthetic whisker, the more trials were needed to relearn the task. We hypothesized that this “transfer” of learning between whiskers might depend on how much the representations of individual whiskers overlap in primary somatosensory cortex. Testing this hypothesis by using 100-electrode cortical recordings, we found that the overlap between the cortical response patterns of two whiskers accounted well for the transfer of learning between them: The correlation between the electrophysiological and behavioral data was very high (r = 0.98). These findings suggest that a topographically distributed memory trace for sensory-perceptual learning may reside in primary sensory cortex.
After finding that the degree of deficit in a learned behavior depended on the size but not on the location of a cortical lesion, Lashley pronounced his influential theory of equipotentiality: “Limited regions may be necessary for learning or retention of a particular activity, but within such regions the parts are functionally equivalent. The engram is represented throughout the region” (ref. 1, p. 62). Since then, many studies have shown that the different zones within a cortical sensory representation are not functionally equivalent (2). However, the problem delineated by Lashley remains relevant because it is still not clear whether neighboring regions within a sensory cortical map function independently or cooperatively during learning and remembering. One way of investigating the problem is to identify a task that depends on sensory cortex and then require subjects to learn this task by using a restricted set of sensory receptors. Later, the subjects are tested on the same task but now are using a different set of receptors. If the entire sensory cortical representation participated equally in the initial learning, then subjects will be able to reacquire the task without any retraining.
The whisker sensory system of rats is well suited to this experimental design. Individual whiskers on the snout project to the contralateral primary somatosensory cortex in a topographic manner (3, 4). Somatosensory cortex plays an essential role in numerous behaviors involving the vibrissal system, including the “gap-crossing” task (5, 6). In this task, the rat uses its whiskers to detect a “goal” platform before crossing the gap to collect a reward (Fig. 1). To investigate the spatial organization of learning, we trained rats to use a single whisker to guide the gap-crossing behavior and then determined how much benefit the initial training provided on retesting, but now with a different whisker available. After finding that the transfer of learning is related to whisker location, we asked whether the topography of the functional projection of the whiskers to cortex might explain the behavioral observations.
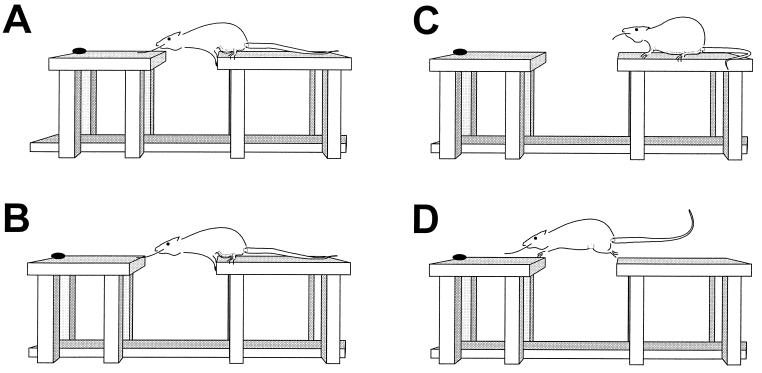
Figure 1.
The gap-crossing task. (A) At 14 cm, the rat can contact the goal platform with its nose and/or whisker. (B) At 16 cm, the rat can contact the goal platform only with its whisker. (C) If the rat fails to utilize the whisker information, it retires to the start platform. (D) Successfully detecting the goal platform, the rat crosses.
MATERIALS AND METHODS
Behavioral Experiment.
Subjects were 28 male Wistar albino rats (350–450 g) housed in standard cages with a natural light-dark cycle. At the beginning of the experiment, we clipped all but one whisker on 24 of the rats (Fig. 2). The intact whisker was from the 3 × 3 grid of B1–3, C1–3, D1–3 on either side of the snout (Fig. 2A). A further four “naïve” rats had all whiskers clipped. The rats were trained on the gap-crossing task (5). The training apparatus consisted of three platforms (11 cm wide × 30 cm long × 34 cm high) covered in black adhesive plastic, with 2-cm-high walls. The platforms were aligned end-to-end. To prevent use of visual information, experiments were conducted under dim red light (<1 lux; Panlux light meter, Gossen, Nuremberg, Germany), invisible to albino rats.
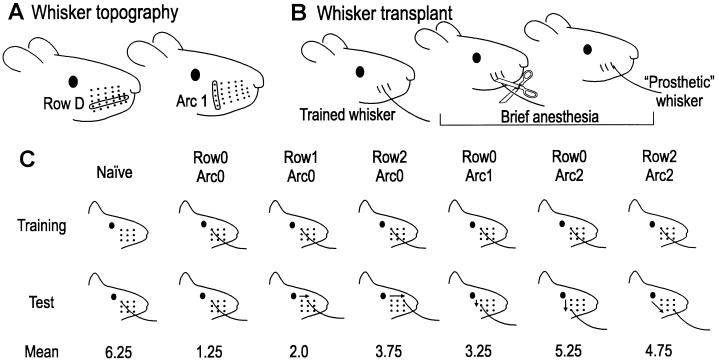
Figure 2.
Whisker topography, prosthesis, and the behavioral outcome. (A) Each whisker is identified by a coordinate system of rows and arcs. One row and one arc are indicated. Caudal whiskers (α, β, γ, δ) located between rows are not shown. (B) Whisker prosthesis procedure. The prosthetic whisker site was within the 3 × 3 grid on the same side as the trained whisker. (C) Relative whisker position within the 3 × 3 grid during training (top row) and testing (middle row). Arrows indicate whisker transposition. Test results are shown in the bottom row.
The rats were trained to cross from the center platform to either of the goal platforms. Five Cocopops (Kellogg’s) served as the reward for crossing. One training session consisted of five successful crossings. Each session included two trials in which the goal platform was removed (once on each side)—these “blank” trials ensured that the rat first contacted the goal platform before attempting to cross. Once a rat reliably crossed a given distance, the gap was widened by 1 cm. Each rat was trained in this manner for two-to-four sessions per day and typically required 7 days to reach criterion. For the 24 rats trained with one intact whisker, the criterion was to cross a whisker-dependent distance (16 cm) on all five trials in a session without attempting to cross on the blank trials. The criterion for the four rats in the naïve group was to cross the maximum nose-reachable distance (14 cm) on all five trials in a session. Once the rat’s performance reached criterion, training was terminated.
Under anesthesia (2% halothane mixed with O2 and NO2), the intact whisker was clipped (except in whiskerless naïve rats) and a “prosthetic” whisker, harvested from those removed before training, was attached to a whisker stub by using fast drying adhesive (SuperAttak Loctite, Milan) and hot glue (Bostik, Middleton, MA). Rats with an intact whisker during training were assigned to six groups (n = 4 per group) based on the location of the prosthetic whisker relative to the trained whisker: same site as the trained whisker (Row0Arc0), one step along the row (Row1Arc0) or along the arc (Row0Arc1), two steps along the row (Row2Arc0) or along the arc (Row0Arc2), or two steps in both dimensions (Row2Arc2). For rats in the naïve group, the prosthetic whisker was attached to a stub within the 3 × 3 grid.
Testing took place 4 hr after attachment of the prosthetic whisker. If the rat failed to cross within 60 s on the first trial, it was removed, and the gap was narrowed to 14 cm to allow it to cross and receive reinforcement. On the second trial, the gap again was set at 16 cm, and this cycle continued: The score was the trial number on which the rat successfully crossed the 16 cm gap. Once they had crossed this gap distance, rats successfully crossed it on subsequent trials, indicating a reliable reacquisition of the behavior.
Physiological Experiment.
The rats used in the physiological experiment were different from those used in the behavioral experiment. In brief, six rats weighing 230–500 g were anesthetized with urethane (i.p., 1.5 g/kg body weight) and were placed in a Narashige stereotaxic apparatus. Body temperature was maintained near 37.5°C. After a craniotomy, a 10 × 10 microelectrode array (Bionic Technologies, Salt Lake City) was implanted through the dura into barrel cortex to a depth of 700–900 μm (Fig. 3A).
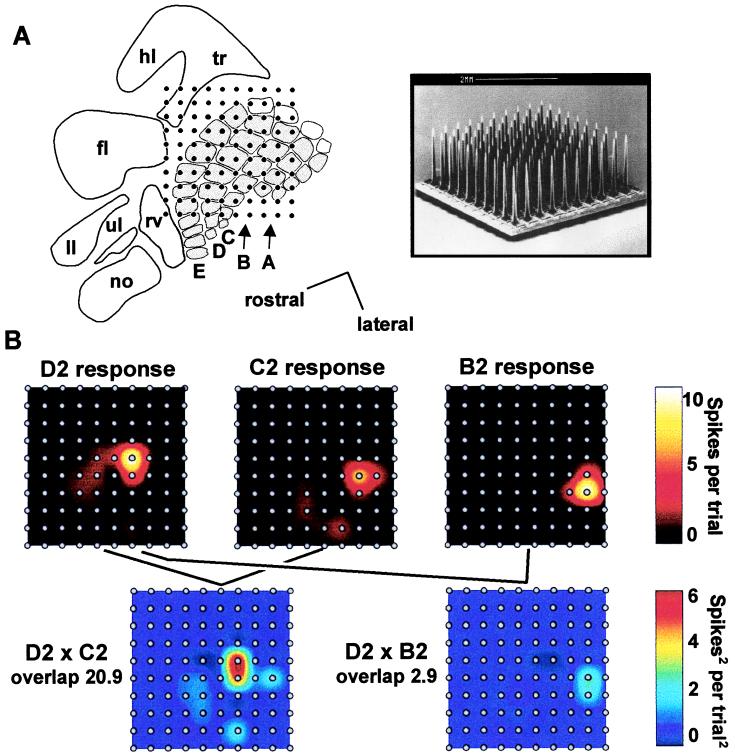
Figure 3.
Physiological experiment. (A) (Left) Cortical position of the microelectrode array in a typical experiment. no, nose; ll, lower lip; ul, upper lip; tr, trunk; hl, hindlimb; fl, forelimb; rv, rostral vibrissae. Barrel rows A–E are indicated; caudal barrels (α, β, γ, δ) are white. (Right) Scanning electron micrograph of 10 × 10 electrode array (courtesy of Bionic Technologies). Scale in both figures is given by the interelectrode distance, 400 μm. (B) (Upper plots) Cortical activity maps for three whiskers. Interpolation was performed between the response values at each electrode. (Lower plots) Overlap between two pairs of activity maps.
The data acquisition system (Bionic Technologies) consisted of a 100-channel amplifier (gain = 5,000, filtered at bandpass 250–7,500 Hz), digital signal processor (30,000 samples/s), and a Pentium personal computer. In off-line analysis, activities of the single-units on each channel were summated. Whiskers were stimulated 3 mm from the base by a piezoelectric wafer (Morgan Matroc, Bedford, OH), which was driven by voltage pulses (A.M.P.I., Jerusalem). The stimulus, an up-down step function of 80-μm amplitude and 100-ms duration, was delivered to each whisker 60 times at 1 or 2 Hz. At each electrode, response per trial was computed as the number of spikes recorded during the 100-ms whisker deflection minus the number recorded during the 100-ms interval preceding whisker deflection, averaged over stimulus trials. The set of responses to a given whisker, across all 100 electrodes, constitutes that whisker’s cortical response pattern. The overlap between two cortical response patterns was quantified by using the formula Σxiyi, where xi and yi are the responses on channel i to two different whiskers, x and y, respectively.
The depth and location of the array placement were examined in histological sections. Subjects were perfused with saline and 4% paraformaldehyde. After postfixation in 20% sucrose, the cortex was removed, flattened, and frozen. The block of tissue was cut in 40-μm sections in the tangential plane and was stained with cresyl violet.
RESULTS
Gap-Crossing Behavior.
In the group whose prosthetic whisker was attached to the stub corresponding to the trained whisker (Row0Arc0), three subjects crossed on the first test trial and one on the second trial. One-tailed paired Student’s t test showed that this performance was not significantly different (P = 0.39) from that of the final training session. This was the only group of rats immediately able to use the prosthetic whisker to guide the behavior. Naïve rats (trained with no whiskers) reached criterion with the “new” prosthetic whisker in an average of 6.25 trials (Fig. 2C). For the other groups, the speed of gap-cross reacquisition—compared with the naïve group—reveals the “transfer” of previous learning. Our main finding is that the degree of transfer was determined by the location of the prosthetic whisker (Fig. 2C). The amount of transfer decreased as the distance between trained and prosthetic whisker increased. Indeed, there was no transfer from previous training if the prosthetic whisker was attached two or more positions from the trained whisker, suggesting that the rats had to learn de novo to use the new whisker in guiding the behavior.
Additional statistical analyses confirmed these observations. Comparing gap-crossing reacquisition time, an analysis of variance testing planned orthogonal contrasts (7) revealed that the naïve group was significantly different from the six whisker-trained groups [F (1, 21) = 17.25, P < 0.001] whereas the Row0Arc0 group was significantly different from the other five whisker-trained groups [F (1, 21) = 13.19, P < 0.005]. Rats with whisker displaced by two positions (either along row or arc) were slower to reacquire the task than rats with whisker displaced by one position [ANOVA, F (1, 21) = 8.56, P < 0.01], and rats with whisker displacement up or down an arc reacquired the task more slowly than did rats with displacement along a row [ANOVA, F (1, 21) = 4.60, P < 0.05]. There was no interaction between direction (row versus arc) and distance (one versus two positions) [F (1, 21) < 1, P > 0.85]. Pairwise Student’s t tests confirmed that the Row0Arc0 group was significantly different from each of the five groups in which the prosthetic whisker was attached to a “nontrained” stub (P < 0.025 for each test). No group with the prosthetic whisker displaced two or more positions from the trained whisker was significantly different from the naïve group (Row2Arc0, P = 0.084; Row0Arc2, P = 0.502; Row2Arc2, P = 0.261).
Representation of the Whiskers in Cortex.
We hypothesize that the degree of “learning transfer” between two whiskers in the gap-crossing task might depend on the spatial relationship between the cortical representations of the two whiskers. To evaluate this proposal, we recorded neural activity from 10 × 10 microelectrode arrays and formed cortical response maps associated with stimulation of individual whiskers. Because interelectrode spacing (400 μm) matches typical barrel-column diameter (Fig. 3A), each barrel-column underlying the array was sampled by at least one electrode. In typical response maps (Fig. 3B, upper plots), it is evident that stimulation of one whisker strongly activated the topographically corresponding barrel-column and more weakly activated surrounding barrel-columns. To determine whether two whiskers engaged some cortical territory in common or, alternatively, engaged completely separate cortical territories, we calculated the degree of overlap between the two 100-channel cortical response patterns. Overlap at a single electrode was computed by multiplying this electrode’s response magnitudes to the two different stimuli (see Materials and Methods). Plotting such data as a map reveals the strength of convergence at each electrode as well as the sites where the representations of two whiskers converge (Fig. 3B, lower plots). As an index of total overlap, the single-channel overlap values were summated across the entire array. Thus, an overlap value of 0 means that no cortical site was activated by both whiskers. In the illustrated case, the overlap between the response maps for whiskers D2 and C2 (20.9 spikes2/trial2) was higher than the overlap between the response maps for whiskers D2 and B2 (2.9 spikes2/trial2) (Fig. 3B, lower plots).
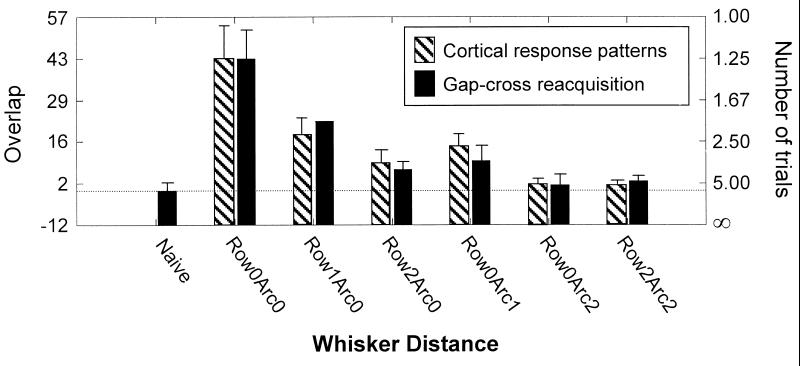
For the entire experimental sample, values of overlap were classified according to the topographic relation between the two whiskers (e.g., Row0Arc1 for whiskers D2 and C2 and Row0Arc2 for whiskers D2 and B2). The number of cortical response pattern comparisons per distance category was Row0Arc0–57; Row1Arc0–35; Row0Arc1–32; Row2Arc0–17; Row0Arc2–18; Row2Arc2–18. In agreement with the pattern of behavioral results, the overlap between cortical activity patterns decreased systematically as a function of the distance between the two whiskers considered. Whiskers displaced by two positions (either along row or arc) elicited cortical activity patterns with lower overlap than did whiskers displaced by one position [ANOVA, F (1, 170) = 8.0, P < 0.01]. The close match between the behavioral and physiological data is highlighted by plotting both gap-cross reacquisition speed (number of trials required) and overlap between cortical activity patterns as a function of row and arc position (Fig. 4). The correlation coefficient between the two data sets is high (r = 0.98). To confirm the robustness of the correlation, many indices of cortical response were examined (e.g., response over various post-stimulus time intervals, peak firing rate); in addition, the overlap between cortical response patterns was measured in various ways (e.g., angle and euclidean distance). Across all indices, correlation with the behavioral dataset was high (r = 0.94–0.98). These observations suggest that the behavioral and physiological experiments uncovered the same neural substrate—the extent to which a common set of cortical columns processes information from two different whiskers.
Figure 4.
Relationship between behavior and cortical sensory responses. Speed of gap-cross reacquisition and response pattern overlap are plotted for different whisker locations. Gap-cross reacquisition is plotted by using an inverse scale. Error bars: standard error of the mean.
DISCUSSION
The brain’s sensory processing systems are modified during perceptual learning (8, 9). If the network that participated in learning and remembering the task were evenly or globally distributed, rats would have utilized the prosthetic whisker to gap-cross without delay, even if it were attached far from its original site. Instead, we found that the amount of benefit obtained from previous training was determined by the spatial arrangement of the sensory receptors. The observed pattern thus suggests that the memory trace related to a specific sensory signal is distributed according to a precise topography. Of course, we are not arguing that the memory trace for the entire behavior is topographically localized. Gap-crossing involves a complex interaction of sensory systems (tactile, proprioceptive, vestibular), integrated with motivational and motor systems, all of which are widely distributed across cortical and subcortical centers. Nonetheless, by isolating one component of the task (the use of whisker information to detect the goal platform), we uncovered discrete aspects of learning that are locally distributed.
The primary somatosensory cortex is the best candidate as the neural substrate for the topography of learning. This region is organized as a map of barrel-columns (3, 4, 10); one whisker from the contralateral snout provides the principal input to each barrel (layer IV of the barrel-column). Somatosensory cortex is essential for learning and performing the task; ablation of this field abolishes the rat’s gap-crossing ability (5). Finally, somatosensory cortex conveys sensory information to multiple motor centers likely to be involved in gap-crossing (11–13).
The available evidence is consistent with a model in which modifications in communication between the layers of barrel-columns, and between neighboring barrel-columns, mediate somatosensory cortical changes during learning (14). It is known that the supragranular layers of barrel cortex (layers I–III) have an essential function in learning: Rats are not able to learn to use their whiskers to gap-cross after selective ablation of the supragranular layers. However, previously trained rats can continue to gap-cross after ablation of those layers (15), signifying that layers IV–VI by themselves can support the behavior once learning has taken place. That layers IV–VI are essential in trained rats is shown by the complete loss of gap-crossing ability after ablation of all layers of barrel cortex (7). During the course of learning, the supragranular layers appear to mediate some transformation in the way that layers IV–VI integrate and distribute sensory information, just as they mediate experience-dependent receptive field modification in layers IV–VI under different conditions (16, 17). The model postulates that modification of layer V output is particularly important because this represents a channel for relaying barrel cortex information to motor centers (11–13).
According to this model, in the horizontal dimension, the neural modifications during initial learning of the task are localized to the set of barrel-columns engaged by the intact whisker during training. During subsequent testing with a prosthetic whisker, a second group of barrel-columns now processes the sensory information. The transfer of previous learning is dictated by the degree to which the second group of barrel-columns overlaps the “trained” group of barrel-columns and thereby participates in the original learning. How widely distributed are the modifications, and what determines their spatial extent? The behavioral data revealed a transfer of initial learning whenever the prosthetic whisker was attached to a stub neighboring the trained whisker. The degree of transfer was higher when the prosthetic whisker was in the same row as the original whisker than when it was in the same arc. Thus, the critical zone of cortical modification, extending in a radius of 1–2 barrel-columns around the barrel-column of the trained whisker and biased in the row-direction, coincides with the territory known to be targeted by axons projecting from the supragranular and infragranular neurons of a single barrel-column (18–20). We therefore suggest that synaptic modifications are concentrated in the barrel-column of the trained whisker and in those surrounding barrel-columns encompassed by, and activated by, the direct intracortical projections of the central barrel-column. Learning can be later transferred to any whisker whose afferent signal is processed within this zone.
To summarize, in the rat whisker system, the neural changes that underlie learning about a specific sensory signal appear to be confined to circumscribed regions of cortex according to the topographic organization of the sensory cortical map. How general is this principle? In primate somatosensory cortex, the hand is represented with a high degree of topographic precision (21). In certain tasks the transfer of learning across skin location is governed by the distance between training site and testing site (22), consistent with the present findings. If the principal of “topographic learning” is also applicable to visual cortex, one might expect that sensory-perceptual learning in some tasks could be restricted to the specific features of the training stimulus. In primate visual cortex, three features of visual stimuli are distributed horizontally across the cortical territory: ocularity, retinotopic location, and orientation (23). Psychophysical experiments in humans and monkeys have indeed shown that a visual task must be relearned after a shift in any of these three parameters (24, 25).
Not every kind of sensory learning is specific to the spatial properties of the stimulus: Some types of complex sensory learning are widely transferred, even across sensory modalities (26). It is tempting to speculate that, for sensory tasks that lead to nontopographic learning, the neural processing of the relevant sensory information takes place principally in a nontopographically organized cortical area.
Acknowledgments
We thank S. Giannotta for technical assistance, G. Mirabella and I. Erchova for participation in some physiological experiments, and A. Treves and J. Nicholls for fruitful discussions. This work was supported by the Whitehall Foundation, the National Institutes of Health, the Telethon Foundation, and the Australian Research Council.
References
- 1.Lashley K S. Psychological Mechanisms in Animal Behavior: Society of Experimental Biology Symposium, No. 4. Cambridge, U.K.: Cambridge Univ. Press; 1950. [Google Scholar]
- 2.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Woolsey T A, Van der Loos H. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 4.Welker C. J Comp Neurol. 1976;166:173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- 5.Hutson K A, Masterton R B. J Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- 6.Guic-Robles E, Jenkins W M, Bravo H. Behav Brain Res. 1992;48:145–152. doi: 10.1016/s0166-4328(05)80150-0. [DOI] [PubMed] [Google Scholar]
- 7.Hays W L. Statistics for the Social Sciences. Rinehart and Winston, New York: Holt; 1972. [Google Scholar]
- 8.Jenkins W M, Merzenich M M, Ochs M T, Allard T, Guic-Robles E. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Leone A, Torres F. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 10.Simons D J. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- 11.Li X G, Florence S L, Kaas J H. Somatosens Mot Res. 1990;7:315–335. doi: 10.3109/08990229009144711. [DOI] [PubMed] [Google Scholar]
- 12.Mercier B E, Legg C R, Glickstein M. Proc Natl Acad Sci USA. 1990;87:4388–4392. doi: 10.1073/pnas.87.11.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izraeli R, Porter L L. Exp Brain Res. 1995;104:41–54. doi: 10.1007/BF00229854. [DOI] [PubMed] [Google Scholar]
- 14.Diamond M. E., Petersen R. S. & Harris J. A. (1999) J. Neurobiol., in press. [PubMed]
- 15.Friedberg M H. Ph.D. thesis. Providence, RI: Brown Univ.; 1991. [Google Scholar]
- 16.Diamond M E, Huang W, Ebner F. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Armstrong-James M, Rema V, Diamond M E, Ebner F F. J Neurophysiol. 1998;80:3261–3271. doi: 10.1152/jn.1998.80.6.3261. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo K L, McCasland J S, Woolsey T A. Exp Brain Res. 1990;82:247–253. doi: 10.1007/BF00231244. [DOI] [PubMed] [Google Scholar]
- 19.Bernardo K L, McCasland J S, Woolsey T A, Strominger R N. J Comp Neurol. 1990;291:231–255. doi: 10.1002/cne.902910207. [DOI] [PubMed] [Google Scholar]
- 20.Hoeflinger B F, Bennett-Clarke C A, Chiaia N L, Killackey H P, Rhoades R W. J Comp Neurol. 1995;354:551–563. doi: 10.1002/cne.903540406. [DOI] [PubMed] [Google Scholar]
- 21.Kaas J H. Brain Res Bull. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 22.Recanzone G H, Jenkins W M, Hradek G T, Merzenich M M. J Neurophysiol. 1992;67:1015–1030. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- 23.Hubel D H, Wiesel T N. Proc R Soc Lond Ser B. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- 24.Fahle M. Perception. 1994;23:411–427. doi: 10.1068/p230411. [DOI] [PubMed] [Google Scholar]
- 25.Karni A, Bertini G. Curr Opin Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 26.Nagarajan S S, Blake D T, Wright B A, Byl N, Merzenich M M. J Neurosci. 1998;18:1559–1570. doi: 10.1523/JNEUROSCI.18-04-01559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]