Abstract
Immunization with a T cell–dependent antigen elicits production of specific memory B cells and antibody-secreting cells (ASCs). The kinetic and developmental relationships between these populations and the phenotypic forms they and their precursors may take remain unclear. Therefore, we examined the early stages of a primary immune response, focusing on the appearance of antigen-specific B cells in blood. Within 1 wk, antigen-specific B cells appear in the blood with either a memory phenotype or as immunoglobulin (Ig)G1 ASCs expressing blimp-1. The memory cells have mutated VH genes; respond to the chemokine CXCL13 but not CXCL12, suggesting recirculation to secondary lymphoid organs; uniformly express B220; show limited differentiation potential unless stimulated by antigen; and develop independently of blimp-1 expression. The antigen-specific IgG1 ASCs in blood show affinity maturation paralleling that of bone marrow ASCs, raising the possibility that this compartment is established directly by blood-borne ASCs. We find no evidence for a blimp-1–expressing preplasma memory compartment, suggesting germinal center output is restricted to ASCs and B220+ memory B cells, and this is sufficient to account for the process of affinity maturation.
The immune response to protein-containing antigen elicits quiescent memory B cells and long-lived antibody-secreting cells (ASCs; for review see reference 1). Memory B cells typically express Ig isotypes downstream of IgM such as IgG or IgA, carry somatic mutations within their variable (V) region genes that preferentially exchange amino acids within the CDR, and differentially express several cell surface markers relative to naive B cells (2–4). Although memory cells show some preference for localization at the site of their formation, they also recirculate (5) and mount a vigorous response to the original antigen after reexposure. In contrast, the population of long-lived ASCs maintains serum titres of high affinity antigen-specific antibody over extended periods (6–8), while neither recirculating nor responding to antigen in any discernible way (9). These cells also differ phenotypically from naive B cells, usually through the down-regulation of surface markers such as CD19, B220, and MHCII (7, 9). Although memory B cells and bone marrow ASCs have been identified as having a common origin in the germinal center (GC), the means by which these compartments are established from B cells that emigrate from the GC remains poorly understood (10, 11).
One model for the formation of the memory compartment (12) proposes that high affinity antibody secreted by GC-derived ASCs feeds back into the GC reaction, competing with centrocytes for binding to antigen localized on the surface of follicular DCs. This infers that formation of a high affinity population of ASCs (now known to reside primarily in the bone marrow) should precede formation of the memory compartment, which agrees with the limited data available on the kinetics of the formation of these two post-GC populations in spleen (10, 11, 13).
The formation of the bone marrow plasma cell populations is also ill-defined. Although it is clear that the vast majority of ASCs in bone marrow are GC derived (10, 11), the means by which they are selected for this fate and the processes of their migration and persistence remain unclear. Two proposals to explain ASC-precursor emigration from the GC are as follows: (a) BCR cross-linking in the GC generates a signal that commits centroblasts to ASC differentiation (14); and (b) bone marrow ASC formation has a post-GC period of clonal selection (11). Neither of these models address the form that GC cells committed to being bone marrow ASCs assume during migration. The bone marrow compartment could be seeded by precursor cells that differentiate upon arrival or alternatively by functional ASCs. Evidence in support of both proposals has been reported, although not with universal acceptance (15). In mice, nonsecreting plasma cell precursor populations have been identified that derive from the GC, retain surface Ig yet lose B220, and, in a recent paper, show an absolute dependence on the plasma cell commitment factor, Blimp-1 (16–19). Alternatively, ASCs have been detected in blood after secondary immunization (20–22), which in humans show a phenotype suggesting they are part of a developmental program ending in bone marrow ASCs (21, 23).
An analysis of GC output from a defined starting point may assist in clarifying the origin and development of the post-GC, effector B cell populations. In this paper, we examine GC output in mice after a single immunization by assessing the appearance and composition of antigen-specific B cells in the blood. We identify cells with characteristics of either memory or ASC lineages in blood and examine their cellular and molecular characteristics. These data suggest both the criteria involved in the selection of GC B cells for emigration and the possible fate of such émigrés. These results have important implications for the regulation of the GC reaction, the mechanism of immunological memory formation, and the establishment of the bone marrow ASC compartment.
Results
Antigen-specific B cells in blood appear early, persist, and are B220+
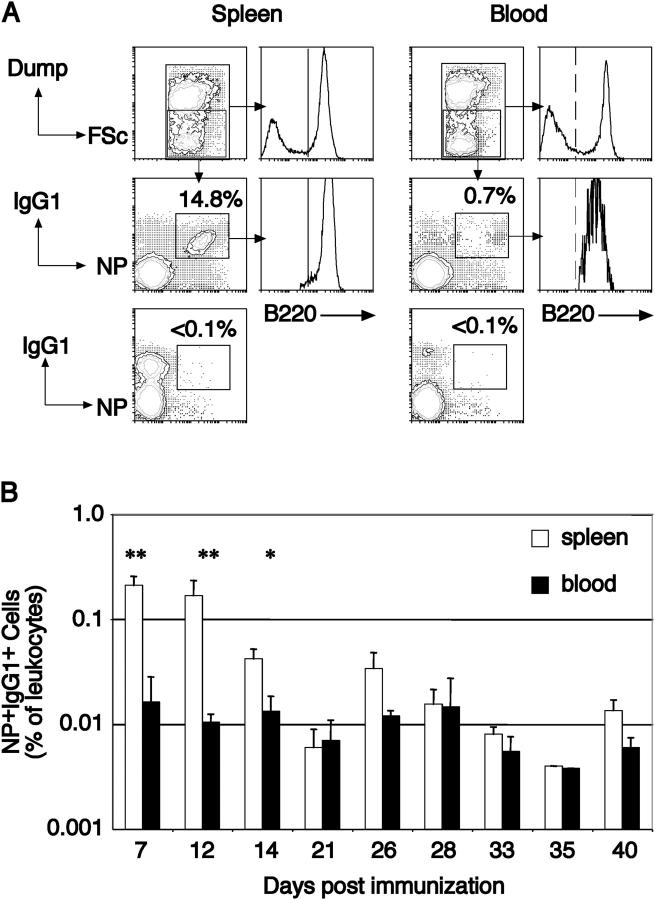
To characterize the development of the post-GC antigen-specific B cell populations, we examined blood for the presence of cellular intermediaries. Animals were examined initially 7 d after primary immunization and had blood and spleens harvested, and leukocytes were recovered and partitioned into populations of cells that did and did not stain with a cocktail of antibodies comprising CD138, Gr-1, F4/80, and both IgM and IgD (Fig. 1 A). Among the negative population, cells were identified that expressed IgG1 and bound the immunizing hapten, (4-hydroxy-3-nitrophenyl)acetyl (NP), coupled in this case to allophycocyanin. Small populations of such cells were seen in both spleen and blood (Fig. 1), and their presence was found to be dependent on immunization with NP-containing antigen. Upon examination, the NP+IgG1+ cells were found to be uniformly positive for B220, in contrast with previous analyses (16). Interestingly, the level of B220 on blood NP-specific cells was consistently lower than that of naive B cells and NP-specific IgG1+ B cells in spleen, although the significance of this observation remains unclear. Therefore, it appears that, with the immunization protocol used in this work, the vast majority of the IgG1+ antigen-specific cells express detectable levels of B220. We examined the kinetics of the formation of B220+NP+IgG1+ B cells in blood after a single immunization, determining their time of appearance and persistence (Fig. 1 B). At regular intervals after primary i.p. immunization, animals were killed and their spleens and blood were examined. Before day 6, no NP-binding IgG1+ cells were detectable in either blood or spleen, but having appeared, antigen-specific B cells remained detectable in blood for the next 5 wk, the duration of the analysis. The frequency of these B cells in blood varied little over the experimental period, suggesting either a stable population or continued output during the time course of the experiment. Although in the early stages of the response, B220+NP+IgG1+ B cells were significantly more common in spleen than in blood, by 21 d after immunization, this difference was no longer apparent (Fig. 1 B). Collectively, these data demonstrate the rapid appearance and subsequent persistence of isotype-switched, antigen-specific B220+ B cells in blood after a single i.p. immunization.
Figure 1.
Identification of antigen-specific B cells in blood. (A) Leukocytes recovered from the spleen and blood of mice immunized 7 d previously were partitioned using flow cytometry into those expressing and not expressing (boxed) a collection of markers, referred to as the Dump-channel: IgM, IgD, Gr-1, and F4/80. Among negative cells, those that had switched isotype to IgG1 and were capable of binding the immunizing hapten were detected by IgG1-specific anti-sera and NP coupled to the fluorescent protein allophycocyanin, revealing a predominant IgG1+ NP-binding population (boxed). Examining the IgG1+NP+ cells in both spleen and blood revealed these cells to be essentially all B220+, shown in comparison to the B220 distribution on all leukocytes (histograms). Mice immunized with KLH in alum do not develop IgG1+NP+ cells in either blood or spleen (lower contours, already gated as Dump−). (B) The kinetics of B220+IgG1+ NP-binding B cells in blood and spleen after primary immunization. Blood and spleen from individual mice were collected at the indicated intervals after a single i.p. immunization and the proportion of B220+ NP-binding IgG1+ cells determined by flow cytometry as depicted in A. The plot shows the average of each tissue with the standard deviation. n ≥ 3 mice for each time point. Significant differences as calculated by Student's t test are indicated. *, P < 0.01; **, P < 0.001.
B220+ antigen-specific B cells in blood have a memory phenotype
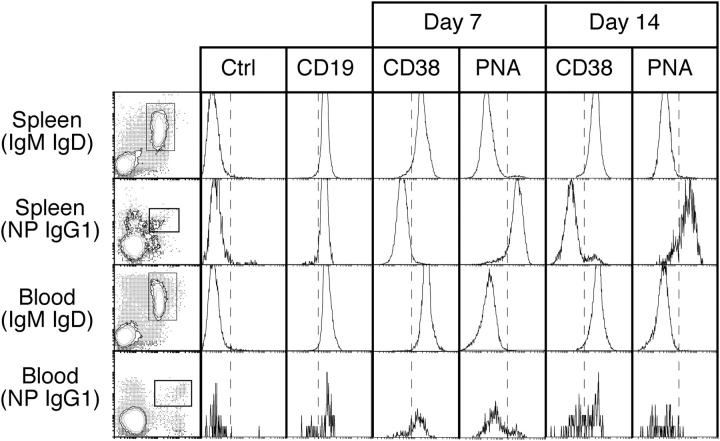
Next, we examined the expression of additional cell surface markers on NP+IgG1+ cells in blood. At days 7 and 14 after immunization, B220+ cells were purified from blood and spleen using B220-specific magnetic bead separation, which consistently produced >99% purity as defined by CD19 expression. The purified B220+ cells were stained with IgM and IgD to exclude naive B cells, IgG1 and NP-allophycocyanin, to reveal the antigen-specific B cells and an additional reagent to examine the expression level of various markers in comparison with naive B cells from the same location. The B220+NP+IgG1+ cells in spleen and blood expressed CD19 at levels equal to naive B cells at all times. However, expression of CD38 and binding of the lectin PNA varied between locations. On days 7 and 14, >80% of B220+NP+IgG1+ cells in spleen had a GC phenotype (Fig. 2), defined as CD38lo and PNAhi (13). However, in blood, the majority of B220+NP+IgG1+ cells at these same time points had the reciprocal memory phenotype, CD38hi and PNAlo (Fig. 2), a phenotype retained at later time points. Furthermore, the NP-specific IgG1+ cells in both spleen and blood were physically larger than their naive B cell counterparts as measured by forward light scatter, consistent with antigen-mediated activation (unpublished data). Neither CD138 nor CD11c were detected on B220+NP+IgG1+ cells in the blood, but these cells did express CD86 at low levels (unpublished data). We conclude that the blood is enriched from very early after immunization for isotype-switched antigen-specific B cells with cell surface characteristics of memory B cells.
Figure 2.
The cell surface phenotype of B220+ NP-binding IgG1+ cells in blood and spleen 7 and 14 d after immunization. B220+ cells were purified mechanically from spleen and blood and stained for surface IgM, IgD, IgG1, and NP. The stains of spleen and blood (IgM, IgD)+ B cells shown in the left column depict before separation, whereas those of NP+IgG1+ B cells depict after separation. Boxed areas indicate the cells analyzed for the parameters depicted by the histograms. The fourth parameter in these analyses is indicated above each column. The dashed line provides a point of reference for comparing samples. The cells were recovered from tissues pooled from two to four mice, and these data are representative of three separate experiments. Ctrl, isotype control antibody.
NP+IgG1+ blood B cells accumulate VH gene somatic mutations
A characteristic of GC-derived B cells is the modification of Ig V region genes by somatic mutation and the subsequent enrichment for amino acid exchanges that enhance affinity. To assist in identifying the developmental origin of the blood NP+IgG1+ cells, we amplified rearranged VH186.2-Cγ1 cDNA from single B220+NP+IgG1+ cells sorted from blood. These PCR products were sequenced to verify the identity of the VH gene and to define the frequency and distribution of somatic mutations (Table I). Blood sequences from different times in the response were compared with the equivalent VH sequences obtained from NP-specific B cells isolated from spleen over the same period. First, we note that the overall recovery of VH-Cγ1 products from single cell cDNAs was essentially equal in blood (52 ± 15.5%) and spleen (58 ± 12.6%). Second, the proportion of these PCR products subsequently confirmed to contain VH186.2 was again equal between blood (84 ± 7.3%) and spleen (80 ± 9.2%). These data, shown for individual time points in Table I, independently support the flow cytometric identification of the NP-binding population in blood as being B cells and being NP specific. We examined the sequences for the appearance, distribution, and frequency of somatic mutations. At day 7, we found an average of 1.8 mutations/VH gene in spleen NP+IgG1+ B cells. However, the corresponding blood sample contained significantly fewer mutations both on average (0.1 mutations/VH gene) and in the proportion of mutated sequences (Table I). Cells recovered at days 10 and 14 showed an increasing frequency of mutations in both blood and spleen, although the frequency in blood was typically less than that of contemporaneous spleen (Fig. 3 A and Table I). By day 28, the average frequency of mutations was approximately equal in both groups. The somatic mutations in the VH genes of antigen-specific B cells in blood indicates a GC origin, whereas the increasing frequency of both mutation and proportion of mutated sequences in a numerically stable population (Fig. 1 B) indicates turnover of the population.
Table I.
VH gene somatic mutation among NP-specific B cells in blood and spleen
| Daya | Tissue | PCR+ b | VH186.2c | Mutation (average)d |
Germline | Position 33 |
|---|---|---|---|---|---|---|
| % | % Y → L | |||||
| 7 | spleen | 21/45 | 16/21 | 1.8 | 25 | 0 |
| blood | 22/35 | 21/22 | 0.1 | 95 | 5 | |
| 10 | spleen | 55/80 | 50/55 | 2.7 | 12 | 50 |
| blood | 54/188 | 45/54 | 1.2 | 47 | 37 | |
| 12 | spleen | ND | ND | ND | ND | ND |
| blood | 27/40 | 24/27 | 2.4 | 33 | 58 | |
| 14 | spleen | 35/80 | 30/35 | 4.4 | 3 | 67 |
| blood | 37/97 | 29/37 | 2.3 | 7 | 48 | |
| 28 | spleen | 43/60 | 29/43 | 5.8 | 7 | 45 |
| blood | 28/45 | 21/28 | 4.4 | 28 | 48 |
Mice were killed at the indicated times after immunization and individual B220+NP+IgG1+ cells were sorted by flow cytometry. Three to eight mice were used per experiment with samples pooled before sorting. Data are from at least two experiments per time point.
The number of successful PCR amplifications of VH186.2-Cγ1 rearrangements from single cell cDNA as a fraction of the number of reactions.
The number of amplified VH186.2-Cγ1 rearrangements confirmed to contain the unique hexamer as a fraction of all sequences.
Average mutation frequency, percent germline sequences, and percent of sequences containing tryptophan to leucine exchange at VH position 33 are for all VH186.2 sequences recovered.
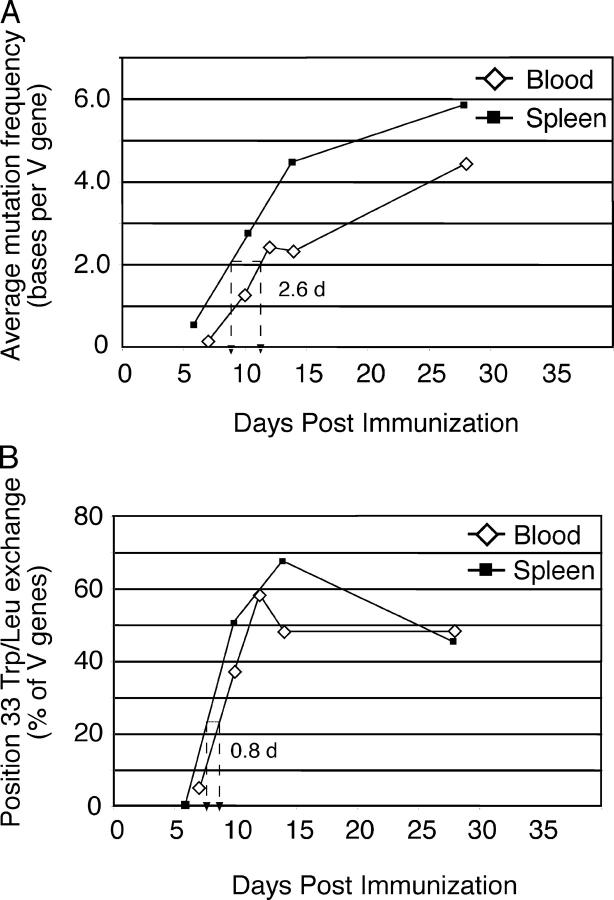
Figure 3.
Evidence for somatic mutation and affinity maturation in blood NP+IgG1+ B cells. Single NP-binding IgG1+ B cells were isolated from blood and spleen at the indicated times (for details, see Table I). Analysis of those VH-Cγ1 rearrangements using VH186.2 provided the values depicted in this figure. (A) The average mutation frequency of VH genes at the indicated times is plotted against the time after immunization. The dashed line indicates the time difference between the two populations required to reach an average of 2.0 mutations per VH gene. Accumulation of mutations in blood cells is delayed by ∼2.5 d compared with spleen. (B) The proportion of VH sequences in blood and spleen NP-specific IgG1+ B cells containing a position 33 Y → L exchange as a function of time. Unlike the mutation frequency, cells bearing affinity-enhancing mutations are almost as common in blood as in spleen at each time point. In both plots, spleen values use square symbols, whereas blood uses diamonds. Cells were recovered from tissues pooled from ≥6 mice at each time point.
Assessing the location of specific mutations in the VH genes from blood and spleen gave further insights into the development of these B cells (Fig. 3 B and Table I). We found that the tryptophan to leucine exchange at position 33 of the heavy chain that typifies affinity maturation in the anti-NP response of C57BL/6 mice (24) appeared in blood B cells at approximately day 10 and increased in representation over time until it reached a plateau by approximately day 28. The frequency of this mutation in spleen NP-specific B cells was consistently similar to that in the blood population, increasing in parallel early and reaching a similar plateau late in the response (Fig. 3 B). However, relative to the overall mutational load, the position 33 replacement was overrepresented in blood compared with spleen, suggesting an enrichment for such cells in this location.
B220+NP+IgG1+ cells respond to CXCL13 but not CXCL12
The appearance of NP-specific B cells in blood early after immunization raises questions concerning their fate. Such cells could either be memory or ASC precursors. A feature of B cell differentiation during an immune response is an alteration in chemokine responsiveness. Cells within the ASC lineage acquire responsiveness to CXCL12 (25, 26), which distinguishes them from cells in the GC (27). Therefore, we sought to use chemokine responsiveness as a marker of B cell differentiation. Leukocytes were prepared from blood and spleen of mice immunized 14 d previously and added to the upper well of Transwell chambers. The level of chemotaxis of B cell subsets toward CXCL12 and CXCL13 was determined using flow cytometry to assess the number of cells that had migrated through the membrane. Thus, migrating cells were assessed for the presence of B220+IgG1+NP+ cells and this number was calculated as a percent of such cells originally placed in the upper well (Table II). We observed strong chemotaxis of antigen-specific IgG1+ B cells toward CXCL13 and essentially none toward CXCL12 (Table II). The propensity of blood-borne NP-specific B cells to migrate toward the follicular chemokine and a corresponding lack of attraction to the bone marrow chemokine suggests a migratory route that returns these B cells to the follicles of secondary lymphoid organs.
Table II.
Chemotaxis of blood-borne NP-specific B cells
| Migration as a percentage of inputa
|
||||
|---|---|---|---|---|
| Chemokineb | B220+IgMIgD+ | B220+IgMIgD− | B220+NP+IgG1+ | Non–B cells |
| % | % | % | % | |
| None | 0.09 ± 0.01 | 0.70 ± 0.14 | 0 | 0.95 ± 0.07 |
| CXCL13 | 8.8 ± 1.6 | 2.9 ± 0.7 | 15.0 ± 4.1 | 1.8 ± 0.3 |
| CXCL12 | 7.8 ± 6.1 | 4.8 ± 4.0 | 0.6 ± 0.8 | 5.3 ± 3.0 |
Cell populations were enumerated by flow cytometry before and after migration. The number migrating through the membrane is expressed as a fraction of input cells of that phenotype. Data are from two experiments each using four wells (CXCL13) and two wells (CXCL12).
CXCL13 present at 800 ng/mL; CXCL12 present at 400 ng/mL.
Differentiation potential of blood leukocytes upon transfer
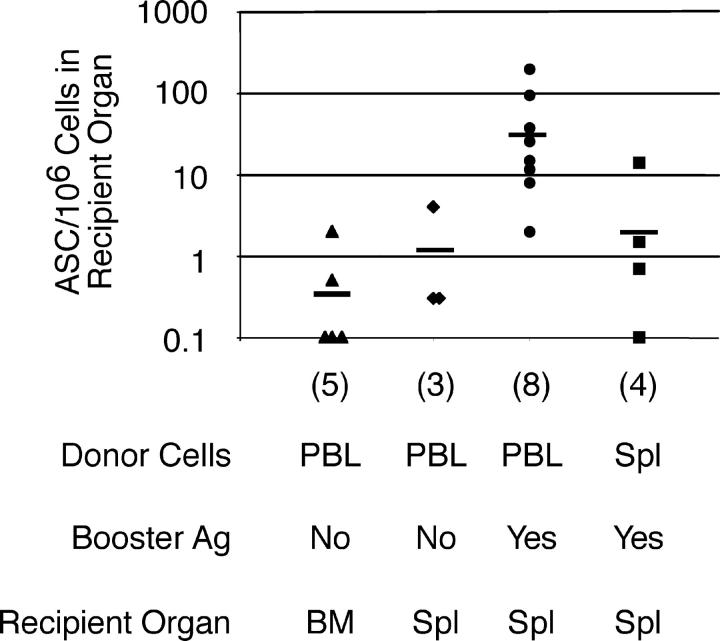
Next, we assessed the developmental potential of blood-borne antigen-specific B cells after transfer into recipients with or without antigen boost. These experiments used CD19 −/− mice as recipients, as they do not produce IgG1 ASCs in the bone marrow after immunization with antigen in alum (references 28, 29 and unpublished data). Transfer of blood leukocytes from mice immunized 14 d previously with NP-KLH in alum resulted in few ASCs appearing in the recipients' bone marrow or spleen 4 d later (Fig. 4). We recovered an average of 0.5 NP-specific IgG1 ASCs per 107 recipient bone marrow cells (Fig. 4), equating to ∼24 such ASCs in the whole animal, given that adult C57BL/6 mice contain 47 × 107 bone marrow cells (30), with an additional 50 such ASCs in spleen (Fig. 4). Because the 107 donor cells contained 103 B220+NP+IgG1+ B cells based on these cells being 0.01% of the population (Fig. 1 B), these data imply that <10% of the B220+ NP-specific population spontaneously differentiated into ASCs if this were the ASC precursor population. The possibility that the ASCs appearing in the recipient arose from an alternative precursor population in blood cannot be excluded (see next paragraph). Next, we examined the potential of blood B cells from NP-KLH immunized mice to respond to reexposure to antigen. In this case, CD19 −/− recipients were primed with human serum albumin (HSA) in alum 14 d before cell transfer from NP-KLH immunized donors. Recipients were boosted with NP-HSA at the time of transfer and, 4 d later, the frequency of NP-specific IgG1 ASCs was determined in the recipients' spleens (Fig. 4). This experiment revealed memory potential in the donor blood leukocytes, in that we observed a significant expansion in the number of NP-specific IgG1 ASCs in the spleens of the recipients after antigen boost (Fig. 4). Collectively, these experiments demonstrate the presence of functional memory B cells in the blood of mice immunized 14 d previously, yet also indicate that there are rare cells in the blood capable of generating ASCs in the bone marrow without deliberate stimulation.
Figure 4.
Blood leukocytes from immunized mice respond to antigen in the presence of T cell help. Leukocytes were purified from the pooled blood or spleen of six mice immunized 14 d previously with NP-KLH. 107 of these cells were transferred to mice previously primed with a single injection of HSA in alum. Recipients were either left untreated or boosted with NP-HSA as indicated. 4 d after transfer, recipients were killed and the frequency of NP-specific IgG1 ASCs determined in bone marrow or spleen are indicated. The horizontal bar shows the average for each condition. The resolution of this assay was 0.1 IgG1 ASCs per 106 plated cells. The number of recipients assessed for each condition is indicated. No NP- specific IgG1 ASCs were detected in either the absence of transferred cells or after transfer of leukocytes from naive mice (not depicted).
Affinity-matured ASCs appear in blood
The apparently limited potential of blood leukocytes to generate bone marrow ASCs upon transfer, coupled with the lack of CD138 expression and chemokine sensitivity of the B220+NP+IgG1+ population, made it unlikely that these cells were committed to the bone marrow ASC compartment. This left the origin of the bone marrow ASCs unclear. This issue was investigated using a recently developed reporter mouse that has GFP targeted to the blimp-1 locus (31). In such blimp-1 gfp/+ mice, cells committed to ASC differentiation as defined by expression of blimp-1 (32) are detectable by flow cytometry for GFP expression in both spleen and bone marrow (Fig. 5 A). After immunization, cells sorted from these locations on the basis of GFP contain NP-specific IgG1 ASCs (Fig. 5 C and reference 31). Therefore, we examined blood at various times after immunization for the presence of ASCs using blimp-1 gfp as a marker. Such a population, expressing simultaneously blimp-1 gfp and CD138, was found in unimmunized mice and changed little in frequency after immunization (Fig. 5, A and B). Identification of these cells in blood allowed us to examine the composition of the population with respect to NP-specific ASC activity and affinity. Cells sorted as blimp-1 gfp CD138+ from mice immunized 0, 7, or 14 d previously were plated in ELISPOT assays to enumerate NP-specific IgG1 ASCs. Such activity was found within the sorted populations in spleen, blood, and bone marrow only after immunization (Fig. 5 C and reference 31). In spleen, the relative and absolute frequency of IgG1+ NP-specific ASCs, both in total and high affinity, declined between 7 and 14 d after immunization, in agreement with previous papers (7). However, the proportion of ASCs in spleen secreting high affinity IgG1 increased from 30 to 60% over this period. In contrast, the frequency of both total and high affinity IgG1 NP-specific ASCs increased in bone marrow between 7 and 14 d after immunization, reflecting the recruitment of high affinity ASCs to this location (Fig. 5 C). In blood, although the overall frequency of NP-specific IgG1 ASCs declined between days 7 and 14, that of high affinity ASCs increased (Fig. 5 B). Therefore, the ASCs in the blood may serve as a repository from which the bone marrow ASC compartment is recruited.
Figure 5.
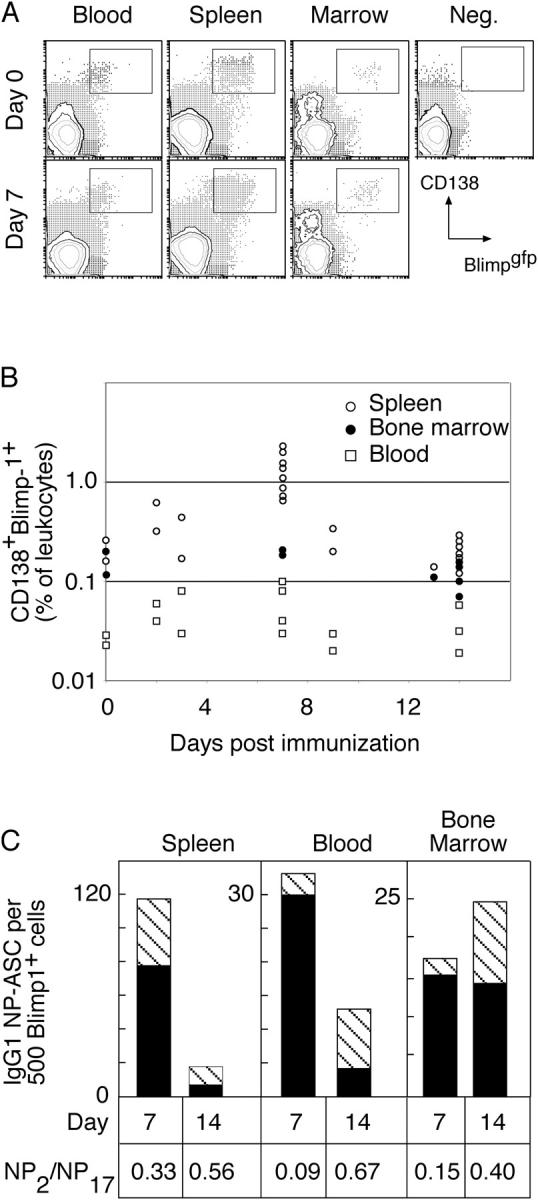
ASCs in blood after immunization revealed by expression of blimp-1. (A) Mice with GFP targeted to one allele of the blimp-1 locus were immunized, and blood, spleen, and bone marrow were collected at the indicated times. Flow cytometric analysis of leukocytes revealed populations of GFP+CD138+ cells in all tissues. (B) The frequency of such cells in individual mice is indicated as a percentage of total blood leukocytes for the different tissues. Data from spleen and bone marrow have been reported previously (reference 31) and are shown here for comparative purposes only. (C) GFP+CD138+ cells were purified by sorting and assessed for the frequency of NP-binding IgG1 ASCs, comparing spleen, blood, and bone marrow populations from the same times. In each case, 500 sort-purified cells were placed in ELISPOT wells coated with either high or low haptenated proteins to detect total or high affinity NP-specific IgG1 ASCs, respectively. The number of spots of each type is plotted and represents the average compiled from four experiments at day 14 and three at day 7, each experiment using tissue pooled from two to four mice. The number of total IgG1 anti-NP ASCs per 500 sorted cells is indicated by the height of the column and the number of high affinity ASCs by the striped section of each column. Unimmunized mice (n = 3) showed no NP- specific IgG1 ASCs in any location. The proportion of total ASCs with high affinity is calculated as NP2/NP17 ASCs. The change in ratio between days 7 and 14 within each population is significantly different (P < 0.05, Student's t test) although the differences between populations at each time are not significantly different.
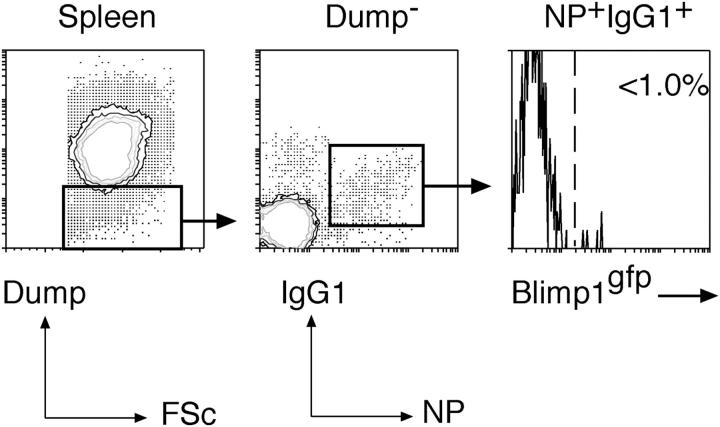
Blimp-1 is not expressed in CD138−NP+IgG1+ cells
Up to this point, our data describe B220+IgG1+ antigen-specific memory B cells in blood and spleen and CD138+Blimp-1+ ASCs in blood, spleen, and bone marrow that include IgG1 anti-NP activity. Previously, another population of GC-derived, antigen-specific but B220− B cells that were localized in spleen and bone marrow and predisposed to generate ASCs upon restimulation has been identified (16). Mice lacking blimp-1 produce very little serum antibody after immunization and lack B220− memory cells despite retaining B220+ memory (19). These results have been interpreted to mean that blimp-1 expression is required for the development of both ASCs and a B220− preplasma memory compartment (19, 33). We examined immunized blimp-1 gfp/+ mice for the presence of NP+IgG1+ cells that were CD138− yet Blimp-1+ as predicted by analysis of the immune response in blimp-1 −/− mice (19, 33). This analysis revealed a clear population of CD138−NP+IgG1+ cells in the spleen at day 14 after immunization but <1% of these cells expressed Blimp-1 (Fig. 6). This is also despite the presence of CD138+Blimp-1+ ASC populations in the spleen, blood, and bone marrow of these same mice (Fig. 5). Thus, in this experimental set-up, blimp-1 expression within the B lineage is restricted to active ASCs and does not occur in other antigen-specific B cells, irrespective of their B220 status.
Figure 6.
CD138-NP+IgG1+ cells appearing in spleen do not express blimp-1. blimp-1 gfp/+ heterozygous mice were immunized with NP-KLH in alum and 14 d later examined for the appearance in the spleen of NP- specific IgG1+ B cells that lacked expression of CD138 in addition to other Dump-channel markers (IgM, IgD, Gr-1, F4/80). However, among these NP+IgG1+ cells, none were found to express blimp-1 as indicated by the absence of GFP+ cells in the gate. One out of two representative experiments is shown.
Discussion
The output of the GC reaction is well defined; it is responsible for the production of a recirculating memory B cell population and a long-lived population of ASCs located primarily in the bone marrow. These populations are enriched, albeit to varying degrees (10), for cells with improved binding to antigen due to the preferential expansion of clones in the GC with appropriate V gene somatic mutations. However, beyond this descriptive statement, little can be said regarding the mechanism by which the memory and ASC compartments are formed. The data presented here address this issue by examining in detail the nature of the cells emigrating from the GC during the early period after primary immunization. We show that representatives of two B cells lineages are detectable in blood, one with characteristics of memory cells and the other of ASCs. The B cells with memory phenotype express B220 and contain V genes with somatic mutations consistent with a GC origin. We also find cells in blood with an ASC phenotype in that they express blimp-1 (a gene associated with commitment to ASC differentiation; reference 32) and, when isolated, secrete antigen-specific IgG1 that shows evidence of affinity maturation. Collectively, our data support a model in which the GC reaction is dynamic with continuous cell output and, even at early stages in the reaction, this output takes the form of ASCs and memory B cells.
GC emigrants with a memory phenotype have several characteristics that provide insight to the GC reaction. First, the cells are enriched for somatic mutations that enhance affinity for the immunizing antigen relative to the overall mutation load when compared with the corresponding splenic population. This enrichment represents ∼2 d of mutation and selection in the GC reaction (Fig. 3) and suggests that affinity is a criterion for emigration. Second, the preferential migration of these GC emigrants toward the follicular chemokine, CXCL13 (34), implies that the NP-specific B cells in blood are likely to return to the follicles of secondary lymphoid organs, including spleen. These observations raise the interesting possibility that GCs are open to immigration; variants formed in one GC, for example, may be able to seed other GC via the blood, thereby equilibrating affinity maturation across the reaction.
The data presented here allow us to speculate on the mechanism of memory formation. From early in the response, the phenotype of the blood antigen-specific B cells was similar to that of memory B cells (Fig. 3). They expressed levels of CD38 and bound levels of the lectin PNA equal to that of naive B cells, both characteristics of memory B cells and the inverse of GC B cells (13, 35, 36). That these cells in blood are antigen experienced is supported by their having isotype switched, being larger, and containing somatically mutated VH genes. However, as the response progressed, the average mutational load of cells in the blood increased, whereas the population size remained constant (Fig. 1 B). Thus, GC-derived B cells constantly entered the circulation at the expense of existing cells in the blood. Furthermore, treatment at day 7 with antibodies that ablate CD4+ T cells, thus abrogating the GC reaction (37), resulted in a 50% reduction in the B220+NP+IgG1+ cells in blood within 3 d (unpublished data). Collectively, these data suggest that early in the response, despite displaying a memory phenotype, the majority of antigen-specific B cells in the blood have a limited life span and presumably either return to the GC or die. However, a proportion of these cells may persist for some time and it may be that the long-lived recirculating memory B cell population is formed by a process of accumulation over the duration of the response rather than in a single “big bang” reaction. It will be of great interest to know whether the retention of cells in the blood is stochastic or selective and whether even at early time points there are a small number of long-lived cells among those already in the blood. Such a process would account for the appearance of low affinity B cells in the memory compartment (10, 38).
Our rationale for examining blood was to identify a population of cells capable of generating bone marrow ASCs. Transferred blood leukocytes from day 14 immunized mice showed very limited potential to differentiate unless simultaneously exposed to antigen in the presence of T cell help, making the major population of NP-binding B cells inefficient intermediates between the GC and the bone marrow ASCs. Therefore, the observation of an ASC population in blood raises the possibility of these cells being the precursors of the bone marrow ASCs. We have yet to test this proposal by transfer of ASCs isolated from blood as the rarity of these cells currently precludes such experiments. If blood ASCs were to give rise to the bone marrow ASCs, the formation of a bone marrow population enriched for high affinity would not necessarily require additional post-GC selection or differentiation, as has been proposed (11, 16, 18). The data presented here support the view that the formation of the bone marrow ASC compartment is achieved by the retention of ASCs in the bone marrow from among cells that emigrate from the GC as ASCs. As the GC reaction proceeds, it becomes enriched for high affinity variants. In vitro studies with both mouse and human B cells show that under conditions mimicking T-dependent stimulation, a proportion of the B cells will differentiate into ASCs with each division (39, 40). Thus, the intrinsic differentiation of GC centroblasts into ASCs that enter the blood will result in an increasing fraction of these cells being high affinity and thereby increasing the likelihood of high affinity ASCs being recruited into the bone marrow ASC compartment. In such a scheme, formation of the bone marrow ASC compartment is stochastic and reflects directly the selective expansion of high affinity cells in the GC. This proposal may explain phenomena associated with the formation of the bone marrow ASCs (11, 14). For example, BCR signaling strength could still influence affinity maturation by influencing the composition of the blood borne ASC pool. Stopping the GC reaction may not block further affinity maturation of the bone marrow ASC compartment as it is drawn from post-GC ASCs already in circulation. It will be of interest to examine the blood ASCs in more detail for their lifespan and their development in situations where affinity maturation is perturbed, as has been reported in Aiolos-deficient mice (41). The blimp-1 gfp reporter mouse will facilitate the identification, isolation, and analysis of ASCs, allowing identification of the factors responsible for their differentiation.
Exit of B cells from the GC has been the subject of several investigations over many years. In an early analysis, Baine and Thorbecke described transfer of antigen reactivity from the contralateral lymph nodes of mice as early as 1 wk after immunization (5). Our results suggest that these cells emigrated from the GC of the ipsilateral (draining) lymph nodes and represented part of the general recirculation of antigen-specific B cells described here. A more recent analysis examined GC emigration in a secondary response and found PNAhi blasts in thoracic duct exudate and evidence for ASCs in blood (20). Curiously, this activity peaked at day 3 after boosting and was undetectable shortly thereafter. Similarly, humans boosted with tetanus toxoid showed a surge in CD38hi cells in blood that, as for the secondary response in mice (20), peaked early after boosting and waned rapidly (21). The systems described here will allow the clear resolution of this critical ASC population during both the primary and secondary response as it overcomes a key previous difficulty of unambiguously identifying ASCs.
Our data support a scheme whereby bone marrow ASCs are derived from GC émigrés that are already ASCs rather than a precursor yet to reach the stage of immunoglobulin secretion (16, 18). We base this on the following five factors. First, NP-specific ASCs with signs of affinity maturation are present in blood during the period when the bone marrow ASC compartment is being established. Second, there is no evidence of a plasma cell precursor population as there are no NP-specific cells expressing blimp-1 that are not active ASCs. Third, previous reports of ASCs in blood after boosting of immunized mice and humans (20, 21, 23) suggest these cells are migratory with bone marrow as a destination. Fourth, i.v. transfer of splenic plasma cells shows some to be retained in bone marrow in a CXCR4-dependent process (26), indicating that blood-borne ASCs have the capacity to be retained in the bone marrow. Finally, blimp-1–expressing cells have been observed within GC (32), indicating that ASCs may arise directly within such structures and subsequently migrate through blood. Collectively, these analyses suggest that the bone marrow ASC compartment is established by the migration through blood of cells committed to the ASC lineage and actively secreting immunoglobulin. How these cells are retained in the bone marrow is of considerable interest. It is interesting to note that B cell niches in bone marrow are disrupted transiently after the introduction of adjuvant (42), suggesting there may be periods during which ASCs present in the blood gain access to survival niches.
Materials and Methods
Mice, antigens, and immunization
C57BL/6 mice were bred and maintained in The Walter and Eliza Hall Institute of Medical Research and used between 8 and 20 wk of age. All animal experimentation protocols were approved by the relevant Animal Ethics Committee. Immunization was with a single i.p. injection of 100 μg NP coupled to KLH in the ratio of 13:1 and prepared as described previously (10), or in control experiments, with 100 μg KLH. The antigen was precipitated onto alum and washed extensively before injection. In some experiments, a secondary response was elicited by intravenous injection of 20 μg NP13-HSA suspended in PBS. CD19−/− mice (28) were maintained in The Walter and Eliza Hall Institute of Medical Research, and were originally a gift from K. Rajewsky (Harvard University, Boston, MA). Construction of the blimp-1 gfp reporter mouse is described previously (31). In brief, an IRES-GFP construct was targeted to exon 6 of the blimp-1 locus resulting in GFP expression coincident with that of blimp-1. In vitro and in vivo experiments showed no difference in the rate or frequency of differentiation of blimp-1 gfp heterozygous B cells into ASCs and all ASC activity lies in the GFP+ cell fraction of heterozygous (blimp-1 gfp/+) mice (31). Lethally irradiated rag2 −/− mice reconstituted with fetal liver cells from homozygous blimp-1 gfp/gfp mice showed a block in plasma cell development and vastly reduced serum immunoglobulin titres consistent with a complete loss of Blimp-1 function. Immunization experiments used mice 8 wk after reconstitution with either blimp-1 gfp/gfp or blimp-1 +/+ fetal liver.
Tissue collection and cell preparation
Spleens and bone marrow were recovered and prepared exactly as described previously (10). Blood was usually collected by cardiac puncture. Animals were asphyxiated with CO2 inhalation and, immediately after the cessation of breathing, had blood removed by cardiac puncture into a heparin-containing syringe. Blood was underlayed with Histopaque (Sigma-Aldrich) and centrifuged at 1,300 revolutions/min at room temperature for 20 min. Leukocytes were collected from the interface, washed extensively, and, where necessary, had residual red blood cells removed by lysis in hypotonic buffer. In trial experiments, blood was sampled from live mice by retro-orbital bleeding through heparin-coated capillary tubes immediately before CO2 asphyxiation and blood collection by cardiac puncture. Leukocytes from both blood samples were stained for NP-specific B cells and compared with no difference in the frequency of such B cells being found in the two samples from any given individual, indicating that CO2 inhalation does not alter the B cell composition of the blood.
Antibodies, flow cytometry, cell sorting, and VH gene sequencing
Single cell suspensions were stained as described previously (10) using the following antibodies: B220 (RA3-6B2), IgM (331.12), IgD (11-26C), goat anti–mouse IgG1 (Southern Biotechnology Associates, Inc.), Mac-1 (M1/70), CD19 (1D3), CD86 (GL1), CD138 (281.1), CD8 (56–7.3), Gr-1, and F4/80. FITC-peanut agglutinin (PNA) was purchased from Vector Laboratories. NP binding was detected using a conjugate of NP to allophycocyanin, made as described previously (43). Individual antigen-specific B cells were sorted and processed for cDNA synthesis and VH gene PCR amplification as described previously (44). Sequencing used ABI Prism Big Dye Terminators v3.1 (Applied Biosystems), run on an Applied Biosystems 3730XL with automated base calling. Sequences identified as VH186.2 by the presence of the hexamer AAACCC at VH73-74 (45) were compared over their entire length to the defined VH186.2 germline sequence (46) to identify the location and frequency of mutations.
Chemokine assay
Leukocytes from blood and spleen were incubated in the upper compartment of 24-well HTS-Transwell chambers (Costar-Corning) at a concentration of 4 × 105 cells/ml. Chemokines (Research Diagnostics) were present in the bottom wells at concentrations of 800 ng/ml (CXCL13) and 400 ng/ml (CXCL12). Plates were incubated for 4 h at 37°C, and the migrant cells in the bottom wells were collected and stained as described before. Migration of antigen-specific B cells was assessed relative to the starting population and to random movement by flow cytometry with a defined number of calibration beads added to each sample to standardize cell enumeration.
Cell transfer experiments
14 d after immunization, mice were killed, and blood and spleen were collected as described before. Single cell suspensions were prepared and assessed for the frequency of NP-specific B cells by flow cytometry. After washing, the cells were resuspended in PBS at a concentration of 108 cells/ml and 100-μl aliquots were injected i.v. into recipient mice. Recipient mice were CD19 −/− (28), immunized 14 d previously with HSA in alum. Some recipients were boosted at the time of transfer with 20 μg NP-HSA, injected i.p. in PBS. 4 d after cell transfer, recipient mice were killed, and spleens and bone marrow were collected. The frequency of IgG1+ NP-specific ASCs was determined by ELISPOT assay using NP-BSA as a plate coat and conducted as described previously (10).
Acknowledgments
The authors are indebted to the B Cell Group for discussion, Prof. K. Rajewsky for CD19−/− mice, and the Flow Cytometry and Animal Facilities of The Walter and Eliza Hall Institute of Medical Research for excellent technical help.
This research was supported by grants from the National Health and Medical Research Council of Australia. E. Blink was supported by an Australian Postgraduate Research Award, D.M. Tarlinton and P.D. Hodgkin were supported by Senior Research Fellowships with the National Health and Medical Research Council (Australia), S.L. Nutt was supported by The Walter and Eliza Hall Institute Metcalf Fellowship, and A. Kallies was supported by the Deutsche Forschungsgemeinschaft.
The authors have no conflicting financial interests.
Abbreviations used: ASC, antibody-secreting cell; GC, germinal center; HSA, human serum albumin; NP, (4-hydroxy-3- nitrophenyl)acetyl; V, variable.
E.J. Blink's present address is Sanquin Research at the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, 1066 CX, Amsterdam, Netherlands.
References
- 1.Zinkernagel, R.M. 2000. What is missing in immunology to understand immunity? Nat. Immunol. 1:181–185. [DOI] [PubMed] [Google Scholar]
- 2.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 3.Klein, U., K. Rajewsky, and R. Kuppers. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangye, S.G., Y.J. Liu, G. Aversa, J.H. Phillips, and J.E. de Vries. 1998. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J. Exp. Med. 188:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baine, Y., and G.J. Thorbecke. 1982. Induction and persistence of local B cell memory in mice. J. Immunol. 128:639–643. [PubMed] [Google Scholar]
- 6.Slifka, M.K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith, K.G., T.D. Hewitson, G.J. Nossal, and D.M. Tarlinton. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26:444–448. [DOI] [PubMed] [Google Scholar]
- 8.Manz, R.A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature. 388:133–134. [DOI] [PubMed] [Google Scholar]
- 9.Manz, R.A., M. Lohning, G. Cassese, A. Thiel, and A. Radbruch. 1998. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10:1703–1711. [DOI] [PubMed] [Google Scholar]
- 10.Smith, K.G., A. Light, G.J. Nossal, and D.M. Tarlinton. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16:2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi, Y., P.R. Dutta, D.M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tew, J.G., J. Wu, D. Qin, S. Helm, G.F. Burton, and A.K. Szakal. 1997. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol. Rev. 156:39–52. [DOI] [PubMed] [Google Scholar]
- 13.Ridderstad, A., and D.M. Tarlinton. 1998. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J. Immunol. 160:4688–4695. [PubMed] [Google Scholar]
- 14.Tarlinton, D.M., and K.G. Smith. 2000. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol. Today. 21:436–441. [DOI] [PubMed] [Google Scholar]
- 15.Bell, J., and D. Gray. 2003. Antigen-capturing cells can masquerade as memory B cells. J. Exp. Med. 197:1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHeyzer-Williams, L.J., M. Cool, and M.G. McHeyzer-Williams. 2000. Antigen-specific B cell memory. Expression and replenishment of a novel B220− memory B cell compartment. J. Exp. Med. 191:1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driver, D.J., L.J. McHeyzer-Williams, M. Cool, D.B. Stetson, and M.G. McHeyzer-Williams. 2001. Development and maintenance of a B220− memory B cell compartment. J. Immunol. 167:1393–1405. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor, B.P., M. Cascalho, and R.J. Noelle. 2002. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 195:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro-Shelef, M., K.I. Lin, L.J. McHeyzer-Williams, J. Liao, M.G. McHeyzer-Williams, and K. Calame. 2003. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 19:607–620. [DOI] [PubMed] [Google Scholar]
- 20.Dilosa, R.M., K. Maeda, A. Masuda, A.K. Szakal, and J.G. Tew. 1991. Germinal center B cells and antibody production in the bone marrow. J. Immunol. 146:4071–4077. [PubMed] [Google Scholar]
- 21.Medina, F., C. Segundo, A. Campos-Caro, I. Gonzalez-Garcia, and J.A. Brieva. 2002. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 99:2154–2161. [DOI] [PubMed] [Google Scholar]
- 22.Bernasconi, N.L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 298:2199–2202. [DOI] [PubMed] [Google Scholar]
- 23.Arce, S., E. Luger, G. Muehlinghaus, G. Cassese, A. Hauser, A. Horst, K. Lehnert, M. Odendahl, D. Honemann, K.D. Heller, et al. 2004. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J. Leukoc. Biol. 75:1022–1028. [DOI] [PubMed] [Google Scholar]
- 24.Cumano, A., and K. Rajewsky. 1986. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 5:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehrli, N., D.F. Legler, D. Finke, K.M. Toellner, P. Loetscher, M. Baggiolini, I.C. MacLennan, and H. Acha-Orbea. 2001. Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur. J. Immunol. 31:609–616. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves, D.C., P.L. Hyman, T.T. Lu, V.N. Ngo, A. Bidgol, G. Suzuki, Y.R. Zou, D.R. Littman, and J.G. Cyster. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleul, C.C., J.L. Schultze, and T.A. Springer. 1998. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J. Exp. Med. 187:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rickert, R.C., K. Rajewsky, and J. Roes. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 376:352–355. [DOI] [PubMed] [Google Scholar]
- 29.Fehr, T., D. Skrastina, P. Pumpens, and R.M. Zinkernagel. 1998. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proc. Natl. Acad. Sci. USA. 95:9477–9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colvin, G.A., J.F. Lambert, M. Abedi, C.C. Hsieh, J.E. Carlson, F.M. Stewart, and P.J. Quesenberry. 2004. Murine marrow cellularity and the concept of stem cell competition: geographic and quantitative determinants in stem cell biology. Leukemia. 18:575–583. [DOI] [PubMed] [Google Scholar]
- 31.Kallies, A., J. Hasbold, D.M. Tarlinton, W. Dietrich, L.M. Corcoran, P.D. Hodgkin, and S.L. Nutt. 2004. Plasma cell ontogeny defined by quantitative changes in Blimp-1 expression. J. Exp. Med. 200:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelin-Duclos, C., G. Cattoretti, K.I. Lin, and K. Calame. 2000. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 165:5462–5471. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro-Shelef, M., and K. Calame. 2004. Plasma cell differentiation and multiple myeloma. Curr. Opin. Immunol. 16:226–234. [DOI] [PubMed] [Google Scholar]
- 34.Gunn, M.D., V.N. Ngo, K.M. Ansel, E.H. Ekland, J.G. Cyster, and L.T. Williams. 1998. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 391:799–803. [DOI] [PubMed] [Google Scholar]
- 35.Coico, R.F., B.S. Bhogal, and G.J. Thorbecke. 1983. Relationship of germinal centers in lymphoid tissue to immunologic memory. VI. Transfer of B cell memory with lymph node cells fractionated according to their receptors for peanut agglutinin. J. Immunol. 131:2254–2257. [PubMed] [Google Scholar]
- 36.Oliver, A.M., F. Martin, and J.F. Kearney. 1997. Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells. J. Immunol. 158:1108–1115. [PubMed] [Google Scholar]
- 37.Vieira, P., and K. Rajewsky. 1990. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 2:487–494. [DOI] [PubMed] [Google Scholar]
- 38.Schittek, B., and K. Rajewsky. 1992. Natural occurrence and origin of somatically mutated memory B cells in mice. J. Exp. Med. 176:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangye, S.G., D.T. Avery, E.K. Deenick, and P.D. Hodgkin. 2003. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 170:686–694. [DOI] [PubMed] [Google Scholar]
- 40.Hasbold, J., L.M. Corcoran, D.M. Tarlinton, S.G. Tangye, and P.D. Hodgkin. 2004. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat. Immunol. 5:55–63. [DOI] [PubMed] [Google Scholar]
- 41.Cortes, M., and K. Georgopoulos. 2004. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J. Exp. Med. 199:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda, Y., K. Yang, S.J. Foster, M. Kondo, and G. Kelsoe. 2004. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 199:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McHeyzer-Williams, M.G., G.J. Nossal, and P.A. Lalor. 1991. Molecular characterization of single memory B cells. Nature. 350:502–505. [DOI] [PubMed] [Google Scholar]
- 44.Smith, K.G., A. Light, L.A. O'Reilly, S.M. Ang, A. Strasser, and D. Tarlinton. 2000. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J. Exp. Med. 191:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss, U., and K. Rajewsky. 1990. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J. Exp. Med. 172:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabat, E.A., T.T. Wu, H.M. Perry, K.S. Gottesman, and C. Foeller. 1991. Sequences of Proteins of Immunological Interest. US National Institutes of Health, Bethesda, MD.