Abstract
Chemerin is a chemotactic agent that was recently identified as the ligand of ChemR23, a serpentine receptor expressed by activated macrophages and monocyte-derived dendritic cells (DCs). This paper shows that blood plasmacytoid and myeloid DCs express functional ChemR23. Recombinant chemerin induced the transmigration of plasmacytoid and myeloid DCs across an endothelial cell monolayer. In secondary lymphoid organs (lymph nodes and tonsils), ChemR23 is expressed by CD123+ plasmacytoid DCs and by CD1a+ DC-SIGN+ DCs in the interfollicular T cell area. ChemR23+ DCs were also observed in dermis from normal skin, whereas Langerhans cells were negative. Chemerin expression was selectively detected on the luminal side of high endothelial venules in secondary lymphoid organs and in dermal endothelial vessels of lupus erythematosus skin lesions. Chemerin+ endothelial cells were surrounded by ChemR23+ plasmacytoid DCs. Thus, ChemR23 is expressed and functional in plasmacytoid DCs, a property shared only by CXCR4 among chemotactic receptors. This finding, together with the selective expression of the cognate ligand on the luminal side of high endothelial venules and inflamed endothelium, suggests a key role of the ChemR23/chemerin axis in directing plasmacytoid DC trafficking.
DCs are potent antigen-presenting cells with a unique ability to induce T and B cell responses as well as immune tolerance (1, 2). Among human peripheral blood DCs, at least two distinct subsets have been defined, based on the expression of CD11c, namely CD11c+ myeloid DCs (M-DC) and CD11c− plasmacytoid DCs (P-DC; reference 1). In addition, a minor, still poorly characterized population of CD11c+ BDCA-3+ DCs has been described previously (3). M-DC express myeloid markers such as CD13 and CD33, the stimulatory receptor Ig-like transcript 1, and can produce high levels of IL-12 (4). Conversely, P-DC have a morphology resembling plasma cells; are devoid of myeloid markers; express high levels of CD4, CD62 ligand, and CD123; and can produce high levels of IFN-α (5–7). This dichotomy observed in human blood is further extended at the tissue level, where P-DC and many M-DC subpopulations can be recognized based on their morphology, location, and phenotypic profile.
M-DC are located in peripheral nonlymphoid tissues at an immature functional state where they exert a sentinel function for incoming antigens. After antigen uptake, in the context of an inflammatory reaction, M-DC leave peripheral sites and reach secondary lymphoid organs by lymphatic vessels (5, 6). In contrast, P-DC are typically absent in peripheral tissues under homeostatic conditions and are believed to leave the circulation to enter secondary lymphoid organs through the interaction with high endothelial venules (HEVs; references 5, 6). However, recently published papers demonstrated P-DC recruitment also in some inflamed skin lesions, such as those associated with systemic lupus erythematosus (LE; references 5, 8), in nasal mucosa during allergic reactions, and in tumors (for review see reference 5).
Differences in distribution and tissue recruitment during inflammatory conditions between M-DC and P-DC may reflect distinct functional properties and be explained by specific migratory mechanisms. Blood DC subsets express a similar pattern of chemotactic receptors, including CCR2, CCR5, CXCR2, CXCR3, CXCR4, and PAFR (9). However, circulating P-DC, in contrast with M-DC, fail to migrate in response to inflammatory chemokines, whereas both subsets respond to lymph node–homing chemokines (i.e., CCL19 and CCL21) after maturation (9). Thus, the only chemotactic factor presently known for immature P-DC in vitro is CXCL12, the ligand of CXCR4. However, the mechanisms leading to the in vivo recruitment of P-DC to normal and inflamed tissues are still poorly understood.
Chemerin is a novel protein identified as the natural ligand of ChemR23, a previously orphan protein G–coupled receptor expressed by immature DCs and macrophages (10). Chemerin was purified from ovarian cancer ascites and found to correspond to the product of the Tig-2 gene. Chemerin is expressed by many tissues, including spleen and lymph nodes, and is secreted as prochemerin, a poorly active precursor protein. Extracellular proteases convert prochemerin into a full agonist of ChemR23 by proteolytic removal of the last six amino acids (10, 11). Previous work has shown that chemerin induces calcium fluxes, mitogen-activated protein kinase activation, and chemotaxis of monocyte-derived immature DCs, whereas LPS- or CD40L-mature DCs do not express functional ChemR23 receptors. The aim of this work was to investigate the functional expression of ChemR23 in human blood DC subsets. Here, we report that ChemR23 is expressed and functional in M-DC and most importantly in P-DC, a property shared only by CXCR4. The ChemR23 ligand, chemerin, is selectively expressed in HEVs and in LE skin lesions, a prototypic inflammatory condition characterized by tissue accumulation of P-DC. These data strongly suggest that the ChemR23/chemerin axis may play a key role in regulating the trafficking of P-DC.
Results and Discussion
Expression and function of ChemR23 in blood MHC class II+ leukocytes
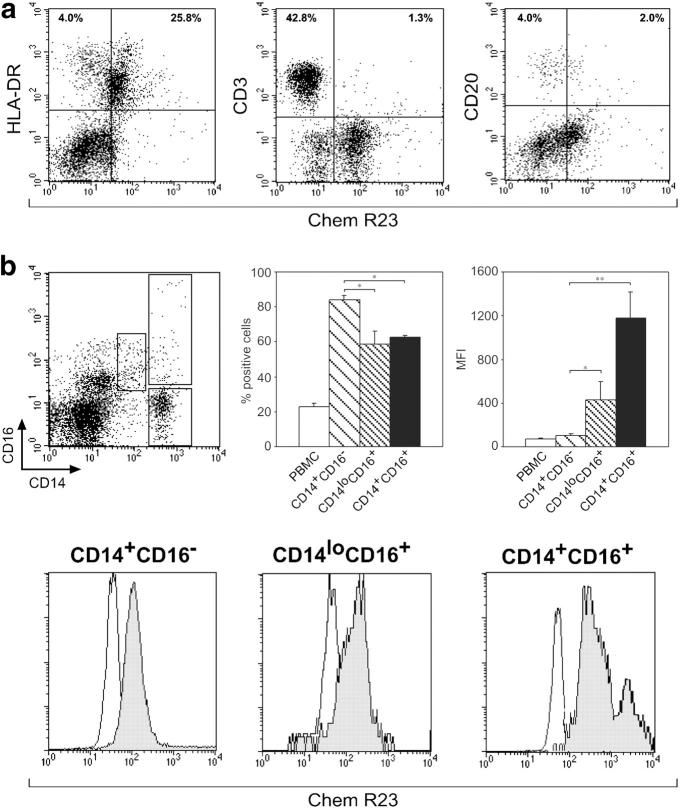
In a previous work, we reported that chemerin represents a new chemotactic factor for monocyte-derived DCs (10). Experiments were performed with the aim to characterize the expression of ChemR23 in circulating leukocytes. Fig. 1 a shows that ChemR23 expression was mainly present in MHC class II+ cells and in a minor subset of CD3+ and CD20+ lymphocytes. Within blood mononuclear cells, MHC class II+ cells were the only cells that migrated in response to chemerin (unpublished data). The expression of ChemR23 was further investigated in MHC-II+ cells. Fig. 1 b shows that ChemR23 was expressed by the three major monocyte subsets, namely CD14+CD16−, CD14+CD16+, and CD14loCD16+. However, a statistically significant difference among the three populations was found both in terms of percentage of positive cells and intensity of membrane staining (mean fluorescence intensity [MFI]), with the predominant CD14+CD16− population having the lowest levels of membrane fluorescence (MFI).
Figure 1.
Expression of ChemR23 in leukocytes. PBMCs were isolated as described in Materials and methods and stained for ChemR23 expression. (a) PBMC subsets were analyzed by FACS analysis. One experiment representative of four independent experiments is shown. (b) PBMCs were analyzed for the expression of CD14 and CD16 and three populations of monocytes were discriminated: CD14+CD16−, CD14loCD16+, and CD14+CD16+. ChemR23 expression was investigated in monocyte subsets. The values represent the mean ± SD of positive cells or intensity of membrane staining (MFI; n = 4; *, P < 0.05; **, P < 0.01 by one-way analysis of variance with Tukey post test). The flow cytometry histograms are the result of one experiment representative of four independent experiments.
The three major blood DC subsets were investigated on the basis of the expression of specific membrane markers: BDCA-1 (M-DC), BDCA-4 (P-DC), and BDCA-3 (3). Fig. 2 a shows that ChemR23 was expressed by ∼40% of the circulating M-DC population and by virtually all P-DC. DC maturation (>85% CD83+ cells), achieved in the presence of LPS (M-DC) or influenza virus (P-DC), did not significantly affect the percentage of ChemR23+ cells, and only induced a decrease of the mean channel fluorescence (Fig. 2 a). On the contrary, ChemR23 was only modestly expressed (<30% positive cells) by BDCA3+ DCs, regardless the state of maturation.
Figure 2.
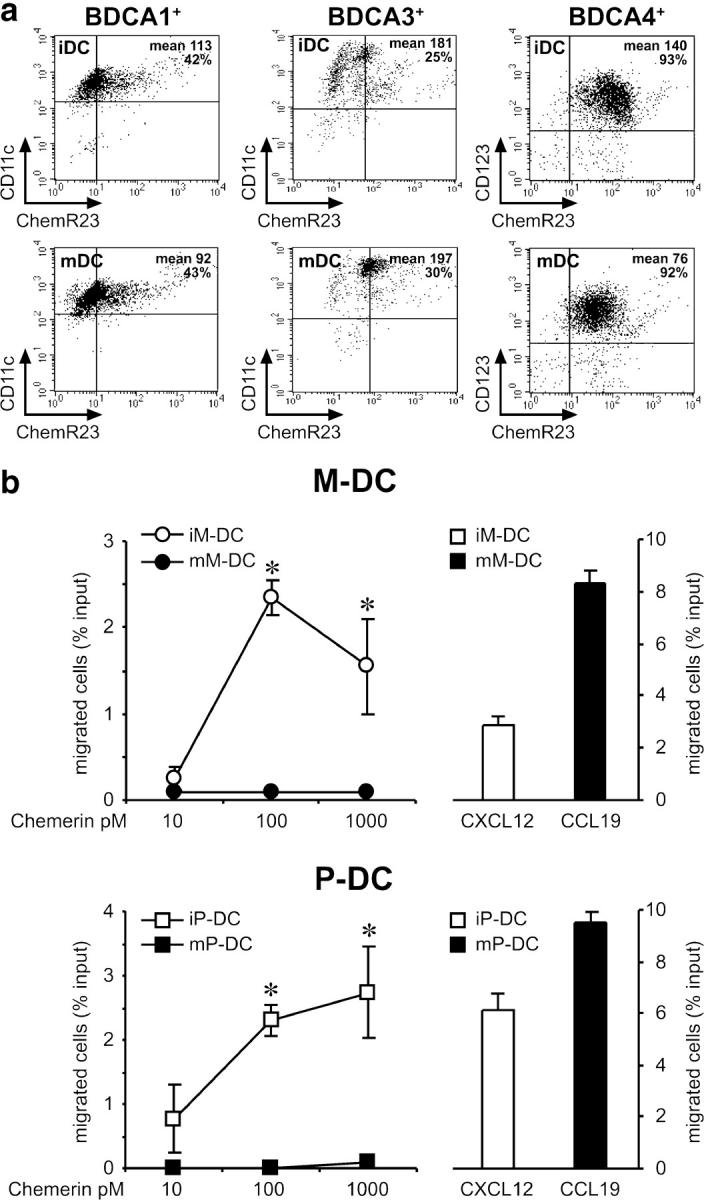
Expression of functional ChemR23 in blood DC subsets. Blood DC subsets were isolated as described in Materials and methods and stained for ChemR23 expression. Maturation of DCs was achieved by incubation with LPS (BDCA-1+), influenza virus (BDCA-4+), or LPS and influenza virus (BDCA-3+) for 24 h. (a) DC subsets were analyzed by FACS analysis. (b) DC subsets were tested for their ability to migrate across an endothelial cell monolayer in response to chemerin or to an optimal concentration (12 nM) of CXCL12 (immature DCs) or CCL19 (mature DCs). The values represent the mean ± SD of triplicate data points and are representative of at least five different experiments performed with independent donors. Note the different scale used in the left versus right panels. (iDC) Immature DCs. (mDC) Mature DCs. *, P < 0.05 by paired Student's t test.
Next, we evaluated the ability of chemerin, the ChemR23 ligand, to induce DC transmigration across an endothelial cell monolayer. Chemerin induced a dose-dependent migration of immature M-DC and P-DC, with a peak observed at 100 pM. Compared with the migration observed in response to an optimal concentration of CXCL12 (12 nM), chemerin induced the migration of a similar percentage of M-DC, whereas P-DC migration was ∼50% of that induced by CXCL12. Therefore, chemerin is characterized by potency ∼100-fold higher (peak chemotactic concentration) and by a similar (M-DC) or slightly reduced (P-DC) efficacy (percentage of migrated cells) as compared with CXCL12 (Fig. 2 b). Chemotaxis of both M-DC and P-DC to chemerin was completely blocked in the presence of an anti-ChemR23 moAb, proving the involvement of ChemR23 in chemerin-induced DC migration (unpublished data). Of note, chemerin-induced migration of M-DC and P-DC was respectively 1.6- and 3.4-fold more efficient when assessed in transmigration assays than using bare filters. This result suggests that chemerin presentation by endothelial cells is required for optimal interaction with ChemR23+ DCs. It was reported previously that CXCR3 ligands increase P-DC migration in response to CXCL12 (12, 13). Therefore, P-DC migration was tested using chemerin in combination with different concentrations of CXCL10 or CXCL12 in the lower wells. P-DC migration to chemerin was never increased by the presence of these two chemokines, indicating that ChemR23 does not cooperate with CXCR3 and CXCR4 for cell migration (unpublished data). Mature M-DC and P-DC did not migrate in response to chemerin (Fig. 2 b), but normally migrated in response to CCL19, one of the CCR7 ligands. It is noteworthy that mature DCs retained high levels of ChemR23 expression, whereas they were unresponsive to the cognate agonist. Thus, the maturation process induces the uncoupling of ChemR23 with no major changes in its membrane expression. All in all, these results show that chemerin represents a new chemotactic factor for immature DCs. Most importantly, ChemR23 represents the only chemotactic receptor, in addition to CXCR4, that is active in immature P-DC.
Chemerin belongs to the cathelicidin/cystatin family of proteins, which includes precursors of bactericidal peptides (cathelicidins), precursors of mediators active on leukocytes through protein G–coupled receptors (prokininogen, cathelicidin precursors), as well as cysteine protease inhibitors (cystatins). Elevated chemerin production was found in ovarian cancer ascites and in the synovial fluids of rheumatoid arthritis patients (10). Prochemerin transcripts were also found in the skin of psoriatic subjects (14). In the bone stromal cell line ST2, the chemerin gene was reported to be induced by 1,25-dihydroxy-vitamin D3 (VitD3) and dexamethasone (15). Therefore, it is likely that chemerin represents an important signal involved in the recruitment of DCs during inflammation and autoimmune diseases, and at tumor sites. The up-regulation of chemerin production by antiinflammatory signals such as VitD3 and dexamethasone postulates a role for chemerin in all the conditions characterized by a “type 2/alternative” state of activation, similar to that present in tumor-associated leukocytes (16).
Expression of ChemR23 by P-DC and M-DC in human tissues
At the tissue level, many DC subpopulations can be recognized based on their morphology, location, and phenotypic profiles. For instance, Langerhans cells express CD1a and Langerin and are exclusively distributed within epithelial surface; in contrast, the so-called “interstitial DCs,” typically represented by dermal DCs, are mainly CD1a− and express DC-SIGN and the mannose receptor. In lymphoid organs, DC subsets are recognizable in B and T cell compartments and include, respectively, germinal center DCs and Langerhans-derived interdigitating DCs in T cell paracortical area (5, 6). The interfollicular area separates nodular lymphoid compartments. The latter is highlighted by the HEVs and populated by many leukocyte populations, including lymphocytes, macrophages, plasma cells, and at least two distinct DC subpopulations, namely CD68+DC-SIGN+ DCs (17) and P-DC (5).
The expression of ChemR23 was investigated by immunohistochemistry in lymph nodes and tonsils. In lymph nodes, numerous ChemR23+ cells with a dendritic morphology were located in the interfollicular compartment (Fig. 3 a). These cells were identified by double immunofluorescence as DC-SIGN+ (Fig. 3 b) and CD1a+ cells (Fig. 3 c), two phenotypic markers that identify distinct nodal myeloid DC populations (17). On the basis of their phenotype and location, these cells are believed to represent the nodal counterpart of peripheral blood DC-SIGN+ DCs (18, 19) that are recruited into the lymph nodes from the blood through the HEVs, or a population of dermal DCs migrated to the lymph node. ChemR23 reactivity was also present on CD123+ and BDCA-2+ P-DC (Fig. 3 d and its inset), either scattered as single cells or in clusters close to HEVs. ChemR23 expression was not restricted to the interfollicular compartment and was also observed in sinus macrophages and in scattered cells within the germinal center (Fig. 3, a and d). This latter population coexpressed CD68+ and represents germinal center macrophages (unpublished data). No reactivity was observed on B and T lymphocytes. These results indicate that ChemR23 expression is retained by P-DC and M-DC after their recruitment to secondary lymphoid tissues.
Figure 3.
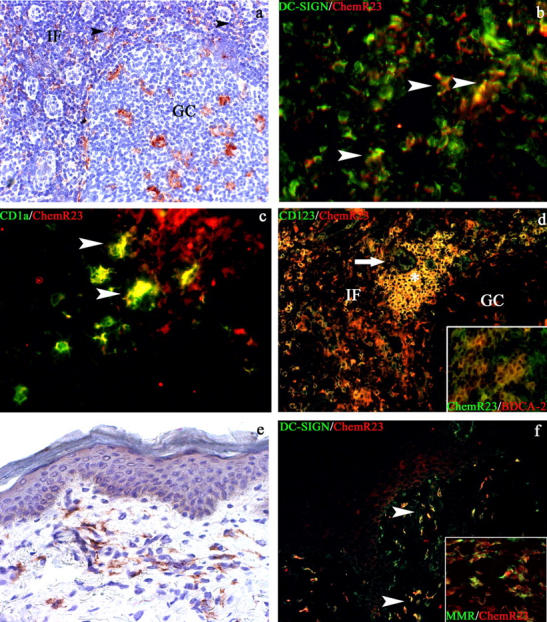
ChemR23 expression in secondary lymphoid organs and normal skin. In secondary lymphoid organs, ChemR23 reactivity is observed in the interfollicular (IF) area and in tingible body macrophages of the germinal center (GC) (a and d). In the interfollicular areas, ChemR23+ cells present a sparse perivenular distribution (a, black arrowheads), a DC morphology, and coexpress DC-SIGN (b, white arrowheads) and CD1a+ (c, white arrowheads). CD123+ChemR23+ plasmacytoid DCs (P-DC; yellow cells) are also observed in the interfollicular area (IF) in proximity to a CD123+ HEV (d), as cluster (d, white asterisk) or as sparse cells coexpressing the P-DC–specific marker BDCA-2 (d, inset). In normal skin, ChemR23 reactivity is observed in dermal cells with fusate/dendritic morphology (e) coexpressing DC-SIGN (f, white arrowheads) and mannose receptor (f, inset). Indirect immunoperoxidase technique was applied (a and e); nuclei were counterstained with hematoxylin. Double immunofluorescence was performed (b–d and f), using Texas red–conjugated secondary antibodies for anti-ChemR23 (b–d, and e) and anti-BDCA-2 (d, inset) and FITC-conjugated secondary antibodies for anti–DC-SIGN (b and f), anti-CD1a (c), anti-CD123 (d), anti-ChemR23 (d, inset), and antimannose receptor (MMR; f, inset). Magnification, 100 (a and d), 200 (e and f), and 400 (b, c, and d and f, insets).
The expression of ChemR23 was also investigated in normal skin. A strong reactivity for ChemR23 was identified in cells located in the upper and intermediate dermis (Fig. 3 e). These ChemR23+ cells displayed fusate/dendritic morphology and coexpressed DC-SIGN (Fig. 3 f) as well as the mannose receptor (Fig. 3 f, inset), thus corresponding to dermal DCs (19). On the contrary, ChemR23 expression was never detected in the cutaneous Langerhans cells (Fig. 3, e and f) and in the Langerhans cell–derived paracortical interdigitating dendritic cells that accumulate in the lymph node during dermatopatic lymphadenitis (reference 20 and not depicted). These observations clearly indicate that chemerin is unlikely to be a relevant chemotactic factor for the homing of Langerhans cell precursors to the skin, as well as for the migration of Langerhans cells to superficial lymph nodes.
Expression of chemerin by HEVs in secondary lymphoid organs
Due to the abundance in ChemR23+ cells in secondary lymphoid organs (lymph nodes and tonsils) and normal skin, the distribution of chemerin was also investigated in these tissues. Chemerin reactivity was primarily expressed in the interfollicular area of lymph nodes and tonsils (Fig. 4 a), the site where the majority of ChemR23+ cells also localize. The strongest reactivity was observed in endothelial cells with cuboidal/cylindrical morphology corresponding to HEVs. The identity of these chemerin+ cells was further confirmed on the basis of the coexpression of Factor VIII, a specific endothelial cell marker (Fig. 4 b, inset), as well as CD123 (Fig. 4 a, inset) and CLA/Heca452 (Fig. 4 b), two markers also expressed by tissue P-DC (5). Of note, in HEVs, the highest expression of chemerin was observed at the luminal side (Fig. 4 b). The presence of P-DC positive for ChemR23 in close proximity of HEVs that express chemerin suggests that chemerin represents an alternative/additional signal to CXCL12 for the recruitment of blood P-DC into lymph nodes across HEVs (21). In this respect, it is intriguing that the pattern of chemerin immunoreactivity resembles that observed with an anti-CXCL12 antibody (reference 12 and unpublished data). In addition to HEVs, chemerin expression was also found on sparse spindle cells located in the perivenular area (Fig. 4 a). Based on their morphology, this population is likely to represent stromal cells; their precise identity is currently under investigation.
Figure 4.
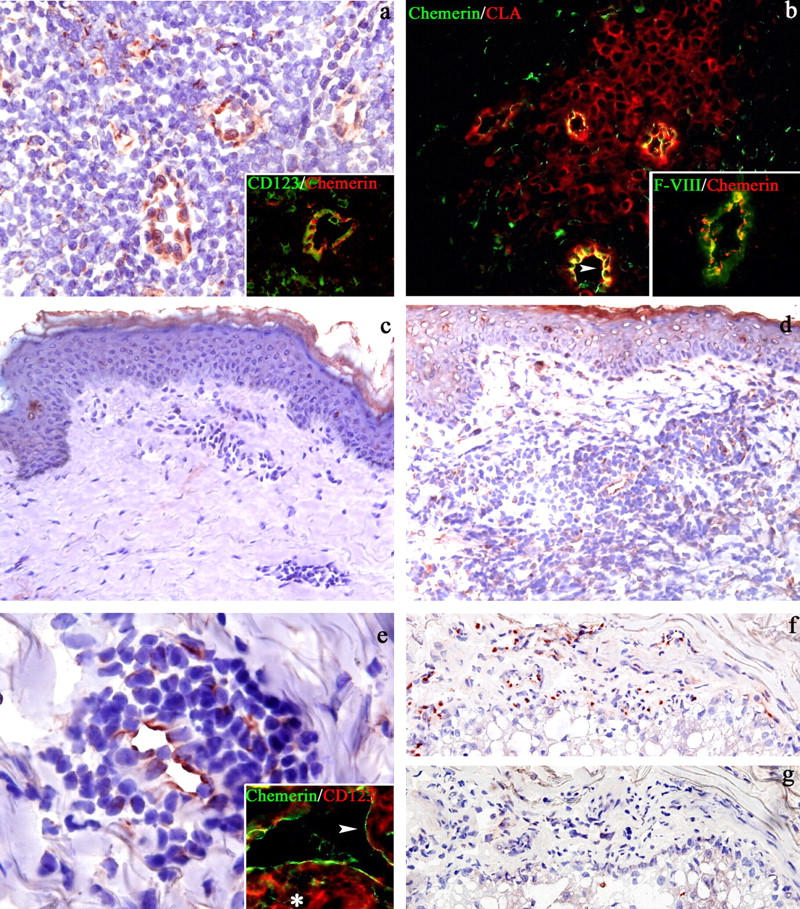
Chemerin expression in secondary lymphoid organs, normal skin, and cutaneous LE lesions. Immunostaining of lymph node sections shows chemerin reactivity on endothelial cells and sparse perivenular spindle cells in the interfollicular area (a and b). Chemerin+ vessels correspond to HEV labeled by CD123 (a, inset), CLA (b), and Factor VIII (b, inset); note the preferential expression of chemerin on the luminal side of HEV (b, arrowhead). Because CLA and CD123 also stain P-DC, these markers also identify P-DC surrounding HEV (a and b). Chemerin is absent in normal skin (c). Chemerin+ cells are numerous in the dermis from a LE case (d). Most of these cells correspond to endothelial cells; in addition, scattered chemerin+ cells with fusate morphology are admixed to the lymphoid infiltrate (d and e). Rare chemerin+ cells are also noticeable in the epidermis (d). Double immunofluorescence demonstrated that, in LE lesions, CD123+ P-DC (e, inset, asterisk) are located around chemerin+ dermal vessels (e, inset; white arrowheads indicate the luminal side). On serial sections obtained from a LE case, chemerin+ endothelial cells (f) did not coexpress the lymphatic endothelial cell marker podoplanin (g). Indirect immunoperoxidase technique was applied (a and c–g); nuclei were counterstained with hematoxylin. Double immunofluorescence was performed (a, inset; b; and e, inset) using Texas red–conjugated secondary antibodies for antichemerin (a and b, insets), anti-CLA (b), anti-CD123 (e, inset) and FITC-conjugated secondary antibodies for anti-CD123 (a, inset), antichemerin (b and e, inset), and anti–Factor VIII (b, inset). Magnification, 200 (b–d, f, g, and a and b, insets); 400 (a); and 600 (e and its inset).
HEVs were shown to produce CCL21, which accumulates at their luminal side. Other chemokines were detected on the surface of HEVs, although they were produced by non-HEV cells (e.g., CCL19, CXCL12, and CCL2; references 22, 23). In vitro experiments performed with five different types of endothelial cells of different origin (i.e., cord blood, dermis, iliac artery, and bone marrow) did not show any production of chemerin by endothelial cells both under basal conditions and after cytokine stimulation (TNF, IL-1, LPS, IFNα, IFNγ, and combinations of these agonists; unpublished data). Therefore, it is unknown at the moment whether chemerin expression is a prerogative of a specialized endothelium of an anatomical compartment, such as that of HEVs, or if chemerin is produced by stromal cells surrounding HEVs, and subsequently transported through the fibroblastic reticular cell network to HEVs (for review see references 22, 23).
Expression of chemerin by dermal endothelial cell of blood vessels in LE
Nonresident DCs, including P-DC, accumulate in the skin in many inflammatory conditions (24). Thus, the expression of chemerin was assessed in tissue sections obtained from normal and inflamed skin. Chemerin expression was completely absent in normal skin, including in endothelial cells of the papillary dermis vessels (Fig. 4 c). These data suggest that chemerin is unlikely to be responsible for skin recruitment of resident DCs in steady-state conditions. In recent years, P-DC were directly implicated in the pathogenesis of LE (5, 8). In LE patients, the number of circulating P-DC is reduced, and these cells are present in the skin lesions, suggesting that P-DC are selectively recruited to the skin (8). Therefore, the expression of chemerin was examined in skin biopsies from nine cases of LE. Chemerin reactivity was observed in six out of the nine cases on the endothelial cells lining dermal vessels (Fig. 4, d–f). These endothelial cells did not express podoplanin (Fig. 4 g), a marker of lymphatic endothelium (25), indicating that they belong to blood vessels. In addition to endothelial cells, rare chemerin+ cells were also observed in the epidermis and in the dermis. In the epidermis, chemerin reactivity was present in scattered Langerhans cells (Fig. 4 d). In the dermis, sparse chemerin+ cells were admixed to lymphoid infiltrates and displayed a fusate/dendritic morphology. These cells were found to correspond to DC-SIGN+ dendrocytes (reference 26 and unpublished data). Notably, in five out of six positive cases, P-DC were present with variable density within the skin lesions and double immunofluorescence staining, demonstrating that CD123+ P-DC are located in close proximity of chemerin+ dermal endothelial cells (Fig. 4 e, inset). This suggests that chemerin local production can direct P-DC to the skin in inflammatory conditions characterized by P-DC accumulation (5).
In summary, this report shows that M-DC and P-DC express ChemR23 in vitro and in vivo. Experiments performed in vitro clearly show that chemerin represents a new chemotactic factor for blood M-DC and most importantly for P-DC. The in vivo distribution of chemerin, located at the luminal side of HEVs and inflamed blood dermal vessels, strongly suggests that chemerin is involved in the migration of P-DC across HEVs under steady-state conditions, and in the accumulation of P-DC observed in inflamed tissues.
Material and Methods
Reagents
Recombinant chemerin was produced and purified as described previously (10). Human chemokines and cytokines used were: CXCL12, CCL19 (PeproTech), IL-1β (Dompè), TNFα (BASF Knoll), IFNγ (Roussel Uclaf), and IFNα (Roferon; Roche). RPMI 1640 was obtained from GIBCO BRL. FCS was obtained from Hyclone. Cytokines and media were endotoxin free as assessed by the Limulus amoebocyte assay (BioWhittaker Inc.). All reagents not otherwise specified were obtained from Sigma-Aldrich.
Peripheral blood DC purification
PBMCs were isolated from buffy coats by Ficoll gradient (Amersham Biosciences) and peripheral blood M-DC and P-DC were magnetically sorted with blood DC Ag (BDCA)-1 and BDCA-4 cell isolation kits (Miltenyi Biotec), respectively, as described previously (9). Blood M-DC and P-DC (106 cells/ml) were cultured in medium containing 1,000 U/ml recombinant human GM-CSF (Mielogen; Schering-Plough) and 10 ng/ml IL-13 (a gift from A. Minty, Sanofi Elf Bio Recherches, Labège, France) or 20 ng/ml IL-3 (ProSpec), respectively. Cells were matured by 24-h incubation with 100 ng/ml LPS (Escherichia coli 055:B5; Sigma-Aldrich), or 20 ng of hemagglutinin/ml inactivated influenza virus strain A/Moscow/10/99 (a gift from T. De Magistris, Istituto Superiore di Sanità, Rome, Italy). BDCA-3+ MDC were isolated with BDCA-3 cell isolation kit (Miltenyi Biotec), cultured in medium containing 1,000 U/ml rhGM-CSF, 10 ng/ml IL-13, and 20 ng/ml IL-3 and matured by 24-h incubation with 100 ng/ml LPS and 20 ng of hemagglutinin/ml inactivated influenza virus (3).
Flow cytometry
The expression of ChemR23 was analyzed by FACS using two monoclonal antibodies (clone 4C7 and 1H2) described previously (10). The expression of ChemR23 on PBMC subsets was analyzed by FACS using: allophycocyanin-conjugated anti-CD3, PE-conjugated anti-CD20, and PE-conjugated anti-HLA-DR (Becton Dickinson); and allophycocyanin-conjugated anti-CD14 and PE-conjugated anti-CD16 (Exalpha). PE-conjugated CD123 (IgG1; clone 9F5) and CD11c (IgG2b; clone S-HCL-3) were obtained from Becton Dickinson. Analysis of fluorescence was performed on a FACStar Plus calibrated with Calibrite beads (Becton Dickinson).
Transendothelial migration assay
Transendothelial migration assay was evaluated using 24-well Costar Transwell chambers (5 μm pore size; Corning). Human umbilical vein endothelial cells (HUVECs; passage <5) were subcultured to confluent monolayers on Transwell inserts and precoated with gelatin. Monolayers were rinsed with chemotaxis medium (RPMI 1640 containing 10% FCS) before use. 100 μl of DC suspension (1–2 × 106 cells/ml) were seeded in the upper chamber and 600 μl of chemoattractant or control medium was added to the lower well. The chamber was incubated at 37°C in humidified atmosphere in the presence of 5% CO2 for 4 h. Migrated cells were recovered from the lower well and counted and the results were expressed as the percentage of input cells. In some experiments, DC subsets were cultured for 24 h in the presence or absence of a maturative stimulus (LPS or influenza virus) and tested for their ability to migrate in response to chemoattractants. PBMCs (10 × 106 cells/ml; 100 μl of cell suspension per well) migration was tested in the same experimental conditions used for DCs. Migrated cells were collected and labeled for FACS analysis.
Chemerin expression in endothelial cells
Chemerin expression was evaluated by RT-PCR. Total RNA samples (1 μg) were reverse transcripted using the SuperScript II Rnase H− Reverse Transcriptase (Invitrogen). PCR was performed on cDNA samples using the following primers: chemerin forward, 5′-ATGCGACGGCTGCTGATCCCTC-3′, chemerin reverse, 5′-TTAGCTGCGGGGCAGGGCCTTG-3′; CXCL10 forward, 5′-GGAACCTCCAGTCTCAGCACC-3′, CXCL10 reverse, 5′-CAGCCTCTGTGTGGTCCATCC-3′; CXCL8 forward, 5′-CGATGTCAGTGCATAAAGACA-3′, CXCL8 reverse, 5′-TGAATTCTCAGCCCTCTTCAAAA-3′; and β-actin forward, 5′-GAAGAGCTACGAGCTGCCTGA-3′, β-actin reverse, 5′-TGATCTTCATTCTGCTGGGTG-3′. Chemerin PCR conditions were as follows: 95°C for 3 min, 95°C for 1 min, 60°C for 1 min, 72°C for 2 min (40 cycles), and 72°C for 5 min. CXCL10/IP-10 PCR conditions were as follows: 95°C for 5 min, 95°C for 30 s, 64°C for 1 min, 72°C for 30 s (22 cycles), and 72°C for 15 min. CXCL8/IL-8 PCR conditions were as follows: 95°C for 5 min, 95°C for 30 s, 58°C for 30 s, 72°C for 30 s (25 cycles), and 72°C for 15 min. β-actin PCR conditions were as follows: 95°C for 4 min, 95°C for 1 min, 50°C for 30 s, 72°C for 1 min (20 cycles), and 72°C for 10 min. Amplified products were subjected to electrophoresis on 2% agarose gels and stained with ethidium bromide. The identity of the chemerin transcript was confirmed by sequencing. HUVECs were cultured as specified before; human iliac artery ECs (27, 28) were grown in E199 with 10% FCS, 10% human serum; human dermal microvascular ECs were obtained from PromoCell GmbH and cultured following the manufacturer's instructions; immortalized human dermal microvascular ECs (29) were grown in E199 with 10% FCS, 10% human serum; human bone marrow microvascular ECs (30) were grown in E199 as HUVECs.
Tissues and reagents
Surgical specimens from human tissues including normal skin (obtained from abdominal plastic surgery), skin biopsies from nine cases of LE, reactive tonsils, and lymph nodes (removed for diagnostic purposes) were analyzed. Each specimen was snap frozen in isopentane precooled to liquid nitrogen temperature and stored at −80°C. Thin cryostat sections were air dried overnight at room temperature and fixed in acetone for 10 min before staining. Tissue expression of ChemR23 was evaluated by immunohistochemistry using two anti-ChemR23 monoclonal antibodies, namely 4C7 (IgG2b; dilution 1:15) and 1H2 (IgG2a; 1:15; reference 10). The indirect streptavidin–biotin complex immunoperoxidase technique was applied using a biotinylated anti-Ig multilinks secondary antibody (1:20 dilution in Tris-HCl buffer, pH 7.4; Biogenex). The 4C7 and 1H2 moAbs properly reacted on frozen sections; no signal was observed in formalin-fixed tissue sections. Tissue expression of chemerin was detected using two mouse monoclonal antibodies (clones 227C and 14G10; both IgG1; dilution 1:30; 2-h incubation), directed against the peptide Cys-QRAGEDPHSFYFPGQFAFS, a common peptide for both active chemerin and prochemerin.
Double immunofluorescence stainings were performed using a set of primary antibodies, including CD123 (clone 7G3; mouse IgG2a; dilution 1:40; BD Biosciences), DC-SIGN (mouse IgG2b; dilution 1:5; BD Biosciences), CLA (clone Heca452; rat IgM; dilution 1:100; BD Biosciences), CD1a (010, IgG1, dilution 1:30; Immunotech), Langerin (provided by G. Trinchieri, Schering-Plough, Dardilly, France), and mannose receptor (clone 3.29B1; mouse IgG1; dilution 1:50). Appropriate Texas red– and FITC-conjugated isotype-specific secondary antibodies were used to reveal the primary antibodies and sections were examined with a fluorescence microscope Olympus BX60, equipped with a Nikon digital camera. Anti–human podoplanin Ab (clone 18H5, dilution 1:30; Abcam) recognizes lymphatic endothelium and was tested on frozen samples of breast carcinoma. This work was conducted in accordance with a protocol approved by the Spedali Civili of Brescia Institutional Ethical Board and informed consent was obtained from all patients.
Acknowledgments
We thank M. Cella for the MR moAb.
F. Gentili is supported by “Fondazione Beretta,” and E. Riboldi is supported by “Centro di studio per il trattamento dello scompenso cardiaco.” We also thank the Associazione Italiana per la Ricerca sul Cancro, Ministero dell'Istruzione Università e Ricerca, the Association for International Cancer Research (grant no. 04-223), and “Fondazione Berlucchi” for financial support to S. Sozzani. This work was also supported by the Belgian program on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, the LifeSciHealth (grant no. LSHB-CT-2003-503337) program of the European Community, the Fonds de la Recherche Scientifique Médicale of Belgium, Télévie, and the Fondation Médicale Reine Elisabeth to M. Parmentier. The authors assume the scientific responsibility. V. Wittamer was supported by a fellowship of the FIRST-Industrie program of the Walloon Region. D. Communi is Research Associate of the Belgian Fonds National de la Recherche Scientifique.
The authors have no conflicting financial interests.
References
- 1.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 2.Steinman, R.M., D. Hawiger, and M.C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711. [DOI] [PubMed] [Google Scholar]
- 3.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D.W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165:6037–6046. [DOI] [PubMed] [Google Scholar]
- 4.Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. [DOI] [PubMed] [Google Scholar]
- 5.Facchetti, F., W. Vermi, D. Mason, and M. Colonna. 2003. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 443:703–717. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., B. Pulendran, R. Steinman, and K. Palucka. 2000. Will the making of plasmacytoid dendritic cells in vitro help unravel their mysteries? J. Exp. Med. 192:F39–F44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14:373–379. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau, J., V. Pascual, and A.K. Palucka. 2004. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 20:539–550. [DOI] [PubMed] [Google Scholar]
- 9.Penna, G., S. Sozzani, and L. Adorini. 2001. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J. Immunol. 167:1862–1866. [DOI] [PubMed] [Google Scholar]
- 10.Wittamer, V., J.D. Franssen, M. Vulcano, J.F. Mirjolet, E. Le Poul, I. Migeotte, S. Brezillon, R. Tyldesley, C. Blanpain, M. Detheux, et al. 2003. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198:977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittamer, V., F. Gregoire, P. Robberecht, G. Vassart, D. Communi, and M. Parmentier. 2004. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J. Biol. Chem. 279:9956–9962. [DOI] [PubMed] [Google Scholar]
- 12.Krug, A., R. Uppaluri, F. Facchetti, B.G. Dorner, K.C. Sheehan, R.D. Schreiber, M. Cella, and M. Colonna. 2002. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J. Immunol. 169:6079–6083. [DOI] [PubMed] [Google Scholar]
- 13.Vanbervliet, B., N. Bendriss-Vermare, C. Massacrier, B. Homey, O. de Bouteiller, F. Briere, G. Trinchieri, and C. Caux. 2003. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J. Exp. Med. 198:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagpal, S., S. Patel, H. Jacobe, D. DiSepio, C. Ghosn, M. Malhotra, M. Teng, M. Duvic, and R.A. Chandraratna. 1997. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Invest. Dermatol. 109:91–95. [DOI] [PubMed] [Google Scholar]
- 15.Adams, A.E., Y. Abu-Amer, J. Chappel, S. Stueckle, F.P. Ross, S.L. Teitelbaum, and L.J. Suva. 1999. 1,25 dihydroxyvitamin D3 and dexamethasone induce the cyclooxygenase 1 gene in osteoclast-supporting stromal cells. J. Cell. Biochem. 74:587–595. [PubMed] [Google Scholar]
- 16.Mantovani, A., S. Sozzani, M. Locati, P. Allavena, and A. Sica. 2002. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23:549–555. [DOI] [PubMed] [Google Scholar]
- 17.Engering, A., S.J. Van Vliet, K. Hebeda, D.G. Jackson, R. Prevo, S.K. Singh, T.B. Geijtenbeek, H. Van Krieken, and Y. Van Kooyk. 2004. Dynamic populations of dendritic cell-specific ICAM-3 grabbing nonintegrin-positive immature dendritic cells and liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive endothelial cells in the outer zones of the paracortex of human lymph nodes. Am. J. Pathol. 164:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soilleux, E.J., L.S. Morris, G. Leslie, J. Chehimi, Q. Luo, E. Levroney, J. Trowsdale, L.J. Montaner, R.W. Doms, D. Weissman, et al. 2002. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J. Leukoc. Biol. 71:445–457. [PubMed] [Google Scholar]
- 19.Geijtenbeek, T.B., D.J. Krooshoop, D.A. Bleijs, S.J. van Vliet, G.C. van Duijnhoven, V. Grabovsky, R. Alon, C.G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353–357. [DOI] [PubMed] [Google Scholar]
- 20.Geissmann, F., M.C. Dieu-Nosjean, C. Dezutter, J. Valladeau, S. Kayal, M. Leborgne, N. Brousse, S. Saeland, and J. Davoust. 2002. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 196:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. [DOI] [PubMed] [Google Scholar]
- 22.von Andrian, U.H., and T.R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878. [DOI] [PubMed] [Google Scholar]
- 23.Miyasaka, M., and T. Tanaka. 2004. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat. Rev. Immunol. 4:360–370. [DOI] [PubMed] [Google Scholar]
- 24.Wollenberg, A., M. Wagner, S. Gunther, A. Towarowski, E. Tuma, M. Moderer, S. Rothenfusser, S. Wetzel, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Invest. Dermatol. 119:1096–1102. [DOI] [PubMed] [Google Scholar]
- 25.Breiteneder-Geleff, S., A. Soleiman, H. Kowalski, R. Horvat, G. Amann, E. Kriehuber, K. Diem, W. Weninger, E. Tschachler, K. Alitalo, and D. Kerjaschki. 1999. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 154:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermi, W., R. Bonecchi, F. Facchetti, D. Bianchi, S. Sozzani, S. Festa, A. Berenzi, M. Cella, and M. Colonna. 2003. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J. Pathology. 200:255–268. [DOI] [PubMed] [Google Scholar]
- 27.de Fouw, N.J., V.W. van Hinsbergh, Y.F. de Jong, F. Haverkate, and R.M. Bertina. 1987. The interaction of activated protein C and thrombin with the plasminogen activator inhibitor released from human endothelial cells. Thromb. Haemost. 57:176–182. [PubMed] [Google Scholar]
- 28.van Hinsbergh, V.W., D. Binnema, M.A. Scheffer, E.D. Sprengers, T. Kooistra, and D.C. Rijken. 1987. Production of plasminogen activators and inhibitor by serially propagated endothelial cells from adult human blood vessels. Arteriosclerosis. 7:389–400. [DOI] [PubMed] [Google Scholar]
- 29.Ades, E.W., F.J. Candal, R.A. Swerlick, V.G. George, S. Summers, D.C. Bosse, and T.J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Invest. Dermatol. 99:683–690. [DOI] [PubMed] [Google Scholar]
- 30.Candal, F.J., S. Rafii, J.T. Parker, E.W. Ades, B. Ferris, R.L. Nachman, and K.L. Kellar. 1996. BMEC-1: a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc. Res. 52:221–234. [DOI] [PubMed] [Google Scholar]