Abstract
Osteoclasts are bone-resorbing cells that play a pivotal role in bone remodeling. Osteoclasts form large multinuclear giant cells by fusion of mononuclear osteoclasts. How cell fusion is mediated, however, is unclear. We identify the dendritic cell–specific transmembrane protein (DC-STAMP), a putative seven-transmembrane protein, by a DNA subtraction screen between multinuclear osteoclasts and mononuclear macrophages. DC-STAMP is highly expressed in osteoclasts but not in macrophages. DC-STAMP–deficient mice were generated, and osteoclast cell fusion was completely abrogated in homozygotes despite normal expression of osteoclast markers and cytoskeletal structure. As osteoclast multinucleation was restored by retroviral introduction of DC-STAMP, loss of cell fusion was directly attributable to a lack of DC-STAMP. Defects in osteoclast multinucleation reduce bone-resorbing activity, leading to osteopetrosis. Similar to osteoclasts, foreign body giant cell formation by macrophage cell fusion was also completely abrogated in DC-STAMP–deficient mice. We have thus identified an essential regulator of osteoclast and macrophage cell fusion, DC-STAMP, and an essential role of osteoclast multinucleation in bone homeostasis.
Osteoclasts are unique bone-resorbing cells, and mice carrying a mutant gene responsible for osteoclast formation exhibit the osteopetrotic phenotype (1, 2). Foreign body giant cells (FBGCs), which are monocyte/macrophage lineage cells, are generated in response to foreign bodies at the site of implantation (3). Both osteoclasts and FBGCs form multinuclear cells by fusion of monocyte/macrophage lineage cells. Mononuclear macrophages recognize each other and fuse to form multinuclear giant cells; thus, cell surface molecules are considered to be fusion-mediating molecules in osteoclasts and macrophages. E-Cadherin and macrophage fusion receptor have been identified as fusion molecules for osteoclasts and macrophages through the use of neutralizing antibodies (4, 5). CD44, CD9, and CD81 are also candidates for cell fusion molecules; however, defects in multinucleation of osteoclasts and macrophages have not been identified by gene targeting (6, 7).
Dendritic cell–specific transmembrane protein (DC-STAMP) is a seven-transmembrane protein originally identified in dendritic cells or IL-4–stimulated macrophages (8, 9). Recently, it has been reported to induce differentiation of osteoclasts (10). However, the role of DC-STAMP in cell–cell fusion and in vivo bone formation remains unknown. We generated DC-STAMP–deficient mice, demonstrating that multinucleation of osteoclasts and macrophages is completely abrogated in DC-STAMP−/− mice.
Results and Discussion
Identification and gene targeting of DC-STAMP
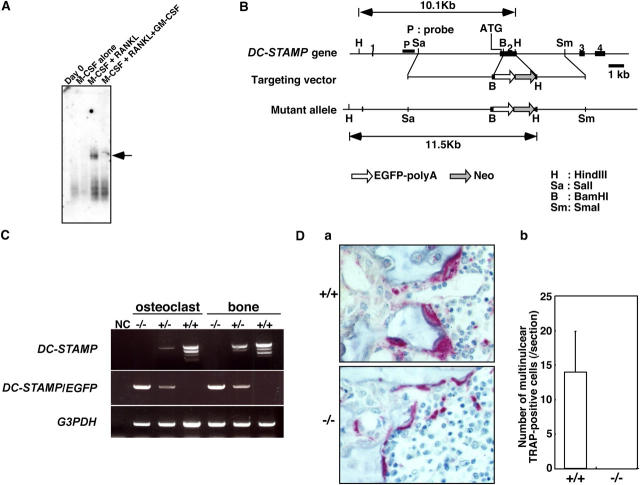
To identify molecules functioning in cell fusion, we undertook subtractive screening between multinuclear osteoclasts and mononuclear macrophages, both of which are derived from common precursor cells. Multinuclear osteoclasts were induced by macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL); macrophages, which do not fuse, were induced by M-CSF alone (11). The screen identified DC-STAMP (clone SU166), also known as IL-4–induced gene (9), which is specifically expressed in osteoclasts among cells of the monocyte/macrophage lineage (Fig. 1 A). Weak expression of DC-STAMP was detected in activated dendritic cells stimulated with granulocyte/M-CSF (GM-CSF) and RANKL (unpublished data), consistent with the fact that DC-STAMP was also cloned from a dendritic cell cDNA library (8). We identified two isoforms of DC-STAMP expressed at equivalent levels in osteoclasts: a full-length form (available from GenBank/EMBL/DDBJ under accession no. AY517483) and a splice variant lacking the third and fourth transmembrane domains and designated DC-STAMP-splice (available from GenBank/EMBL/DDBJ under accession no. AY517484; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20050645/DC1).
Figure 1.
Identification and gene targeting of DC-STAMP. (A) Specific expression of DC-STAMP (arrow) in osteoclasts (M-CSF + RANKL) was determined among macrophages (M-CSF alone), immature dendritic cells (M-CSF + RANKL + GM-CSF), and osteoclasts derived from common precursor cells by Northern blot analysis. (B) The endogenous DC-STAMP locus (DC-STAMP gene), targeting vector, and targeted locus (mutant allele) are shown. Exons, represented by black boxes, are numbered. The ATG start codon is located in exon 2. The neomycin resistance gene (Neo) and EGFP-polyA sequence are indicated. The probe used for Southern hybridization is indicated (P). The EGFP-polyA sequence and Neo cassette were inserted into exon 2 to yield a construct encoding a DC-STAMP (1–55)–EGFP chimeric protein. (C) RT-PCR analysis of the expression of DC-STAMP and the chimeric DC-STAMP-EGFP genes in osteoclasts and bones from mice of the indicated genotypes. (D) TRAP staining in tibias of 8-wk-old DC-STAMP+/+ or DC-STAMP−/− mice (a) and the number of multinuclear TRAP-positive cells (b). Multinuclear osteoclast formation was abrogated in DC-STAMP−/− mice. Values represent SD.
GM-CSF is a potent inhibitor of osteoclastogenesis: cells treated with GM-CSF differentiate into immature dendritic cells that do not fuse even in the presence of M-CSF and RANKL (12). Expression of DC-STAMP was abolished in the presence of GM-CSF (Fig. 1 A), suggesting that DC-STAMP regulates cell fusion. To determine its function, we undertook gene targeting of DC-STAMP (Fig. 1 B and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20050645/DC1). The ATG start codon of DC-STAMP is located in exon 2. The targeting vector was constructed to insert an enhanced green fluorescent protein (EGFP) sequence into exon 2 such that EGFP was in frame with amino acid Leu55 of DC-STAMP. This mutation resulted in deletion of the second through fifth transmembrane domains, resulting in mutation of both the full-length and splice variant form of DC-STAMP. Heterozygotes were mated, and DC-STAMP–nulls were generated at Mendelian ratios as determined at 4–8 wk of age (−/−, 78 [23.3%]; +/−, 179 [53.4%]; and +/+, 78 [23.3%]). Targeted disruption of both the full-length and splice variant forms of DC-STAMP was confirmed by RT-PCR analysis using common primer sets to detect both forms (Fig. 1 C).
Multinucleation is abrogated in DC-STAMP −/− osteoclasts
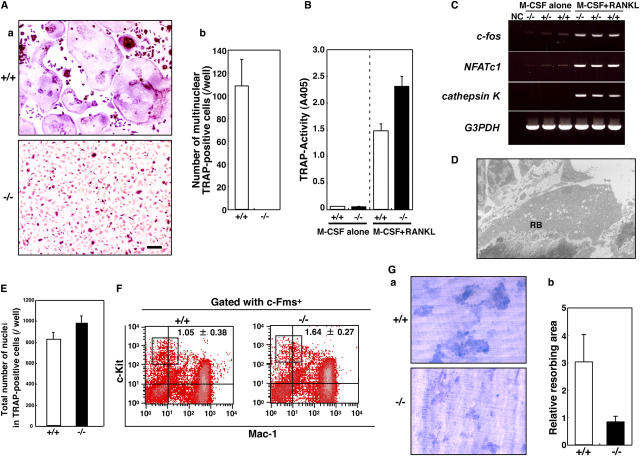
Notably, no multinuclear osteoclasts were identified in bone sections of DC-STAMP homozygotes (Fig. 1 D). To confirm these observations, we performed in vitro culture of osteoclasts with an osteoclast marker. Tartrate-resistant acid phosphatase (TRAP)–positive multinuclear osteoclasts were generated from mononuclear cells derived from wild-type mice in the presence of M-CSF and RANKL, but multinucleation was completely abrogated in osteoclasts derived from DC-STAMP−/− mouse mononuclear cells (Fig. 2 A). Interestingly, mononuclear TRAP-positive cells were present in DC-STAMP−/− mice as shown in Fig. 2 A, suggesting that osteoclastogenesis occurs without cell fusion in DC-STAMP−/− osteoclasts. In fact, the total TRAP activity of TRAP-positive cells derived from DC-STAMP−/− mice was higher than that seen in wild-type mice (Fig. 2 B).
Figure 2.
Lack of multinucleation in DC-STAMP−/−osteoclasts. (A) TRAP expression was induced, but multinucleation was completely abrogated in DC-STAMP−/− osteoclasts. TRAP staining (a) and the number of multinuclear TRAP-positive cells (b) are shown. Values represent SD. Bar, 50 μm. (B) TRAP solution assay of macrophages (M-CSF alone) and osteoclasts (M-CSF + RANKL). (C) Expression of c-fos, NFATc1, or cathepsin K in macrophages (M-CSF alone) or osteoclasts (M-CSF + RANKL) derived from DC-STAMP+/+, DC-STAMP+/−, or DC-STAMP−/− mice was analyzed by RT-PCR. NC, no template control. (D) Ruffled border formation was detected in osteoclasts of DC-STAMP−/− tibial sections under electron microscopy. RB, ruffled border. (E) Total number of nuclei in cultured osteoclasts. Values represent SD. (F) The percent frequency of a population of osteoclast precursor cells (boxes, c-Fms+c-Kit+Mac-1low) is shown as the mean ± SD. (G) Resorbing lacunae were visualized by toluidine blue O staining (a), and relative resorbing areas were scored (b). Values represent SD.
RANK signals play an important role in osteoclast differentiation, including multinucleation and bone resorption via up-regulating NFATc1 expression (13–15). The expression of osteoclast markers and transcription factors required for osteoclast differentiation, including RANK and NFATc1, was induced by M-CSF and RANKL in DC-STAMP−/− cells, as well as in wild-type cells (Fig. 2 C and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20050645/DC1). Ruffled borders and actin rings, which are highly specific cytoskeletal features seen in osteoclasts, were formed in DC-STAMP−/− osteoclasts, in further support of the fact that DC-STAMP is not involved in mononuclear osteoclast development (Fig. 2 D and not depicted).
Multinuclear cell formation is dependent on cell density. The total number of nuclei in cultures of osteoclasts was comparable between DC-STAMP−/− and wild-type mice, indicating that inhibition of multinucleation was not caused by reduced cell density in DC-STAMP−/− cell cultures (Fig. 2 E). Furthermore, the proportion of osteoclast precursor cells (c-Fms+c-Kit+Mac1low) in DC-STAMP−/− mice was equivalent to that seen in wild-type mice (Fig. 2 F), indicating that osteoclast differentiation from precursors to mononuclear osteoclasts was not affected. Osteoclasts derived from DC-STAMP−/− mice were mononuclear and exhibited bone-resorbing activity (Fig. 2 G); however, the bone-resorbing area exhibited in cultures of DC-STAMP−/− osteoclasts was small compared with wild-type cells (Fig. 2 G), even though the total number of nuclei was equal (Fig. 2 E), suggesting that multinucleation enhances the resorbing efficiency of osteoclasts.
Bone mass is increased in DC-STAMP −/− mice
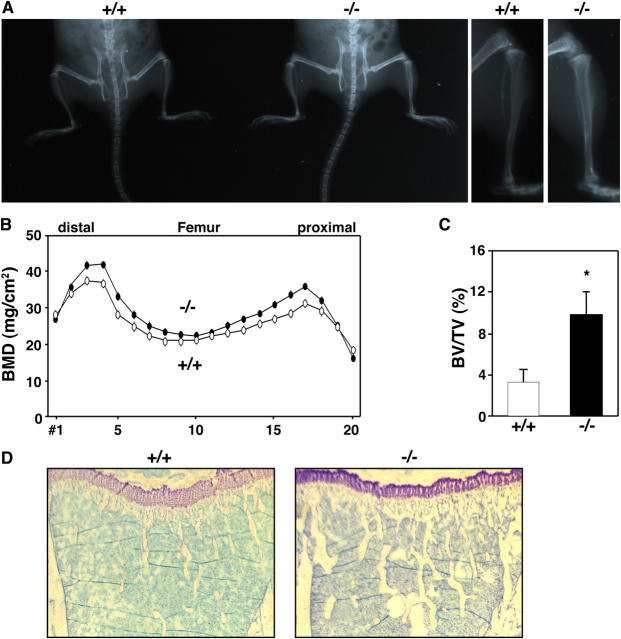
Decreased bone resorption seen in DC-STAMP−/− compared with wild-type mice was observed as increased bone mass (Fig. 3). Soft x-ray analysis showed an elevated radioopacity in DC-STAMP−/− mice (Fig. 3 A). Bone mineral density (BMD) and bone volume per tissue volume (BV/TV) was increased in DC-STAMP−/− mice compared with wild-type mice (Fig. 3, B and C). Furthermore, trabecular bone mass was increased in DC-STAMP−/− mice (Fig. 3 D), indicating that loss of osteoclast cell fusion leads to an osteopetrotic phenotype. However, because tooth eruption and formation of the bone marrow cavity was normal in DC-STAMP−/− mice, such osteopetrosis was relatively mild compared with mouse mutants exhibiting strong osteopetrotic phenotypes such as c-Fos knockout or op/op mice (2, 16). Collectively, our observations indicate that bone-resorbing activity is stimulated by cell fusion and its disruption affects the bone as indicated by osteopetrosis.
Figure 3.
Increased bone mass in DC-STAMP−/− mice. (A) Soft x-ray analysis. Elevated radioopacity was observed in DC-STAMP−/− mice. (B) Increased BMD (mg/cm2) was detected in DC-STAMP−/− mice (closed circles) compared with DC-STAMP+/+ mice (open circles) by the dual energy x-ray absorptiometry method measured in 20 longitudinal divisions of femurs from 8-wk-old female mice. (C) Bone morphometric analysis of tibia from 8-wk-old mice. The percent BV/TV was elevated in DC-STAMP−/− mice compared with wild-type mice (*, P < 0.01). Values represent SD. (D) Increased numbers of trabeculae were observed in tibial sections of DC-STAMP−/− mice by toluidine blue staining.
DC-STAMP is indispensable for cell fusion of osteoclasts
To exclude the possibility that the lack of cell fusion seen in DC-STAMP−/− mice could be caused by reduced expression of other factors, we analyzed expression of factors known to function in cell fusion (4–6, 17). Such factors, which included macrophage fusion receptor, E-cadherin, meltrin-α, CD44, and CD47, were expressed similarly in DC-STAMP−/− as in wild-type mice (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20050645/DC1). Integrin αvβ3 plays a role in osteoclast adhesion and differentiation (18, 19); however, the expression level of adhesion molecules was unchanged in DC-STAMP−/− relative to wild-type osteoclasts (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20050645/DC1).
To confirm that DC-STAMP is indispensable for osteoclast fusion, DC-STAMP was transduced into osteoclast progenitor cells isolated from DC-STAMP−/− mice using retrovirus infection. This treatment effectively rescued osteoclast cell fusion, indicating that DC-STAMP is required for osteoclast cell fusion (Fig. 4 A). Transduction of the splice variant failed to induce cell fusion (Fig. 4 A). Currently, the function of that construct is unclear.
Figure 4.
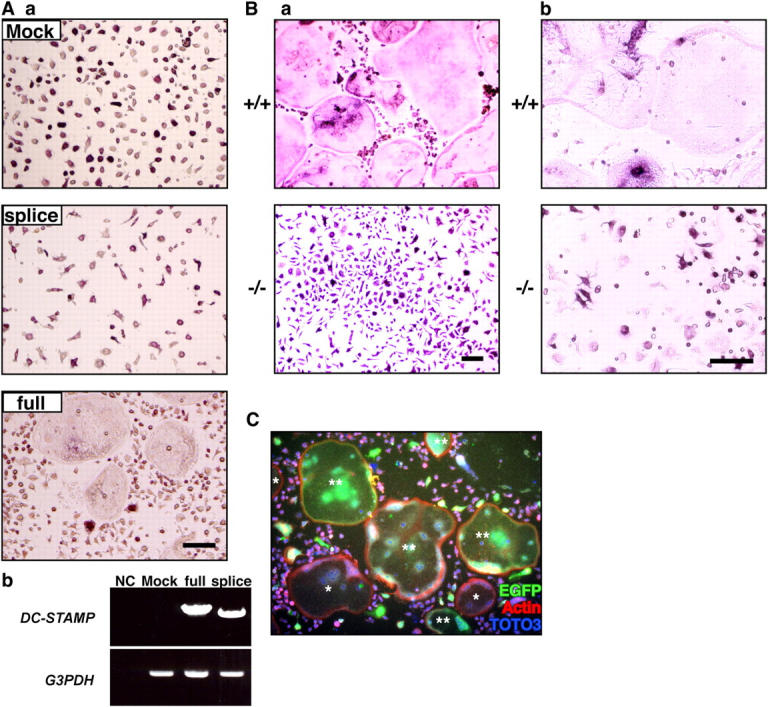
Rescue of cell fusion in DC-STAMP−/− osteoclasts. (A) Osteoclast precursors from DC-STAMP−/− mice were transduced by retrovirus expressing full-length (full) or a splice variant form (splice) of DC-STAMP (a). The indicated form of DC-STAMP expressed via retroviral infection was detected in DC-STAMP−/−osteoclasts by RT-PCR analysis (b). NC, no template control. Bar, 50 μm. (B) No multinuclear TRAP-positive cells were induced in DC-STAMP−/− cells by high density culture (a) or cocultivation with osteoblasts (b). Bar, 50 μm. (C) 2 × 104 cells/well of M-CSF–dependent osteoclast precursors from DC-STAMP−/− and DC-STAMP+/+ mice were mixed and cultured in the presence of M-CSF and RANKL for 4 d in 96-well culture plates. Cells were stained by rabbit anti-EGFP antibody followed by Alexa 488 (EGFP)–conjugated anti–rabbit IgG antibody with rhodamine-conjugated phalloidin for F-actin staining and TOTO3 for nuclear staining. Note the formation of EGFP-positive multinuclear cells. *, EGFP-negative multinuclear cells; **, EGFP-positive multinuclear cells.
DC-STAMP is a member of the seven-transmembrane receptor family, and it may function as a chemokine receptor; if that were the case, loss of cell fusion could be caused by reduced cell contact caused by impaired cell migration. To test this hypothesis, cells were cultured at high density to induce migration-independent cell contact (18). Dramatic multinucleation was induced in osteoclasts derived from wild-type mice, whereas no fusion occurred in DC-STAMP−/− osteoclasts, indicating that fusion is inhibited even under cell-to-cell contact conditions (Fig. 4 B). Osteoblasts play a key role in osteoclast differentiation through cytokines such as M-CSF and RANKL or cell-adhesion signals. In the case of DC-STAMP−/− mice, the fusion signal was not rescued in vivo or by cocultivation with osteoblasts (Fig. 4 B). High doses of M-CSF have been reported to restore the osteoclastogenesis defect seen in integrin β3−/− mice or DAP12−/− mice in vitro (19, 20). Long-term culture or high doses of M-CSF and RANKL did not induce cell fusion, and multinucleation was still completely abrogated in DC-STAMP−/− osteoclasts (unpublished data).
Next, we asked how cell fusion is mediated through DC-STAMP. Supernatants of wild-type osteoclast cultures did not induce fusion in DC-STAMP−/− osteoclasts, and supernatants from DC-STAMP−/− osteoclast cultures did not inhibit multinucleation of wild-type osteoclasts (unpublished data). Thus, cell fusion induced by DC-STAMP is not mediated via unknown soluble factors but by direct interaction between DC-STAMP and a putative ligand expressed by mononuclear osteoclasts. In that case, a homogeneous population of osteoclast precursors likely serves as founder and fusion-competent cells, similar to myoblasts. To address this possibility, mixed cultures of osteoclast precursors isolated from DC-STAMP−/− and wild-type mice were created. Interestingly, multinuclear EGFP-expressing cells were induced in these cultures in the presence of M-CSF plus RANKL, suggesting that fusion between EGFP-positive DC-STAMP−/− cells and wild-type osteoclast precursor cells was induced (Fig. 4 C). These data indicate two possibilities: (a) a putative DC-STAMP ligand is expressed on osteoclasts, or (b) expression of DC-STAMP is required only in fusion founder cells and not in both founder and fusion-competent cells. Heterogeneous EGFP expression in DC-STAMP–EGFP knock-in cells during the course of osteoclast differentiation (unpublished data) supports the latter possibility. Thus, DC-STAMP might be involved in cell–cell interactions in a receptor–ligand fashion.
Multinucleation is abrogated in DC-STAMP−/− FBGCs
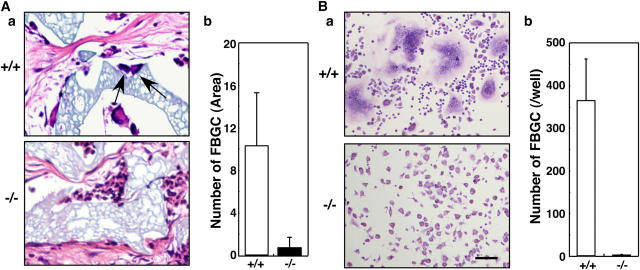
DC-STAMP may also be required to form other monocyte-derived multinucleated giant cells, such as FBGCs. FBGCs are induced by fusion of macrophages in response to foreign bodies at the site of implantation (3). Interestingly, multinucleation of FBGCs induced by implantation of foreign bodies was also inhibited in DC-STAMP−/− mice (Fig. 5 A). FBGCs are formed in vitro from monocyte progenitors by stimulation with cytokines such as IL-3 and IL-4 (21). FBGC formation by combined IL-3 and IL-4 treatment was abrogated in DC-STAMP−/− cells (Fig. 5 B), indicating that DC-STAMP is universally required for monocyte lineage cell fusion. The evidence that DC-STAMP is overexpressed in giant cell tumors, which are primary bone neoplasm-containing, multinucleated osteoclast-like giant cells compared with normal tissues (22), supports involvement of DC-STAMP in pathological giant cell formation.
Figure 5.
Macrophage cell fusion is abrogated in DC-STAMP−/− mice. (A) Histological analysis of implants in DC-STAMP−/− and DC-STAMP+/+ mice. Hematoxylin and eosin staining (a) and the number of FBGCs (b) are shown. Arrows indicate FBGCs. (B) FBGCs were induced and stained with May-Gruenwald Giemsa (a), and the number of FBGCs containing more than three nuclei was scored (b). Values represent SD. Bar, 50 μm.
DC-STAMP was originally isolated from dendritic cells, which do not fuse (8). We are now evaluating the function of dendritic cells in DC-STAMP–deficient mice. Dendritic cells differentiate from the same precursors as osteoclasts, and stimulation of dendritic cell differentiation inhibits osteoclast formation, including multinucleation (12). These findings suggest that expression of a putative DC-STAMP ligand might require osteoclast induction stimuli, or that intracellular events after DC-STAMP binding to such a ligand in osteoclasts play an important role in cell fusion. DC-STAMP has been reported to be expressed on the surface of osteoclasts (10) and in the endoplasmic reticulum of DC-STAMP–transduced dendritic cells or 293 cells (23). Phagosomes fuse with the endoplasmic reticulum in dendritic cells (24), suggesting that DC-STAMP may function in such a cytoplasmic membrane fusion. Thus, differences in DC-STAMP localization may explain differences of multinucleation between osteoclasts/FBGCs and dendritic cells, both of which express DC-STAMP. Identification of a DC-STAMP ligand should resolve these questions.
Materials and Methods
Targeted disruption of the DC-STAMP gene
The EGFP-polyA gene was inserted into the BamHI site in exon 2 in frame with the juxtamembrane region of the first transmembrane domain of DC-STAMP. Exon 2 was replaced by EGFP-polyA and the PGK-Neo gene to yield a targeting vector. The Ethics Review Committee for Animal Experimentation of Keio University approved the experimental protocol.
Analysis of skeletal morphology
BMD (mg/cm2) and bone radiographs of 8-wk-old DC-STAMP+/+ and DC-STAMP−/− littermates were measured by the dual energy x-ray absorptiometry method using a DCS-600R (Aloka Co. Ltd.) and a soft x-ray apparatus (Softex Co. Ltd.), respectively. BV/TV was determined by bone morphometric analysis.
Cell culture
Macrophages and osteoclasts were induced in the presence of M-CSF and RANKL or by cocultivation of osteoclast precursor cells with osteoblasts as previously described (11). Osteoclastogenesis was evaluated by either TRAP staining or a TRAP solution assay as previously described (18). For the pit formation assay, retroviral transduction, and FBGC formation assay, unfractionated BM cells were pretreated with 50 ng/ml M-CSF for 2 d, and M-CSF–dependent cells were harvested and used as progenitor cells. The resorbing lacunae were visualized by toluidine blue O staining (15), and the relative resorbing area was scored under a microscope (IX70; Olympus). Preparation of retrovirus was as previously described (12). For FBGC induction, M-CSF–dependent progenitors were cultured in the presence of 100 ng/ml IL-3 (Wako) and 100 ng/ml IL-4 (R&D Systems) for 4 d, and May-Gruenwald Giemsa staining was performed.
Immunohistochemical staining
Cells cultured in dishes were fixed with 4% paraformaldehyde/PBS and stained with the anti-EGFP antibody (Mo Bio Laboratories, Inc.), followed by Alexa 488–conjugated anti–rabbit IgG antibody (Invitrogen) with TOTO3 (Invitrogen) for nuclear staining, and F-actin was stained by rhodamine-conjugated phalloidin (Invitrogen). For electron microscopic analysis, ultrathin sections were stained with uranyl acetate and lead citrate and observed with an electron microscope (H-7500; Hitachi).
In vivo FBGC formation
Ivalon surgical product (M-PACT, 10 × 10 × 0.5 mm; Eudora) was implanted s.c. in DC-STAMP−/− and DC-STAMP+/+ mice. After 12 d, implants were harvested, and histological analyses were performed by hematoxylin and eosin staining. Multinuclear cells containing more than three nuclei that adhered to implants were scored as FBGC in five independent fields per section isolated from four independent implanted mice.
RT-PCR analysis
Total RNA was extracted from cultured macrophages or osteoclasts using an RNeasy mini kit (QIAGEN). First-strand cDNA was prepared, and PCR was performed as previously described (18). Primer sets used to detect various molecules are as previously described (12, 15, 18).
Online supplemental material
Figs. S1 and S2 describe the amino acid sequence information of DC-STAMP and the generation of DC-STAMP/EGFP knock-in mice, respectively. Fig. S3 shows the expression of transcription factors, Fig. S4 depicts candidate molecules for cell fusion, and Fig. S5 shows adhesion molecules in DC-STAMP−/−, +/−, or +/+ osteoclasts. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050645/DC1.
Acknowledgments
We thank K. Matasuo and H. Saya for helpful discussions and Y. Sato and A. Kumamkubo for technical support.
T. Suda was supported by a grant-in-aid from the Specially Promoted Research of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The authors have no conflicting financial interests.
M. Yagi and T. Miyamoto contributed equally to this work.
References
- 1.Boyce, B.F., T. Yoneda, C. Lowe, P. Soriano, and G.R. Mundy. 1992. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Invest. 90:1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grigoriadis, A.E., Z.Q. Wang, M.G. Cecchini, W. Hofstetter, R. Felix, H.A. Fleisch, and E.F. Wagner. 1994. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 266:443–448. [DOI] [PubMed] [Google Scholar]
- 3.Kyriakides, T.R., M.J. Foster, G.E. Keeney, A. Tsai, C.M. Giachelli, I. Clark-Lewis, B.J. Rollins, and P. Bornstein. 2004. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am. J. Pathol. 165:2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbalaviele, G., H. Chen, B.F. Boyce, G.R. Mundy, and T. Yoneda. 1995. The role of cadherin in the generation of multinucleated osteoclasts from mononuclear precursors in murine marrow. J. Clin. Invest. 95:2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saginario, C., H. Sterling, C. Beckers, R. Kobayashi, M. Solimena, E. Ullu, and A. Vignery. 1998. MFR, a putative receptor mediating the fusion of macrophages. Mol. Cell. Biol. 18:6213–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries, T.J., T. Schoenmaker, W. Beertsen, R. van der Neut, and V. Everts. 2005. Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J. Cell. Biochem. 94:954–966. [DOI] [PubMed] [Google Scholar]
- 7.Takeda, Y., I. Tachibana, K. Miyado, M. Kobayashi, T. Miyazaki, T. Funakoshi, H. Kimura, H. Yamane, Y. Saito, and H. Goto. 2003. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J. Cell Biol. 161:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartgers, F.C., J.L. Vissers, M.W. Looman, C. van Zoelen, C. Huffine, C.G. Figdor, and G.J. Adema. 2000. DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur. J. Immunol. 30:3585–3590. [DOI] [PubMed] [Google Scholar]
- 9.Staege, H., A. Brauchlin, G. Schoedon, and A. Schaffner. 2001. Two novel genes FIND and LIND differentially expressed in deactivated and Listeria-infected human macrophages. Immunogenetics. 53:105–113. [DOI] [PubMed] [Google Scholar]
- 10.Kukita, T., N. Wada, A. Kukita, T. Kakimoto, F. Sandra, K. Toh, K. Nagata, T. Iijima, M. Horiuchi, H. Matsusaki, et al. 2004. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 200:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai, F., T. Miyamoto, O. Ohneda, T. Inada, T. Sudo, K. Brasel, T. Miyata, and D.M. Anderson. 1999. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J. Exp. Med. 190:1741–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto, T., O. Ohneda, F. Arai, K. Iwamoto, S. Okada, K. Takagi, D.M. Anderson, and T. Suda. 2001. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 98:2544–2554. [DOI] [PubMed] [Google Scholar]
- 13.Takayanagi, H., S. Kim, T. Koga, H. Nishina, M. Isshiki, H. Yoshida, A. Saiura, M. Isobe, T. Yokochi, J. Inoue, et al. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 3:889–901. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo, K., D.L. Galson, C. Zhao, L. Peng, C. Laplace, K.Z. Wang, M.A. Bachler, H. Amano, H. Aburatani, H. Ishikawa, et al. 2004. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 279:26475–26480. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto, K., T. Miyamoto, Y. Sawatani, N. Hosogane, I. Hamaguchi, M. Takami, K. Nomiyama, K. Takagi, and T. Suda. 2004. Dimer formation of receptor activator of nuclear factor kappaB induces incomplete osteoclast formation. Biochem. Biophys. Res. Commun. 325:229–234. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida, H., S. Hayashi, T. Kunisada, M. Ogawa, S. Nishikawa, H. Okamura, T. Sudo, L.D. Shultz, and S. Nishikawa. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345:442–444. [DOI] [PubMed] [Google Scholar]
- 17.Han, X., H. Sterling, Y. Chen, C. Saginario, E.J. Brown, W.A. Frazier, F.P. Lindberg, and A. Vignery. 2000. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J. Biol. Chem. 275:37984–37992. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto, T., F. Arai, O. Ohneda, K. Takagi, D.M. Anderson, and T. Suda. 2000. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood. 96:4335–4343. [PubMed] [Google Scholar]
- 19.Faccio, R., S. Takeshita, A. Zallone, F.P. Ross, and S.L. Teitelbaum. 2003. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J. Clin. Invest. 111:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faccio, R., W. Zou, G. Colaianni, S.L. Teitelbaum, and F.P. Ross. 2003. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J. Cell. Biochem. 90:871–873. [DOI] [PubMed] [Google Scholar]
- 21.McNally, A.K., and J.M. Anderson. 1995. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am. J. Pathol. 147:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 22.Skubitz, K.M., E.Y. Cheng, D.R. Clohisy, R.C. Thompson, and A.P. Skubitz. 2004. Gene expression in giant-cell tumors. J. Lab. Clin. Med. 144:193–200. [DOI] [PubMed] [Google Scholar]
- 23.Eleveld-Trancikova, D., V. Triantis, V. Moulin, M.W. Looman, M. Wijers, J.A. Fransen, A.A. Lemckert, M.J. Havenga, C.G. Figdor, R.A. Janssen, et al. 2004. The dendritic cell-derived protein DC-STAMP is highly conserved and localizes to the endoplasmic reticulum. J. Leukoc. Biol. 77:337–343. [DOI] [PubMed] [Google Scholar]
- 24.Guermonprez, P., L. Saveanu, M. Kleijmeer, J. Davoust, P. Van Endert, and S. Amigorena. 2003. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 425:397–402. [DOI] [PubMed] [Google Scholar]