Abstract
Bone remodeling, a coupled process involving bone resorption and formation, is initiated by mechanical signals and is controlled by local and systemic factors that regulate osteoblast and osteoclast differentiation and function. An excess of resorption over formation leads to the bone loss and increased propensity to fracture that is characteristic of osteoporosis. A newly described inhibitor of osteoblast differentiation, Ciz, interferes with bone morphogenic protein signaling. As a consequence, Ciz-deficient mice develop increased bone mass.
The term osteoporosis refers to a group of disorders characterized by low bone mass, poor bone quality, and an increased propensity to fracture (1). Throughout life, bone is continuously being remodeled with resorption of old bone (catabolic process) performed by osteoclasts and deposition of new bone (anabolic process) performed by osteoblasts. Bone remodeling is not a random process and takes place in focal remodeling units comprising osteoblasts, osteoclasts, and their precursors, in which resorption and formation are coupled. Bone resorption is likely the initial event that occurs in response to local mechanical stress signals. The reduction in bone mass in osteoporosis results from an imbalance between resorption and formation in which the rate of resorption exceeds that of formation. It has been suggested that osteoporosis results from a failure of resident bone cells to respond appropriately to mechanical load–bearing signals and to control bone mass and architecture (2). In the majority of conditions that lead to osteoporosis, such as age-associated gonadal hormone deficiency, remodeling rates are high, and although bone formation and resorption are both increased, the rate of bone formation is insufficient to keep up with resorption. Indeed, pharmacologic suppression of bone resorption with estrogens or bisphosphonates, the most widely used approach to treat osteoporosis, converts a high bone remodeling state to a lower remodeling state. This results in a net increase in bone mineral density and a reduction in fracture rate despite the coupled decrease in bone formation. There is evidence, however, that the apparent increase in bone mass takes place not by increased deposition of bone (inorganic mineral phase plus organic matrix) but by a slow increase in deposition of more mineral phase within a previously mineralized matrix (3). Alternatively, an increase in bone mass and reduction in fracture rate can also be achieved by intermittent administration of parathyroid hormone, which increases both bone formation and resorption with formation outpacing resorption (4). It is not yet certain, however, whether the anabolic actions of parathyroid hormone are critically linked to its catabolic actions. Thus, the search is still on for anabolic agents that increase bone formation without affecting resorption or, better still, agents that increase formation yet suppress resorption. The identification of Cas-interacting zinc finger protein (CIZ), in this issue, as a protein that inhibits bone formation without affecting resorption suggests that agents designed to inhibit CIZ might fit the bill (5).
Controls of osteoblast differentiation and function
Studies of the pathophysiology and genetics of skeletal cell differentiation have provided insight into the mechanism by which osteoblasts arise from mesenchymal precursors and osteoclasts arise from hematopoietic precursors (6, 7). The differentiation of osteoblasts is controlled by the transcription factor Runx2 and the transcription factor osterix, which functions downstream of Runx2 (6–8). In the absence of either Runx2 or osterix, no osteoblasts are formed. Mice and humans that carry only a single copy of Runx2 have abnormalities that are specific to certain bones rather than the entire skeleton. Formation and proliferation of preosteoblast cells requires signaling through the Wnt–frizzled–Lrp5 (low density lipoprotein receptor–related protein)–β-catenin signaling pathway (9), and LRP5 deficiencies lead to the development of osteoporosis in both mice and humans (10, 11). The function of mature osteoblasts, including the ability to synthesize extracellular matrix proteins, also requires LRP5 as well as the signaling protein ATF4 (Fig. 1, reference 12).
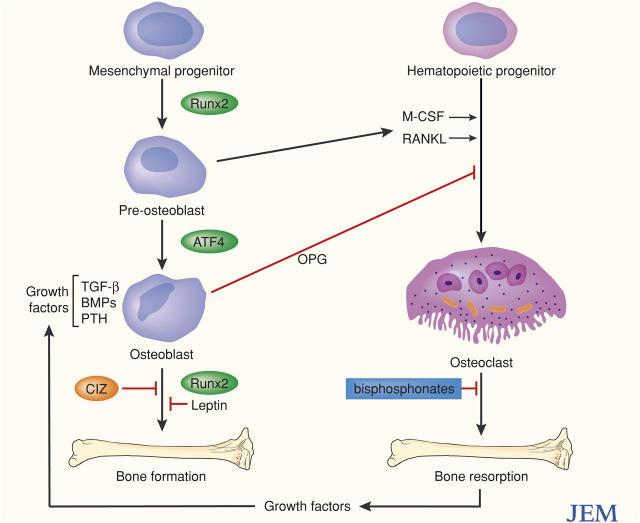
Figure 1.
Cells and ligands in skeletal remodeling. Interactions in bone formation and resorption among osteoblasts and osteoclasts and some factors that modulate their differentiation and function. OPG, osteoprotegerin.
Bone morphogenetic proteins (BMPs), members of a family of secreted growth factors, provide important tissue-specific signals to preosteoblasts that are essential for full osteogenic differentiation (13, 14). Thus, it has been possible to use a reporter system driven by the BMP-2 promoter to screen libraries of chemical compounds and natural products for potential bone anabolic agents (14). One class of drugs identified was the cholesterol-reducing statins, widely used to treat patients with heart disease. There is evidence for interactions among the pathways described. The Wnt/β-catenin signaling pathway, for example, synergizes with the BMP-2 signaling pathway (15). Antagonists and inhibitors also function. The embryonic head inducer, Dickkopf1, binds to Lrp5/6, blocks binding of Wnts, and inhibits signaling through the β-catenin pathway (12). Thus, mutations in human LRP5 that interfere with the Dickkopf1 inhibition appear to cause a high bone mass phenotype (16). There are also suggestions that some of the anabolic effects of parathyroid hormone (PTH) are exerted through suppression of Dickkopf1 expression (17). Leptin, a circulating factor, which binds receptors in the hypothalmus, is another important inhibitor of bone formation. In a startling series of studies, it has been demonstrated that leptin has a powerful antiosteogenic function that is exerted via pathways distinct from those used to regulate body weight (6, 7, 18).
Controls of osteoclast differentiation and function
Osteoclast differentiation requires the binding of macrophage colony-stimulating factor to its receptor as well as the binding of the soluble differentiation factor receptor activator of NF-κB ligand (RANKL) to its receptor (RANK) on osteoclast precursor cells (19). One of the first factors to be cloned that regulates osteoclast differentiation was osteoprotegerin. Osteoprotegerin, originally identified as a novel secreted member of the TNFR super family, was later found to inhibit spontaneous or induced bone resorption and cause osteopetrosis, the converse of osteoporosis. Osteoprotegerin acts as a decoy receptor that binds to RANKL and prevents it from interacting with its receptor (Fig. 1).
RANKL and osteoprotegerin, which are both produced by osteoblasts at different stages of maturity (20), account for some of the signals in osteoblast–osteoclast communication. In conjunction with macrophage colony-stimulating factor, the RANK–RANKL–osteoprotegerin system regulates osteoclast differentiation. Thus, mice and humans deficient in osteoprotegerin have a high rate of bone loss (increased bone resorption that exceeds formation; reference 21). As in other high turnover states, anti-resorptive agents can still reduce both bone formation and resorption and compensate for osteoprotegerin deficiency. The signal(s) that couples resorption and formation remains elusive, although several of the anabolic ligands, such as BMPs and TGF-β, are stored in bone matrix as bone is formed and are released at sites of bone resorption and can thus act on osteoblasts and precursors in the vicinity. Signals from osteoblasts to osteoclasts can be provided by RANKL and osteoprotegerin. In this case, preosteoblasts express a high level of RANKL relative to osteoprotegerin, which stimulates osteoclast differentiation and function. More mature osteoblasts, by contrast, express high levels of osteoprotegerin relative to RANKL, which inhibits osteoclast differentiation and function (20).
The role of CIZ in bone formation
The protein p130cas(Cas) is a docking protein that localizes to focal adhesion plaques (22). Signal transduction in response to mechanical stress in both osteoblasts and osteoclasts is mediated by interaction of integrins in the focal adhesion complex with components of the extracellular matrix. Because the SH3 domain of Cas binds to focal adhesion-associated tyrosine kinase (FAK), it was assumed that localization of Cas to focal adhesions involves FAK. Cas also localizes to focal adhesions in FAK-null cells, and therefore a search was launched for other binding partners. A novel ligand of Cas was identified and named CIZ (Cas-interacting zinc finger protein; reference 22). CIZ is expressed in different mesenchymal cells, including osteoblasts. When overexpressed, CIZ activates the transcription of several genes encoding matrix metalloproteinases. Localization of CIZ to focal adhesions makes it an attractive candidate for integrating mechanical signals, such as those induced by fluid sheer stress, and osteoblast function, but its pathophysiological role had not been investigated in vivo.
In this issue, Morinobu et al. (5) report that Ciz−/−mice have increased trabecular bone mass and conclude that Ciz normally suppresses osteoblast activity through its interactions with BMPs. In Ciz−/−mice, the increased trabecular bone mass was associated with increased rates of bone formation based on the established method of dynamic histomorphometry, which measures the distance between timed systemic pulses of calcein, a fluorescent marker that binds to newly mineralized bone. Bone resorption was not affected in Ciz−/−mice, indicating no suppression of remodeling. Furthermore, in bone marrow cultures from Ciz−/−mice, levels of alkaline phosphatase activity (a marker for functioning osteoblasts) were increased and, in long-term cultures, more mineralized nodules were formed. Nodule formation serves as a cell culture model for deposition of mineralized bone matrix. Consistent with the results of the histomorphometry, Morinobu et al. (5) found that there was no difference in the generation of osteoclasts in Ciz−/−and wild-type marrow cultures. Based in part on earlier work (23), the authors postulated that the effects of Ciz deficiency could be exerted through the BMP pathway and, indeed, they demonstrated that effects of BMP-2 on alkaline phosphatase activity were greater in bone marrow cultures from Ciz−/−mice compared with wild-type mice. Finally, they showed that more orthotopic bone is produced in vivo after intracranial injection of recombinant BMP-2 in Ciz−/−mice than is produced in wild-type mice.
With respect to mechanisms of BMP antagonism, Morinobu et al. (5) hypothesized that Ciz acts by interfering with BMP receptor signaling by acting as an inhibitory Smad protein (Smads belong to a family of proteins that relay signals from the BMP receptor to target genes in the nucleus) rather than interfering with BMP binding to its receptor. Another factor (Tob) also inhibits osteoblast function by acting as a Smad suppressor (24). Whether Ciz can inhibit osteoblast function by affecting the Wnt–β–catenin signaling pathway in synergy with BMP signaling remains to be shown.
The study of the Ciz-deficient mice is a further illustration of the progress over the past two decades in understanding skeletal biology. It is possible that molecular and genetic observations, including those described here, will lead to identification of useful new therapeutic targets for treatment of common bone diseases such as osteoporosis. Even though anti-resorptive agents have great efficacy, there is potential usefulness for the development of anabolic agents that could increase bone formation without affecting resorption, as mentioned earlier. It is conceivable that drugs designed to antagonize CIZ could help to achieve this goal.
References
- 1.Rodan, G.A., L.G. Raisz, and J.P. Bilezikian. 2002. Pathophysiology of osteoporosis. Principles of Bone Biology. J.P. Bilezikian, L.G. Raisz, and G.A. Rodan, editors. San Diego, Academic Press. 1275–1289.
- 2.Lanyon, L., and T. Skerry. 2001. Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J. Bone Miner. Res. 16:1937–1947. [DOI] [PubMed] [Google Scholar]
- 3.Delmas, P.D. 2000. How does antiresorptive therapy decrease the risk of fracture in women with osteoporosis? Bone. 27:1–3. [DOI] [PubMed] [Google Scholar]
- 4.Neer, R.M., C.D. Arnaud, J.R. Zanchetta, R. Prince, G.A. Gaich, J.Y. Reginster, A.B. Hodsman, E.F. Eriksen, S. Shalom, H.K. Genant, O. Wang, and B.H. Mitlak. 2001. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344:1434–1441. [DOI] [PubMed] [Google Scholar]
- 5.Morinobu, M., T. Nakamoto, K. Hino, K. Tsuji, Z.-J. Shen, K. Nakashima, A. Nifuji, H. Yamamoto, H. Hirai, and M. Noda. 2005. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J. Exp. Med. 201:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karsenty, G., and E.F. Wagner. 2002. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2:389–406. [DOI] [PubMed] [Google Scholar]
- 7.Karsenty, G. 2003. The complexities of skeletal biology. Nature. 423:316–318. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima, K., X. Zhou, G. Kunkel, Z. Zhang, J.M. Deng, R.R. Behringer, and B. de Crombrugghe. 2002. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 108:17–29. [DOI] [PubMed] [Google Scholar]
- 9.He, X., M. Semenov, K. Tamai, and X. Zeng. 2004. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 131:1663–1677. [DOI] [PubMed] [Google Scholar]
- 10.Gong, Y., R.B. Slee, N. Fukai, G. Rawadi, S. Roman-Roman, A.M. Reginato, H. Wang, T. Cundy, F.H. Glorieux, D. Lev, M. Zacharin, K. Oexle, J. Marcelin et al. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 107:513–523. [DOI] [PubMed] [Google Scholar]
- 11.Kato, M., M.S. Patel, R. Levasseur, I. Lobov, B.H. Chang, D.A. Glass II, C. Hartmann, L. Li, T.H. Hwang, C.F. Brayton, R.A. Lang, G. Karsenty, and L. Chan. 2002. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 157:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang, X., K. Matsuda, P. Bialek, S. Jacquot, H.C. Masuoka, T. Schinke, L. Li, S. Brancorsini, P. Sassone-Corsi, T.M. Townes, et al. 2004. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 117:387–398. [DOI] [PubMed] [Google Scholar]
- 13.Chen, D., M. Zhao, and G.R. Mundy. 2004. Bone morphogenetic proteins. Growth Factors. 22:233–241. [DOI] [PubMed] [Google Scholar]
- 14.Mundy, G.R. 2002. Directions of drug discovery in osteoporosis. Annu. Rev. Med. 53:337–354. [DOI] [PubMed] [Google Scholar]
- 15.Mbalaviele, G., S. Sheikh, J.P. Stains, V.S. Salazar, S.L. Cheng, D. Chen, and R. Civitelli. 2005. β-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J. Cell. Biochem. 94:403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyden, L.M., J.M. Mao, J. Belsky, L. Mitzner, A. Farhi, M.A. Mitnick, D. Wu, K. Insogna, and R.P. Lifton. 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346:1513–1521. [DOI] [PubMed] [Google Scholar]
- 17.Guo, J., F.R. Bringhurst, and H.M. Kronenberg. 2004. α1 collagen promoter-directed overexpression of Dkk1 in mice causes dwarfism and very short limbs. J. Bone Miner. Res. 19:S6. [Google Scholar]
- 18.Elefteriou, F., S. Takeda, K. Ebihara, J. Magre, N. Patano, C.A. Kim, Y. Ogawa, X. Liu, S.M. Ware, W.J. Craigen, et al. 2004. Serum leptin level is a regulator of bone mass. Proc. Natl. Acad. Sci. USA. 101:3258–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teitelbaum, S.L., and F.P. Ross. 2003. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4:638–649. [DOI] [PubMed] [Google Scholar]
- 20.Gori, F., L.C. Hofbauer, C.R. Dunstan, T.C. Spelsberg, S. Khosla, and B.L. Riggs. 2000. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 141:4768–4776. [DOI] [PubMed] [Google Scholar]
- 21.Whyte, M.P., S.E. Obrecht, P.M. Finnegan, J.L. Jones, M.N. Podgornik, W.H. McAlister, and S. Mumm. 2002. Homozygous deletion of osteoprotegerin and juvenile Paget disease. N. Engl. J. Med. 347:175–184. [DOI] [PubMed] [Google Scholar]
- 22.Nakamoto, T., T. Yamagata, R. Sakai, S. Ogawa, H. Honda, H. Ueno, N. Hirano, Y. Yazaki, and H. Hirai. 2000. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol. Cell. Biol. 20:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen, Z.J., T. Nakamoto, K. Tsuji, A. Nifuji, K. Miyazono, T. Komori, H. Hirai, and M. Noda. 2002. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J. Biol. Chem. 277:29840–29846. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida, Y., A. von Bubnoff, N. Ikematsu, I.L. Blitz, J.K. Tsuzuku, E.H. Yoshida, H. Umemori, K. Miyazono, T. Yamamoto, and K.W. Cho. 2003. Tob proteins enhance inhibitory Smad-receptor interactions to repress BMP signaling. Mech. Dev. 120:629–637. [DOI] [PubMed] [Google Scholar]