Abstract
Improving approaches for hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) mobilization is clinically important because increased numbers of these cells are needed for enhanced transplantation. Chemokine stromal cell derived factor-1 (also known as CXCL12) is believed to be involved in retention of HSCs and HPCs in bone marrow. AMD3100, a selective antagonist of CXCL12 that binds to its receptor, CXCR4, was evaluated in murine and human systems for mobilizing capacity, alone and in combination with granulocyte colony-stimulating factor (G-CSF). AMD3100 induced rapid mobilization of mouse and human HPCs and synergistically augmented G-CSF–induced mobilization of HPCs. AMD3100 also mobilized murine long-term repopulating (LTR) cells that engrafted primary and secondary lethally-irradiated mice, and human CD34+ cells that can repopulate nonobese diabetic-severe combined immunodeficiency (SCID) mice. AMD3100 synergized with G-CSF to mobilize murine LTR cells and human SCID repopulating cells (SRCs). Human CD34+ cells isolated after treatment with G-CSF plus AMD3100 expressed a phenotype that was characteristic of highly engrafting mouse HSCs. Synergy of AMD3100 and G-CSF in mobilization was due to enhanced numbers and perhaps other characteristics of the mobilized cells. These results support the hypothesis that the CXCL12-CXCR4 axis is involved in marrow retention of HSCs and HPCs, and demonstrate the clinical potential of AMD3100 for HSC mobilization.
Hematopoietic stem cells (HSCs) give rise to blood-forming cells through intermediates that are termed hematopoietic progenitor cells (HPCs), including common myeloid (e.g., CFU–granulocyte erythroid macrophage, megakaryocyte [GEMM]/CFU-Mix), lymphoid progenitors, and more lineage-restricted progenitors (e.g., CFU–granulocyte macrophage [GM], CFU-G, CFU-M, burst-forming unit–erythroid [BFU-E]) (1–3). Movement of HSCs/HPCs between marrow (their main site of production) and blood is a physiological process (4, 5); few HSCs/HPCs circulate in blood. Increased concentrations of HSCs/HPCs are present in cord blood (6); postbirth, these cells can be mobilized in increased numbers to the blood (7–9).
Granulocyte colony-stimulating factor (G-CSF) is the “gold-standard” to mobilize HSCs/HPCs for transplantation (7, 8). However, broad interindividual variability exists in this mobilization (10); variability also is seen in different mouse strains (11, 12). Poor HSC/HPC mobilization in response to G-CSF is apparent in heavily-treated patients who have cancer and genetic disorders, such as Fanconi's anemia (7, 8, 13). In part, this may reflect a decreased pool of HSCs and/or HPCs. Other agents have been used to mobilize and enhance G-CSF–induced mobilization (7). One mobilizing cytokine, stem cell factor, was withdrawn from clinical development in the United States because of toxicity concerns (14); hence, the search for other agents to mobilize HSCs/HPCs, alone and with G-CSF.
SDF-1/CXCL12 and its receptor, CXCR4, are implicated in chemotaxis (15–18), homing (19–21), survival/antiapoptosis of HSCs/HPCs (22, 23). SDF-1/CXCL12-CXCR4 may be involved in retention of HSCs and HPCs within the marrow (16, 20); this suggests that antagonizing interactions of marrow produced SDF-1/CXCL12 with CXCR4 expressed on HSCs and HPCs, or changing the SDF-1/CXCL12 gradient between marrow and blood might be useful as a HSC/HPC mobilizing strategy (24–27). However, the role of altered SDF-1/CXCL12 gradients in mobilization and on functional CXCR4 expression in G-CSF mobilized cells has not been established definitively, and none of these preclinical (24–27) efforts has been adapted for clinical HSC/HPC mobilization. As a further proof of principle that antagonizing SDF-1/CXCL12-CXCR4 interactions results in mobilization of HSCs/HPCs and that this antagonism can result in clinically useful effects, we evaluated the capacity of AMD3100—a bicyclam that specifically and reversibly blocks SDF-1/CXCL12 binding to, and signaling through, CXCR4 (28–30)—to mobilize HSCs and HPCs in mice and man alone, and in combination with G-CSF. We and others reported that AMD3100 mobilizes CD34+ cells and HPCs in man (31–33) and more recently reported that AMD3100 enhances G-CSF–induced mobilization of CD34+ cells in man (34). However, HSCs make up an extremely small percentage of the CD34+ population, and it is not yet clear whether AMD3100 mobilizes functionally effective HSCs, alone and in combination with G-CSF in mouse and man.
RESULTS
AMD3100 effects on murine HPCs, without and with G-CSF
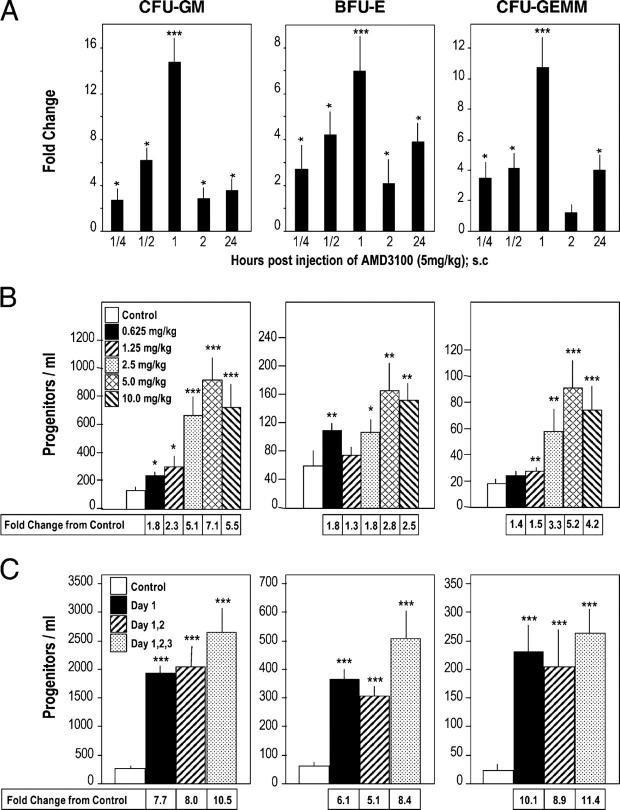
We evaluated the effects of AMD3100 on mobilization of CFU-GM, BFU-E, and CFU-GEMM to the blood of C3H/HeJ mice (Fig. 1, A–C). Although no endotoxin was detected in the samples of AMD3100 that were used, we chose C3H/HeJ mice as our first test mouse strain because these mice are relatively insensitive to endotoxin. Using a dose of 5 mg/kg AMD3100 s.c., peak mobilization of HPC occurred 1 h postinjection (Fig. 1 A). Data from various time points were reproduced in two or three additional experiments in terms of relative peak changes with control colony numbers of: 74–290 CFU-GM, 17–49 BFU-E, and 10–26 CFU-GEMM. Maximal mobilization of HPC at 1 h was noted with 2.5–10.0 mg/kg AMD3100 s.c. (Fig. 1 B). Sterile pyrogen-free saline (control medium) or AMD3100 (5 mg/kg s.c.) was injected on day 1, days 1 and 2, or days 1–3 and the blood of these mice was evaluated 1 h after injection of control medium or AMD3100 (Fig. 1 C). HPC mobilization was similar each day (no significant difference between the three AMD3100 groups) which suggested that within this timing period, the HPC-mobilizing capacity of AMD3100 is not desensitized. AMD3100 also was a potent mobilizer of HPCs in other strains of mice, as noted below.
Figure 1.
Time and dose response effects of AMD3100 as a mobilizer of HPC (CFU-GM, BFU-E and CFU-GEMM) to the blood of mice. (A) Time course of mobilization in response to a single s.c. injection of 5 mg/kg AMD3100 into C3H/HeJ mice; results are for one full experiment (5 mice/group and time 0 control numbers of CFU-GM, BFU-E, and CFU-GEMM per ml of blood were 78 ± 18, 17 ± 2, and 10 ± 2, respectively). (B) Dose response analysis at 1 h post s.c. injections of 0.625, 1.25, 2.5, 5.0, or 10 mg/kg AMD3100 into C3H/HeJ mice; results are average of 7 mice/group from a total of two separate experiments. (C) Influence of multiple injections of 5 mg/kg AMD3100 or saline given on day 1; days 1 and 2; or days 1, 2, and 3 given 24 h apart, and analyzed 1 h after the last injection into C3H/HeJ mice; results are average of 14 mice for control group for different timed saline injections and 8 mice for each test group from a total of two complete experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Broad interindividual variability exists in mobilization of HSCs/HPCs in different strains of mice (11, 12), and man (7, 8, 10). In some patients, G-CSF is a poor mobilizer (7, 8, 13). Thus, we evaluated the mobilizing capability of AMD3100 in comparison with G-CSF, and in combination with G-CSF, in strains of mice that were reported to differ in responsiveness to G-CSF. C57Bl/6 mice respond poorly to mobilizing effects of G-CSF compared with DBA/2 mice (11, 12). Mice were injected s.c. with control medium (sterile pyrogen-free saline) or 2.5 μg G-CSF per mouse, twice a day for 2 d (35) and 18 h after the last control injections were injected s.c. with control medium or 5 mg/kg AMD3100. Mice were bled 1 h later, and numbers of HPCs (Fig. 2 A) were calculated per ml of blood. HPC from C57Bl/6 and C3H/HeJ mice responded less-well to the mobilizing capacity of G-CSF than DBA/2 mice, consistent with studies of others (11, 12). AMD3100 mobilized CFU-GM and a single injection of AMD3100 synergized with 2 d of G-CSF to enhance mobilization of HPC, although maximum mobilization with AMD3100 plus G-CSF was significantly greater in DBA/2, compared with either C57/Bl/6 or C3H/HeJ mice (P < 0.001) (Fig. 2 A). Synergism in mobilization of CFU-GM was also seen in C57Bl/6 mice when G-CSF was administered to mice twice a day for 4, instead of 4, d before injection of AMD3100 (Fig. 2 B). With 4 d of G-CSF, the effects of AMD3100 plus G-CSF were additive to slightly greater than additive on mobilization of BFU-E and CFU-GEMM.
Figure 2.
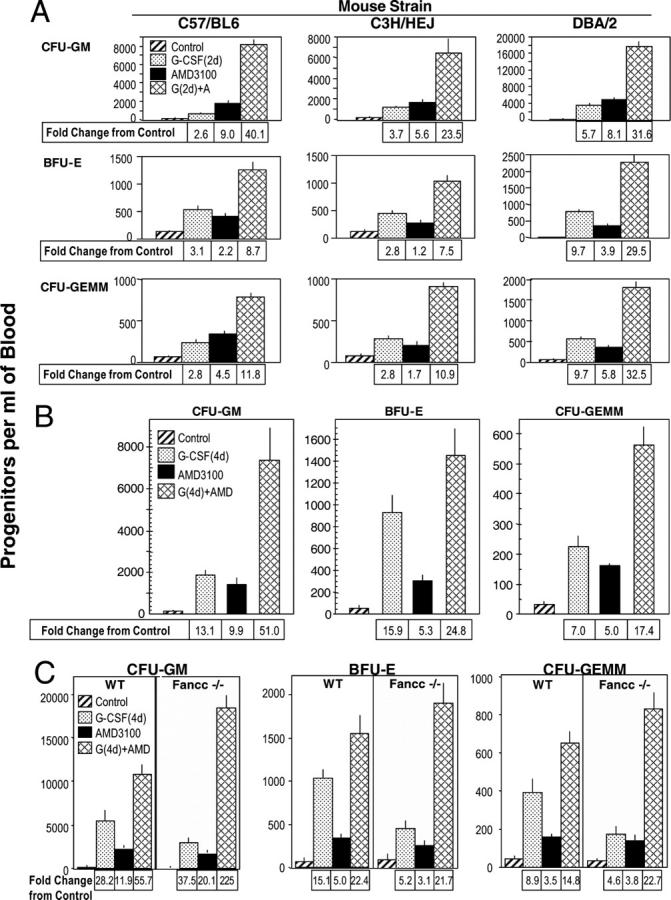
Comparative AMD3100 enhancement of G-CSF–induced mobilization of CFU-GM, BFU-E, and CFU-GEMM in: (A) 2 d G-CSF: C57Bl/6, C3H/HeJ, DBA/2, (B) 4 d G-CSF: C57Bl/6, and (C) 4 d of G-CSF: Fancc−/− and +/+ mice. The results shown in A are an average from a total of three experiments of 15 C57Bl/6, 15 DBA/2, and 11 C3H/HeJ mice. Results in B are for one experiment of 5 mice/group. Results shown in C are the averages of 15 wild-type and 8 Fancc−/− mice per group averaged from a total of two experiments. For parts A–C, all experimental points within a group are at least P < 0.001 compared with the control for the specific progenitor cell of each mouse strain.
Many patients who have Fanconi's anemia are poor responders to HPC mobilization with G-CSF (13). G-CSF was a less effective mobilizer of HPC in Fanconi's anemia complementation C group gene (Fancc)−/− compared with Fancc+/+ WT mice (Fig. 2 C; in these studies, G-CSF was administered twice a day for 4 d). AMD3100 as one injection by itself mobilized similar numbers of HPCs in Fancc−/− and WT mice. Of interest is that the synergy in mobilization of HPCs in Fancc−/− mice was greater (P < 0.001) than effects seen in WT mice. The reasons for this enhanced synergy in Fancc−/− mice are not known. The results in Fig. 2 demonstrate that a single injection of AMD3100 greatly enhances G-CSF–induced mobilization.
AMD3100 effects on murine HSCs, without and with G-CSF
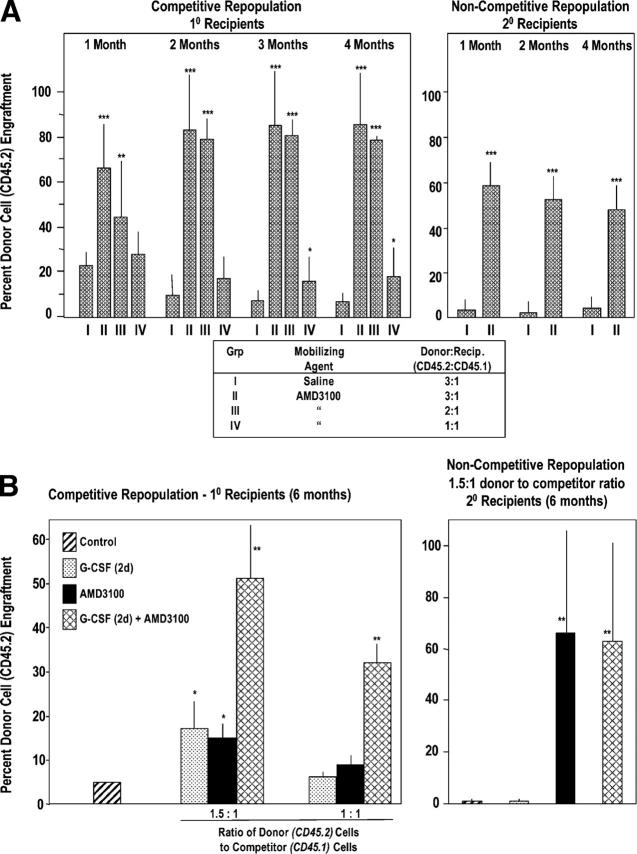
Although mobilization of HPC may be of use for short-term repopulation in a transplant setting, HSCs are required for long-term repopulation. To assess mobilization of murine HSCs, we used a competitive repopulating assay (Fig. 3) in which dilutions of donor blood cells that contain long-term repopulating (LTR) cells compete with recipient marrow cells for engraftment in lethally-irradiated recipients (36, 37). Donor chimerism was assessed using CD45 congenic mice in which donor cells were C57Bl/6 (CD45.2+) and recipients were B6.BoyJ (CD45.1+). Cells mobilized by AMD3100 contained LTR activity (Fig. 3 A, left). At 2–4 mo posttransplant, 3:1 and 2:1 ratios of AMD3100-mobilized donor/recipient competitor cells sustained a greater than eight-fold higher chimerism compared with cells that were mobilized from control medium–treated donor mice; a 1:1 ratio of cells still produced significantly enhanced engraftment of AMD3100-mobilized cells. To test self-renewal of AMD3100-mobilized LTR cells, marrow cells that were obtained from competitively engrafted mice at 4 mo posttransplantation with a 3:1 ratio of donor/recipient cells were injected into lethally-irradiated secondary mice in a noncompetitive assay (Fig. 3 A, right). All secondary mice survived and ≥50% of hematopoietic cells that engrafted the secondary mice from cells of primary mice that received cells that were mobilized with AMD3100 were of donor origin. Thus, AMD3100 rapidly mobilized LTR, self-renewing mouse HSCs.
Figure 3.
AMD3100 mobilizes competitive repopulating mouse HSCs with self-renewal capacity (A) and G-CSF synergizes this effect (B). (A, left) 18 C57Bl/6 mice (CD45.2) serving as donors were injected s.c. with 0.1 ml saline, and 36 C57Bl/6 mice were injected with 0.1 ml AMD3100 at 5 mg/kg (=∼100 μg/mouse). Blood was collected 1 h later and LDMNCs isolated. BM from nonirradiated B6.BoyJ (CD45.1) mice served as competitor cells. The ratio of donor (CD45.2) blood cells to competitor (CD45.1) BM cells was set as the number of LDMNC blood cells in 3 donor mice to a constant number of 0.5 × 106 competitor BM cells equaling 3:1. A 2:1 ratio was the number of LDMNCs in blood of 2 donor mice to 0.5 × 106 competitor cells, and a 1:1 ratio was LDMNC in 1 donor mouse to 0.5 × 106 competitor cells. Results are based on analysis of transplantation of 6 lethally irradiated recipients per test group of i.v. infused cells. Right panel: BM cells were removed from femurs of primary mice 4 mo after injection of 3:1 saline or 3:1 AMD3100:competitor cell mixture and 2.5 × 106 marrow cells from 3 mice of each group injected separately into 3 lethally secondary irradiated B6.BoyJ mice in a noncompetitive assay. Results are shown as percent CD45.2 C57Bl/6 donor cell chimerism in CD45.1 B6.BoyJ recipients. *P < 0.05; **P < 0.01; ***P < 0.001 compared with group 1 at each time point. (B) G-CSF and AMD3100 were administered as in Figure 2, part A, with G-CSF given twice a day for 2 d. Left panel: competitive repopulation in primary recipients 6 mo post transplant. The results are an average of mouse cells from five donors into six recipients each. Right panel: secondary repopulation in a noncompetitive assay 6 mo after transplant. Results are an average of 3 donor mice with cells transplanted into three recipients each. *P < 0.01 or **P < 0.001 compared with control.
The combined effects of AMD3100 and G-CSF on C57Bl/6 mice were assessed on mobilization of LTR cells. As shown in Fig. 3 B (left panel), AMD3100 or G-CSF (two times per day for 2 d) induced essentially equal mobilization of long-term marrow competitive repopulatory (LTMCR)–HSCs (see 1.5:1 donor/competitor ratio data). The combination of AMD3100 plus G-CSF was synergistic; effects were seen most clearly with 1:1 donor/competitor cells, whereas no increase in chimerism was seen with only AMD3100 or G-CSF, but significant increases were seen with AMD3100 plus G-CSF. 6 mo after the transplants, cells were removed from the marrows of competitively engrafted mice and injected into lethally irradiated secondary recipient mice in a noncompetitive assay (Fig. 3 B, right). CD45.2 donor cell engraftment (>60%) of the secondary mice was apparent 6 mo after the secondary transplant for cells that initially were mobilized with AMD3100 alone or in combination with G-CSF. In the context of what is likely a suboptimal schedule of G-CSF (2 d of G-CSF), AMD3100 enhances mobilization of a LTMCR-HSC with self-renewal capacity.
AMD3100 effects on human HPCs and SRC, with and without G-CSF
Studies in Fig. 1 led, in part, to the first clinical evaluations of AMD3100 for mobilization of CD34+ cells and HPCs in healthy human volunteers (31, 32); studies in Fig. 2 led to the first clinical evaluation of the combination of G-CSF and AMD3100 for mobilization of CD34+ cells in healthy donors (34). Peak mobilization of CFU-GM, BFU-E, and CFU-GEMM occurred in humans 6 h after i.v. injection of AMD3100. We tested whether AMD3100 could be administered to humans over two successive days, 24 h apart, and still mobilize HPCs. Results shown in Fig. 4 A demonstrate significant mobilization of HPCs within 6 h of each injection of AMD3100 given once a day on day 1, and days 1 and 2. The repetitive AMD3100 mobilizing capacity for human HPCs is similar to that for mouse HPCs (Fig. 1 C).
Figure 4.
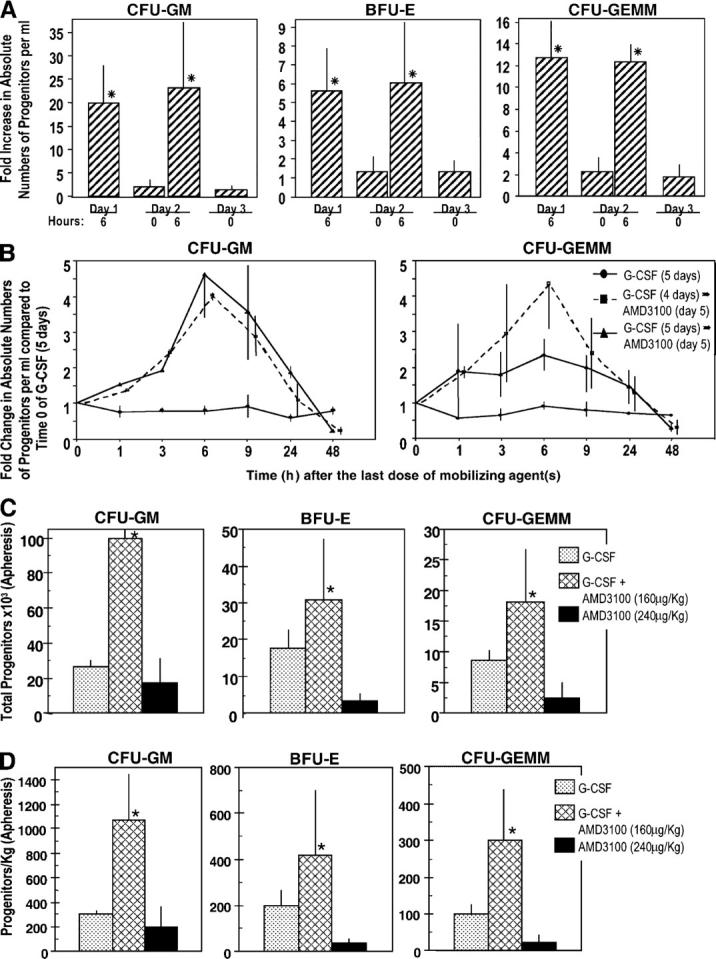
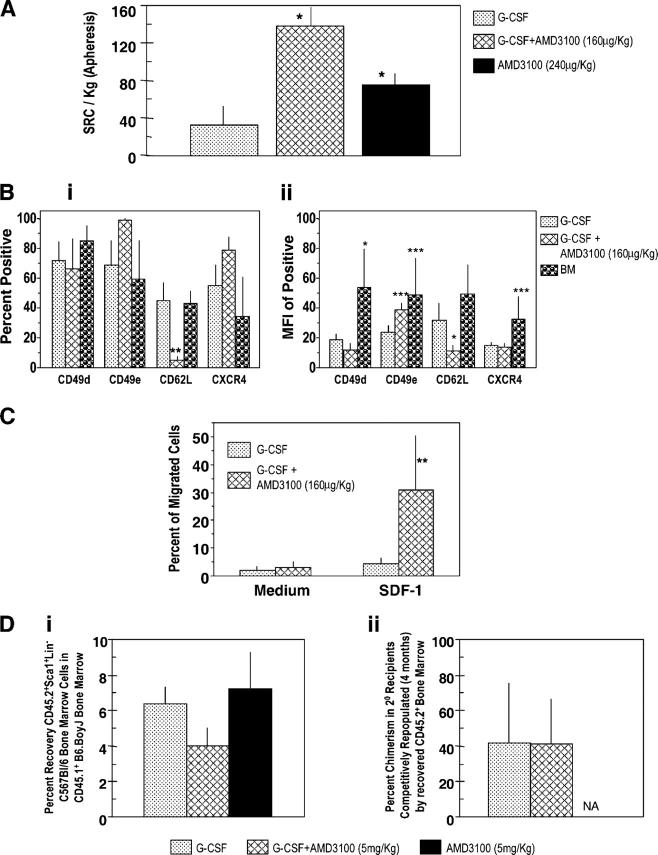
Influence of AMD3100, alone and with G-CSF, on circulating HPCs in healthy human volunteers. (A) Influence of multiple injections of AMD3100 alone. Three volunteers were injected i.v. with AMD3100 (80 μg/kg) on the first day (day 1) and again at 24 h. Progenitors/ml blood were assessed preinjection (day 1, 0 h) and at the noted time intervals. Fold changes are calculated based on the following day 1, 0 h progenitors/ml for three donors: CFU-GM (387, 78, 201), BFU-E (712, 78, 217), and CFU-GEMM (77, 29, 93) grown in methylcellulose cultures in the presence of Epo, stem cell factor, and interleukin-3. The fold changes for CFU-GM also include progenitor cells/ml as calculated in agar culture medium with GM-CSF and stem cell factor (control numbers for three donors: 108, 18, 54). *P < 0.001 compared with 0 h counts of that particular day. (B) Influence of AMD3100 on mobilization of circulating HPCs in healthy human volunteers receiving G-CSF or G-CSF plus AMD3100 (160 μg/kg). The results shown are the mean plus range of fold increases of the absolute numbers of CFU-GM and CFU-GEMM per ml of blood for two healthy volunteers each without apheresis. Fold changes are based on the following numbers of G-CSF day 5, 0 h control progenitor cells/ml: group I: G-CSF (CFU-GM: 34403 and 27194; CFU-GEMM: 14363 and 10083); group II: G-CSF (4 d) plus AMD3100 (day 5; CFU-GM: 11968 and 2675; CFU-GEMM: 3366 and 510), and group III: G-CSF (5 d) plus AMD3100 (day 5; CFU-GM: 2698 and 2380; CFU-GEMM: 1028 and 1238). (C) Results of apheresis after mobilization of circulating HPCs. Results are expressed as total progenitors collected (in thousands) for donors receiving G-CSF (n = 3), G-CSF + AMD3100 (160 μg/kg; n = 3), or AMD3100 (240 μg/kg; n = 4). *P < 0.01 for G-CSF– or AMD3100 versus G-CSF + AMD3100-mobilized cells. (D) Results of apheresis shown in C but expressed as progenitors mobilized per kg donor. *P < 0.05 for same comparisons as in C.
To test the mobilizing effects of G-CSF plus AMD3100, healthy volunteers received 4 d of G-CSF (10 μg/kg/d s.c. × 1) and then were randomized into three groups of two donors each. One group was randomized to receive the same amount of G-CSF on day 5, the second group received only AMD3100 (160 μg/kg s.c. × 1) on day 5, and the third group received G-CSF and AMD3100 on day 5. HPC numbers per milliliter were evaluated just before the last treatment on day 5 of G-CSF, AMD3100, or the combination of G-CSF plus AMD3100, and also 1–48 h after the last treatment. Peak fold change in numbers of CFU-GM and CFU-GEMM per milliliter of blood occurred at 6 h (Fig. 4 B), when AMD3100 was given on day 5 after 4 d of G-CSF administration and resulted in a greater than fourfold increase in HPCs compared with 5 d of administration of G-CSF. No further increase was seen when G-CSF was given for 5 d with the addition of AMD3100 on the fifth day. These results are comparable to the enhanced mobilization of CD34+ cells that is seen with AMD3100 and G-CSF (34). Thus, the combination of AMD3100 with G-CSF greatly enhances mobilization of human HPCs, effects which are similar to murine HPCs.
In further experiments, normal donors whose HPCs were mobilized by multiple injections of G-CSF, or one injection of AMD3100 (160 μg/kg) after multiple injections of G-CSF as noted directly above, were apheresed (34). Total numbers of HPCs apheresed and progenitors/kg donor after apheresis are shown in Fig. 4 (C and D, respectively). Unfortunately, we did not have access to samples from donors who were mobilized by only 160 μg AMD3100/kg. However, we compared these results with apheresis samples from donors who were mobilized with one injection of 240 μg AMD3100/kg. Mobilization of CD34+ cells with 240 μg AMD3100/kg is equal to or slightly better than with a 160 μg/kg dose (31). As seen in Fig. 4 C and D, significantly enhanced numbers of HPCs were seen in apheresed samples from donors who were mobilized with the combination of G-CSF plus AMD3100 compared with either G-CSF or AMD3100 (higher dose than used with G-CSF), these effects were greater than additive, data were similar to mobilization of CD34+ cells (34).
To evaluate mobilization of HSCs in humans, we used the SRC assay (38, 39). We first compared numbers of SRCs per 106 CD34+ cells obtained after administration of 10 μg/kg G-CSF once a day for 5 d compared with that mobilized 6 h after a single injection of 80 μg/kg AMD3100 (without G-CSF). The frequency of SRCs per 106 CD34+ cells for four donors who were mobilized as in Fig. 4 (C and D) by 5 d of G-CSF was 3.35 ± 2.04 compared with 12.68 ± 6.36 for four donors who were mobilized by one injection of 80 μg/kg AMD3100. In four experiments, in which a different G-CSF–mobilized sample was compared with each AMD3100-mobilized sample, the increase in frequency of SRCs per 106 CD34+ cells for AMD3100- compared with G-CSF–mobilized blood was 4.5 ± 3.4-fold. We then compared numbers of SRCs from apheresed blood of donors who were mobilized by 5 d of G-CSF; 5 d of G-CSF plus one injection of 160 μg/kg AMD3100; or one injection of 240 μg/kg AMD3100. The greatest number of SRCs/kg were found in the group that was mobilized by the combination of G-CSF plus AMD3100 (Fig. 5 A). Human cell chimerism in NOD/severe combined immunodeficiency (SCID) mice that received cells that were mobilized by AMD3100 plus G-CSF was higher than with G-CSF; effects were more apparent with limiting numbers of cells (2.0 vs. 0.3% chimerism at 75,000 cells and 3.0% vs. 1.5% at 150,000 cells, respectively, for G-CSF plus AMD3100 compared with G-CSF alone; n = 11–16 mice). Human cell chimerism information was not available for AMD3100 alone.
Figure 5.
Influence of AMD3100, G-CSF, and the combination of G-CSF plus AMD3100 on mobilization of NOD-SCID SRCs from normal human volunteers (apheresis samples), surface expression of adhesion molecules and chemotaxis of CD34+ cells, and homing of mobilized murine Sca1+Lin− cells. (A) SRCs per kg in apheresis samples from G-CSF– (n = 3), G-CSF + AMD3100- (160 μg/kg; n = 3), and AMD3100- (240 μg/kg; n = 4) mobilized circulating blood. Each set of test samples was assayed simultaneously in limiting dilutions in conditioned NOD-SCID mice. For every sample, four different cell concentrations were used and four mice were transplanted with each cell concentration. Mice were assayed for chimerism 8 wk later; those that demonstrated >0.2% chimerism (total CD45+ cells in BM) were considered to be positive. Percentage of negative mice were used to calculate SRC frequencies. (B, i) Expression of CD49d (VLA-4), CD49e (VLA-5), CD26L (L-selectin), and CXCR4 on mobilized CD34+ cells (mean ± 1SEM of percent positive cells of five to seven different G-CSF samples, three AMD3100 plus G-CSF samples, and four BM samples). (B, ii) Mean fluorescent intensity (MFI) of positive samples. Same number of samples evaluated as in B, i except G-CSF group has a different number (n =7). (C) CD34+ cells isolated from G-CSF (n = 6) and G-CSF plus AMD3100 (n = 3) mobilized peripheral blood were assessed for chemotaxis to SDF-1/CXCL12 (100 ng/ml) and results expressed as percentage of migrated cells. Data for each sample were collected in duplicate. (D, i) Homing of CD45.2+ Sca1+Lin− BM cells from C57Bl/6 mice into lethally irradiated CD45.1+ B6.BoyJ BM from samples mobilized with G-CSF (2 times/d for 4 d as in Fig. 2B; n = 3), G-CSF + AMD3100 (5 mg/kg; n = 4), and AMD3100 (5 mg/kg; n = 3). Homing was assessed as in Materials and methods. (D, ii) Competitive repopulation of CD45.2+ BM cells recovered after homing shown in D, i. Results of chimerism are after 4 mo in 2° irradiated B6.BoyJ recipients. For A–D, significant differences compared with G-CSF: *P < 0.001; **P < 0.01; ***P < 0.05; all other values are P > 0.05. NA, data not depicted.
Antigen expression and chemotaxis of human CD34+ cells
Adhesion molecules and chemotactic responsiveness to SDF-1/CXCL12 have been implicated in HSC/HPC homing (9). We examined expression of CD49d very late antigen (VLA)-4, CD49e (VLA-5), CD62L (L-selectin), and CXCR4 on CD34+ cells (Fig. 5 B) as percentage of positive cells (Fig. 5 B, i) and expression levels (Fig. 5 B, ii), and the chemotactic response of these cells to SDF-1/CXCL12 (Fig. 5 C). The percentage of CD34+ cells that expressed CD62L (Fig. 5 B, i) and the level of CD62L expression (Fig. 5 B, ii) was significantly lower in the G-CSF plus AMD3100-mobilized cells compared with G-CSF-mobilized cells. There was a trend toward a higher percentage for CD49e-expressing cells (Fig. 5 B, i), but the expression level of CD49e on the dual mobilized positive cells was significantly higher (Fig. 5 B, ii). This mirrors the phenotype of highly engrafting murine BM cells (40). No significant differences were apparent in expression of CD49d or CXCR4 on the differently mobilized CD34+, cells although there was a trend to a higher percentage of CXCR4+ cells (Fig. 5 B, i and ii). Percent positive G-CSF–mobilized CD34+ cells were similar to that of CD34+ human BM cells, although the expression levels of CD49d and CXCR4 on BM was greater than that on G-CSF–mobilized cells.
CD34+ cells that were mobilized by AMD3100 plus G-CSF were more responsive to the in vitro migratory effects of SDF-1/CXCL12 than those that were mobilized by only G-CSF (Fig. 5 C). The combination of AMD3100 plus G-CSF induces mobilization of more CD34+ cells than G-CSF or AMD3100 alone (34). However, the chemotaxis data opened up the possibility that the enhanced numbers of SRC cells that were mobilized by the combination of G-CSF plus AMD3100 was due, in part, to enhanced homing of the G-CSF plus AMD3100-mobilized CD34+ cells. Homing of human CD34+ cells (77–87% pure) that were collected from apheresed samples of two mice each after mobilization with G-CSF, G-CSF + AMD3100 (160 μg/kg), and AMD3100 was assessed in conditioned NOD/SCID BM after 20 h. No significant difference was noted in recovery of human CD34+ cells (1.5 ± 0.2%, 1.2 ± 1.0%, and 1.8 ± 0.2%, respectively) for the three groups. Because the percentage of human homed cells was low, and the results were based on phenotype only, we also assessed the homing of mobilized murine Sca+Lin− cells (C57Bl/6) into lethally irradiated B6.BoyJ mice (Fig. 5 D). There was significantly decreased homing of G-CSF plus AMD3100-mobilized cells compared with those that were mobilized with only G-CSF (Fig. 5 D, i). When these phenotypically homed cells were analyzed for competitively repopulating capacity to prove that the phenotyped cells contained stem cells, the competitive repopulating ability of the G-CSF plus AMD3100-mobilized cells was similar to that of G-CSF–mobilized cells (Fig. 5 D, ii). Numbers of Sca1+c-kit+Lin− cells/ml blood that were mobilized by the combination of G-CSF plus AMD3100 demonstrated an approximately threefold increase compared with those mobilized only by G-CSF. Numbers of Sca1+c-kit+Lin− cells (in thousands) per ml for G-CSF plus AMD3100 compared with G-CSF were 31.4 ± 10.9 and 10.1 ± 3.4, respectively (P < 0.01). Thus, we could not link enhanced numbers of SRCs/HSCs that were mobilized by G-CSF plus AMD3100 to enhanced homing of G-CSF plus AMD3100-mobilized cells.
DISCUSSION
Stem cell transplantation is curative for many life-threatening disorders. The first source of transplantable HSCs was BM (41). Subsequently, mobilized peripheral blood (7, 8) and cord blood (6) were found to contain HSCs. By the 1990s, the majority of autologous and allogeneic HSC transplants following myeloablative therapy in patients who had hematolymphoid malignancies or solid tumors were done with cytokine-mobilized HSCs; G-CSF was the most frequently used inducer of HSC/HPC mobilization (7, 8). However, there is large individual variability in responsiveness of normal donors and patients to the HSC/HPC-mobilizing capacity of G-CSF (7, 8, 10, 13, 42–45), and various cytokines and other agents have been evaluated alone and in combination with G-CSF to enhance HSC/HPC mobilization.
One potential mechanism for retaining HSCs/HPCs in the marrow is the attraction of CXCR4-expressing HSCs/HPCs to marrow stromal cell–produced SDF-1/CXCL12 (16, 20), a possibility that was substantiated by additional experimental evidence (24–27). Thus, we postulated that known specific antagonism of SDF-1/CXCL12 binding to CXCR4 (28, 29) could be used clinically for mobilization of HSCs/HPCs. That AMD3100 could mobilize human CD34+ HPCs was reported recently (31–33). The impetus to pursue the clinical studies of AMD3100 as a mobilizer of human CD34+ cells and HPCs was, in large part, a consequence of our initial studies that demonstrated that AMD3100 could mobilize HPCs rapidly in mice and synergized with G-CSF to enhance this mobilization. These studies are reported for the first time in Fig. 1 A–C and Fig. 2 A–C respectively.
Although mobilization of HPCs and CD34+ cells in response to AMD3100 is of interest, proof that AMD3100 also mobilizes HSCs is of paramount importance if AMD3100 is to be a useful source of long-term engrafting cells. That AMD3100 is a rapid mobilizer of murine HSCs and human SRCs is seen in Figs. 3 and 5. Mouse data that are shown in Fig. 3 are definitive proof that AMD3100 mobilizes a LTR cell with self-renewal capacity.
Although expression of CD34 is considered clinically to be a surrogate measure of stem cells, CD34+ cells constitute a relatively heterogenous group of cells, of which HPCs can constitute 10–50% of the population, depending on how pure the CD34+ population is and the level of expression of CD34 on the isolated cells. The HSC content of CD34+ cells is very small (<1%), and not all HSCs that are inherent within the CD34+ population may be LTR cells. Thus, although phenotypic markers are of some use, it is the functional attributes of mobilized cells from donors that are of greatest relevance and importance, especially when one considers using these cells for clinical transplantation. The assay that is best recognized as defining a functionally competent human HSC involves human cell engraftment of sublethally irradiated immune-deficient mice, such as NOD-SCID mice (38, 39). Only an extremely small percentage of SRCs are found in the population of CD34+CD38− human cells (46) and this phenotype is a minor fraction of total CD34+ cells. It is apparent from our current studies that human SRCs are mobilized by AMD3100, which suggests the importance of the SDF-1/CXCL12-CXCR4 axis for retention of SRCs in the human marrow microenvironment. If AMD3100 is to be used by itself for clinical mobilization, the data shown in Fig. 1 C (for murine HPCs) and in Fig. 4 A (for human HPCs) are of relevance because they demonstrate that numbers of mobilized cells can be increased by consecutive collections after repeated injections of AMD3100.
The combined use of G-CSF and AMD3100 may have the greatest clinical impact, and our recent study has demonstrated that G-CSF plus AMD3100 mobilizes greater numbers of CD34+ cells/kg donor body weight than either G-CSF or AMD3100 (34). That a single injection of AMD3100 synergized with multiple additions of G-CSF for HPCs and LTMCR- and self-renewing–HSCs in mice and for HPCs and SRCs in man suggests that the mechanisms that are inherent in G-CSF and AMD3100 mobilization of HSCs and HPCs are likely different, or do not overlap completely. Extensive efforts have gone into understanding the interacting elements that are involved in the mobilization of HSCs and HPCs, including that of the SDF-1/CXCL12-CXCR4 axis (9). Adhesion molecules have been implicated in homing, retention, and mobilization of HSCs and HPCs (9). It is of interest that the expression profile of the dual AMD3100/G-CSF–mobilized CD34+ human cells (Fig. 5 B) mirrors the phenotype of highly engrafting murine BM cells (40). The functional and mechanistic relevance of this murine HSC and human CD34+ phenotype remains to be determined. It did not seem that enhanced engrafting capability of the G-CSF plus AMD3100-mobilized cells was due to enhanced homing of these cells (Fig. 5 D, i and ii) within the context of the specific timing and experimental procedure that we used. However, more intricate homing studies that can determine exactly where homed cells arrive in the microenvironmental niche are needed to answer definitively whether homing played a role. Unfortunately, this technology is not yet available. The highly engrafting nature of the dual mobilized cells makes it clear that the AMD3100 mobilization process does not interfere with the functional capacity of the mobilized cells; this is consistent with the antagonistic and rapidly reversible effects of AMD3100 (28, 29).
Efforts are moving quickly to evaluate cells that are mobilized by the combination of G-CSF and AMD3100 in a clinical setting. The side effects of single-dose administration of AMD3100 to normal donors are very minor (31–34). AMD3100 does not cause bone pain when administered alone, or increase G-CSF–related bone pain when given in combination with G-CSF. The results presented here suggest that dual AMD3100/G-CSF, and AMD3100 alone, mobilize high-quality populations of HSCs that should provide stable long-term hematopoietic engraftment.
MATERIALS AND METHODS
Cells
Peripheral blood was obtained from C57Bl/6, DBA/2, and C3H/HeJ mice that were purchased from Harlan Laboratories or Jackson ImmunoResearch Laboratories. Congenic C57Bl/6 (CD45.2+) and B6.SJL-Ptrca Pep3b/BoyJ (B6.BoyJ:CD45.1) mice were purchased from Jackson ImmunoResearch Laboratories. Fancc−/− and +/+ (WT) mice originally were obtained from M. Buchwald (Hospital for Sick Children and University of Toronto, Toronto, Ontario, Canada) (47). NOD-SCID mice were purchased from Jackson ImmunoResearch Laboratories. The Institutional Animal Care and Use Committee of the Indiana University School of Medicine approved all experimental procedures with the mice used. Mice were bred and/or maintained at the animal facilities of the Indiana University School of Medicine. Blood cells from healthy human volunteers (ages 24–33 yr) were obtained following protocols approved by the Human Subjects Committee/Institutional Review Boards of the University of Washington, Indiana University School of Medicine, and the Scientific Advisory Committee for the Clinical Research Center of the University of Washington Medical Center. Informed consent was obtained from all volunteers before enrollment in study protocols. All subjects had normal blood cell counts, normal liver and kidney functions, were normal on physical examination, and were not taking regular medication. Normal electrocardiogram findings were required (31, 32, 34). Each subject was admitted to the Clinical Research Center for injections of AMD3100 (AnorMed, Inc.), blood sampling, and clinical observation. Human BM also was obtained with Institutional Review Board approval and informed consent at Indiana University. LDMNCs were obtained from mouse and human blood/BM by density cut procedures using murine lympholyte and Ficoll Hypaque (Amersham Biosciences), respectively. Human CD34+ cells were isolated first into a LDMNC fraction and then purified by positive selection with a Magnetic Affinity Cell Separation CD34+ isolation kit (Miltenyi Biotec; purity of isolated cells was >90% CD34+ unless otherwise noted).
HPC assays
Mouse and human cells were assayed for CFU-GM, BFU-E, and CFU-GEMM using ingredients and cytokine combinations as noted elsewhere (3, 22, 23). For mouse HPCs, cells were cultured in 1% methylcellulose with 30% FBS (Hyclone) with 1 U/ml rhu Epo, 5% (vol/vol)) pokeweed mitogen mouse spleen cell conditioned medium, and 50 ng/ml recombinant murine stem cell factor (= steel factor). For human HPCs, we used methylcellulose, 30% FBS, 1 U/ml rhu Epo, 10 ng/ml rhu GM-CSF, 10 ng/ml rhu IL-3, and 50 ng/ml rhu stem cell factor. Colonies were scored after 14 d for human cells and after 7 d for mouse cells in a humidified atmosphere with 5% CO2 and lowered (5%) O2. Cytokines were purchased from R&D Systems, except for Epo which was purchased from Amgen Corp. Pokeweed mitogen mouse spleen cell conditioned medium was prepared as described previously (3).
Mouse HSC and human SRC assays
The mouse LTR cell assay detects functional and competitive stem cells (36). The LTR cell assay and phenotyping for donor (CD45.2+) and recipient (CD45.1+) cells was performed as previously described (23, 37), and as noted in the legend to Fig. 3. Recipient mice were given a lethal dose of irradiation (950 cGy) before i.v. injection of cells. Additionally, BM cells recovered from lethally irradiated mice repopulated by donor cells were injected i.v. into lethally irradiated secondary mice without the injection of competitor cells as noted in the legend to Fig. 3.
The human SRC assay was performed in sublethally irradiated (300 cGy) NOD-SCID mice as described previously (48), using limiting dilution analysis (49).
Flow cytometry and chemotaxis assay
Quantitation of surface antigens was performed in the Flow Cytometry Core Facility of the Indiana University School of Medicine and the National Cancer Institute–Designated Indiana University Cancer Center. FITC-conjugated anti–mouse CD45.2 and anti–mouse C45.1 (BD Biosciences) was used to assay mouse donor cell chimerism of transplanted B6.BoyJ mice (23), whereas anti–human CD45 (FITC, clone 2D1; BD PharMingen) was used to assess human donor cell chimerism of NOD-SCID mice (45). CD34 (PE, clone 8G12), CD49e (PE, clone 11A1), CD62L (FITC, clone Dreg56), and CXCR4 (PE, clone 12G5) antibodies were purchased from BD Biosciences. CD49d (FITC, clone HP2/1) antibody was purchased from Coulter-Immunotech. Chemotaxis assay was done as described previously (50) using SDF-1/CXCL12 that was purchased from R&D Systems.
Methods for stem cell homing studies
Fresh cells.
A known volume of mobilized peripheral blood was obtained from 10 C57Bl/6 mice/group. Low density cells were obtained, enumerated, and phenotyped by flow cytometry using biotinylated Sca-1 developed with SA-PerCPCy5.5, lineage markers conjugated to APC (CD3, B220, Gr-1), and cKit-FITC. Cells were analyzed using a FACSCaliber (Becton Dickinson), and all antibodies were obtained from BD Biosciences.
Homing studies.
Low-density mobilized peripheral blood PB from 10 C57Bl/6 donor mice/group was pooled and 40 × 106 cells were transplanted via tail vein injections into one to four B6.BoyJ recipients/group (10 wk old). Mice were killed 11 h later, BM isolated, red cells lysed, and cells phenotyped by flow cytometry using CD45.2-FITC, biotinylated Sca-1 developed with SA-PerCPCy5.5, and lineage markers conjugated to APCs (CD3, B220, Gr-1). Recovery of primitive BM-homed donor cells was calculated as described previously (51, 52). Frequency of donor cells, and Sca-1+Lin− cells—falling within a light scatter gate, including lymphocytes and large granular cells—was determined for each mouse. Frequencies were multiplied by the total number of cells in each tissue, then divided by the number of cells in the original graft to calculate the recovery of transplanted cells. Numbers of BM cells that were harvested from tibias and femurs were considered to represent 18.7% of total murine marrow (53).
Transplantation studies.
A fraction of BM-homed donor cells from the primary B6.BoyJ recipients was assessed for long-term competitive repopulating potential in lethally-irradiated secondary B6.BoyJ recipients (51, 52). A volume of marrow from one primary mouse that contained 3 × 105 donor C57Bl/6 cells was transplanted along with 105 competitor low density bone marrow of B6.BoyJ origin into one B6.BoyJ recipient within 2–3 h of receiving lethal irradiation in a split dose of 650cGy + 300cGy, 4 h apart. Mice were bled from the tail vein monthly for analysis of donor-derived hematopoiesis by determining the percentage of CD45.2+CD45.1− PB leucocytes.
Human to NOD/SCID homing.
Homing of CD34+ cells was assessed as described (54). In brief, test samples, with a known percentage of CD34+ cells were transplanted i.v. into 10–12-wk-old NOD/SCID mice conditioned with 300 cGy total body irradiation to deliver 2.5 × 106 CD34+ cells per mouse. Approximately 20 h later, BM cells were collected and the percentage of human cells was determined by direct measurement of total human CD45+ cells.
Statistical analysis
Results are expressed as mean ± 1 SD unless otherwise noted. Statistical differences between groups was determined for in vitro colony forming cell (HPC) studies using Student's t test. An unpaired two-sided Student's t test was used to evaluate significant differences in chimerism for in vivo transplantation studies.
Acknowledgments
These studies were supported by Public Health Service grant nos. RO1 DK53574 and HL67384 (to H.E. Broxmeyer); R01 HL63219 and a project in P01 HL53586 (to D.W. Clapp); R01 HL55716 (to E.F. Srour); Core Center of Excellence in Molecular Hematology no. P50 DK49218; a University of Washington Clinical Center grant; and research funding from AnorMed, Inc. (to D.C. Dale, H.E. Broxmeyer, and E.F. Srour). B. Graham-Evans was supported by minority supplement no. RO1 DK53674. T.B. Campbell was supported by a predoctoral slot on National Institutes of Health training grant no. T32 DK07519 (to H.E. Broxmeyer).
G. Calandra and G. Bridger have shares in AnorMed, Inc. After the research studies were completed, D.C. Dale agreed to serve on the Scientific Advisory Board of AnorMed, Inc. The authors have no other potential conflicting financial interests.
Abbreviations used: BFU-E, burst-forming unit–erythroid; Epo, erythropoietin; GEMM, granulocyte erythroid macrophage, megakaryocyte; GM, granulocyte macrophage; HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell; LDMNC, low-density mononuclear cell; LTR, long-term repopulating; rhu, recombinant human; NOD, nonobese diabetic; SDF, stromal cell–derived factor; SRC, SCID repopulating cell; TMCR, long-term marrow competitive repopulating; VLA, very late antigen.
C.M. Orschell and D.W. Clapp contributed equally to this work.
References
- 1.Kondo, M., A.J. Wagers, M.G. Manz, S.S. Prohaska, D.C. Scherer, G.F. Beilhack, J.A. Shizuru, and I.L. Weissman. 2003. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21:759–806. [DOI] [PubMed] [Google Scholar]
- 2.Manz, M.G., K. Akashi, and I.L. Weissman. 2004. Biology of hematopoietic stem and progenitor cells. Thomas' Hematopoietic Cell Transplantation. 3rd ed. K.G. Blume, S.J. Forman, and F.R. Appelbaum, editors. Blackwell Publishing Ltd., Malden, MA. 69–95.
- 3.Cooper, S., and H.E. Broxmeyer. 1996. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin and the potent co-stimulating cytokines steel factor and Flt-3 ligand. Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, W. Strober, and R. Coico, editors. John Wiley & Sons, Inc., NY. Supp. 18, 6.4.1–6.4.12.
- 4.Wright, D.E., A.J. Wagers, A.P. Gulati, F.L. Johnson, and L.L. Weissman. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science. 294:1933–1936. [DOI] [PubMed] [Google Scholar]
- 5.Abkowitz, J.L., A.E. Robinson, S. Kale, M.W. Long, and J. Chen. 2003. The mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 102:1249–1253. [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer, H.E., and F.O. Smith. 2004. Cord blood hematopoietic cell transplantation. Thomas' Hematopoietic Cell Transplantation. 3rd ed. K.G. Blume, S.J. Forman, and F.R. Appelbaum, editors. Blackwell Publishing Ltd., Malden, MA. 550–564.
- 7.Ng-Cashin, J., and T. Shen. 2004. Mobilization of autologous peripheral blood hematopoietic cells for support of high-dose cancer therapy. Thomas' Hematopoietic Cell Transplantation. 3rd ed. K.G. Blume, S.J. Forman, and F.R. Appelbaum, editors. Blackwell Publishing, Ltd., Malden, MA. 576–587.
- 8.Schmitz, N. 2004. Peripheral blood hematopoietic cells for allogeneic transplantation. Thomas' Hematopoietic Cell Transplantation. 3rd ed. K.G. Blume, S.J. Forman, and F.R. Appelbaum, editors. Blackwell Publishing, Ltd., Malden, MA. 588–598.
- 9.Papayannopoulou, T. 2004. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 103:1580–1588. [DOI] [PubMed] [Google Scholar]
- 10.Roberts, A.W., E. DeLuca, C.G. Begley, R. Basser, A.P. Grigg, and D. Metcalf. 1995. Broad inter-individual variations in circulating progenitor cell numbers induced by granulocyte colony-stimulating factor therapy. Stem Cells. 13:512–516. [DOI] [PubMed] [Google Scholar]
- 11.Roberts, A.W., S. Foote, W.S. Alexander, C. Scott, L. Robb, and D. Metcalf. 1997. Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood. 9:2736–2744. [PubMed] [Google Scholar]
- 12.De Haan, G., A. Ausema, M. Wilkens, G. Molineux, and B. Dontje. 2000. Efficient mobilization of haematopoietic progenitors after a single injection of pegylated recombinant human granulocyte colony-stimulating factor in mouse strains with distinct marrow-cell pool sizes. Br. J. Haematol. 110:638–646. [DOI] [PubMed] [Google Scholar]
- 13.Croop, J.M., R. Cooper, C. Fernandez, V. Graves, S. Kreissman, H. Hanenberg, F.O. Smith, and D.A. Williams. 2001. Mobilization and collection of peripheral blood CD34+ cells from patients with Fanconi anemia. Blood. 98:2917–2921. [DOI] [PubMed] [Google Scholar]
- 14.Hu, J., and C.E. Dunbar. 2002. Update on hematopoietic stem cell gene transfer using non-human primate models. Curr. Opin. Mol. Ther. 4:482–490. [PubMed] [Google Scholar]
- 15.Aiuti, A., I.J. Webb, C. Bleul, T. Springer, and J.C. Gutierrez-Ramos. 1997. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 185:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, C.H., and H.E. Broxmeyer. 1998. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor and the bone marrow environment. Blood. 91:100–110. [PubMed] [Google Scholar]
- 17.Jo, D.-Y., S. Rafii, T. Hamada, and M.A.S. Moore. 2000. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J. Clin. Invest. 105:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright, D.E., E.P. Bowman, A.J. Wagers, E.C. Butcher, and L.L. Weissman. 2002. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 195:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peled, A., I. Petit, O. Kollet, M. Magid, T. Ponomaryov, T. Byk, A. Nagler, H. Ben-Hur, A. Many, L. Shultz, O. Lider, R. Alon, D. Zipori, and T. Lapidot. 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 283:845–848. [DOI] [PubMed] [Google Scholar]
- 20.Lapidot, T., and O. Kollet. 2002. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/β2mnull mice. Leukemia. 16:1992–2203. [DOI] [PubMed] [Google Scholar]
- 21.Christopherson, K.W., II, G. Hangoc, C. Mantel, and H.E. Broxmeyer. 2004. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 305:1000–1003. [DOI] [PubMed] [Google Scholar]
- 22.Broxmeyer, H.E., S. Cooper, L. Kohli, G. Hangoc, Y.H. Lee, C. Mantel, D.W. Clapp, and C.H. Kim. 2003. Transgenic expression of stromal cell derived factor-1/CXCL12 enhances myeloid progenitor cell survival/anti-apoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J. Immunol. 170:421–429. [DOI] [PubMed] [Google Scholar]
- 23.Broxmeyer, H.E., L. Kohli, C.H. Kim, Y. Lee, C. Mantel, S. Cooper, G. Hangoc, M. Shaheen, X. Li, and D.W. Clapp. 2003. Stromal cell derived factor-1/CXCL12 enhances survival/anti-apoptosis of hematopoietic stem and myeloid progenitor cells: direct effects mediated through CXCR4 and Gαi proteins. J. Leuk. Biol. 73:630–638. [DOI] [PubMed] [Google Scholar]
- 24.Shen, H., T. Cheng, I. Olszak, E. Garcia-Zepeda, Z. Lu, S. Hermann, R. Fallon, A.D. Luster, and D.T. Scadden. 2001. CXCR-4 desensitization is associated with tissue localization of hematopoietic progenitor cells. J. Immunol. 166:5027–5033. [DOI] [PubMed] [Google Scholar]
- 25.Hattori, K., B. Heissig, K. Tashiro, T. Honjo, M. Tateno, J.H. Shieh, N.R. Hackett, M.S. Quitoriano, R.G. Crystal, S. Rafii, and M.A. Moore. 2001. Plasma elevation of stromal cell-derived factor-1 induced mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 97:3354–3360. [DOI] [PubMed] [Google Scholar]
- 26.Sweeny, E.A., H. Lortat-Jacob, G.V. Priestley, B. Nakamoto, and T. Papayannopoulou. 2002. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 99:44–51. [DOI] [PubMed] [Google Scholar]
- 27.Petit, I., M. Szyper-Kravitz, A. Nagler, M. Lahav, A. Peled, L. Habler, T. Ponomaryov, R.S. Taichman, F. Arenzana-Seisdedos, N. Fujii, et al. 2002. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3:687–694. [DOI] [PubMed] [Google Scholar]
- 28.De Clercq, E. 2003. The bicyclam AMD3100 story. Nat. Rev. Drug Discov. 2:581–587. [DOI] [PubMed] [Google Scholar]
- 29.Hatse, S., K. Princen, G. Bridger, E. De Clercq, and D. Schols. 2002. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 527:255–262. [DOI] [PubMed] [Google Scholar]
- 30.Joo, E.K., H.E. Broxmeyer, H.J. Kwon, H.B. Kang, J.S. Kim, J.S. Lim, Y.K. Choe, I.S. Choe, P.K. Myung, and Y. Lee. 2004. Enhancement of cell survival by stromal cell–derived factor-1/CXCL12 involves activation of CREB and induction of Mcl-1 and c-Fos in factor-dependent human cell line MO7e. Stem Cells Dev. 13:563–570. [DOI] [PubMed] [Google Scholar]
- 31.Liles, W.C., H.E. Broxmeyer, E. Rodger, B. Wood, K. Hubel, S. Cooper, G. Hangoc, G. Bridger, G.W. Hensen, G. Calandra, and D.C. Daly. 2003. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 102:2728–2730. [DOI] [PubMed] [Google Scholar]
- 32.Hubel, K., W.C. Liles, H.E. Broxmeyer, E. Rodger, B. Wood, S. Cooper, G. Hangoc, R. MacFarland, G.J. Bridger, G.W. Henson, G. Calandra, and D.C. Dale. 2004. Leukocytosis and mobilization of CD34+ hematopoietic progenitor cells by AMD3100, a CXCR4 antagonist. Supportive Cancer Therapy. 1:165–172. [DOI] [PubMed] [Google Scholar]
- 33.Devine, S.M., N. Flomenberg, D.H. Vesole, J. Liesveld, D. Weisdorf, K. Badel, G. Calandra, and J.F. DiPersio. 2004. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J. Clin. Oncol. 22:1095–1102. [DOI] [PubMed] [Google Scholar]
- 34.Liles, W.C., E. Rodger, H.E. Broxmeyer, C. Dehner, K. Badel, G. Calandra, J. Christensen, B. Wood, T.H. Price, and D.C. Dale. 2004. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with G-CSF by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 45:295–300. [DOI] [PubMed] [Google Scholar]
- 35.Broxmeyer, H.E., S. Cooper, G. Hangoc, J.-L. Gao, and P.M. Murphy. 1999. Dominant myelopoietic effector functions mediated by chemokine receptor CCR1. J. Exp. Med. 189:1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison, D. 1980. Competitive repopulation: a new assay for long term stem cell functional capacity. Blood. 55:77–81. [PubMed] [Google Scholar]
- 37.Haneline, L., H.E. Broxmeyer, S. Cooper, G. Hangoc, M. Carreau, M. Buchwald, and D.W. Clapp. 1998. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac −/− mice. Blood. 91:4092–4098. [PubMed] [Google Scholar]
- 38.Dick, J.E., M. Bhatia, O. Gan, U. Kapp, and J.C. Wang. 1997. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells. 15:199–203. [DOI] [PubMed] [Google Scholar]
- 39.Bodine, D. 2004. Animal models for the engraftment and differentiation of human hematopoietic stem and progenitor cells. Cord Blood: Biology, Immunology, Banking, and Clinical Transplantation. H.E. Broxmeyer, editor. American Association of Blood Banking, Bethesda, MD. 47–64.
- 40.Orschell-Traycoff, C.M., K. Hiatt, R.N. Dagher, S. Rice, M.C. Yoder, and E.F. Srour. 2000. Homing and engraftment potential of Sca-1(+)lin(−) cells fractionated on the basis of adhesion molecule expression and position in cell cycle. Blood. 96:1380–1387. [PubMed] [Google Scholar]
- 41.Thomas, E.D. 2004. A history of bone marrow transplantation. Thomas' Hematopoietic Cell Transplantation. 3rd ed. K.G. Blume, S.J. Forman, and F.R. Appelbaum, editors. Blackwell Publishing Ltd., Malden, MA. 3–8.
- 42.Grigg, A.P., A.W. Roberts, H. Raunow, S. Houghton, J.E. Layton, A.W. Boyd, K.M. McGrath, and D. Maher. 1995. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 86:4437–4445. [PubMed] [Google Scholar]
- 43.Anderlini, P., D. Przepiorka, C. Seong, T.L. Smith, Y.O. Huh, J. Lauppe, R. Champlin, and M. Korbling. 1997. Factors affecting mobilization of CD34+ cells in normal donors treated with filgrastim. Transfusion. 37:507–512. [DOI] [PubMed] [Google Scholar]
- 44.Holm, M. 1998. Not all healthy donors mobilize hematopoietic progenitor cells sufficiently after G-CSF administration to allow for subsequent CD34 purification of the leukapheresis product. J. Hematol. 7:111–113. [DOI] [PubMed] [Google Scholar]
- 45.de la Rubia, J., C. Arbona, F. de Arriba, C. del Canizo, S. Brunet, C. Zamora, M.A. Diaz, J. Bargay, J. Petit, J. de la Serna, et al. 2002. Analysis of factors associated with low peripheral blood progenitor cell collection in normal donors. Transfusion. 42:4–9. [DOI] [PubMed] [Google Scholar]
- 46.Bhatia, M., J.C. Wang, U. Kapp, D. Bonnet, and J.E. Dick. 1997. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. USA. 94:5320–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen, M., D.J. Tomkins, W. Auerbach, C. McKerlie, H. Youssoufian, L. Liu, O. Gan, M. Carreau, A. Auerbach, T. Groves, et al. 1996. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconia anemia. Nat. Genet. 12:448–451. [DOI] [PubMed] [Google Scholar]
- 48.Wilpshaar, J., J.H. Falkenburg, X. Tong, W.A. Noort, R. Breese, D. Heilman, H. Kanhai, C.M. Orschell-Traycoff, and E.F. Srour. 2000. Similar repopulating capacity of mitotically active and resting umbilical cord blood CD34+ cells in NOD/SCID mice. Blood. 96:2100–2107. [PubMed] [Google Scholar]
- 49.Taswell, C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614–1619. [PubMed] [Google Scholar]
- 50.Plett, P., S. Frankovitz, F. Wolber, R. Abonour, and C. Orschell-Traycoff. 2002. Treatment of circulating CD34+ cells with SDF-1α or anti-CXCR4 antibody enhances migration and NOD/SCID repopulating potential. Exp. Hematol. 30:1061–1069. [DOI] [PubMed] [Google Scholar]
- 51.Plett, P.A., S.M. Frankovitz, and C.M. Orschell-Traycoff. 2002. In vivo trafficking, cell cycle activity, and engraftment potential of phenotypically defined primitive hematopoietic cells after transplantation into irradiated or nonirradiated recipients. Blood. 100:3545–3552. [DOI] [PubMed] [Google Scholar]
- 52.Plett, P.A., S.M. Frankovitz, and C.M. Orschell. 2003. Distribution of marrow repopulating cells between bone marrow and spleen early after transplantation. Blood. 102:2285–2291. [DOI] [PubMed] [Google Scholar]
- 53.Boggs, D. 1984. The total marrow mass of the mouse: a simplified method of measurement. Am. J. Hematol 16:277–286. [DOI] [PubMed] [Google Scholar]
- 54.Jetmore, A., P.A. Plett, X. Tong, F.M. Wolber, R. Breese, R. Abonour, C.M. Orschell-Traycoff, and E.F. Srour. 2002. Homing efficiency, cell cycle kinetics and survival of quiescent and cycling human CD34+ cells transplanted into conditioned NOD/SCID mice. Blood. 99:1585–1593. [DOI] [PubMed] [Google Scholar]