Abstract
Pancreatic islet transplantation is a highly promising approach for the treatment of insulin-dependent diabetes mellitus. However, the procedure remains experimental for several reasons, including its low efficiency caused by the early graft loss of transplanted islets. We demonstrate that Gr-1+CD11b+ cells generated by transplantation and their IFN-γ production triggered by Vα14 NKT cells are an essential component and a major cause of early graft loss of pancreatic islet transplants. Gr-1+CD11b+ cells from Vα14 NKT cell–deficient (Jα281−/−) mice failed to produce IFN-γ, resulting in efficient islet graft acceptance. Early graft loss was successfully prevented through the repeated administration of α-galactosylceramide, a specific ligand for Vα14 NKT cells, resulting in dramatically reduced IFN-γ production by Gr-1+CD11b+ cells, as well as Vα14 NKT cells. Our study elucidates, for the first time, the crucial role of Gr-1+CD11b+ cells and the IFN-γ they produce in islet graft rejection and suggests a novel approach to improving transplantation efficiency through the modulation of Vα14 NKT cell function.
Patients with insulin-dependent diabetes mellitus regularly fail to achieve insulin independence after transplantation of islets from a single donor pancreas because of early graft loss (1, 2). Early islet loss is estimated to occur in ≤50% of the transplanted islet mass (3) and occurs at similar frequencies in both allogeneic and syngeneic combinations in a mouse model, possibly because of stress, inflammation, or innate responses caused by transplantation. To analyze the mechanisms of early islet loss, the syngeneic islet transplantation model is advantageous because allogeneic immune responses are avoidable.
After transplantation into the recipient liver, islet grafts are lodged together in the periphery of the portal vein as emboli, causing ischemic degeneration of the corresponding areas. This may trigger innate immune responses resulting in the release of inflammatory cytokines harmful to islet grafts. Insulin-secreting β cells have been shown to be susceptible in vitro to exposure to proinflammatory cytokines such as IFN-γ, TNF-α, and IL-1β (4), and it is likely that proinflammatory cytokines are also a major cause of islet graft failure in vivo.
Vα14 NKT cells belong to a distinct subset of lymphocytes bridging the innate and acquired immune systems and play an important role in the immediate responses, because Vα14 NKT cells produce large amounts of IFN-γ immediately after activation, which in turn activates NK cells and other cells in the innate immune system and CD4 Th1 cells and CD8 cytotoxic T cells in the acquired immune system (5–9). Thus, Vα14 NKT cells serve as a front line defense against various bacteria, viruses, fungi, and intracytoplasmic parasites (5, 6).
Because Vα14 NKT cells are relatively abundant in the liver, the site of choice for islet transplantation, and because IFN-γ is believed to be an important factor in the destruction of islet β cells, we hypothesized that Vα14 NKT cells in the liver might be involved in islet graft failure. We demonstrate that, in mice, IFN-γ–producing Gr-1+CD11b+ cells and Vα14 NKT cells are major components of the early graft loss of transplanted islets and the low efficiency of pancreatic islet transplants. Our findings provide an approach to improve transplant efficiency through the modulation of Vα14 NKT cell function.
Results AND Discussion
Vα14 NKT cells are responsible for early islet graft loss
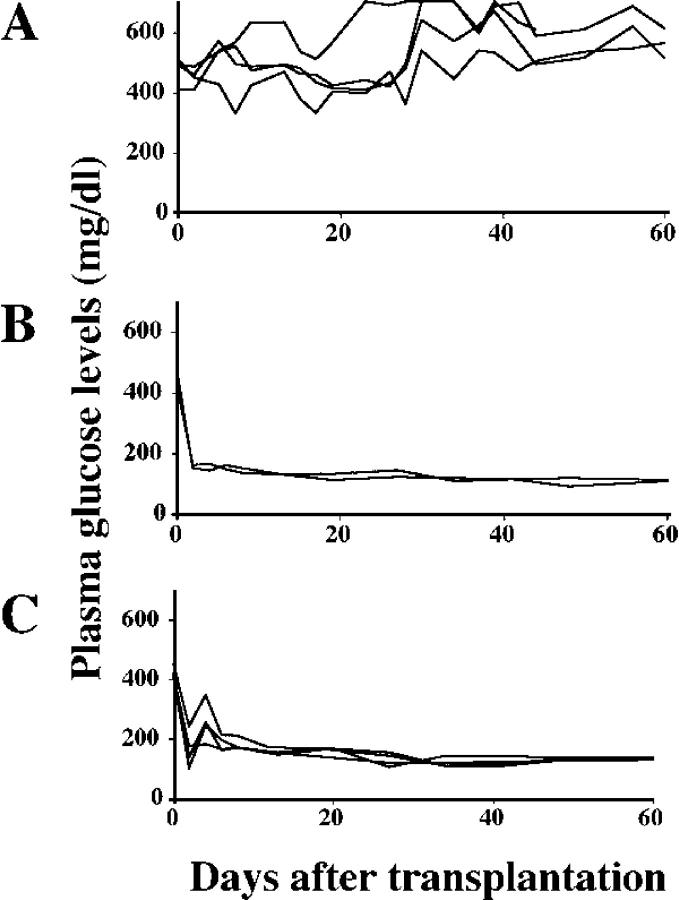
To test the possibility that Vα14 NKT cells are involved in islet graft failure, we attempted to establish an experimental system of islet transplantation. We first examined the minimum number of donor islets required to ameliorate hyperglycemia in streptozotocin (STZ)-induced diabetic mice. C57BL/6 mice became hyperglycemic and diabetic soon after the i.v. injection of STZ (Fig. 1 A). In STZ-induced diabetic mice, 400 syngeneic islets transplanted into the liver were found to be sufficient to achieve normoglycemia (Fig. 1 B, left), whereas normoglycemia could not be achieved by the transplantation of 200 syngeneic islets, the number of islets that can be isolated from a single mouse pancreas (Fig. 1 C, left). In agreement with biochemical data, intact islets with well-granulated β cells were histologically seen in mice receiving 400 islets, whereas damaged islets with degranulated β cells were observed in mice receiving 200 islets (Fig. 1, B and C, right).
Figure 1.
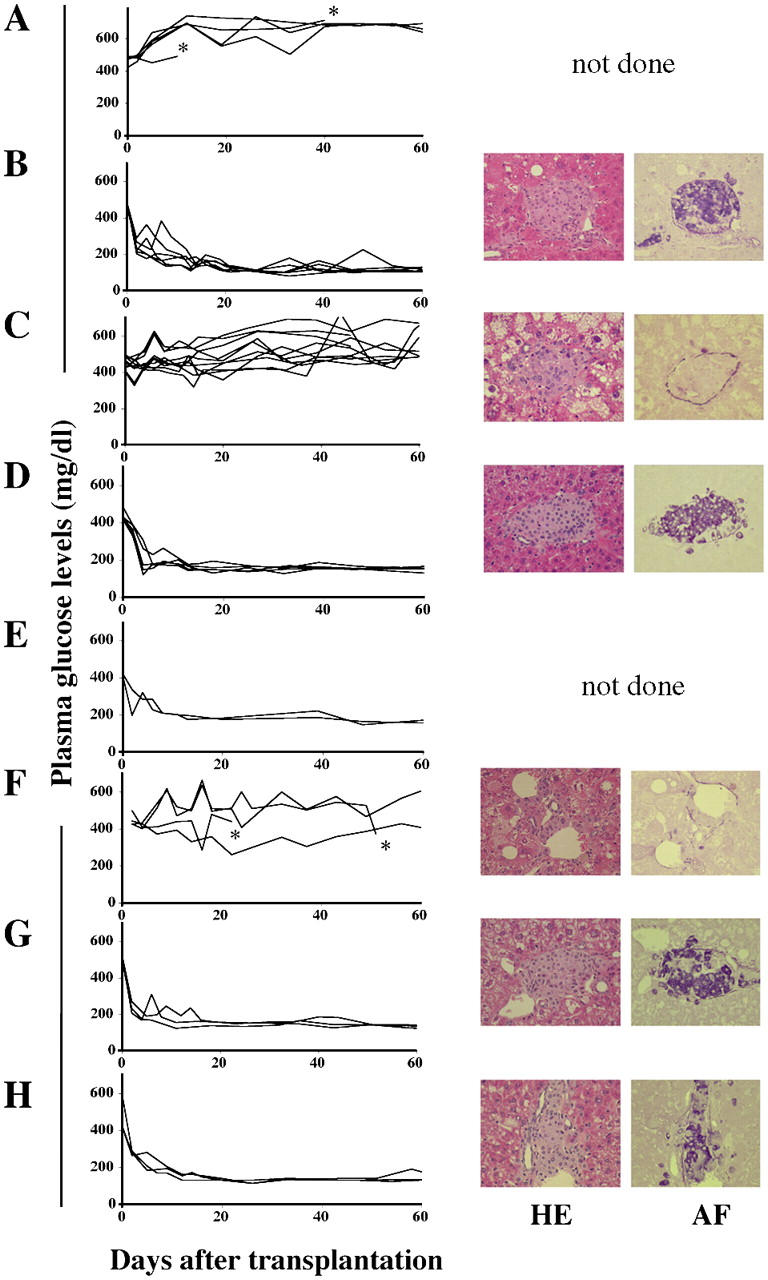
Vα14 NKT cells are responsible for the early loss of syngeneic islet grafts. (A) Hyperglycemia in C57BL/6 mice after i.v. injection with STZ. (B) Normoglycemia in STZ-induced diabetic mice transplanted with 400 islets. (C) Hyperglycemia in STZ-induced diabetic mice transplanted with 200 islets. (D and E) Normoglycemia in STZ-induced diabetic Jα281−/− mice transplanted with 200 (D) or 100 (E) islets. (F–H) STZ-induced diabetic Jα281−/− mice transplanted with 200 islets were injected i.p. with 5 × 106 hepatic mononuclear cells from WT (F), Jα281−/− (G), or INF-γ–deficient (H) mice. Individual lines represent the nonfasting plasma glucose levels of each animal (left panels). The difference in the rate of euglycemia in diabetic WT mice after transplantation of 400 (n = 6) or 200 (n = 10) islets is statistically significant (P < 0.001). The asterisks indicate that the animals died because of severe diabetes. The right panels show photomicrographs of islet grafts in the liver examined on day 60 after transplantation. HE, hematoxylin and eosin; AF, aldehyde fuchsin. Original magnification = 100.
We then investigated whether Vα14 NKT cells are involved in early islet graft loss. All diabetic Jα281−/− mice became normoglycemic by day 11 after transplantation of 200 or even 100 islets (Fig. 1, D and E, left). Histological examination also clearly demonstrated the existence of intact islet grafts with well-granulated β cells (Fig. 1 D, right), suggesting that Vα14 NKT cells are responsible for graft rejection of transplanted islets.
To prove the deleterious effects of Vα14 NKT cells on grafted islets, 5 × 106 liver mononuclear cells isolated from naive WT, Jα281−/−, or IFN-γ–deficient mice were injected at the time of transplantation into the portal vein of diabetic Jα281−/− mice receiving 200 syngeneic islets. Diabetic Jα281−/− mice injected with cells from WT mice remained hyperglycemic 60 d after transplantation (Fig. 1 F, left), whereas those with cells from Jα281−/− or IFN-γ–deficient mice became normoglycemic (Fig. 1, G and H, left). Histological examination also proved the existence of intact islets in the recipients of cells from Jα281−/− or IFN-γ–deficient mice, but not from WT mice (Fig. 1, F–H, right). The results suggest that Vα14 NKT cells and IFN-γ are essential components of early graft failure.
Gr-1+CD11b+ cells induced by transplantation are effector cells for early graft loss
To elucidate cellular changes associated with islet graft failure, hepatic mononuclear cells were isolated periodically after islet transplantation and examined by FACS using α-galactosylceramide (α-GalCer)–loaded CD1d (α-GalCer–CD1d) tetramers. FACS analysis showed that the percentage of α-GalCer–CD1d tetramer+ Vα14 NKT cells initially decreased from 10.3% at 0 h to ∼5% at 6 h but then surged to 19.2% at 24 h after islet transplantation (Fig. 2 A, left). The findings indicate the activation of Vα14 NKT cells that down-modulate their receptor expression, resulting in the failure to detect Vα14 NKT cells by α-GalCer–CD1d tetramers, as reported previously (10–12). Furthermore, Vα14 NKT cells obtained at 2–6 h after transplantation produced inflammatory cytokines, such as IFN-γ and IL-1β (Fig. 2 A), suggesting that Vα14 NKT cells are activated by transplantation. After the disappearance of Vα14 NKT cells, Vα14 NKT cells expand rapidly, as shown in Fig. 2 A (24 h) and as previously reported (10–12). Interestingly, the proportion of α-GalCer–CD1d tetramer− cells producing high levels of INF-γ increased markedly from 0.5 to 14.3% at 6 h after islet transplantation (Fig. 2 A, left). The α-GalCer–CD1d tetramer− cells were further gated, analyzed for Gr-1 and CD11b expression in relation to IFN-γ production, and determined to be Gr-1+CD11b+ cells (Fig. 2 B). Gr-1+ CD11b+ cells were CD11c−, CD86−, MHC class II−, F4/80+/−, and CD68+/− (Fig. 2 C) and were neutrophils in morphology (Fig. 2 D), indicating that these cells are neutrophils. The neutrophils that infiltrated the transplanted islet (Fig. 2 D) are likely to be the effector cells, because the injection of antibodies against Gr-1 or CD11b prevented the rejection of islet transplants (Fig. 3).
Figure 2.
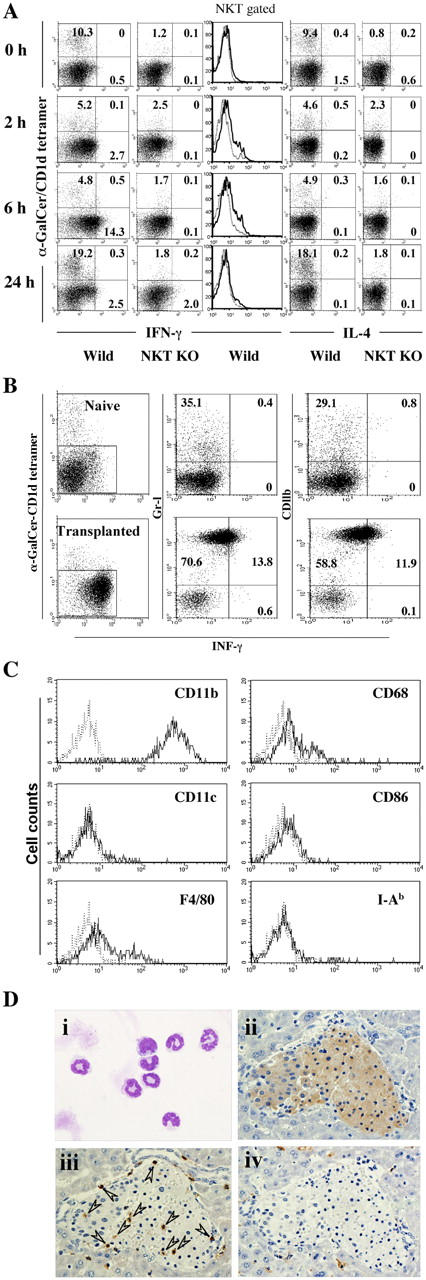
INF-γ–producing Gr-1+CD11b+ cells generated after syngeneic islet transplantation. (A) Requirement of Vα14 NKT cells for induction of IFN-γ–producing cells. Liver mononuclear cells isolated from WT or Jα281−/− mice 2, 6, and 24 h after the transplantation of 200 islets were examined by FACS with PE–α-GalCer–CD1d tetramers and allophycocyanin-conjugated anti–IFN-γ (left) or allophycocyanin-conjugated anti–IL-4 (right). Gated CD3+α-GalCer–CD1d tetramer+ cells (solid line) were also investigated for their IFN-γ production compared with the negative control (dotted line). (B) IFN-γ–producing Gr-1+CD11b+ cells. α-GalCer–CD1d tetramer− cells from the livers of naive mice (top) and mice 6 h after the transplantation of 200 syngenic islets (bottom) were further gated (left) and analyzed for Gr-1 (PerCP) and CD11b (FITC) expression in relation to IFN-γ production by intracytoplasmic staining (middle and right). (C) Surface phenotypes of Gr-1+CD11b+ cells. α-GalCer–CD1d tetramer− cells in B were further analyzed by FACS. Their surface expression (solid line) was compared with the control (dotted line). The numbers in B represent the percentages of cells in the corresponding areas. Representative data from two to three experiments are shown. (D) Histological findings of Gr-1+CD11b+ cells isolated from the liver 6 h after transplantation. (i) May-Grunwald-Giemsa staining. Original magnification = 500. (ii–iv) Immunohistochemical staining on insulin (ii), Gr-1 (iii), and F4/80 (iv). The arrowheads indicate infiltrated Gr-1+ cells into the transplanted islet. Original magnification = 400.
Figure 3.
Prevention of diabetes with transplantation of 200 syngeneic islets and anti–Gr-1 or anti-CD11b antibody treatment. STZ-induced diabetic mice transplanted with 200 islets were injected intraportally with control antibody (A), anti–Gr-1 mAb (B), or anti-CD11b mAb (C).
In marked contrast to WT mice, the increase in IFN-γ–producing Gr-1+CD11b+ cells was not seen in Jα281−/− mice (Fig. 2 A, left), suggesting that the activation of Vα14 NKT cells after islet transplantation triggers the induction of IFN-γ production by Gr-1+CD11b+ cells. Note that no increase in IL-4–producing cells was observed regardless of the presence or absence of Vα14 NKT cells (Fig. 2 A, right).
It is well known that soon after activation with α-GalCer or IL-12, Vα14 NKT cells produce IFN-γ, which, in turn, stimulates IFN-γ secretion by other cells, such as NK cells or CD8 T cells (9, 13, 14). In fact, a single injection of α-GalCer dramatically augmented IFN-γ production in Gr-1+CD11b+ cells (see Fig. 5 D), suggesting that Vα14 NKT cells indeed activate Gr-1+CD11b+ cells to produce IFN-γ. It is thus likely that increased IFN-γ production by Gr-1+CD11b+ cells is a direct consequence of Vα14 NKT cell activation triggered by islet transplantation, although molecules responsible for the activation of Vα14 NKT cells remain to be determined.
Figure 5.
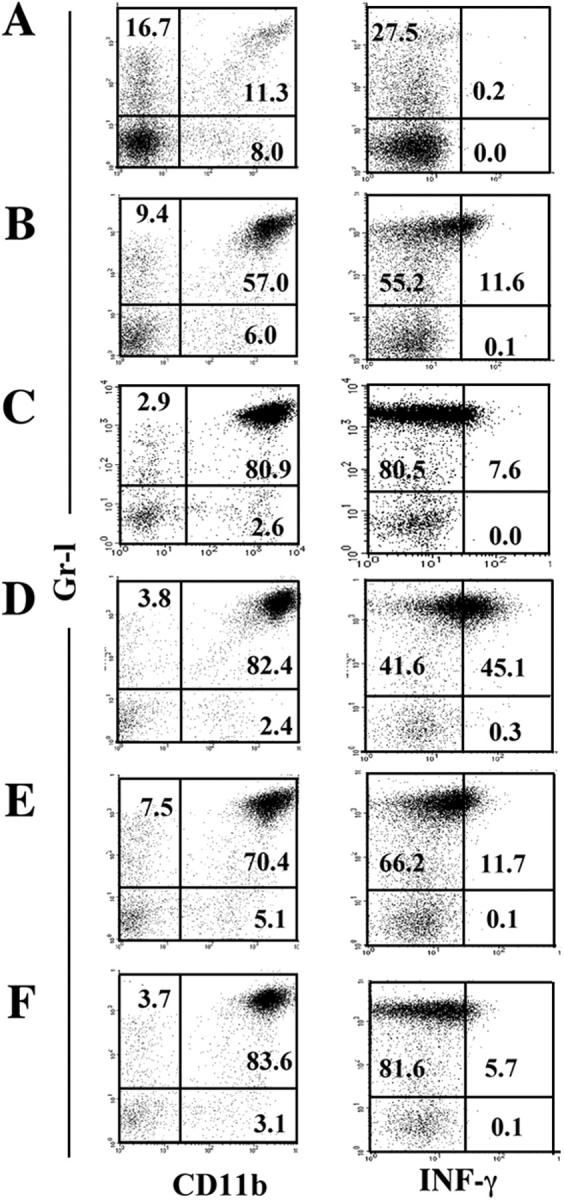
Effects of α-GalCer treatment on the production of IFN-γ by Gr-1+CD11b+ cells. Liver mononuclear cells from naive mice (A) and transplant recipients of WT (B and D–F) or Jα281−/− (C) mice isolated 6 h after transplantation of 400 or 200 syngeneic islets were examined by FACS. Mice transplanted with 400 islets were injected once with vehicle (B and C) or 100 μg/kg α-GalCer (D). Similarly, mice transplanted with 200 islets were injected three times with vehicle (E) or 100 μg/kg α-GalCer (F) on days 15, 11, and 7 before being injected i.v. with STZ 3 d before transplantation. The numbers in the figures represent the percentages of cells in the corresponding areas. Representative data from two to three experiments are shown.
Similar to IFN-γ–producing Gr-1+CD11b+ cells, IL-4–producing Gr-1+CD11b+ cells induced by aluminum hydroxide, when antigens precipitated by aluminum hydroxide are used for immunization, play an important role in B cell activation (15). Despite the similarities in antigen independence and importance in the immune responses of these two populations, several apparent differences are noted, such as the fact that IL-4–producing Gr-1+CD11b+ cells are evident 6 d after immunization, whereas IFN-γ–producing Gr-1+CD11b+ cells appear soon (6 h) after transplantation. The findings suggest functional diversity, although it is unclear whether the two populations are the same or different cells producing different cytokines at different time points.
Prevention of early graft loss by modulation of Vα14 NKT cell function
In agreement with previously reported data (16), Vα14 NKT cells secreted large amounts of IFN-γ immediately after stimulation with α-GalCer but stopped producing IFN-γ after repeated α-GalCer stimulation (Fig. 4 A). Therefore, we hypothesized that repeated α-GalCer injections would modify IFN-γ production by Vα14 NKT cells and thus affect graft survival. To test this possibility, STZ-induced diabetic WT mice that had received 400 islets were treated once with vehicle at the time of transplantation; all mice became normoglycemic (Fig. 4 B). When diabetic WT mice that had received 400 islets were treated with a single 100-μg/kg injection of α-GalCer, none of the mice became normoglycemic even 60 d after transplantation (Fig. 4 C). In contrast, when WT mice were treated three times with 100 μg/kg α-GalCer on days 15, 11, and 7 before the induction of diabetes before islet transplantation, the transplantation of only 200 islets restored normoglycemia in all recipient mice within 10 d (Fig. 4 E). Mice treated with vehicle instead of α-GalCer remained hyperglycemic (Fig. 4 D). In agreement with our prediction, the results demonstrated that repeated α-GalCer injections before islet transplantation resulted in the down-regulation of IFN-γ production, which positively affected islet engraftment.
Figure 4.
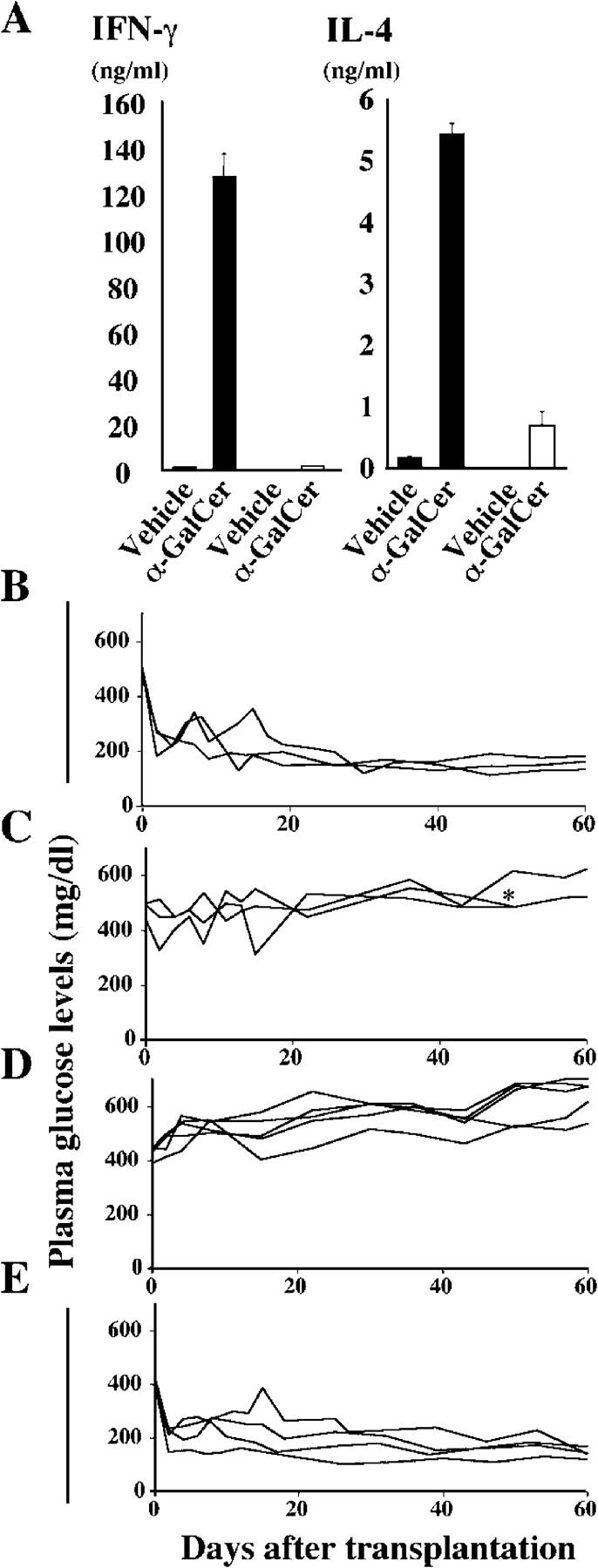
Prevention of syngeneic islet graft failure by repeated α-GalCer treatment. (A) Cytokine production of Vα14 NKT cells. 2 × 104 purified liver Vα14 NKT cells/well from mice injected i.p. one (closed bar) or three (open bar) times with α-GalCer or vehicle were cultured with 100 ng/ml α-GalCer or 104 vehicle-pulsed DCs/well and measured for their cytokines by ELISA. Values represent mean ± SD. (B and C) Rejection of islet graft by single α-GalCer stimulation. 400 syngenic islets were grafted into STZ-induced diabetic C57BL/6 mice treated with a single i.p. injection of vehicle (B) or 100 μg/kg α-GalCer (C) at the time of islet transplantation. (D and E) Protection of islet graft failure by repeated α-GalCer stimulation. Mice transplanted with 200 syngenic islets were treated three times with vehicle (D) or 100 μg/kg α-GalCer (E) on days 15, 11, and 7 before being injected with STZ 3 d before the transplantation. Individual lines represent the nonfasting plasma glucose levels of each animal. The asterisk indicates that the animal died because of severe diabetes.
IFN-γ production by Gr1+CD11b+ cells that were triggered by Vα14 NKT cells
We investigated the effect of α-GalCer on the production of IFN-γ by Gr-1+CD11b+ cells. FACS analysis revealed that Gr-1+CD11b+ cells expanded rapidly after islet transplantation (Fig. 5 B) compared with nontransplanted controls (Fig. 5 A). The influx of Gr-1+CD11b+ cells is independent of Vα14 NKT cells because increased numbers of Gr-1+CD11b+ cells were detected in Jα281−/− mice after islet transplantation (Fig. 5 C), suggesting that Gr-1+CD11b+ cells are generated by transplantation. However, IFN-γ production by Gr-1+ CD11b+ cells was dramatically augmented by a single injection of α-GalCer (Fig. 5 D) but diminished to a level equivalent to the vehicle-treated control (Fig. 5 E) when repeated injections of α-GalCer were given (Fig. 5 F). Thus, IFN-γ production by Gr-1+CD11b+ cells is totally dependent on the activation status of Vα14 NKT cells. The findings on the protection of disease development by repeated α-GalCer injections were also reported in a type I diabetes model (17). It has been reported that IFN-γ is an essential molecule in the destruction of islets, causing the development of diabetes in a mouse model of virus-induced insulin-dependent diabetes mellitus (18). Furthermore, IFN-γ has been shown to induce nitric oxide–mediated toxicity to islet β cells in vitro in combination with IL-1 (19). It is thus likely that IFN-γ produced by Gr-1+CD11b+ cells might similarly affect islet β cells. Overall, our data suggest that the modulation of Vα14 NKT cell function by repeated α-GalCer stimulation in vivo manipulates IFN-γ production by Gr-1+CD11b+ cells and prevents immediate graft failure and the early loss of islet grafts, thus contributing to successful pancreatic islet transplantation.
Concluding remarks
Our results obtained in a mouse model have important clinical implications. Similar to Vα14 NKT cells in mice, human Vα24 NKT cells also release large quantities of INF-γ on activation (20), suggesting that the early graft loss of islets in humans is similarly mediated by Vα24 NKT cells. If this assumption is correct, treatment regimens aimed at the down-regulation of INF-γ production by human Vα24 NKT cells might help to prevent the early loss of islet grafts in clinical settings, even though Vα24 NKT cells are less frequent in the human liver (21), and, thus, might help to increase the efficiency of pancreatic islet transplants.
MATERALS AND METHODS
Animals.
C57BL/6 mice were purchased from Charles River Laboratories and C57BL/6 Jα281−/− mice (10–15 wk old) raised in our facility were used (9). C57BL/6 IFN-γ–deficient mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan) (22). Experimental plans were approved by the committee on institutional animal care and use at Fukuoka University and RIKEN.
Diabetes induction.
Diabetes was induced by an i.v. injection of 180 mg/kg STZ (Sigma-Aldrich) (23).
Islet isolation.
Islets isolated by collagenase digestion were centrifuged in Ficoll-Conray gradients (24, 25) and transplanted via the portal vein into the liver of STZ-treated mice (26).
α-GalCer treatment.
α-GalCer was synthesized in our laboratory (27). Mice were injected i.p. one or three times with 100 μg/kg α-GalCer or 0.025% vehicle (wt/vol) before islet transplantation.
Flow cytometry and staining.
The following mAbs were used: anti–mouse FcRγII/III (2.4G2), FITC-conjugated anti-CD3ɛ (145-2C11), FITC- or PE-conjugated anti-CD11b (M1/70), allophycocyanin-conjugated anti–IFN-γ (XMG1.2), allophycocyanin-conjugated anti–IL-4 (11B11), PerCP-conjugated anti–Gr-1 (Rb6-8c5), PerCP-Cy5.5–anti–Gr-1 (86-8C5), FITC–anti-CD11c (HL3), FITC–anti-CD86 (GL-1), FITC–anti–I-Ab (AF6-120.1), and controls (BD Biosciences). FITC–anti-F4/80 (A3-1) and FITC–anti-CD68 (FA-11) were obtained from SEROTEC. PE–α-GalCer–CD1d tetramers were prepared as previously described (28). For intracellular staining, cells were incubated with anti-FcRγII/III and neutravidin (Invitrogen), surface stained, fixed, permeabilized, stained with mAbs, and analyzed on a flow cytometer (FACSCalibur; Becton Dickinson). 10,000 viable cells were analyzed.
Cell sorting and transfer.
Hepatic mononuclear cells were prepared as described previously (25). Gr-1+CD11b+ cells and Vα14 NKT cells were sorted by MoFlo (DakoCytomation) with purity ≥99 and ≥98%, respectively. Splenic DCs were purified by MACS with anti-CD11c–coupled magnetic beads (Miltenyi Biotec) with purity ≥95%.
Antibody treatment.
mAbs (10 μg/mouse) against Gr-1 or CD11b were i.p. injected into STZ-diabetic mice receiving 200 islets at the time of transplantation.
Histology.
The liver and pancreas from transplant recipients were fixed in Bouin's solution, processed, and embedded in paraffin. Sections were stained with hematoxylin and eosin and aldehyde fuchsin. Immunohistochemistry was performed by a streptavidin–biotin–peroxidase complex method (29).
Acknowledgments
This work was supported by a grant (0211001) from the Central Research Institute of Fukuoka University (to Y. Yasunami); a Health Science Research Grant from the Ministry of Health, Labour and Welfare, Japan (to Y. Yasunami); a Grant-in-Aid (15390386) for Scientific Research (B) from the Japan Society for the Promotion of Science (to Y. Yasunami); and a Grant-in-Aid (13307011) for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to M. Taniguchi).
The authors have no conflicting financial interests.
References
- 1.Ricordi, C., and T.B. Strom. 2004. Clinical islet transplantation: advances and immunological challenges. Nat. Rev. Immunol. 4:259–268. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro, A.M., J.R. Lakey, E.A. Ryan, G.S. Korbutt, E. Toth, G.L. Warnock, N.M. Kneteman, and R.V. Rajotte. 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343:230–238. [DOI] [PubMed] [Google Scholar]
- 3.Ryan, E.A., J.R. Lakey, B.W. Paty, S. Imes, G.S. Korbutt, N.M. Kneteman, D. Bigam, R.V. Rajotte, and A.M. Shapiro. 2002. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 51:2148–2157. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovitch, A. 1993. Roles of cytokines in IDDM pathogenesis and islet β-cell destruction. Diabetes Rev. 1:215–240. [Google Scholar]
- 5.Brigl, M., L. Bry, S.C. Kent, J.E. Gumperz, and M.B. Brenner. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4:1230–1237. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483–513. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi, M., K. Seino, and T. Nakayama. 2003. The NKT cell system: bridging innate and acquired immunity. Nat. Immunol. 4:1164–1165. [DOI] [PubMed] [Google Scholar]
- 8.Wilson, S.B., and T.L. Delovitch. 2003. Janus-like role of regulatory iNKT cells in autoimmune disease and tumor immunity. Nat. Rev. Immunol. 3:211–222. [DOI] [PubMed] [Google Scholar]
- 9.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 278:1623–1626. [DOI] [PubMed] [Google Scholar]
- 10.Harada, M., K. Seino, H. Wakao, S. Sakata, Y. Ishizuka, T. Ito, S. Kojo, T. Nakayama, and M. Taniguchi. 2004. Down-regulation of invariant Vα14 antigen receptor in NKT cells upon activation. Int. Immunol. 16:241–247. [DOI] [PubMed] [Google Scholar]
- 11.Wilson, M.T., C. Johansson, D. Olivares-Villagomez, A.K. Singh, A.K. Stanic, C.R. Wang, S. Joyce, M.J. Wick, and L. Van Kaer. 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. USA. 100:10913–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe, N.Y., A.P. Uldrich, K. Kyparissoudis, K.J. Hammond, Y. Hayakawa, S. Sidobre, R. Keating, M. Kronenberg, M.J. Smyth, and D.I. Godfrey. 2003. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J. Immunol. 171:4020–4027. [DOI] [PubMed] [Google Scholar]
- 13.Smyth, M.J., N.Y. Crowe, D.G. Pellicci, K. Kyparissoudis, J.M. Kelly, K. Takeda, H. Yagita, and D.I. Godfrey. 2002. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 99:1259–1266. [DOI] [PubMed] [Google Scholar]
- 14.Fujii, S.-i., K. Shimizu, C. Smith, L. Bonifaz, and R.M. Steinman. 2003. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan, M.B., D.M. Mills, J. Kappler, P. Marrack, and J.C. Cambier. 2004. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 304:1808–1810. [DOI] [PubMed] [Google Scholar]
- 16.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014–2025. [DOI] [PubMed] [Google Scholar]
- 17.Sharif, S., G.A. Arreaza, P. Zucker, Q.S. Mi, J. Sondhi, O.V. Naidenko, M. Kronenberg, Y. Koezuka, T.L. Delovitch, J.M. Gombert, et al. 2001. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat. Med. 7:1057–1062. [DOI] [PubMed] [Google Scholar]
- 18.von Herrath, M.G., and M.B. Oldstone. 1997. Interferon-γ is essential for destruction of β cells and development of insulin-dependent diabetes mellitus. J. Exp. Med. 185:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas, H.E., R. Darwiche, J.A. Corbett, and T.W. Kay. 2002. Interleukin-1 plus γ-interferon–induced pancreatic β-cell dysfunction is mediated by β-cell nitric oxide production. Diabetes. 51:311–316. [DOI] [PubMed] [Google Scholar]
- 20.Exley, M., J. Garcia, S.P. Balk, and S. Porcelli. 1997. Requirement for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J. Exp. Med. 186:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenna, T., L. Golden-Mason, S.A. Porcelli, Y. Koezuka, J.E. Hegarty, C. O'Farrelly, and D.G. Doherty. 2003. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J. Immunol. 171:1775–1779. [DOI] [PubMed] [Google Scholar]
- 22.Tagawa, Y., K. Sekikawa, and Y. Iwakura. 1997. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice: role for IFN-γ in activating apoptosis of hepatocytes. J. Immunol. 159:1418–1428. [PubMed] [Google Scholar]
- 23.Schein, P.S., and R.W. Bates. 1968. Plasma glucose levels in normal and adrenalectomized mice treated with streptozotocin and nicotinamide. Diabetes. 17:760–765. [DOI] [PubMed] [Google Scholar]
- 24.Sutton, R., M. Peters, P. McShane, D.W. Gray, and P.J. Morris. 1986. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 42:689–691. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka, K., Y. Yasunami, Y. Ikehara, T. Nagai, S. Kodama, T. Maki, A. Tomita, T. Abo, and S. Ikeda. 1997. Expansion of intermediate T cell receptor cells expressing IL-2Rα−β+, CD8α +β+, and lymphocyte function-associated antigen-1+ in the liver in association with intrahepatic islet xenograft rejection from rat to mouse: prevention of rejection with anti-IL-2Rβ monoclonal antibody treatment. Transplantation. 64:633–639. [DOI] [PubMed] [Google Scholar]
- 26.Kemp, C.B., M.J. Knight, D.W. Scharp, P.E. Lacy, and W.F. Ballinger. 1973. Transplantation of isolated pancreatic islets into the portal vein of diabetic rats. Nature. 244:447. [DOI] [PubMed] [Google Scholar]
- 27.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.-R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibao, K., H. Takano, Y. Nakayama, K. Okazaki, N. Nagata, H. Izumi, T. Uchiumi, M. Kuwano, K. Kohno, and H. Itoh. 1999. Enhanced coexpression of YB-1 and DNA topoisomerase IIα genes in human colorectal carcinomas. Int. J. Cancer. 83:732–737. [DOI] [PubMed] [Google Scholar]