Abstract
The SH2 domain containing leukocyte phosphoprotein of 76 kD (SLP-76) is critical for pre-TCR–mediated maturation to the CD4+CD8+ double positive (DP) stage in the thymus. The absolute block in SLP-76null mice at the CD4−CD8−CD44−CD25+ (double-negative 3, DN3) stage has hindered our understanding of the role of this adaptor in αβ TCR-mediated signal transduction in primary thymocytes and peripheral T lymphocytes. To evaluate the requirements for SLP-76 in these events, we used a cre-loxP approach to generate mice that conditionally delete SLP-76 after the DN3 checkpoint. These mice develop DP thymocytes that express the αβ TCR on the surface, but lack SLP-76 at the genomic DNA and protein levels. The DP compartment has reduced cellularity in young mice and fails to undergo positive selection to CD4+ or CD8+ single positive (SP) cells in vivo or activation-induced cell death in vitro. A small number of CD4+SP thymocytes are generated, but these cells fail to flux calcium in response to an αβ TCR-generated signal. Peripheral T cells are reduced in number, lack SLP-76 protein, and have an abnormal surface phenotype. These studies show for the first time that SLP-76 is required for signal transduction through the mature αβ TCR in primary cells of the T lineage.
The complex process by which immature T lymphocyte precursors differentiate into CD4 and CD8-bearing αβ T lymphocytes requires productive signaling at multiple stages during thymic development. The SH2 domain containing leukocyte phosphoprotein of 76 kD (SLP-76) is an adaptor protein that normally is expressed in all hematopoietic lineages, except mature B lymphocytes (1–3). SLP-76 and the adaptor molecule linker of activated T cells (LAT) are key components of the pre-TCR proximal signaling machinery (1). Mice lacking expression of either of these molecules because of targeted deletion lack development of T cells after the double negative (DN) 3 stage (4, 5). In the absence of SLP-76, DN3 cells are unable to transduce a productive signal and have impaired allelic exclusion (6). Unlike RAG-deficient mice, the DN3 block even is seen following in vivo anti-TCR treatment, which indicates that SLP-76 is essential for the pre-TCR to couple with its signaling machinery (4).
There are differences in composition of the pre-TCR and αβ TCR complexes that suggest distinct requirements for proximal signal transduction. For example, CD3ɛ, but not CD3δ, is required for progression from DN to double positive (DP) thymocytes, presumably because of differential pre-TCR requirements for these associated signaling molecules (7). Expression of the pre-TCR localizes to lipid rafts and results in phosphorylation of ZAP-70 and receptor internalization in a ligand-independent manner, whereas the mature TCR requires interaction with an MHC–peptide ligand to initiate these downstream events (8). In addition, although targeted deletion of protein kinase C-Θ leads to defects in optimal TCR-induced proliferation and IL-2 production of peripheral T lymphocytes, its absence does not impair progression through the pre-TCR checkpoint (DN3) or positive and negative selection (9). To examine the requirement for SLP-76 expression on thymocyte development beyond the DN3 stage, we have generated mice that conditionally delete SLP-76 using a cre-loxP approach. Breeding these animals with CD4cre transgenic mice (10), we show that when SLP-76 is deleted between the DN and DP stages, SLP-76–deficient CD4+CD8+ DP thymocytes fail to generate the signals that are needed for positive selection or antigen-induced clonal deletion.
RESULTS AND DISCUSSION
Strategy for conditional inactivation of the SLP-76 gene
To examine the role of SLP-76 in thymocyte development after the DN3 checkpoint, we generated mice in which exon 3 of the gene was flanked by loxP sites (floxed) (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20051128/DC1). Aberrant splicing of exon 2 directly to exon 4 or 5 results in a frameshift mutation that ensures an early translational stop codon. Appropriately targeted embryonic stem (ES) cells were used to generate chimeric mice, and germline transmission was confirmed (unpublished data). Targeted mice expressing the floxed allele are termed SLP-76F mice. Initial experiments examining homozygous SLP-76F/F mice confirmed that introduction of the targeting vector before cre expression did not confer a T cell phenotype in the thymus or peripheral compartments. We found no difference in thymocyte cell surface phenotype or cellularity when SLP-76F/F mice were compared with littermate controls (Fig. S1, B and C). In addition, the peripheral lymphoid compartment showed no difference in T lymphocyte cellularity or in the up-regulation of activation markers after TCR-mediated stimulation (unpublished data).
SLP-76 inactivation after the DN3 stage blocks the appearance of CD4 single positive and CD8 single positive (SP) thymocytes
Due to the absolute block in T cell development at the DN3 stage, conventional SLP-76–deficient mice lack any thymocytes beyond this maturational stage (4, 5). Thus, signal transduction by way of a mature αβ TCR complex in primary cells lacking SLP-76 has been impossible to assess. To bypass the DN3 checkpoint, we mated SLP-76F/F mice with the well-characterized CD4cre transgenic mouse (10). In this mouse, the cre recombinase is not expressed until after the DN3 checkpoint. We reasoned that developing T cell precursors would express normal levels of SLP-76 at the DN3 stage, which would allow for an appropriate pre-TCR signal with progression to the DN4 stage and potentially beyond. Between the DN and DP stages, developing thymocytes would begin to express the transgenic cre recombinase, which would result in deletion of the SLP-76 gene and a population of thymocytes that was deficient in SLP-76 protein. We reasoned that if SLP-76 is required for TCRαβ-mediated signals, conditional deletion before the DP stage would have a deleterious effect on thymocyte selection.
We assessed adult SLP-76F/FCD4cre mice for thymocyte cellularity and CD4 and CD8 phenotype. Thymi from adult SLP-76F/FCD4cre mice contain DP cells (Fig. 1 A), which indicated that our strategy of cre expression after DN3 allowed for progression past the blockade seen in conventional SLP-76null mice. However, there is a dramatic reduction in the percentage of CD4SP thymocytes; these cells account for <1% of the thymic cell population. The relative percentage of CD8SP thymocytes is reduced by 40–60% versus littermate controls. Whereas most cells in the CD8SP compartment of WT mice are mature HSAloTCR-βhi cells, most of the CD8SP cells in the SLP-76F/FCD4cre thymus are heat stable antigen (HSA)hiTCR-βlo-int; this indicates that they are immature CD8SP cells (Fig. 1 B). The CD4 CD8 density plot in SLP-76F/FCD4cre mice is consistent in mice from 3 wk to 6 mo of age (unpublished data).
Figure 1.
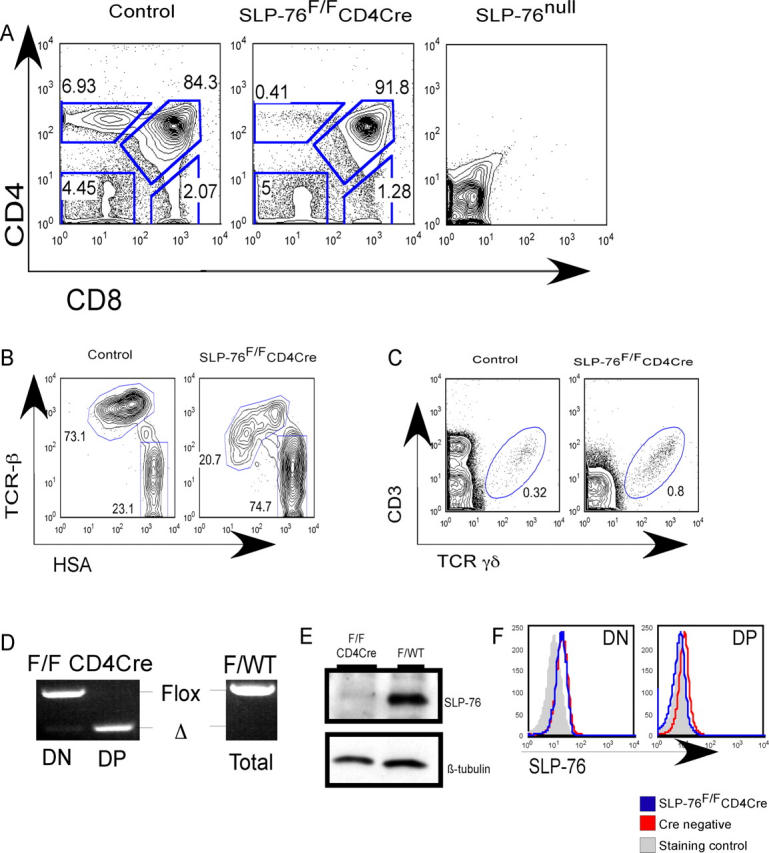
Conditional deletion of SLP-76 after DN3 results in the appearance of DP thymocytes. (A) Representative FACS analysis of thymocytes from adult SLP-76F/FCD4cre mice and littermate controls. Similar analysis of a conventional SLP-76null thymus is shown for comparison. (B) Representative FACS analysis of CD8SP gated adult thymocytes for CD24 (HSA) and TCR-β levels. (C) Representative FACS analysis of thymocytes from 3-wk-old mice for γδ TCR population. Numbers represent the percentage of cells in the indicated regions. (D) PCR analysis of DN and DP thymocyte DNA. Bands representing the floxed and deleted alleles are indicated. DNA isolated from a littermate control, amplified in the same experiment and run on the same gel, is shown for comparison. (E) Western blot analysis for SLP-76 protein levels in DP cells from SLP-76F/FCD4cre and littermate control. β tubulin from the same blot is shown as a loading control. (F) Representative FACS analysis for SLP-76 levels of cells gated on DN and DP cells. The shaded line graph is a staining control using mature B220+ lymphocytes that are known to lack SLP-76 (1).
Adult and young mice were evaluated for thymic cellularity. There was no statistical difference in overall, DN, or DP cellularity in adult SLP-76F/FCD4cre mice relative to littermate controls (Table I, top). However, there was a statistically significant reduction in CD4SP and mature CD8SP thymocyte numbers seen in the SLP-76F/FCD4cre mice. In contrast to older mice, overall thymic cellularity was decreased by ∼50% in mice that were younger than 5 wk of age (Table I, bottom). DN cellularity was equivalent in the young SLP-76F/FCD4cre and littermate controls, but absolute cellularity of DP, CD4SP, and CD8SP cells was reduced significantly.
Table I.
Thymocyte cellularity of SLP-76F/FCD4cre and littermate controlsm
|
|
CD8SPa
|
|
|||||
|---|---|---|---|---|---|---|---|
| Total cellularity | DN | DP | CD4SP | Matureb , c | ISPb , c | Total | |
| Adult, 13–17 wk old | |||||||
| WT (n = 10) | 86.9 ± 26.8 | 2.2 ± 0.7 | 63.9 ± 22.1 | 5.9 ± 2.0 | 1.17 ± 0.20 | 0.52 ± 0.32 | 2.46 ± 1.2 |
| SLP-76F/F CD4cre (n = 7) | 77.1 ± 41.5 | 3.2 ± 1.2 | 59.4 ± 35.4 | 0.63 ± 0.59 | 0.12 ± 0.20 | 0.40 ± 0.07 | 1.4 ± 1.3 |
| p-valued | 0.56 | 0.05 | 0.76 | <0.001 | 0.02 | 0.67 | 0.1 |
| Young, 3–5 wk old | |||||||
| WT (n = 3) | 214 ± 33.4 | 3.9 ± 0.4 | 169.1 ± 26.4 | 17.1 ± 4.1 | 3.8 ± 1.0 | 0.41 ± 0..8 | 4.8 ± 0.7 |
| SLP-76F/F CD4cre (n = 5) | 108.3 ± 11.4 | 4.0 ± 0.1 | 92.3 ± 12.1 | 0.9 ± 0.1 | 0.3 ± 0.2 | 0.47 ± 0.04 | 1.3 ± 0.4 |
| p-valued | <0.01 | 0.67 | 0.001 | <0.001 | <0.001 | 0.39 | <0.001 |
Thymocytes were stained with a cocktail of directly conjugated antibodies, including those specific for CD4 and CD8. In some experiments, antibodies specific for CD24 (HSA) and TCR β antibodies were included. Cell counts are divided by 106. Cells were subjected to FACS and gated on live cells based on FSC/ SSC profile. Total cellularity was determined by using a hemocytometer and trypan blue exclusion of dead cells. Absolute numbers of cells in each subset were determined by multiplying the total number of cells × % live gated × % subset. Numbers of thymi assessed are shown in the first column unless otherwise indicated. WT, littermate control.
For top three rows, to determine CD8SP subsets, CD4−CD8+ gated cells were assessed for expression of HSA and TCRβ as shown in Fig. 1 B. HSAloTCRβint-hi are defined as mature. HSAhiTCRβlo-int are defined as immature CD8SP cells.
For top three rows, mice were 15 wk of age (n = 2).
For bottom three rows,
ice were 5 wk of age (n = 2).
Statistical significance was assessed using a two-tailed Student's t test.
We next assessed the thymic production of γδ T cells. Because these cells never express CD4, we hypothesized that production of γδ T cells would be unaffected in SLP-76F/F CD4cre mice. These animals have approximately twice the relative percentage of γδ TCR+ T cells compared with 5-wk-old control littermates (Fig. 1 C) and similar absolute numbers (not depicted). These data confirm that the phenotype seen in the SLP-76F/FCD4cre mice is restricted to the αβ TCR lineage and is dependent on expression of CD4 at some time during development.
To confirm effective cre-mediated recombination of the SLP-76 locus, we used high-speed FACS to isolate DP thymocytes from SLP-76F/FCD4cre mice and littermate controls expressing a WT SLP-76 allele. PCR of genomic DNA isolated from DN thymocytes amplifies the floxed allele with almost no evidence of the deleted allele (Fig. 1 D). In contrast, PCR of genomic DNA isolated from SLP-76F/F CD4cre DP thymocytes amplifies the deleted allele without evidence of the germline floxed SLP-76 allele.
We next used two methods to assess SLP-76 protein levels. First, whole-cell lysates of FACS-sorted DP thymocytes were used for Western blot analysis (Fig. 1 E). DP thymocytes normally express moderate levels of SLP-76 protein. In contrast, the SLP-76F/FCD4cre DP thymocytes express no detectable SLP-76 protein. Next, we assessed SLP-76 protein levels on a single-cell basis using FACS analysis with polyclonal anti–SLP-76 antisera. DN thymocytes from SLP-76F/FCD4cre mice express SLP-76 at levels equivalent to littermate control mice (Fig. 1 F). DN3 and DN4 subpopulations contain normal levels of SLP-76 protein (unpublished data). Consistent with the Western blot results, DP thymocytes from SLP-76F/FCD4cre mice contain lower levels of the adaptor. Size and granularity of DP cells from SLP-76F/FCD4cre and littermate mice are equivalent as assessed by forward and side scatter profiles (unpublished data). Although we cannot rule out that a small number of cells escape cre-mediated deletion or that some cells express low levels of SLP-76 protein, we conclude that the floxed SLP-76 gene undergoes efficient recombination between the DN3 and DP stage and that DP thymocytes effectively lack SLP-76 protein.
DP thymocytes lacking SLP-76 cannot flux calcium and do not undergo positive selection or activation-induced cell death
Based on cell surface phenotype, we hypothesized that DP thymocytes lacking SLP-76 would be unable to transduce the appropriate TCR-mediated signals that are needed for positive selection. As an initial assessment of TCR-mediated signaling, we examined calcium flux in DP cells using a FACS-based, single-cell assay. DP thymocytes from littermate controls have an increase in intracellular calcium concentration after CD3/CD4 cocross-linking (Fig. 2 A). In contrast, DP cells from SLP-76F/FCD4cre mice failed to flux calcium (Fig. 2 A).
Figure 2.

SLP-76-deficient DP thymocytes cannot transduce TCR-mediated signals. (A). TCR-induced calcium flux was measured in DP-gated thymocytes as described in Materials and methods. (B) Representative FACS of thymocytes from 11-wk-old mice stained with HSA and TCR β. Numbers represent the percentage of cells in the indicated regions. (C) Representative FACS of DP-gated thymocytes from SLP-76F/FCD4cre and control mice. (D) Thymocytes were left unstimulated or were stimulated with plate-bound anti-CD3, plate-bound CD3 plus CD28, or dexamethasone for 14 h. Acquired cells were gated on DP cells and assessed for exclusion of TOPRO-3. The percentage of TOPRO-3− DP cells relative to total DP cells is shown as percent viable. The reduction in viability of control cells stimulated with CD3 plus CD28 was statistically significant (*P < 0.05). (E) Thymocytes were left unstimulated or were stimulated for 2 h then stained for CD4, CD8, and nur77. Line graphs are gated on DP cells.
SLP-76F/FCD4cre thymocytes transit the β selection checkpoint, have normal numbers of DP thymocytes in adults, but lack mature SP thymocytes. DP αβ TCR thymocytes are believed to undergo one of three TCR signal-dependent fates based on the strength or duration of the TCR:MHC–peptide interaction. A strong or prolonged signal leads to negative selection of potentially autoreactive cells, a weak or transient signal results in positive selection and maturation to the SP pool, and a total lack of signal results in death by neglect. We tested the ability of SLP-76–deficient DP thymocytes to undergo each of these fates.
We assessed cell surface expression of markers known to be altered in response to a TCR-mediated positively selecting signal. Specifically, as cells mature, the CD24 (HSA) marker is down-regulated (11). Thymocytes from SLP-76F/FCD4cre mice lack normal numbers of the HSAloTCRβhi cells that have undergone positive selection (Fig. 2 B). In addition, stimulation by way of the TCR results in up-regulation of CD5 and CD69 and of the TCR from TCRint to TCRhi levels. DP thymocytes lacking SLP-76 fail to increase expression of any of these markers (Fig. 2 C). The lack of CD5 and CD69 up-regulation, lack of TCRhiHSAlo cells, and a lack of SP thymocytes (Fig. 1 A) strongly argue for an absence of efficient positive selection, regardless of TCR specificity.
To address the ability of SLP-76–deficient DP thymocytes to undergo negative selection, we assessed activation-induced cell death in vitro. Single-cell suspensions of thymocytes from SLP-76F/FCD4cre and littermate controls were stimulated overnight with plate-bound anti-CD3 or a combination of anti-CD3 plus anti-CD28 to induce a stronger signal. Background cell death of DP cells was not statistically different in the two groups. Littermate control DP thymocytes had no statistically significant increase in cell death in response to anti-CD3 alone. However, stimulation with a combination of anti-CD3 and anti-CD28 promoted a significant increase in cell death either 14 or 20 h of stimulation (Fig. 2 D and not depicted, respectively). In contrast, stimulation with anti-CD3 alone or anti-CD3 in combination with anti-CD28 did not result in augmentation of cell death over background in DP thymocytes from SLP-76F/FCD4cre mice. Dexamethasone, which causes death of DP thymocytes in a TCR-independent fashion, was effective at inducing apoptosis, regardless of the expression of SLP-76.
An early marker of cells that are destined for TCR-induced cell death is expression of the nur77 gene product (12). Nur77 is up-regulated in vivo and in vitro in cells that are destined for the apoptotic pathway, and is believed to be directly downstream of TCR-mediated signals (12). We stimulated thymocyte suspensions with anti-CD3 or a combination of anti-CD3 and CD28 for 2 h and assessed nur77 levels in DP cells (Fig. 2 E). A substantial proportion of DP thymocytes isolated from WT littermates up-regulated nur77 expression at this time point. A majority of DP thymocytes lacking SLP-76 showed no up-regulation of this TCR-induced nuclear protein.
The rare SLP-76–deficient CD4SP thymocytes and peripheral T cells develop abnormally
Despite the overall decrease in SP cellularity, there are CD4SP and CD8SP cells. We next asked whether the cells that had matured into the SP populations had undergone normal lineage commitment. We gated on CD69+ cells or HSAlo cells and evaluated their CD4/CD8 ratio. This ratio is ∼3:1 in HSAlo-gated cells from littermate control mice. However, in the SLP-76F/FCD4cre mice, the ratio is inverted. CD8SP cells predominate in the HSAlo subset (Fig. 3 A). In addition, TCR levels on the selected cells are not uniformly high, as seen in the controls (Fig. 1 B and Fig. 3 D for CD8SP and CD4SP, respectively). Because loss of SLP-76 protein is unlikely to be synchronous in all cells, we believe that most CD4SP and mature CD8SP cells in the SLP-76F/FCD4cre thymi are the result of TCR-mediated positive selection from the DP stage in a cell that can no longer generate new SLP-76 protein because of gene deletion. During the transition from DP to SP, these cells lose the remainder of their SLP-76 through normal protein turnover.
Figure 3.

Post DP T cells have an altered CD4/CD8 ratio and cannot flux calcium in response to TCR cross-linking. Representative FACS analysis of (A) HSAlo-gated thymocytes, (B) live-gated lymph node cells, and (C) CD4-gated splenocytes. Relative percentages of cells within the gated regions are shown. (D) Intracellular staining for SLP-76 and TCR-β in CD4SP thymocytes. Staining controls are mature B220+ cells with SLP-76 antiserum and PE-labeled isotype control for TCR-β. (E) TCR-induced calcium flux from SLP-76F/FCD4cre mice and control animals was performed in CD4SP-gated cells as described in Materials and methods.
We also evaluated for the presence of mature αβ T cells in the periphery of the SLP-76F/FCD4cre mice. Consistent with the few CD4SP and CD8SP cells in the thymus, the relative percentage and absolute number of CD4 and CD8 T cells are reduced in the spleen and lymph node (Fig. 3 B). In addition, the CD4/CD8 ratio is altered in the lymph node and spleen. In control animals the CD4/CD8 ratio is ∼2:1, but is reversed in SLP-76F/FCD4cre mice. This finding is consistent with an increase in the relative production of CD8 cells in the thymus or enhanced survival of these cells relative to the CD4 cells. Flow cytometry revealed that the CD4 and CD8 cells lack intracellular SLP-76 protein and have low levels of surface TCR (unpublished data). Although most littermate control CD4 splenic T cells are naive, the cell surface phenotype of splenic CD4 cells in the SLP-76F/FCD4cre mice is skewed toward that of effector/memory cells—low in CD62L and high in CD44 expression (Fig. 3 C).
Although there were only a few cells available (and recognizing that they do not have the identical cell surface phenotype of CD4 peripheral T cells from WT animals), there were sufficient numbers of CD4 SP thymocytes from the SLP-76F/FCD4cre mice for us to test their ability to transduce a TCR-mediated signal. To confirm that these cells lack SLP-76 protein, we used intracellular FACS (Fig. 3 D, left) and found that most lack SLP-76 protein. However, there are some CD4SP cells that express relatively normal levels of the adaptor; these few cells presumably have not deleted the SLP-76 gene or protein turnover in these cells has not yet resulted in complete loss. Assessment of cell surface TCR levels shows that although CD4SP cells from normal mice express only high levels of TCR, CD4SP cells from SLP-76F/FCD4cre mice have a more heterogeneous and lower distribution (Fig. 3 D, right). Recognizing these potential confounders, we were able to assess the generation of TCR-mediated signals at a single-cell level by examining calcium flux in CD4SP cells from WT controls versus those with SLP-76 deleted. WT cells demonstrated an increase in intracellular calcium concentration after TCR cross-linking (Fig. 3 E). Conversely, CD4SP cells lacking SLP-76 show an inability to flux calcium in response to the same stimulus. Similar to results in CD4SP thymocytes, cross-linking of the αβ TCR on peripheral CD4 cells does not result in an increase of intracellular calcium (unpublished data). To address the issue of low cell surface TCR levels, we used a range of TCR cross-linking reagent concentrations, and found no increase in intracellular calcium, regardless of the dose used in the SLP-76–deficient DP thymocytes.
One interesting finding in these studies is that the decreased thymic cellularity found in young SLP-76F/FCD4cre mice normalizes by the time that the mice reach adulthood. That adult mice exhibit preserved thymic cellularity is consistent with data from ZAP-70 (13), TCRα (14, 15), and MHC class (I × II) knockout mice (16). In each of these models, normal pre-TCR signals at the DN3 stage allow transition through this developmental checkpoint. In ZAP-70 knockouts, the pre-TCR signal is provided by the syk kinase, which is not expressed in DP thymocytes (17, 18). ZAP-70−/− DP cells express a mature αβ TCR on the surface, but are unable to transduce a signal, and thus, die by neglect. In the case of TCRα knockouts, we argue that the maintenance of thymic cellularity is due to the continued expression of the pre-TCR (19), which is able to transduce a signal, and thus, up-regulates CD5. However, because these cells are unable to express a mature αβ TCR, they cannot transit to CD4 or CD8 SP thymocyte subsets. Although thymocytes from MHC knockouts have a preserved αβ TCR and normal signaling machinery, there is no ligand for the receptor, and cells die by neglect at the DP stage. Therefore, we were surprised that in young SLP-76F/FCD4cre mice, overall and DP cellularity are decreased, whereas DN cellularity remains normal.
It is possible that a continuous pre-TCR signal is required for normal proliferation and survival during the DN→DP transition, and that deletion of SLP-76 during this transition results in a loss of pre-TCR signaling in the SLP-76F/FCD4cre mice. However, we used continuous and pulse labeling with bromodeoxyuridine and found no difference in the percentage of cells that incorporated bromodeoxyuridine (unpublished data). It also is possible that DN and DP cells are proliferating at a normal rate but that a continuous pre-TCR signal is required for survival during the proliferative phase. Experiments using Bcl-xL transgenic mice are underway to address this hypothesis.
CD5 is a cell surface marker whose transcription is regulated by TCR-mediated signals (20). Levels of CD5 are developmentally regulated; they are low in DN cells, increase to an intermediate level in the DP population, and further increase on cells that have up-regulated TCR levels and committed to the CD4 or CD8 lineage (21). TCR-mediated signals are required for normal up-regulation in DP cells, because MHC class (I × II)−/− DP cells have levels of CD5 between those found on DN and WT DP cells (CD5lo-int). The CD5lo-int levels are reminiscent of those found in the SLP-76F/FCD4cre DP cells, which suggests that lack of SLP-76 at the DP stage has the same effect on CD5 levels as a lack of TCR ligand. This finding is in contrast to TCRα−/− cells, in which DP cells are all CD5int, but lack the CD5hi population because of a lack of αβ TCR signals. Because CD5 levels correlate directly with TCR affinity (20), we hypothesize that the abnormal levels on the SLP-76–deficient DP cells is due to the absence of signals by way of a mature αβ TCR, rather than a lack of persistent pre-TCR–derived signaling as in the TCRα knockout.
A small number of cells leak through and are positively selected into the CD4SP and CD8SP compartments of the thymus and periphery in the SLP-76 conditionally deleted mice. We hypothesize that these cells retain low levels of SLP-76 protein at the DP stage. Gene deletion occurs before the DP stage; using PCR analysis, we see no evidence of the germline SLP-76F allele by the DP stage. However, the half-life of SLP-76 protein in developing thymocytes is unknown and likely depends on a combination of the rate of protein degradation and the dilutional effect of several cell divisions. These processes likely result in a small number of DP cells that retain SLP-76 at a low level. Thus, any αβ TCR will transduce an attenuated signal relative to the same TCR expressed with normal levels of SLP-76. When gating on CD69hi or CD24lo cells, we saw an abnormal skewing toward CD8SP cells; this suggested that the DP cells that were being positively selected received such an attenuated signal. These data support a quantitative signaling model of lineage commitment but do not discriminate between strength of signal and the kinetic (i.e., duration of signal) models (22, 23). If sustained signals are required for CD4 commitment and SLP-76 levels are not maintained, TCR and CD4 may remain engaged without transducing a continuous signal resulting in CD8 commitment. It is possible that rather than coreceptor down-regulation leading to cessation of signal, normal degradation of SLP-76 without renewal (due to gene deletion) terminates the TCR:CD4-generated signal, and results in an increased relative percentage of CD8SP cells in the thymus.
The appearance of a few SP cells allowed us to evaluate signal transduction that was mediated by the αβ TCR in the absence of SLP-76. CD4SP thymocytes and CD4 peripheral T cells from SLP-76F/FCD4cre mice fail to flux calcium in response to TCR cross-linking (Fig. 3 E and not depicted). One potential explanation is that CD4 cells in the SLP-76F/FCD4cre mice express lower overall levels of TCR. We do not believe that this is the only explanation, because there are some CD4SP thymocytes and CD4 peripheral, SLP-76–deficient cells that express intermediate to high levels of TCR. Our favored hypothesis is that SLP-76 protein expression is required to nucleate a “signalosome” that includes SLP-76, LAT, and phospholipase C γ1; the absence of the complex results in an inability to flux calcium and presumably translocates NFAT to the nucleus in response to TCR cross-linking (1, 24).
These data demonstrate that expression of SLP-76 protein is required for productive αβ TCR signal transduction leading to positive or negative selection. A lack of SLP-76 at the DP stage of thymic development leads to arrest without excess cell death. A small number of mature CD4SP and CD8SP cells are produced but at an abnormal CD4/CD8 ratio, which indicates altered lineage commitment. DP and the few CD4SP cells that escape largely lack SLP-76 protein, and are unable to flux calcium in response to anti-TCR signals. Because of the abnormalities in thymic development and the abnormal cell surface phenotype of peripheral T cells, experiments on naive T cells are difficult to interpret in these mice. Evaluation of the role of SLP-76 in a normal peripheral T cell compartment will require additional experiments utilizing temporally regulated cre activity.
MATERIALS AND METHODS
Generation of SLP-76F/+ embryonic stem cells.
To generate the pflox–SLP-76 vector, the 2-kb EcoR1/Xba1 including exon 2 was isolated, blunt-ended with Klenow, and subcloned into the HpaI site of pOSDupdel (a gift from B. Yang, University of Iowa, Iowa City, IA). For the 3′ arm, the 4-kb region between Xba1 and BamH1 was subcloned into pBS, a 34-bp loxP site was blunt-end ligated into the Stu1 site, and the fragment was excised with Xho1 and ligated into the Xho1 site of pOSDupdel. Linearized DNA was electroporated into R1 ES cells (A. Nagy, Mt. Sinai Hospital, Toronto, Canada). Homologous recombinants were selected in the presence of G418 and ganciclovir. DNA from each colony was digested with BamH1 and analyzed by Southern blotting using the 5′ external- and 3′ internal-probes shown in Fig. S1.
Mice.
An SLP-76F/+ ES clone was injected into C57BL/6 blastocysts. Chimeric mice were generated, mated with C57BL/6 females, and the offspring were interbred. SLP-76F/F mice were crossed with CD4cre (10) transgenic mice to generate SLP-76F/FCD4cre mice. All animal work was done in compliance with the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Antibodies.
Antibodies used for flow cytometric analysis, including anti-CD4, anti-CD8α, anti–TCR-β, anti-CD3, anti-CD5, anti-CD69, anti-CD24 (HSA), anti-nur77, anti–TCR-γδ, anti-B220, anti–mouse Ig, and isotype controls, were purchased from BD Biosciences.
Flow cytometry.
For surface staining, cells were washed in FACS buffer (PBS containing 1% FCS and 0.002% sodium azide) and then stained in FACS buffer, antibodies, and 10–20% rat serum for 30 min at 4°C. Cells were then washed, resuspended in FACS buffer, and acquired on a FACSCalibur using CellQuest software (BD Biosciences). Data were analyzed with FlowJo software (Tree Star, Inc.). Dead cells were excluded based on forward and side scatter characteristics. For intracellular staining of SLP-76, cells were stained for cell surface markers and washed. Cells were fixed, permeabilized, and stained with PE–anti-SLP-76 (25) for 30 min at 4°C using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. For intracellular staining with nur77, cells were fixed in 4% paraformaldehyde for 10 min at room temperature and washed in FACS buffer. Ice-cold methanol was added drop wise, and cells were incubated for 30 min at 4°C followed by two washes in FACS buffer. Cells were stained with a cocktail containing PerPC-Cy5.5 CD8α, APC CD4, and unlabeled nur77 for 30 min at 4°C, washed, and incubated with FITC anti–mouse IgG for an additional 30 min at 4°C.
Calcium flux.
Thymocytes were labeled with FITC–anti-CD8, PerCP–anti-CD4 (RMA4-5 clone), biotin–anti-CD3 (2C11 clone), and biotin–anti-CD4 (RMA 4–4 clone) antibodies and loaded with 2 μg/ml of Indo-1 (Molecular Probes) at 30°C for 30 min. Calcium influx was measured by flow cytometry on a BD-LSR (Becton Dickinson). Baseline levels were measured for 30 s, at which time streptavidin (Molecular Probes) was added at various doses as a cross-linker. Events were collected for an additional 6.5 min. Ionomycin was added to all samples for the last 30 s. Calcium flux was measured as a ratio of FL5/FL4 fluorescence.
Western blot.
1 million DP thymocytes, isolated using a Cytomation MoFlo cell sorter, were lysed in 1% Nonidet P-40 lysis buffer (1% Nonidet-40, 150 mM NaCl, and 50 mM Tris, pH 7.4) with protease inhibitors. Proteins were resolved by SDS-PAGE and transferred to a Trans-Blot Nitrocellulose membrane (Bio-Rad Laboratories). The membrane was probed with sheep-anti–SLP-76 anti-sera (25) and β-tubulin.
Stimulation-induced cell death.
Freshly isolated thymocytes were stimulated with plate-bound anti-CD3, anti-CD3 plus CD28, or dexamethasone in tissue culture plates coated with 250 μl of PBS containing 10 μg/ml of anti-CD3 ± 50 μg/ml of anti-CD28 for 14 or 20 h. Cells were stained with anti-CD4, anti-CD8, and TOPRO-3 (Molecular Probes), then immediately acquired by FACS.
Online supplemental material.
Fig. S1 describes the construction of the targeting vector and normal thymic phenotype in SLP-76F mice lacking Cre. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20051128/DC1.
Acknowledgments
We thank A. Bhandoola, T. Laufer, M. Jordan, and J. Punt for helpful discussions and critical review of the manuscript. We thank M.C. Simon and the Abramson Family Cancer Research Institute Transgenic Core for the ES cell blastocyst injection to generate chimeric SLP-76F/+ mice.
This work was supported, in part, by grants from the National Institutes of Health (to G.A. Koretzky and J.S. Maltzman).
The authors have no conflicting financial interests.
References
- 1.Jordan, M.S., A.L. Singer, and G.A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4:110–116. [DOI] [PubMed] [Google Scholar]
- 2.Jackman, J.K., D.G. Motto, Q. Sun, M. Tanemoto, C.W. Turck, G.A. Peltz, G.A. Koretzky, and P.R. Findell. 1995. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J. Biol. Chem. 270:7029–7032. [DOI] [PubMed] [Google Scholar]
- 3.Su, Y.W., and H. Jumaa. 2003. LAT links the pre-BCR to calcium signaling. Immunity. 19:295–305. [DOI] [PubMed] [Google Scholar]
- 4.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F.W. Alt, and R.S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 94:229–238. [DOI] [PubMed] [Google Scholar]
- 5.Clements, J.L., B. Yang, S.E. Ross-Barta, S.L. Eliason, R.F. Hrstka, R.A. Williamson, and G.A. Koretzky. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 281:416–419. [DOI] [PubMed] [Google Scholar]
- 6.Aifantis, I., V.I. Pivniouk, F. Gartner, J. Feinberg, W. Swat, F.W. Alt, H. von Boehmer, and R.S. Geha. 1999. Allelic exclusion of the T cell receptor beta locus requires the SH2 domain-containing leukocyte protein (SLP)-76 adaptor protein. J. Exp. Med. 190:1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes, S.M., E.W. Shores, and P.E. Love. 2003. An architectural perspective on signaling by the pre-, alphabeta and gammadelta T cell receptors. Immunol. Rev. 191:28–37. [DOI] [PubMed] [Google Scholar]
- 8.Panigada, M., S. Porcellini, E. Barbier, S. Hoeflinger, P.A. Cazenave, H. Gu, H. Band, H. von Boehmer, and F. Grassi. 2002. Constitutive endocytosis and degradation of the pre-T cell receptor. J. Exp. Med. 195:1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun, Z., C.W. Arendt, W. Ellmeier, E.M. Schaeffer, M.J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P.L. Schwartzberg, and D.R. Littman. 2000. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 404:402–407. [DOI] [PubMed] [Google Scholar]
- 10.Lee, P.P., D.R. Fitzpatrick, C. Beard, H.K. Jessup, S. Lehar, K.W. Makar, M. Perez-Melgosa, M.T. Sweetser, M.S. Schlissel, S. Nguyen, et al. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 15:763–774. [DOI] [PubMed] [Google Scholar]
- 11.Lucas, B., F. Vasseur, and C. Penit. 1993. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2′-deoxyuridine-pulse-labeled thymocytes. Correlation with T cell receptor expression. J. Immunol. 151:4574–4582. [PubMed] [Google Scholar]
- 12.Cho, H.J., S.G. Edmondson, A.D. Miller, M. Sellars, S.T. Alexander, S. Somersan, and J.A. Punt. 2003. Cutting edge: identification of the targets of clonal deletion in an unmanipulated thymus. J. Immunol. 170:10–13. [DOI] [PubMed] [Google Scholar]
- 13.Mombaerts, P., A.R. Clarke, M.A. Rudnicki, J. Iacomini, S. Itohara, J.J. Lafaille, L. Wang, Y. Ichikawa, R. Jaenisch, M.L. Hooper, et al. 1992. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 360:225–231. [DOI] [PubMed] [Google Scholar]
- 14.Negishi, I., N. Motoyama, K. Nakayama, S. Senju, S. Hatakeyama, Q. Zhang, A.C. Chan, and D.Y. Loh. 1995. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 376:435–438. [DOI] [PubMed] [Google Scholar]
- 15.Liu, X., and R. Bosselut. 2004. Duration of TCR signaling controls CD4-CD8 lineage differentiation in vivo. Nat. Immunol. 5:280–288. [DOI] [PubMed] [Google Scholar]
- 16.Grusby, M.J., H. Auchincloss Jr., R. Lee, R.S. Johnson, J.P. Spencer, M. Zijlstra, R. Jaenisch, V.E. Papaioannou, and L.H. Glimcher. 1993. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl. Acad. Sci. USA. 90:3913–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong, Q., L. White, R. Johnson, M. White, I. Negishi, M. Thomas, and A.C. Chan. 1997. Restoration of thymocyte development and function in zap-70−/− mice by the Syk protein tyrosine kinase. Immunity. 7:369–377. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, A.M., I. Negishi, S.J. Anderson, A.C. Chan, J. Bolen, D.Y. Loh, and T. Pawson. 1997. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc. Natl. Acad. Sci. USA. 94:9797–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trop, S., M. Rhodes, D.L. Wiest, P. Hugo, and J.C. Zuniga-Pflucker. 2000. Competitive displacement of pT alpha by TCR-alpha during TCR assembly prevents surface coexpression of pre-TCR and alpha beta TCR. J. Immunol. 165:5566–5572. [DOI] [PubMed] [Google Scholar]
- 20.Niederberger, N., L.K. Buehler, J. Ampudia, and N.R. Gascoigne. 2005. Thymocyte stimulation by anti-TCR-{beta}, but not by anti-TCR-{alpha}, leads to induction of developmental transcription program. J. Leukoc. Biol. 77:830–841. [DOI] [PubMed] [Google Scholar]
- 21.Azzam, H.S., A. Grinberg, K. Lui, H. Shen, E.W. Shores, and P.E. Love. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188:2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosselut, R., T.I. Guinter, S.O. Sharrow, and A. Singer. 2003. Unraveling a revealing paradox: Why major histocompatibility complex I-signaled thymocytes “paradoxically” appear as CD4+8lo transitional cells during positive selection of CD8+ T cells. J. Exp. Med. 197:1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer, A. 2002. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr. Opin. Immunol. 14:207–215. [DOI] [PubMed] [Google Scholar]
- 24.Singer, A.L., S.C. Bunnell, A.E. Obstfeld, M.S. Jordan, J.N. Wu, P.S. Myung, L.E. Samelson, and G.A. Koretzky. 2004. Roles of the proline-rich domain in SLP-76 subcellular localization and T cell function. J. Biol. Chem. 279:15481–15490. [DOI] [PubMed] [Google Scholar]
- 25.Clements, J.L., S.E. Ross-Barta, L.T. Tygrett, T.J. Waldschmidt, and G.A. Koretzky. 1998. SLP-76 expression is restricted to hemopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J. Immunol. 161:3880–3889. [PubMed] [Google Scholar]


