Abstract
The switch in immunoglobulin (Ig) heavy chain class is preceded by germline transcription and then mediated by a DNA recombination event. To study germline transcription and class switch recombination we used transgenic mice with a 230-kilobase bacterial artificial chromosome that included a rearranged VDJ gene and the entire heavy chain constant region locus. In addition to several lines with intact transgenes, we identified two lines in which the heavy chain locus transgene lacked Cα and everything 3′ of it, including the regulatory elements HS3a, HS1-2, HS3b, and HS4. B cells from both lines with the truncated transgenes make abundant transgenic (Tg) VDJCμ transcripts and IgM protein. Deletion of the 3′ end of the locus results in dramatically reduced expression of both germline transcripts and switched VDJCH transcripts of the γ3, γ2b, γ2a, and ɛ genes. In addition, the transgenes lacking the 3′ end of the locus express reduced amounts of γ1 germline transcripts and 2–3% of the amount of Tg IgG1 in tissue culture compared with intact transgenes. Finally, switch recombination to γ1 is undetectable in the transgenes lacking the 3′ elements, as measured by digestion circularization–polymerase chain reaction or by the expression of VDJCγ1 transcripts.
Switch recombination between the μ and γ, ɛ, or α heavy chain genes, and the germline transcription that precedes it, are likely to be regulated by cis-acting elements within the heavy chain locus (1, 2). Many experiments implicate promoter region sequences, and other elements associated with individual heavy chain genes in this regulation (3). A group of enhancers 3′ of the Cα gene is known to control switch recombination to some isotypes (2). These elements are associated with DNase I hypersensitive (HS) sites (4–6), and hence, are termed HS3a, HS1-2, HS3b, and HS4 (in the order that they appear in the heavy chain constant region locus). These elements are strong B cell specific enhancers, especially in combination with one another (5, 7–17). A spontaneous deletion of all four elements has been associated with a loss of immunoglobulin expression by a cell line (18). Replacement of individual HS sites with a promoter/neomycin resistance gene cassette down-regulates the expression of some isotypes in tissue culture or in vivo (19–21). Finally, deletion of HS3b and HS4 from the germline reduces dramatically the expression of γ3 and γ2b, and reduces somewhat the expression of γ2a, ɛ, and α. However, expression of μ and γ1 was reduced by <50% by the HS3b + HS4 deletion (22).
However, the “clean” deletion of HS3a or HS1-2, without replacement by the promoter/neomycin resistance cassette, does not alter expression of any heavy chain isotype, and, with the aforementioned exception (22), it has been difficult to delete more than one of the HS sites from the mouse genome. We have investigated the regulation of germline transcription and switch recombination by the 3′ HS sites using a transgene of the entire heavy chain constant region locus, including the four HS sites named above. We identified two independent lines of transgenic (Tg) mice in which the transgene was truncated before insertion, and therefore, the integrated construct lacks all four HS sites. We report here the effect of these spontaneous deletions on the expression of Tg μ and four γ genes.
RESULTS
Composition of transgenes
In independent microinjections of three constructs that differ slightly (see Materials and methods), we produced mice with a transgene of the entire constant region locus, and tested all Tg lines for the presence and copy number of eleven elements at locations throughout the locus. We found that most lines included one to four complete copies of the transgene, in that all 11 Tg elements were present in genomic DNA (Fig. 1 A and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20041988/DC1). In the majority of cases, the copy number of the elements was consistent across the locus; some lines had more copies of some elements (e.g., line 551 had two copies of most Cγ genes and the 3′ enhancers), but had only one copy of one or more elements (the VDJ exon for line 551). However, we identified two lines (1001 and 822) that lacked the entire 3′ end of the locus, including the Sα segment (Fig. 1 A). After analyzing all 11 gene segments, we found that line 1001 had two copies of the VDJ exon, Sγ3, Sγ1, one copy of Sγ2b and Sγ2a, but lacked all of the genes downstream of γ2a. Line 822 had two copies of the VDJ exon, and one copy of the four Sγ segments and Cɛ (e.g., Fig. 1 B and Fig. S1).
Figure 1.
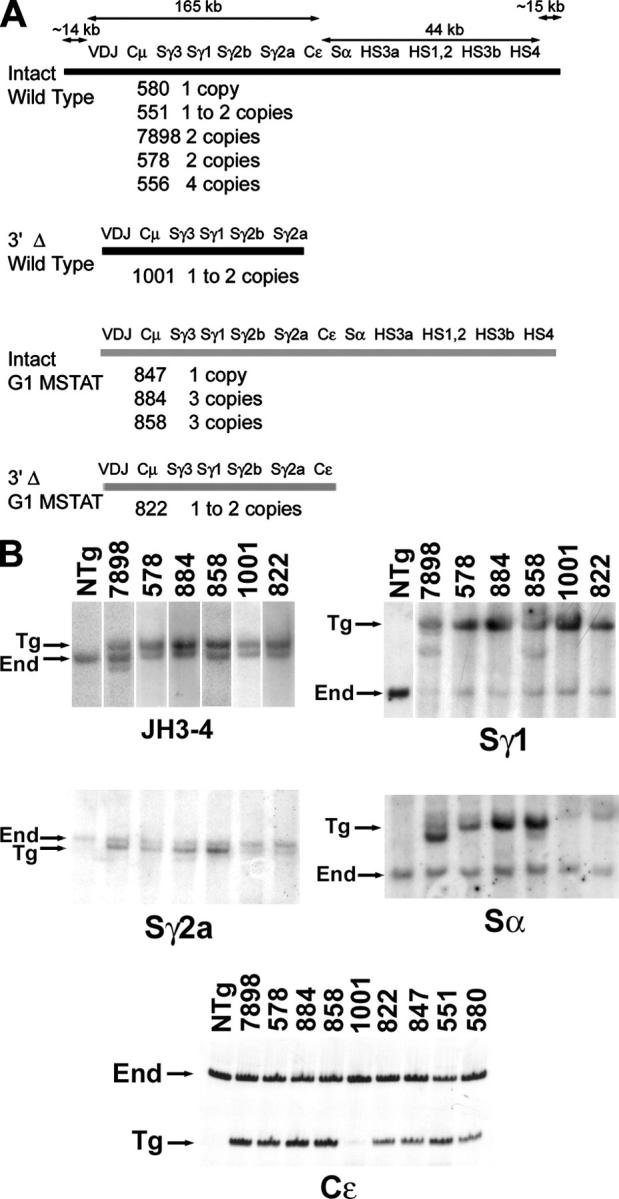
Structure of the transgenes of the heavy chain constant region locus. (A) The DNA segments included are indicated for Tg mice with a wild-type γ1 gene (black lines) or the mutated Stat6 binding site in the γ1 promoter region (gray lines). The number designation and transgene copy number of each Tg line are indicated below each type of transgene. (B) Southern hybridization (JH3-4 and the various S segments, indicated below each set of lanes) or PCR results (Cɛ) define the presence and copy number of the transgene. In the “Sα” panel, the faint band in each sample above the Tg “Sα” fragment is the cross-hybridizing Sμ fragment.
Expression of Tg μ, γ3, γ2b, and ɛ transcripts
Other investigators have shown that deletion of HS3b and HS4, or replacement of various 3′ enhancers with the neo gene driven by a strong promoter, reduces the expression of the γ3, γ2b, γ2a, and ɛ genes (19, 21, 22). Expression of the μ gene was reduced only slightly or not at all (19, 20, 22). However, a heavy chain locus lacking all four 3′ HS sites has not been derived using the technology of homologous recombination in embryonic stem cells. We tested the expression of germline transcripts and postswitch transcripts of heavy chain genes in the transgenes that included the four 3′ HS sites and other transgenes that had deleted the 3′ end of the locus, including all four HS sites. To control for transgene expression from line 1001, we used lines 551, 7898, 580, 578, or 556, because these lines have a wild-type γ1 gene within their arsonate (ARS)/Igh transgene. To control for line 822, we used lines 847, 884, or 858, which share with line 822 the STAT6 site mutation in the γ1 promoter region (see Materials and methods).
We tested the expression of the μ, γ3, and γ2b genes in RNA that was derived from T depleted splenocytes that were cultured in LPS. The deletion of the 3′ end of the Tg heavy chain locus had a minimal effect on expression of Cμ transcripts (Fig. 2 A). All Tg lines expressed approximately equal amounts of VDJCμ transcripts, whether the lines included an intact heavy chain locus (7898, 578, or 858) or lacked the 3′ end of the locus (1001 and 822).
Figure 2.

Transgenic heavy chain expression as determined by RT-PCR. (A) Transgenic VDJCμ expression. Two amounts of cDNA, differing by fivefold, were tested. Line 858 has three copies of the Tg μ gene; the other four lines have two copies. (B) Transgenic γ3 expression. Lines 822, 580, 551, and 847 have one copy of the transgene; the other lines have two or three copies. The expression of Tg γ3 germline transcripts by B cells from lines 847, 551, and 580 is documented in Fig. S3 (available at http://www.jem.org/cgi/content/full/jem.20041988/DC1). (C) Transgenic γ2b expression. The HPRT expression for some of these LPS samples is presented in Fig. 3 C. (D) Transgenic ɛ expression. For γ3, γ2b, and ɛ, the same amount of cDNA was used for IμCH and for Tg VDJCH amplifications.
To detect Tg Cγ or Cɛ germline transcripts, we amplified Tg and End germline transcripts, using oligonucleotides that were exactly complementary to the transgene and the End genes. We then cut the PCR products with restriction enzymes that would distinguish the transgene and End genes. In contrast with Tg VDJCμ expression, we were unable to detect Tg germline γ3 transcripts in lines lacking the 3′ end of the heavy chain locus (1001 and 822; Fig. 2 B). To estimate the amount of switch recombination to Tg γ3, we measured the expression of Tg VDJCγ3 transcripts. As a positive control for the expression of postswitch transcripts, we amplified cDNAs using primers in the Iμ sequence and in the Cγ3 gene (Fig. 2 B). These primers anneal perfectly to the End genes and the transgene, and amplify RNA products from End and Tg γ3 genes that have undergone switch recombination (23). Thus, the IμCγ3 RT-PCR ensured that switch recombination and expression of postswitch transcripts, at least from the End genes, had taken place. In addition, we used the amount of IμCγ3 transcripts (and other IμCH transcripts, see next paragraph) to normalize samples for variability in B cell preparations and response to cytokines and activators in tissue culture. To the extent possible, we equalized the amount of IμCγ3 transcripts for each cDNA sample, and then tested the same concentrations of cDNA for Tg VDJCγ3 transcripts (Fig. 2 B). Hence, the various RNA samples were approximately equalized for amount of switch recombination. Transgenes that included most of the 3′ end of the heavy chain locus also expressed postswitch VDJCγ3 transcripts from the transgene, whether they had three copies of this gene (lines 884 and 858), two copies (line 7898), or one copy (lines 551, 847, and 580; Fig. 3 B). However, lines 1001 and 822 did not express Tg VDJCγ3 transcripts. This was despite the fact that RNA samples from these lines included transcripts from the End locus that had switched to Cγ3 (IμCγ3).
Figure 3.
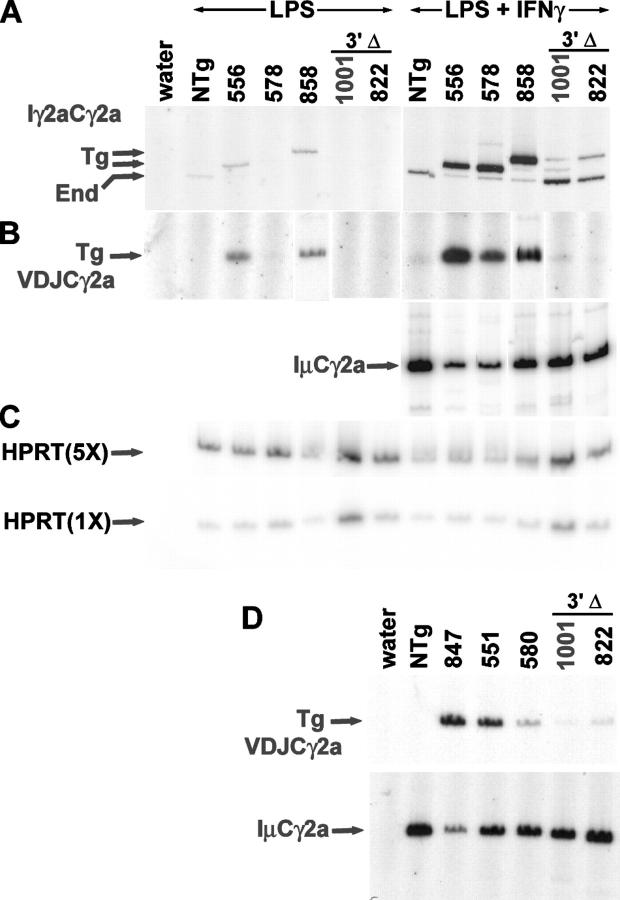
Transgenic γ2a expression. (A) RT-PCR for germline transcripts. The size difference between germline transcripts of the End gene and the transgene in lines 556, 578, and 1001 without the four-bp insertion in Iγ2a is noted by the lower Tg arrow. The size difference between germline transcripts of the End gene and the transgene with the four-bp insertion in Iγ2a in lines 858 and 822 with the four-bp insertion is noted by the upper Tg arrow. (B and D) RT-PCR for Tg VDJCγ2a and IμCγ2a transcripts. Lines 556, 578, and 858 have two or three copies of the ARS/Igh transgene. Lines 1001 and 822 have two Tg VH copies, but one Tg γ2a copy. Lines 847, 551, and 580 have one intact copy of the ARS/Igh transgene, but line 551 has two copies of Tg γ2a. (C) RT-PCR for HPRT transcripts. Although we normalized the LPS + IFNγ samples using IμCγ2a expression, we present HPRT transcripts to allow comparison of RNA levels between LPS and LPS + IFNγ samples. HPRT transcripts were amplified beginning with two cDNA concentrations, one fivefold greater than the other. The amount of cDNA that was used for each founder line was identical for germline, Tg VDJCγ2a, and IμCγ2a transcripts. The more abundant HPRT transcripts were amplified from smaller amounts of cDNA, but the relative quantities of the cDNA samples were consistent among the HPRT amplifications and the other three reactions.
Parallel results were obtained when the γ2b and ɛ genes were analyzed. Although the transgenes with an intact 3′ end expressed germline transcripts and switched VDJC transcripts well for the Cγ2b and Cɛ genes, the transgenes in lines 1001 and 822—lacking most of the 3′ end—did not (Fig. 2, C and D). Line 822 does express a small amount of Tg γ2b and ɛ germline transcripts, although it expresses barely detectable or undetectable Tg VDJCγ2b and VDJCɛ transcripts (Fig. 2, C and D).
Expression of Tg γ2a transcripts
To investigate expression of γ2a germline transcripts from the transgene, we cultured T depleted splenocytes from nonTg (NTg) and Tg mice in LPS or in LPS + IFNγ. IFNγ induces the expression of γ2a germline transcripts and the secretion of IgG2a (24, 25); we observed increased amounts of both germline transcripts (Fig. 3 A) and Tg VDJCγ2a transcripts (Fig. 3 B) from B cells that were cultured in LPS + IFNγ. As noted by the arrows in Fig. 3 A, γ2a germline transcripts from the End gene migrate slightly faster than those from the transgene. In addition, there is greater expression of γ2a germline transcripts from the “a” allele associated with the transgene than from the “b” allele of the End gene (compare, for example, the LPS + IFNγ samples for NTg and for line 578, with two copies of the transgene). We do not know the basis for this increased expression, but we also have observed the same magnitude of increased expression of germline transcripts by the “a” allele of the End γ2a gene of BALB/c mice compared with expression from the “b” allele of C57BL/6 mice (unpublished data). B cells from lines 1001 and 822 express a small amount of IFNγ-inducible Tg γ2a germline transcripts, at most 5% of the amount of Tg γ2a germline transcripts from lines 556, 578, or 858 with transgenes with an intact 3′ end.
B cells from lines 1001 and 822 express abundant IμCγ2a transcripts and demonstrate good switch recombination of the End γ2a genes (Fig. 3, B and D). However, in comparison with B cells with one (Fig. 3 D) or two copies (Fig. 3 B) of the Tg γ2a gene, B cells with a deletion of the 3′ end of the transgene express reduced amounts of Tg VDJCγ2a transcripts. For example, the VDJCγ2a transcript signal (by PhosphorImager analysis) for line 1001 is 14% that of line 580.
Expression of Tg IgG1 and γ1 transcripts
In studies from other investigators, none of the disruptions or deletions of the 3′ enhancers have reduced the expression of the γ1 gene by more than 50% (19, 21, 22). We tested B cells with the two transgenes lacking the 3′ end of the locus for secretion of IgG1 with the Tg (“a”) allotype. As reported (26), intact transgenes produce abundant Tg IgG1 when exposed to IL-4 and either LPS or CD40L (Fig. 4 A). The induction by IL-4 is 10- to 100-fold. However, IL-4 does not induce Tg IgG1 secretion by B cells from lines 1001 and 822; this results in much smaller absolute amounts of IgG1a (Fig. 3 A). The mutation in the Stat6 binding site in Tg lines 847 and 858 does not alter the ability of B cells from these lines to express Tg IgG1 in tissue culture (26). Hence, the reduced expression of Tg IgG1 by B cells from line 822 is unlikely to be a result of the Stat6 site mutation in the γ1 gene of the transgene. The cultures of B cells from lines 1001 and 822 do express abundant IgG1 from the End locus, and when the results are expressed as Tg IgG1 divided by total IgG1 in the culture, B cells from lines 1001 and 822 secrete 2–3% of the amount of Tg IgG1 secreted by B cells with an intact transgene (black bars in Fig. 4 B). We assayed Tg IgM expression from LPS cultures set up in parallel with the LPS + IL-4 and CD40L + IL-4 cultures, and expressed the data as Tg IgM divided by total IgM in the cultures (Fig. 4 B, gray bars). B cells from lines 1001 and 822 express abundant Tg IgM; on average, the same amount of Tg IgM as B cells with an intact transgene. B cells from lines 1001 and 822 that were cultured in LPS + IL-4 also secreted similar amounts of IgM compared with B cells from lines 7898, 578, and 884 that were cultured in LPS + IL-4 (unpublished data).
Figure 4.
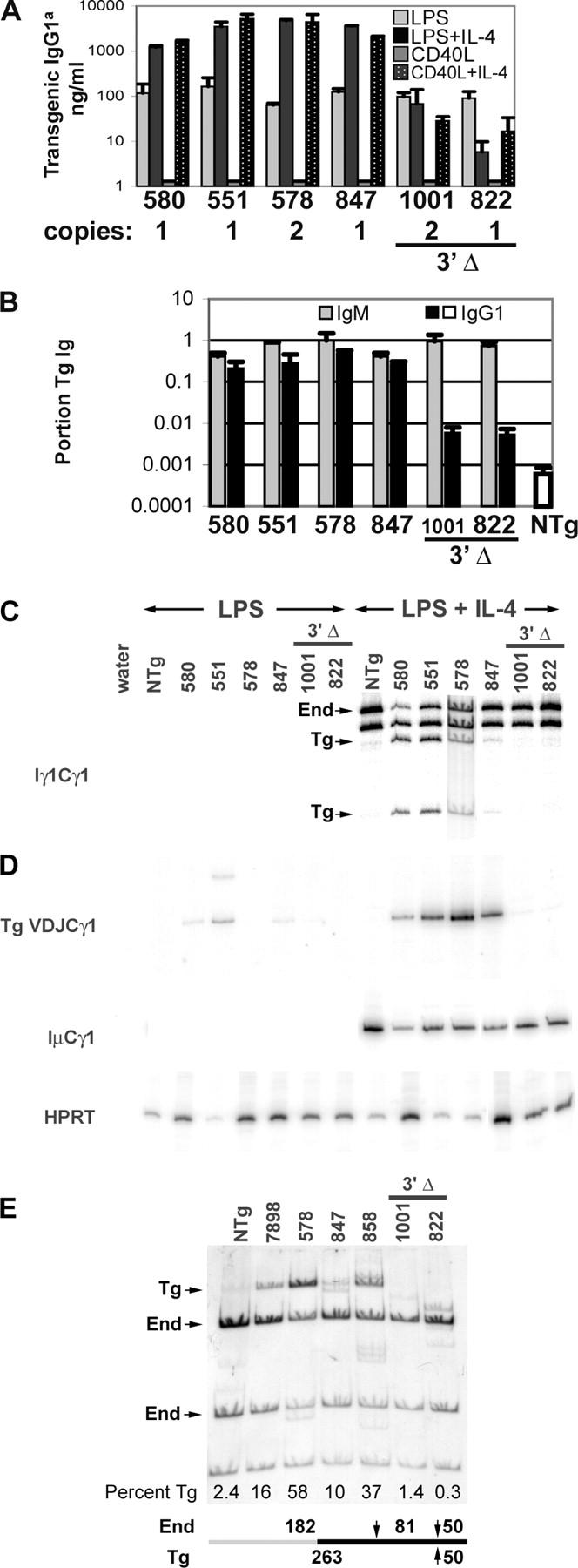
Transgenic IgG1 expression. (A) Means of quadruplicate samples, with SD error bars, for a single set of cultures. Supernatant fluids were tested for IgG1 with the Tg allotype by ELISA. The signals for Tg IgG1 in lines 1001 and 822, for all four culture conditions, are near the limit of detection, and therefore, can fluctuate between 0 and 20 ng/ml. In particular, the relative signals for line 822 B cells that were cultured in LPS compared with LPS + IL-4 are not representative of the average twofold (not statistically significant) reduction in LPS + IL-4 compared with LPS alone. Transgenic γ1 copy number for each line is given directly below the line number. (B) The portion of Tg IgM was calculated by dividing the amount of AD8+ (Tg idiotype) IgM by total IgM for LPS cultures. Means, with SEM bars, are presented for two to four independent cultures. The portion of Tg IgG1 was calculated by dividing the amount of IgG1a by the amount of total IgG1 for LPS + IL-4 or CD40L + IL-4 cultures. Means, with SEM bars, are presented for 5 to 11 cultures. As a negative control, the mean IgG1a/total IgG1 value from LPS + IL-4 and CD40L + IL-4 cultures of NTg B cells is presented as an open box. (C) Endogenous and Tg germline transcripts were identified by TaqI-digestion of RT-PCR products of RNA from B cells induced with LPS + IL-4 (23). The line 578 sample was derived from an independent experiment. B cells from line 847 express smaller amounts of γ1 germline transcripts as a result of the mutation of the Stat6 site in the γ1 promoter (26). (D) Transgenic VDJCγ1 and IμCγ1 transcripts were determined by RT-PCR, using the same amount of cDNA for the two reactions. RT-PCR for HPRT used less cDNA, but the relative amounts for each sample were held constant. (E) DC-PCR for Tg γ1 switch recombination. Transgenic and End recombination products were distinguished by MboI digestion. MboI sites are indicated by arrows at the bottom of the figure. Sequences flanking the 5′ portion of Sμ are indicated by a gray bar and those flanking the 3′ portion of Sγ1 are indicated by a black bar. These DNA segments are joined at the junction of the gray and black bars by ligation at EcoRI ends to form a circle. “Percent Tg” was calculated by dividing the cpm in the Tg band by the sum of the cpm in the Tg and the two End bands.
PCR-based tests for γ1 nucleic acids confirmed these results for IgG1 protein secretion (Fig. 4, C–E). Transgenic germline transcripts (Fig. 4 C) and VDJCγ1 transcripts (Fig. 4 D) were barely detectable in B cells from lines 1001 and 822; the level of expression was at or slightly above the background level that was detected in cDNA from NTg B cells. Switch recombination of the End γ1 genes occurred in the same cell samples, as revealed by abundant IμCγ1 transcripts. These results with RNA were confirmed at the DNA level by a variation of the digestion circularization (DC)-PCR assay (27). We performed DC-PCR on DNA samples from B cells that were treated with LPS + IL-4, and then digested the final products with MboI, which differentiates the transgene and End genes. We detected the 263-bp fragment (“Tg”) in DNA from B cells with an intact transgene (lines 7898, 578, 847, and 858); this indicated switch recombination by the transgene (Fig. 4 E). The amount of the Tg recombination varied from ∼10% of the total SμSγ1 fragments (line 847 with one copy of the transgene) to greater than 50% of the total SμSγ1 fragments (line 578 with two copies of the transgene). However, the Tg fragment was not detected when DNA from B cells whose transgenes lacked the 3′ end of the heavy chain locus (1001 and 822) was tested (Fig. 4 E).
DISCUSSION
Loss of the 3′ end of the heavy chain locus and heavy chain gene expression
We have found that, relative to transgenes with an intact heavy chain constant region locus, transgenes lacking the 3′ end of the locus express markedly reduced amounts of postswitched transcripts from the γ3, γ2b, γ2a, and ɛ genes (Figs. 2 and 3). However, Tg μ expression was reduced marginally, if at all (Figs. 2 and 4 B). Qualitatively, these results are similar to experiments that use replacement of single 3′ enhancer elements (19–21) or the deletion of HS3b and HS4 (22). Although it is a little difficult to compare our results with those of the HS3b + HS4 deletion because the assays for switch recombination are different, it seems that the loss of the entire 3′ end of the locus in a transgene results in quantitatively more profound reduction in the expression of γ2a and ɛ. Also similar to other studies of alterations of the 3′ enhancers (19, 21, 22), loss of the 3′ end of the heavy chain locus in a transgene resulted in dramatically reduced amounts of germline transcripts of the γ3, γ2b, γ2a, and ɛ genes (Figs. 2 and 3). One limitation of our study is that both truncated transgenes lacked Cα, so we could not study the effect of the truncations on α gene expression. Only one of the two truncated transgenes retained Cɛ, so our study of the expression of ɛ is limited to a single founder line lacking the 3′ end of the locus. It will be important to determine the effect of deletion of all four 3′ HS sites on the expression of the α heavy chain gene, and, in more founder lines, on the ɛ heavy chain gene.
In the study by Pinaud et al., expression of γ1 germline transcripts and switch recombination was not reduced by deletion of HS3b and HS4 from the germline (22). Likewise, insertions of a neo cassette into HS1-2 or HS3A did not alter γ1 expression (19, 21). In our Tg system, loss of the 3′ end of the locus is correlated with loss of γ1 expression that is as profound as the other isotypes that were studied (Fig. 4). It is a formal possibility that the IgG1 expression was unaffected in the study by Pinaud et al. (22), but was reduced to 2–3% of wild-type levels in our study (Fig. 4) because the former involved the End locus, whereas ours involved a transgene inserted into an ectopic locus. However, the copy number dependence and insertion site independence of Tg IgG1 expression in vitro argues against this possibility (26). It seems more likely that the larger deletion in our study leads to the loss in γ1 expression. From the study by Pinaud et al., the 6-kb deletion of HS3a + HS4 does not alter expression of IgG1 (22). The truncation of the transgenes in lines 822 and 1001 by ∼60 kb results in a dramatic reduction in Tg IgG1 expression (Fig. 4). Therefore, deletion of the additional 54 kb reveals elements that are important for γ1 gene expression (HS3a, HS1-2, or other unidentified elements). Redundancy among the 3′ enhancer elements could allow γ1 gene expression in a heavy chain locus with much of the 3′ end deleted, if some critical elements were present (e.g., HS3b and HS4). A more directed series of deletions, which are feasible by modification of the ARS/Igh bacterial artificial chromosome in Escherichia coli, will be useful to define better the role of each element in the regulation of heavy chain germline transcription and switch recombination.
Enhancement of germline transcription
Our results support the notion that the 3′ HS elements are enhancers of a small amount of transcriptional activity. This enhancer activity has been observed readily in tissue culture cells and normal B cells by transfection experiments and in conventional transgenes (5, 7–17). In our study, it is best identified for the γ2a and ɛ genes, where a small amount of germline transcripts are observed in lines 1001 and 822. Addition of the 3′ end of the heavy chain locus (e.g., in lines 578 and 858) results in expression of γ2a and ɛ germline transcripts that are at least 10-fold more abundant. The amount of these germline transcripts in lines lacking the 3′ end of the locus is entirely consistent with the amount of cytokine-inducible germline transcripts from a 10-kb γ2a transgene (14) and from transient transfection of constructs with the ɛ germline promoter (16). Thus, the promoter regions for the individual heavy chain genes may direct a small amount of regulated germline transcription, which is enhanced dramatically by the 3′ HS sites. Synergy between two or more 3′ HS sites may be particularly important for this enhancement (11–15).
MATERIALS AND METHODS
Transgenes and Tg mice.
The construction of the 230-kb ARS/Igh transgene with the entire constant region locus has been described previously (26). The transgene is derived from strain 129 DNA (Igha). Injection of C57BL/6xSJL F2 eggs and backcrosses to C57BL/6 maintained the End loci as Ighb. Lines 551 and 7898 were derived from the first microinjection of ARS/Igh DNA. The transgene in these two lines has a wild-type γ1 gene and a four-bp insertion at the EcoRI site in Iγ2a. Lines 556, 578, 580, and 1001 were derived from a second microinjection. The transgene in these four lines includes a wild-type γ1 gene and lacks the four-bp insertion at the EcoRI site in Iγ2a. Lines 847, 858, 884, and 822 were derived from a third microinjection. These lines have mutations of four consecutive bp in the Stat6 binding site at −123 bp 5′ of the promoter for germline transcripts of the γ1 gene and a 4-bp insertion at the EcoRI site in Iγ2a (26). Lines 556, 578, 580, 1001, 551, and 7898 are designated “wild-type,” which refers to their γ1 genes. Lines 847, 884, 858, and 822 are designated “MSTAT,” which refers to their γ1 genes. All transgenes have a 4-bp insertion in the BglII site in Iγ1.
The presence and copy number for the following elements in the transgene was determined as described previously (26): JH3-4/Eμ, Sγ3, Sγ1, Sγ2a, Sα, HS3a, HS1-2, HS3b, and HS4. Sγ2b was tested using a HindIII digest probed with nick-translated pSγ2b/E6.6 (28). The Tg HindIII fragment is 10 kb long; the End HindIII fragment is 11.5 kb long (29). DNA samples from Tg mice were tested for the presence of Tg Cɛ by PCR of tail DNA in the presence of 32P-dATP, using the primers 5′-GAGCTGTCAACATCACTGACCC-3′ (5′) and 5′-GAGACATCATTTAGGATGTGGCC-3′ (3′). Denaturation was at 95°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min for 30 cycles, followed by a 10 min extension at 72°C. Digestion of the 169-bp product with NsiI yielded the uncut fragment (End), and 108- and 61-bp fragments (transgene). Copy number was determined by PhosphorImager quantification of the Tg and End bands. The End band represented an internal standard of two copies.
Assay of germline transcripts and switch recombination.
B cells were prepared from various Tg mice and cultured with various combinations of LPS, CD40L, IFNγ, and IL-4 as described previously (30). RNA and cDNA were prepared from those B cells. For all RT-PCR reactions (in the presence of 32P-dATP), denaturation was at 95° for 1 min, annealing for 1 min, and extension at 72° for 1 min for 30 cycles, followed by a 10 min extension at 72°. The following primers were used to specifically prime in each heavy chain constant region: Cμ: 5′-AATGGTGCTGGGCAGGAAGT-3′, Cγ3: 5′-TACTGGGCTTGGGTATTCTAG-3′, Cγ1: 5′-GCATGATGGGAAGTTCACTGACTG-3′, Cγ2b: 5′-GGGCATTTGTGACACTCCTTGCA-3′ (VDJ and Iμ transcripts) or 5′-AGATGGT-TCTCTCGATGGGTGA-3′ (germline transcripts), Cγ2a: 5′-GTCCACCTTGGTGCTGCTT-3′, and Cɛ: 5′-GAGACATCATTTAGGATGTGGCC-3′. For the amplification of IμCH transcripts, the constant region primers were paired with the Iμ primer: 5′-CACCCATCCACCTGGCTGCTCA-3′. Annealing was at 67°. For the amplification of Tg VDJ transcripts, each constant region primer was paired with a primer (5′-GTCTATTTCTGTGCAAGATCGAAT-3′) that spans the VD junction of the transgene, including four N nucleotides (31). Annealing was at 69°. The VDJCH and IμCH RT-PCRs were semi-quantitative by examination of various cDNA dilution series (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20041988/DC1).
For the amplification of germline transcripts each C region primer was paired with a primer for its respective I exon. After amplification, products were digested with restriction enzymes that distinguished the End and transgenes by polymorphisms between the I and/or C exons. Iγ3 primer: 5′-TGATGGGATATATCAGGATACC-3′, 61° annealing. Digestion of the 537-bp product with PstI results in a 196-bp fragment (End), 127-bp and 69-bp fragments (transgene), and 121-bp and 145-bp fragments (both genes). Iγ1 primer: 5′-GACGGCTGCTTTCACAGCTT-3′, 58° annealing. Digestion of the 578-bp product with TaqI results in a 317-bp fragment (End), 217-bp and 100-bp fragments (transgene), and a 261-bp fragment (both genes). Iγ2b primer: 5′-GAGCACTGGGCCTTTCCAGAACTA-3′, 65° annealing. Digestion of the ∼880-bp product with HpaII results in a 761-bp fragment (transgene), 691-bp and 82-bp fragments (End), and a 108-bp fragment (both genes). Iγ2a primer: 5′-GCTGATGTACCTACCTGAGAGAG-3′, 62° annealing. For lines 556, 578, 580, and 1001, γ2a germline transcripts were distinguished by a mobility difference between the “a” (Tg) allele and the “b” allele (End). For other lines, a mobility difference that was due to the four bp insertion in Iγ2a was used to distinguish transcripts of the transgene from those of the End genes. Iɛ primer: 5′-GCCTGGGAGCCTGCACAGG-3′, 60° annealing. Digestion of the 478-bp product with NsiI results in the uncut fragment (End), and 381-bp and 63-bp fragments (transgene). Hypoxanthine phosphoribsyl transferase (HPRT) transcripts were amplified as described previously (14).
DNA samples from Tg B cells cultured with LPS + IL-4 for 4 d were digested with EcoRI and ligated to form circles (27). DC-PCR products were amplified in the presence of 32P-dATP using the primers 5′-GGAGACCAATAATCAGAGGGAAG-3′ (5′ Sμ) and 5′-GGTCCAGTTGAGTGTCTTTAGAG-3′ (3′ Sγ1) for 35 cycles with annealing at 63°. The resulting 313-bp product was digested with MboI. Fragment sizes were 263 bp (Tg), 181 bp and 82 bp (End), and 50 bp (both genes).
Online supplemental material
Fig. S1 documents the semi-quantitative nature of several RT-PCRs. Fig. S2 presents additional data that are used in determining the transgene content for various Tg lines. Fig. S3 presents additional data on germline transcription of the γ3 gene in specific Tg lines. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20041988/DC1.
Acknowledgments
We thank Drs. M. Berton, E. Kraig, E. Max, and J. Stavnezer for their comments on this text. We thank W. Filipiak, G. Gavrilina, and M. Van Keuren for assistance in preparing Tg mice.
This work was supported by a grant from the Midwest Affiliate of the American Heart Association (no. 051127Z) and by grants from the National Cancer Institute, no. CA39068 (to W.A. Dunnick) and no. CA46952 in support of the University of Michigan Transgenic Animal Model Core.
The authors have no conflicting financial interests.
Abbreviations used: ARS, arsonate; DC, digestion circularization; End, endogenous; HPRT, hypoxanthine phosphoribsyl transferase; HS, hypersensitive; I, the 5′ most exon of heavy chain germline transcripts; NTg, nonTg; S, switch region; Tg, transgenic.
References
- 1.Manis, J.P., M. Tian, and F.W. Alt. 2003. Mechanism and control of class-switch recombination. Trends Immunol. 23:31–39. [DOI] [PubMed] [Google Scholar]
- 2.Birshtein, B.K., C. Chen, S. Saleque, J.S. Michaelson, M. Singh, and R.D. Little. 1997. Murine and human 3′ IgH regulatory sequences. Curr. Top. Microbiol. Immunol. 224:73–80. [DOI] [PubMed] [Google Scholar]
- 3.Stavnezer, J. 2000. Molecular processes that regulate class-switching. Curr. Top. Microbiol. Immunol. 245:127–168. [DOI] [PubMed] [Google Scholar]
- 4.Giannini, S.L., M. Singh, C.-F. Calvo, G. Ding, and B.K. Birshtein. 1993. DNA regions flanking the mouse Ig 3′ α enhancer are differentially methylated and DNase I hypersensitive during B cell differentiation. J. Immunol. 150:1772–1780. [PubMed] [Google Scholar]
- 5.Madisen, L., and M. Groudine. 1994. Identification of a locus control region (LCR) in the immunoglobulin heavy chain locus that deregulates c-myc expression in plasmacytoma and Burkitt's lymphoma cells. Genes Dev. 8:2212–2226. [DOI] [PubMed] [Google Scholar]
- 6.Michaelson, J.S., S.L. Giannini, and B.K. Birshtein. 1995. Identification of 3′ α-HS4, a novel Ig heavy chain enhancer element regulated at multiple stages of B cell differentiation. Nucleic Acids Res. 23:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettersson, S., G.P. Cook, M. Bruggemann, G. Williams, and M.S. Neuberger. 1990. A second B cell specific enhancer 3′ of the immunoglobulin heavy-chain locus. Nature. 344:165–168. [DOI] [PubMed] [Google Scholar]
- 8.Dariavach, P., G.T. Williams, K. Campbell, S. Pettersson, and M.S. Neuberger. 1991. The mouse IgH 3′ enhancer. Eur. J. Immunol. 21:1499–1504. [DOI] [PubMed] [Google Scholar]
- 9.Mattias, P., and D. Baltimore. 1993. The immunoglobulin heavy chain locus contains another B-cell specific 3′ enhancer close to the α constant region. Mol. Cell. Biol. 13:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberson, R., S.L. Giannini, B.K. Birshtein, and L.A. Eckhardt. 1991. An enhancer at the 3′ end of the mouse immunoglobulin heavy chain locus. Nucleic Acids Res. 19:933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong, J., S. Stevens, R.G. Roeder, and L.A. Eckhardt. 1998. 3′ IgH enhancer elements shift synergistic interactions during B-cell development. J. Immunol. 160:4896–4903. [PubMed] [Google Scholar]
- 12.Arulampalam, V., C. Furebring, A. Samuelsson, U. Lendahl, L. Lundkvist, C. Borrebaeck, and S. Pettersson. 1996. Elevated expression of a rearranged immunoglobulin transgene in mice links the regulation of IgH expression to the IgH 3′ enhancer. Int. Immunol. 8:1149–1157. [DOI] [PubMed] [Google Scholar]
- 13.Chauveau, C., E. Pinaud, and M. Cogne. 1998. Synergies between regulatory elements of the immunoglobulin heavy chain locus and its palindromic 3′ locus control region. Eur. J. Immunol. 28:3048–3056. [DOI] [PubMed] [Google Scholar]
- 14.Collins, J.T., and W.A. Dunnick. 1999. Interferon-γ regulated germline transcripts are expressed from γ2a transgenes independently of the heavy chain 3′ enhancers. J. Immunol. 163:5758–5762. [PubMed] [Google Scholar]
- 15.Chauveau, C., E.A. Jansson, S. Muller, M. Cogne, and S. Pettersson. 1999. Ig heavy chain 3′ HS1-4 directs correct spatial position-independent expression of a linked transgene to B lineage cells. J. Immunol. 163:4637–4641. [PubMed] [Google Scholar]
- 16.Laurencikiene, J., V. Deveikaite, and E. Severinson. 2001. HS1,2 enhancer regulation of germline ɛ and γ2b promoters in murine B lymphocytes: evidence for specific promoter-enhancer interactions. J. Immunol. 167:3257–3265. [DOI] [PubMed] [Google Scholar]
- 17.Shi, X., and L.A. Eckhardt. 2001. Deletional analysis reveals an essential role for the hs3b/hs4 IgH 3′ enhancer pair in an Ig-secreting but not an earlier B cell line. Int. Immunol. 13:1003–1012. [DOI] [PubMed] [Google Scholar]
- 18.Gregor, P.D., and S.L. Morrison. 1986. Myeloma mutant with a novel 3′ flanking region: loss of normal sequence. Mol. Cell. Biol. 6:1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cogne, M., R. Lansford, A. Bottaro, J. Zhang, J. Gorman, F. Young, H.-L. Cheng, and F.W. Alt. 1994. A class switch control region at the 3′ end of the immunoglobulin heavy chain locus. Cell. 77:737–747. [DOI] [PubMed] [Google Scholar]
- 20.Lieberson, R., J. Ong, X. Shi, and L.A. Eckhardt. 1995. Immunoglobulin gene transcription ceases upon deletion of a distant enhancer. EMBO J. 24:6229–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manis, J.P., N. van der Stoep, M. Tian, R. Ferrini, L. Davidson, A. Bottaro, and F.W. Alt. 1998. Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. J. Exp. Med. 188:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinaud, E., A.A. Khamlichi, C. Le Morvan, M. Drouet, V. Nalesso, M. Le Bert, and M. Cogne. 2001. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity. 15:187–199. [DOI] [PubMed] [Google Scholar]
- 23.Li, S.C., P.B. Rothman, J. Zhang, C. Chan, D. Hirsh, and F.W. Alt. 1994. Expression of Iμ-Cγ hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int. Immunol. 6:491–497. [DOI] [PubMed] [Google Scholar]
- 24.Snapper, C.M., C. Peschel, and W.E. Paul. 1988. IFN-γ stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 140:2121–2127. [PubMed] [Google Scholar]
- 25.Collins, J.T., and W. Dunnick. 1993. Germline transcripts of the murine immunoglobulin γ2a gene: structure and induction by IFN-γ. Int. Immunol. 5:885–891. [DOI] [PubMed] [Google Scholar]
- 26.Dunnick, W.A., J. Shi, K.A. Graves, and J.T. Collins. 2004. Germline transcription and switch recombination of a transgene containing the entire heavy chain constant region locus: effect of a mutation in a STAT6 binding site in the γ1 promoter. J. Immunol. 173:5531–5539. [DOI] [PubMed] [Google Scholar]
- 27.Chu, C.C., W.E. Paul, and E.E. Max. 1992. Quantitation of immunoglobulin heavy chain switch recombination (Sμ-Sγ1) by novel digestion-circulation polymerase chain reaction method. Proc. Natl. Acad. Sci. USA. 89:6978–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hummel, M., J.K. Berry, and W. Dunnick. 1987. Switch region content of hybridomas: the two spleen cell Igh loci tend to rearrange to the same isotype. J. Immunol. 138:3539–3548. [PubMed] [Google Scholar]
- 29.Shimizu, A., N. Takahashi, Y. Yaoita, and T. Honjo. 1982. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 28:499–506. [DOI] [PubMed] [Google Scholar]
- 30.Elenich, L.A., C.S. Ford, and W.A. Dunnick. 1996. The γ1 heavy chain gene includes all the cis-acting elements necessary for expression of properly regulated germline transcripts. J. Immunol. 157:176–82. [PubMed] [Google Scholar]
- 31.Durdik, J., R.M. Gerstein, S. Rath, P.F. Robbins, A. Nisonoff, and E. Selsing. 1989. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc. Natl. Acad. Sci. USA. 86:2346–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]