Abstract
The integrin CD103 is highly expressed at mucosal sites, but its role in mucosal immune regulation remains poorly understood. We have analyzed the functional role of CD103 in intestinal immune regulation using the T cell transfer model of colitis. Our results show no mandatory role for CD103 expression on T cells for either the development or CD4+CD25+ regulatory T (T reg) cell–mediated control of colitis. However, wild-type CD4+CD25+ T cells were unable to prevent colitis in immune-deficient recipients lacking CD103, demonstrating a nonredundant functional role for CD103 on host cells in T reg cell–mediated intestinal immune regulation. Non–T cell expression of CD103 is restricted primarily to CD11chighMHC class IIhigh dendritic cells (DCs). This DC population is present at a high frequency in the gut-associated lymphoid tissue and appears to mediate a distinct functional role. Thus, CD103+ DCs, but not their CD103− counterparts, promoted expression of the gut-homing receptor CCR9 on T cells. Conversely, CD103− DCs promoted the differentiation of IFN-γ–producing T cells. Collectively, these data suggest that CD103+ and CD103− DCs represent functionally distinct subsets and that CD103 expression on DCs influences the balance between effector and regulatory T cell activity in the intestine.
Malfunction of regulatory circuits within the immune system can lead to pathological immune responses and tissue destruction. One example of this is inflammatory bowel disease (IBD), which involves chronic intestinal inflammation and affects a substantial number of people in the western world. A well-described mouse model that mimics some of the features observed in human IBD is the T cell transfer model of colitis. Here, predominantly naive CD4+CD45RBhigh T cells from nonmanipulated animals are transferred into immunodeficient recipients (1, 2). Many of the transferred T cells expand and differentiate into Th1 cells, inducing an IBD-like syndrome associated with severe tissue destruction in the colon that is driven by the enteric flora (for review see reference 3).
Although almost any leukocytic cell type can influence the outcome of an immune response under appropriate circumstances, an increasing amount of data suggests that CD4+ T reg cells play a central role in the control of peripheral immune reactivity, be it against self- or nonself-antigens (4–6). Indeed, many of the properties of T reg cells were first established in the T cell transfer model of colitis. Thus, the transfer of T reg cells to recipients of CD4+CD45RBhigh T cells not only prevents colitis (2), but also cures recipients with established disease (7). T reg cell function in this as well as in many other experimental systems is enriched in the naturally activated CD45RBlowCD25+ subset of CD4+ cells (8, 9). There is evidence that both IL-10 (10, 11) and TGF-β (12) are involved in T reg–mediated control of colitis; however, the exact mechanisms by which these cells interfere with pathogenic immune responses are not completely understood.
Comparison of the gene expression profile of CD4+CD25+ T cells with that of CD4+CD25− T cells revealed several molecules overexpressed by the former (13, 14), raising the possibility that these molecules may play a role in T reg cell function. Among the transcripts identified as being highly expressed in CD4+CD25+ T cells was that of the integrin CD103 (αE integrin). The corresponding molecule can be found on the cell surface of both CD4 and CD8 T cells (15) but has also been shown to be expressed by DCs (16–19), a principal APC population (20). CD103 can bind, paired with the β7 chain, to E-cadherin (21). E-Cadherin is expressed on a variety of cell types, including intestinal epithelial cells, keratinocytes, and Langerhans cells, an epidermal APC subset (22–24). Because of the high frequency of CD103-expressing T cells in the gut, initial hypotheses proposed that this molecule serves to facilitate the localization of these cells in this organ. However, more recent evidence not only indicates that there may be another, as of yet unidentified ligand for CD103 (25, 26) but also shows that T cell–expressed αEβ7 is not necessary for the localization of transgenic CD8+ T cells in the intestine (27).
It was previously reported that mAbs against CD103 reduced intestinal inflammation in IL-2–deficient animals treated with OVA linked to 2,4,6-trinitrophenol (28), whereas another study suggested that CD103 may be useful to identify CD4+CD25− T reg cells (29). Recently, a study by Banz et al. indicated that the major regulatory functions observed with CD4+CD25+ T cells may be attributed to the CD103+ subset (30). Furthermore, expression of CD103 on CD4+CD25+ T cells seems to be required for their retention at the site of Leishmania major infection (31). In light of these data, we decided to investigate the contribution of CD103 to intestinal homeostasis using the T cell transfer model of colitis. The results show that CD103 plays an essential role in mucosal immune regulation because T reg cell–mediated suppression of colitis was lost in CD103-deficient recipients.
Results
CD103 is not required for the induction of colitis by CD4+CD45RBhigh T cells
The role of the αE integrin, or CD103, in the localization of T cells in the intestine is currently unclear. To investigate the contribution of CD103 to the migration and accumulation of CD4 T cells in the intestine, we transferred FACS-sorted CD4+CD45RBhigh T cells from WT or CD103-deficient animals into C.B.-17-SCID recipients.
As expected, the WT population induced severe wasting and colitis in the recipients (for examples of different degrees of colitis, see Fig. 1), and high numbers of T cells accumulated in both the intestine and peripheral lymphoid organs of the animals (Fig. 2 A). Although the T cells did not express CD103 at the time of transfer, at the time of death ∼20% of the recovered CD4+ T cells expressed CD103 in both the mesenteric LN (MLN) and the lamina propria (LP) of the recipients, and CD103 expression was also detected on splenic T cells from the recipients (unpublished data). When stimulated with anti-CD3 mAbs in vitro, many of the T cells isolated from the LP produced IFN-γ (Fig. 2 B).
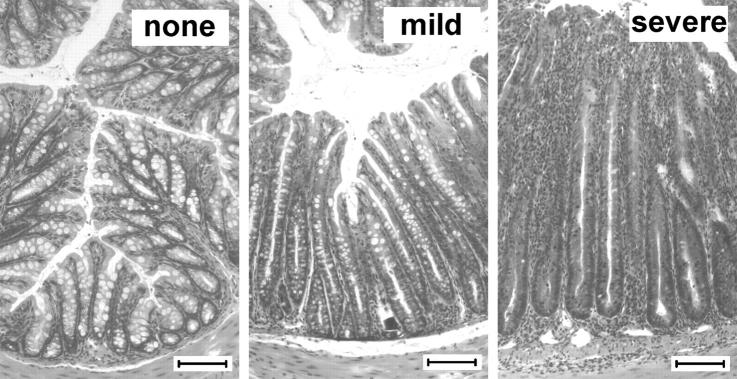
Figure 1.
Histopathological definition of colitis as applied throughout this study. Animals were transferred with CD4 T cell subsets and killed after 8–12 wk or when reaching 80% of their initial weight. Representative photomicrographs show hematoxylin and eosin–stained sections of colons from C.B.-17-SCID recipients of CD4 T cell subsets and represent different definitions of colitis (none, mild, and severe). Bar, 100 μm.
Figure 2.
CD103 is not required for the development of colitis. C.B.-17-SCID mice received 4 × 105 sorted CD4+CD45RBhigh T cells. At death, hosts were analyzed for various parameters. (A) T cell numbers recovered from various organs. Symbols represent individual mice. Data are pooled from three independent experiments. The horizontal lines represent the mean. (B) Intracytoplasmic cytokine production by T cells from the LP after overnight stimulation with anti-CD3 antibodies. The numbers shown indicate the percentage of cells in that gate. Data are representative of three individual animals per group. (C) Cumulative colitis incidence in various recipients of CD103−/− CD4+CD45RBhigh T cells. Data are pooled from seven independent experiments.
Transfer of the corresponding T cell population from CD103-deficient donors yielded essentially the same results as observed with T cells from WT donors with regard to the incidence of wasting and colitis, the accumulation of T cells in the spleen, MLN, and LP, and the production of Th1 cytokines (Fig. 2). These data show that CD4 T cells need not express CD103 in order to accumulate in the LP and induce a Th1-mediated colitis in lymphocyte-deficient mice.
As mentioned above, CD103 is not exclusively expressed by T cells. It was therefore possible that CD103 may play an essential role on non–T cells for directing the accumulation of T cells in the intestine. However, development of colitis was similar in BALB/c-RAG−/− or CD103−/− BALB/c-RAG−/− hosts after WT or CD103−/− T cell transfers, ruling out this possibility (Fig. 2 C and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20040662/DC1). Thus, CD103 is neither required for the accumulation of CD4+ T cells in the intestine nor is it essential for the induction of colitis in lymphocyte-deficient recipients.
Anti-CD103 mAb abrogates suppression of colitis by CD4+CD45RBlow T cells
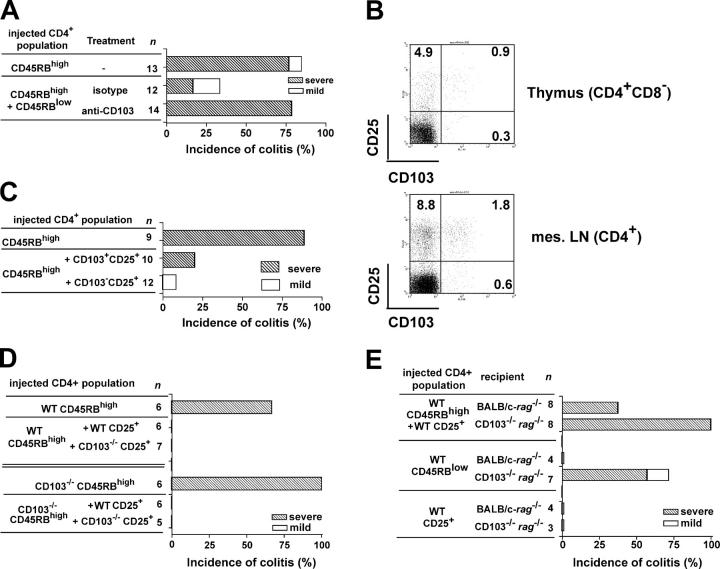
Because the lack of CD103 expression on CD4 T cells does not hamper their ability to induce colitis, it was possible that the previously observed beneficial effect of monoclonal anti-CD103 antibodies on the colitis development in IL-2–deficient animals (28) revealed an involvement of CD103 in T reg cell function. To test this, we transferred disease-inducing CD4+CD45RBhigh T cells together with protective CD4+CD45RBlow T cells from WT donors into C.B.-17-SCID recipients and subsequently treated the animals with a chimeric mAb against CD103 or an isotype control. The chimeric antibody contained a mouse IgG1-Fc section instead of the original rat IgG2a component, thus eliminating the possibility that the antibody itself would lead to the deletion of the cells binding it. Although the administration of anti-CD103 antibodies did not influence the development of colitis in animals injected with CD4+CD45RBhigh T cells alone (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20040662/DC1), animals receiving both CD4+ CD45RBhigh and CD4+CD45RBlow T cells and treated with anti-CD103 antibodies had a significantly higher degree of colitis as compared with the isotype-treated control group (P < 0.007; Fig. 3 A). The anti-CD103–treated recipients showed high numbers of CD4 T cells in lymphoid organs and the LP, with a high frequency of Th1 cytokine–producing cells in the LP of the large intestine, similar to recipients of CD4+CD45RBhigh T cells alone (unpublished data). In contrast, isotype-treated animals remained healthy throughout the experimental period of 8–10 wk. Thus, although CD103 is not essential for the disease progression, antibodies against CD103 can abrogate suppression of colitis by T reg cells in the CD4+CD45RBlow T cell pool.
Figure 3.
Protection from colitis requires expression of CD103 by host cells. Mice received 4 × 105 CD4+CD45RBhigh T cells with 1 or 2 × 105 CD4+CD45RBlow or CD4+CD25+ T cells. (A) Colitis incidence in C.B.-17-SCID recipients of the indicated CD4 T cell populations. Antibodies were given i.p. twice weekly for 8 wk (0.5mg/injection). Data are pooled from four independent experiments. (B) Representative CD103 expression by CD4 T cells from WT animals (n = 8). The numbers shown indicate the percentage of cells in that gate. (C) Colitis incidence in C57BL/6-RAG−/− recipients of CD4+CD45RBhigh T cells and sorted CD103+ and CD103−CD4+CD25+ cell subsets. Data are pooled from two independent experiments. (D) Cumulative colitis incidence in C.B.-17-SCID recipients after transfer of the indicated CD4 T cell subsets. Data are pooled from two independent experiments. (E) Colitis development in RAG−/− recipients of WT CD4 T cell subsets as indicated. Data are pooled from five independent experiments.
CD103 expression by T reg cells is not essential for their function
Several reports showed that, in this experimental system, the CD25+ subpopulation of the CD4+CD45RBlow T cell pool appears to contain the bulk of T reg cell activity (9, 11). Moreover, the majority of thymic and peripheral CD4+CD103+ T cells in normal animals coexpress CD25 (Fig. 3 B). Having identified that T reg cell activity requires CD103, it was possible that this regulatory activity was concentrated in the CD4+CD25+CD103+ subset. However, after separating splenic CD103+ and CD103− subsets of naturally occurring CD4+CD25+ T cells and coinjecting them with CD4+CD45RBhigh T cells into SCID recipients, there were no obvious differences in the protective capacity of these peripheral T cell subsets (Fig. 3 C), indicating that expression of CD103 is not a definitive marker for T reg cells. Interestingly, the vast majority of the progeny of the transferred CD25+ population expressed CD103 at the end of the experiment irrespective of their phenotype at the time of transfer (unpublished data), raising the possibility that CD103 plays a role in T reg cell function after expansion in vivo. To address this directly, the function of CD103−/− CD4+CD25+ T cells was examined. CD4+CD25+ T cells were present in a similar frequency in the spleens of CD103−/− mice as WT controls (unpublished data) and were able to prevent the colitis induced by either WT or CD103−/− CD4+CD45RBhigh T cells (Fig. 3 D), demonstrating that T cell–expressed CD103 is not essential for the generation and maintenance of T reg cells or for their ability to inhibit intestinal inflammation.
CD103 expression on non–T cells is essential for T reg cell function in vivo
The lack of any demonstrable role for CD103 on T cells in T reg cell activity suggests that the ability of anti-CD103 mAb to abrogate T reg cell function may be an effect mediated via CD103 expression on non–T cells. To test this, we repeated the transfer experiments, this time using CD103-deficient RAG−/− recipients. Strikingly, the protection from colitis was completely abolished when the host lacked expression of CD103 (P < 0.04 when compared with BALB/c-RAG−/− recipients; Fig. 3 E). There was no evidence of increased colitis incidence in CD103−/−RAG−/− recipients of CD4+CD45RBhigh cells (Fig. S1), suggesting that impaired T reg cell activity is not attributable to an enhanced colitogenic T cell response. In addition, even CD4+CD45RBlow T cells from WT donors, when transferred on their own, led to intestinal pathology in a large fraction of CD103−/− hosts, whereas they did not induce colitis in BALB/c-RAG−/− recipients (Fig. 3 E). This confirmed the reported presence of potentially pathogenic T cells in the previously activated/memory T cell pool (32, 33). The only WT CD4+ T cell population that reliably did not induce colitis in CD103−/− hosts was the CD25+ subset (Fig. 3 E).
In conclusion, although T reg cells themselves do not require CD103 to perform their function, CD103 is mandatory on non–T cells for the proper function of immune regulatory activity because protection from colitis is overcome when this molecule is absent from the host. However, the requirement for CD103 expression on host cells is likely to be a feature of local immune regulation rather than general CD4+CD25+ T reg cell function, as CD4+CD25+ T reg cells were able to suppress proliferation of CD4+CD25− T cells in vitro in the presence of CD103−/− APCs (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20040662/DC1).
Non–T cell expression of CD103 is restricted to the CD11chigh pool
CD103 has been shown to be expressed by a variety of cell types of hematopoietic origin but has not been found on any nonhematopoietic cells (34). Indeed, immunofluorescent staining of colon tissue sections from unreconstituted Balb/c Rag−/− animals showed that virtually all CD103+ cells costained with the hematopoietic cell-specific surface marker, CD45 (unpublished data). FACS analysis showed that the CD103-expressing cells in the spleen, MLN, and colon of BALB/c-RAG−/− hosts were almost exclusively CD11chigh DCs (Fig. 4 A). Consistent with a previous study (17), further analysis revealed that CD103+ DCs in the spleen were B220−, Gr1low, and expressed similarly high levels of MHC class II as their CD103−CD11chigh counterparts (Fig. 4 B). Splenic CD103+ DCs were also CD11b−, and the majority, though not all, expressed CD8α (Fig. 4 B). It was notable that splenic CD103+ DCs were larger than the CD103− subset, perhaps indicating a more activated physiological state (Fig. 4 B). The distribution of CD103 on DCs in the MLN and colon, though similar to each other, differed slightly from that found in the spleen. In both organs, populations of CD103+CD11b+ and CD103+CD8a− DCs were more prominent than in the spleen (Fig. 4 C). CD103+ DCs were present at the highest density in the MLN, accounting for 53 ± 6% of total CD11chigh DCs (Fig. 4 D). In contrast, this subset represented only 28 ± 5% of DCs in the spleen. Enrichment of the CD103+ DC subset in the MLN and colon was more pronounced in immunocompetent Balb/c mice, where CD103+ DCs accounted for <10% of DCs in the spleen (unpublished data). CD103+ DCs in immunocompetent Balb/c mice were, however, phenotypically similar to those in Balb/c Rag−/− mice (unpublished data). In situ analysis showed CD103+ DCs in the colonic LP and in isolated lymphoid clusters in colitic as well as protected T cell–restored RAG−/− mice (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20040662/DC1). The latter site has been shown to contain a high frequency of proliferating colitogenic and T reg cells (7).
Figure 4.
Phenotypic characteristics of CD11chighCD103+ cells. Spleens, MLN, or colons from BALB/c-RAG−/− or WT animals were digested with collagenase before the distribution of cell surface molecules was investigated. (A) Representative staining of CD11c and CD103 on splenic cell preparations from nonreconstituted Balb/c Rag−/− animals (n = 4) showing that the majority of CD103+ cells are CD11chigh. The majority of CD103+ cells in the MLN and colon were also CD11chigh. (B) Representative staining of various cell surface molecules on splenic preparations from nonreconstituted BALB/c-RAG−/− animals (n = 4). FSC, forward scatter. (C) Representative staining of CD11b, CD8α, and CD103 on MLN and colon preparations from nonreconstituted Balb/c Rag−/− animals (n = 6, pooled into three groups). Positioning of quadrants reflects isotype controls. The numbers shown in A–C indicate the percentage of cells in that gate. (D) Percentage of CD11chigh cells in the spleen (n = 7), MLN (n = 10, pooled into five groups), and colon (n = 10, pooled into five groups) expressing CD103, as determined by FACS analysis. Values represent mean ± SD.
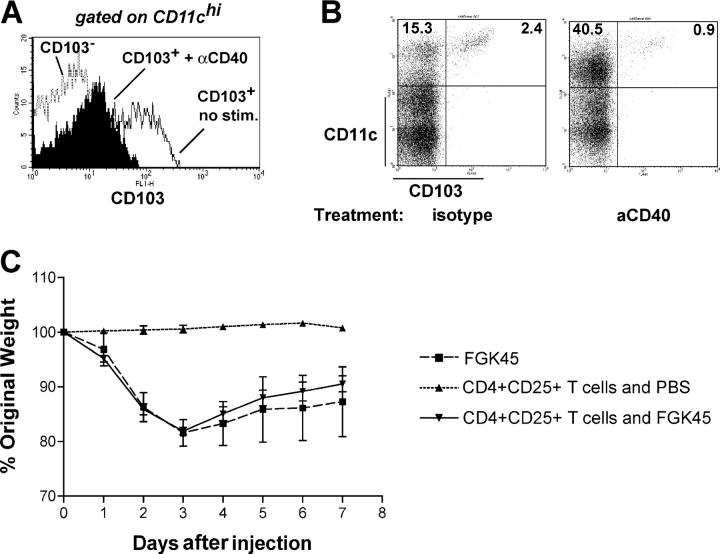
Although the CD103+ DC subset appears to be phenotypically distinct, expression of CD103 itself is not fixed and is dependent on external signals. By screening a panel of activation stimuli, we found that stimulation of FACS-sorted CD103+ DCs with anti-CD40 mAbs reduced the expression of CD103 on these DCs. This was not caused by cell death, as there was similar cell viability in anti-CD40 stimulated and unstimulated CD103+ DC cultures, but rather reflected a genuine down-regulation of the CD103 molecule (Fig. 5 A and not depicted). A similar effect could also be observed 3 d after injecting the mAb into RAG-deficient animals (Fig. 5 B). Interestingly, RAG-deficient mice injected with anti-CD40 develop a wasting disease and colitis (unpublished data), and we found that this could not be prevented by transfer of CD25+ T cells (Fig. 5 C). Other activation stimuli such as LPS did not lead to the disappearance of CD103 from the cell surface (unpublished data).
Figure 5.
Effect of stimulation through CD40 on CD103 expression. (A) FACS-sorted CD11chigh subpopulations were incubated overnight with 5 μg/ml anti-CD40 mAbs before assessing the expression of CD103. Representative stainings of two independent experiments per group. Further experiments with less stringently sorted CD11chigh populations gave similar results. (B) C57BL/6-RAG−/− mice were injected i.p. with 200 μg anti-CD40 or isotype control mAbs. 3 d later, spleens were analyzed for the presence of CD103+ and CD11c+ cells. The numbers shown indicate the percentage of cells in that gate. Stainings are representative of three animals per group. (C) Wasting disease in mice treated with anti-CD40 in the presence or absence of CD4+CD25+ cells. Wasting was accompanied by colitis. Transfer of CD4+CD25+ T cells 28 d before injection of anti-CD40 did not result in any improvement in wasting or in the severity of colitis. Data is representative of three similar experiments. Values represent mean ± SD.
Impaired T reg cell activity in CD103−/−RAG−/− mice does not seem to be attributable to a gross alteration in DC subsets, as there were similar numbers of CD11chigh DCs in the colon, MLN, and spleen of CD103−/−RAG−/− as in RAG−/− mice, with no detectable changes in the CD11b+ and CD8α+ subsets (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20040662/DC1). Furthermore, DCs from both Balb/c and CD103−/− donors were able to stimulate T cell proliferation equally (Fig. S5)
Functional characterization of CD103+ DCs
We next investigated whether the distinct phenotypic characteristics of the CD11chighMHC class IIhighCD103+ cell population corresponded with any differences in their ability to activate T cells. DCs derived from the gut and MLN have previously been shown to be able to support the expression of gut-homing receptors such as CCR9 and α4β7 on T cells (35–38).
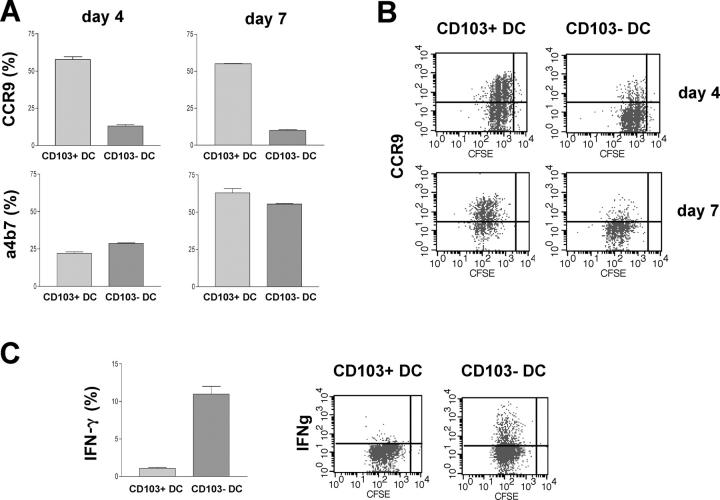
CD11chigh cells obtained from MLN of Balb/c mice were sorted into CD103+ and CD103− subsets and cultured with carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled naive CD4+ T cells purified from DO11.10 SCID mice. T cells went through similar rounds of division after culture with either DC subset, indicating that both subsets have a similar capacity to present antigen to T cells (unpublished data). Strikingly, however, T cells cultured with CD103+ DCs were phenotypically distinct from those cultured with CD103− DCs, with a much higher percentage expressing the gut-homing chemokine receptor, CCR9, by day 4 of culture (Fig. 6, A and B). Because the kinetics of up-regulation of α4β7 in vitro are often slow, we also analyzed the T cells after a further 3-d expansion in the presence of IL-2. At this time point, the difference in CCR9 expression was maintained, but the proportion of α4β7-expressing cells was still similar, irrespective of which DC subset had been present. The failure of CD103− DCs to induce CCR9 was not caused by an inability to completely activate T cells, as a greater proportion of T cells cultured with CD103− DCs expressed IFN-γ after restimulation with anti-CD3, and this was reflected in the levels of IFN-γ in the culture supernatants at day 4 (Fig. 6 C and not depicted). We were unable to detect IL-10 production by T cells, irrespective of which DC population they were cultured with (unpublished data).
Figure 6.
Functional characteristics of CD11chigh CD103+ cells. MACS-sorted, CFSE-labeled CD4+ cells from DO11.10 SCID donors were incubated with FACS-sorted CD103+CD11chigh or CD103−CD11chigh DCs from MLN of Balb/c donors and OVA peptide. T cells were analyzed by FACS for expression of CCR9 and α4β7 at day 4 of culture and, after a further 3 d of expansion in IL-2. At day 7 of culture, T cells were restimulated overnight with platebound anti-CD3 mAb before intracellular cytokines were assessed. (A) Mean percentage ± SD of T cells expressing CCR9 and α4β7. Data from one of two independent experiments. (B) Representative staining of CCR9 on T cells. (C) Mean percentage ± SD of T cells producing IFN-γ and representative stainings. Data from one of two independent experiments.
Discussion
To date, the mechanisms of T reg cell function and their interaction with other immune system components are incompletely characterized. We have newly identified a critical function for CD103 in the regulation of intestinal immune responses. The data show that CD103 was not required for the accumulation of CD4 T cells in the intestine and the induction of colitis, whereas T reg cells deficient in this molecule were also still able to suppress IBD. In contrast, the absence of CD103 on DCs in RAG−/− hosts led to abrogation of T reg cell activity indicating that CD103 expression by DCs is crucial in the maintenance of a healthy balance between effector and regulatory immune cell functions. Strikingly, the ability of MLN-derived DCs to imprint expression of CCR9, a gut-homing receptor, on T cells was a unique feature of the CD103+ subset, further illustrating the importance of this DC subset in intestinal immunity.
Despite being first identified ∼20 yr ago (39, 40), the function of CD103 in immune regulation has not been extensively studied. CD103 was found to be expressed by intestinal intraepithelial T cells, suggesting that it was important in the localization of CD8+ T cells to epithelial surfaces (41, 42). There is also evidence that CD103 plays a functional role in CD8+ T cell responses, being required for destruction of islet allografts (43) and for the induction of intestinal graft-versus-host disease pathology (44). In addition, treatment with anti-CD103 mAb ameliorated TNP-OVA–induced colitis in IL-2–deficient mice, suggesting that CD103 may also play a role in CD4+ T cell–mediated colitis (28). However, in this study we have failed to confirm a role for CD103 in the development of colitis. Indeed, colitis developed normally in the absence of CD103 expression on colitogenic T cells and host APCs, clearly establishing that CD103 expression is dispensable for CD4+ T cell accumulation in the intestine and development of Th1 cell–mediated colitis.
More recently, CD103 has been suggested to mark regulatory T cell subsets with enhanced T reg cell activity compared with their CD103− counterparts (29, 30). CD103+ T reg cells showed an increased capacity to accumulate in inflammatory sites, and there is evidence that CD103 expression by CD4+CD25+ T reg cells plays a functional role in retention of T reg cells in the inflamed skin (31). However, our findings that CD103-deficient CD4+CD25+ T cells retain the ability to inhibit intestinal inflammation indicate that CD103 expression by T reg cells is not absolutely essential for their function in vivo. The requirement for CD103 expression on T reg cells for control of skin but not intestinal inflammation suggests that mechanisms of T reg cell–mediated control are determined, at least in part, by tissue-specific features of the inflammatory response being regulated.
Although CD103 expression may be redundant for T reg cells themselves, it is required on innate immune cells in the RAG−/− hosts to maintain T reg cell–mediated protection from T cell–induced colitis. CD103 expression in immune-deficient mice is restricted primarily to a population of CD11chighMHC class IIhigh DCs with discrete phenotypic and functional properties. CD103+ DCs are not distributed evenly throughout secondary lymphoid tissue, with a higher proportion present in the MLN than in the spleen. The CD103+ DC subset has a similar subset composition in the colon as in the MLN, suggesting a link between CD103+ DCs in these two sites. Notably, Huang et al. have reported the presence of CD103+ DCs containing fragments of apoptotic epithelial cells in the rat MLN (45). Importantly, MLN-derived CD103+ DCs but not their CD103− counterparts led to the induction of the intestinal homing receptor CCR9 on antigen-activated CD4+ T cells. Our results are consistent with a recent study showing that CD103 marks DCs in the MLN that have migrated from the intestine and that the ability of MLN-derived DCs to imprint a gut-homing phenotype on CD8+ T cells is a unique feature of the CD103+ subset (see Johansson-Lindbom et al. on p. 1063 of this issue). In that study, induction of both CCR9 and α4β7 was restricted to CD103+ DCs, whereas we found that both CD103+ and CD103− DCs from the MLN were able to induce α4β7 expression on CD4 cells. Collectively, these results suggest that expression of CCR9 and α4β7 is differentially regulated on CD4 cells. Although the mechanism is unknown, it may reflect quantitative differences in the requirement for retinoic acid, which has been shown to be responsible for the ability of gut-associated lymphoid tissue (GALT)–derived DCs to imprint both CCR9 and α4β7 expression on T cells (38). Indeed, there is evidence that induction of α4β7 on T cells in vitro is less dependent on the presence of GALT-derived DCs than CCR9 (46, 47), and it may be that other co-stimulatory pathways can contribute to α4β7 expression on CD4 cells. Indeed, blockade of CD134–CD134L interactions prevented the up-regulation of α4β7 expression on CD4 cells in vivo, and this latter pathway may be involved in CD103− DC-induced α4β7 induction, particularly as expression of CD134L on in vivo–activated DCs is restricted to the CD8α− CDllb+ DC subset (48), the majority of which are CD103− (Fig. 4).
Although CD103+ DCs induced the expression of a gut-homing phenotype on CD4+ T cells, they were inefficient at inducing the production of IFN-γ or IL-10 by T cells. In contrast, CD103− DCs led to the differentiation of T cells secreting IFN-γ but not IL-10. This functional difference was not the consequence of differences in the ability of either DC subset to activate T cells, as both induced similar rounds of antigen-induced cell division. TGF-β has been shown to induce CD103 expression on T cells (44, 49), and expression of CD103 on DCs may also be a mark of their exposure to TGF-β in the gut. Conditioning of CD103+ DCs in the immunosuppressive environment of the gut may explain their inability to drive secretion of effector cytokines by CD4+ T cells. In fact, DCs found in the GALT appear to be functionally distinct from their splenic counterparts and may have a tolerogenic role under steady-state conditions (50). Peyer's patch DCs, for example, differed from splenic DCs in their ability to produce high levels of IL-10 and induce the differentiation of IL-4– and IL-10–producing T cells (51). It may be advantageous, under physiologic conditions, that gut-derived DCs induce T cell expression of gut-homing receptors without differentiation into inflammatory cytokine-secreting effector cells, preventing the development of effector responses to gut-derived antigens. This may change during an intestinal infection, where it will be important for effector cells to be able to home to the inflamed intestine. In this regard it will be of interest to determine whether there are differences in the ability of CD103+ DCs isolated from the MLN of colitic mice to induce the differentiation of IFN-γ–secreting T cells.
Impaired T reg cell activity in RAG−/− mice lacking CD103 suggests that CD103 expression not only defines functionally distinct subsets of DCs but that CD103 itself plays a functional role in regulating the balance of effector and regulatory responses in the intestine. DCs have been shown to play an important role in driving CD4+CD25+ T reg cell expansion in vitro and in vivo. (7, 11, 52–54). During cure of colitis, proliferating T reg cells are found adjacent to DCs in the secondary lymphoid tissue and locally in the intestine (7), suggesting that DCs may also drive T reg cell expansion in tissues. In view of this, several mechanisms can be envisaged for how CD103 expression on DCs may modulate T reg cell function. First, CD103, via interaction with its ligand E-cadherin, may facilitate the retention of DCs in the intestine, influencing their conditioning in this immunosuppressive environment. Such “gut-conditioned” DCs may then favor regulatory as opposed to effector cell responses, allowing CD4+CD25+ T reg cells to dominate and suppress the colitogenic effector cell response. In the absence of CD103, gut conditioning is altered and DCs more efficiently drive the differentiation of colitogenic Th1 responses, as is observed with the CD103− DC subset isolated from WT mice. Alternatively, the involvement of CD103 in T reg cell function may be indirect, involving interactions among DCs themselves. Thus, it has been shown that ligation of E-cadherin can inhibit DC maturation (55), raising the possibility that CD103 on DCs may influence the functional state of other DCs, leading to different T cell responses. Whatever the mechanism by which CD103 expression on DCs effects intestinal T reg cell responses, it does not appear to play a general role in T reg cell function, as CD4+CD25+ T reg cells retain the ability to suppress T cell activation in vitro in cultures containing CD103−/− APCs.
CD103 expression on DCs appears to be tightly regulated, which is consistent with its important role in determining the balance between effector and regulatory T cells. The finding that CD103 can be down-regulated via acute CD40 stimulation provides a mechanism by which activated CD40L+ T cells may overcome suppression by T reg cells. In support of this, CD4+CD25+ T cells, although capable of regulating the innate inflammatory response (56), could not prevent wasting disease and colitis induced by administration of an anti-CD40 mAb to RAG-deficient recipients, which is consistent with the findings that T reg cell control of DC functions can be abrogated by CD40-mediated signals (57).
In conclusion, we show that CD103, though dispensable for T cells, is critically important in the regulation of intestinal immune responses through its expression on DCs, raising the possibility that this molecule may be a useful therapeutic target for the treatment of IBDs.
Material and Methods
Mice.
BALB/c, BALB/c-RAG−/−, CD103−/− BALB/c-RAG−/−, CD103−/−, C.B.-17-SCID, C57BL/6, Ly5.1+-C57BL/6, C57BL/6-RAG−/−, and DO11.10-SCID TCR-transgenic mice were maintained in specific pathogen-free animal facilities at the University of Oxford in microisolator cages with filtered air. All genetically modified strains were extensively backcrossed onto the appropriate genetic background. Experiments were performed according to the United Kingdom Animals (Scientific Procedures) Act of 1986.
Antibodies.
The following antibodies were used for cell purification: anti–mouse CD8 (clone YTS169); MHC class II (TIB120); Mac-1 (M1/70); B220 (RA3-6B2); FITC-conjugated anti–mouse CD45RB (16A); biotinylated anti–mouse CD25 (7D4); CyChrome-conjugated anti–mouse CD4 (RM4-5); and PE-conjugated streptavidin. For FACS analysis, appropriately biotinylated or fluorochrome-conjugated antibodies against mouse CD103 (M290), CD3 (145-2C11), TCR-β (H57), CD25 (7D4), CD4 (RM4-5), CD11c (HL-3), B220 (RA3-6B2), Ly-6G (Gr-1), CD11b (M1/70), CD40 (3/23), CD80 (16-10A1), CD86 (GL-1), MHC class II (2G9), CD8α (Ly-2), α4β7 (DATK32), and CD45.2 (104) were purchased from BD Biosciences. Anti–mouse CD40 (FGK45) was affinity purified from hybridoma supernatants. Anti–mouse CCR9 (K629) was as described previously (58).
For in vivo use, anti–mouse CD103 (chimeric clone M290, mouse IgG1) and an isotype control (clone 101.4) were used containing <3 endotoxin U/mg of protein. These antibodies were obtained from Celltech R&D.
For histology, purified antibodies against CD11c (HL-3) and CD103 (M290) and an FITC-conjugated antibody against MHC class II were purchased from BD Biosciences. Peroxidase-conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories.
Purification of T cell subsets.
CD4 T cell subsets were isolated from spleens as described previously (2). In brief, single cell suspensions were depleted of CD8+, MHC class II+, Mac-1+, and B220+ cells by negative selection using sheep anti–rat–coated Dynabeads (Dynal). The resulting CD4-enriched cells were stained with anti-CD4, anti-CD25, and anti-CD45RB or anti-CD103 mAb. Subpopulations of CD4 cells were generated by three-color sorting on a cell sorter (MoFlo; DakoCytomation). The resulting populations were routinely >98% pure on reanalysis.
T cell reconstitution and antibody treatment.
Recipient mice were injected i.p. with sorted CD4 T cell subpopulations in PBS. Recipients were aged 8–12 wk, and donors were aged 8–16 wk. If not stated otherwise, mice received 4 × 105 CD4+CD45RBhigh cells alone or in combination with 1 or 2 × 105 cells of other CD4+ subpopulations. Experiments typically lasted between 8 and 14 wk, with mice being monitored for changes in weight and the development of clinical signs of colitis. Chimeric anti-CD103 mAb or isotype control mAb (1 mg) in PBS were injected i.p. the day after T cell reconstitution. The treatment was continued with 0.5-mg mAb injections i.p. twice weekly for 8 wk. For experiments involving treatment with anti-CD40, immunodeficient Rag1−/− mice received 105 CD4+CD25+ T cells i.p. After 28 d, these mice and an unreconstituted control group were injected with 200 μg FGK45 i.p. Mice were weighed daily and killed after 7 d. Mice given FGK45 developed a wasting disease and colitis characterized histologically by epithelial cell hyperplasia and a leukocytic infiltrate within the LP (unpublished data). Transfer of CD4+CD25+ T cells did not result in any improvement in wasting or histological signs of colitis.
Histology.
Colons were removed from mice at death and fixed in buffered 3.7% formalin. 6-μm paraffin-embedded sections were cut and stained with hematoxylin and eosin. Inflammation was scored on a scale of 0–4, representing no (grades 0 and 1), mild (grade 2), and severe colitis (grades 3 and 4), as described previously (30).
Additional tissue samples were snap frozen and cryocut. Sections were acetone fixed and blocked with donkey serum. Endogenous peroxidase activity was quenched using 0.5% H2O2 and 1% sodium azide. Staining with anti–mouse CD11c was followed with a peroxidase-conjugated anti–hamster secondary and tyramide amplification. Staining with anti–mouse CD103 was followed with a peroxidase-conjugated anti–rat secondary and tyramide amplification. CD103 staining was combined with staining for MHC class II using a directly FITC-labeled antibody.
LP cell preparation and intracellular cytokine analysis.
LP lymphocytes were purified as previously described (59). In brief, colons were cut into 0.5–1-cm pieces and incubated in Ca- and Mg-free PBS containing 10% heat-inactivated FCS (Invitrogen) and 5 mM EDTA to remove the epithelial layer. The remaining tissue was further digested with 100 U/ml collagenase/dispase (Sigma-Aldrich), and the LP cells were then layered on a Percoll gradient (GE Healthcare). The lymphocyte-enriched population was recovered after centrifugation at 600 g for 20 min at the 40–75% interface. Cells were stained for FACS analysis or for detection of cytokines and were cultured overnight in RPMI 1640 (Invitrogen) with 10% FCS, 2 mM l-glutamine, 0.05 mM 2-ME, and 100 U/ml each of penicillin and streptomycin (RPMI complete) in 24-well flatbottom plates coated with 5 μg/ml anti–mouse CD3ɛ (clone 145-2C11). 10 μg/ml Brefeldin A (Sigma-Aldrich) was added for the final 2–4 h of incubation, and surface and cytoplasmic staining was performed as described previously (60). Labeled cells were stained with antibodies against IFN-γ (XMG1.2) and TNF-α (MP6-XT22) and analyzed on a FACSCalibur (BD Biosciences) using CellQuest software (BD Biosciences).
Preparation of CD11chigh subsets.
Pooled or single spleens or mesenteric LN were cut into small fragments and incubated in PBS with 10% FCS and 1–2 mg/ml collagenase type II (Worthington Biochemicals) with 50 Kunitz U/ml DNase I (Sigma-Aldrich) for 25 min at 37°C on a shaking incubator. After adding EDTA for a further 5 min, the solution was filtered through a nylon mesh and washed in PBS with 0.1% BSA. Cells were then stained for FACS analysis or purified for functional analysis. For the latter, cell suspensions were incubated with an anti-FcR antibody (clone 24G2), followed by anti-CD11c MACS beads (Miltenyi Biotec). CD11c+ cells were then positively selected on an LS MACS column. Cells were labeled with anti-CD11c–PE, anti-CD103–biotin, and streptavidin–FITC and sorted on a MoFlo sorter to >97% purity.
T cell differentiation assay.
CD4 T cells were isolated from splenic single cell suspensions of DO11.10-SCID mice by labeling with magnetic anti-CD4 beads (Miltenyi Biotec) and separating the cell populations using MACS columns according to the manufacturer's instructions. The positively selected CD4+ T cells (>90% pure) were CFSE labeled using a Vybrant CFDA SE Cell Tracer Kit (Invitrogen) and resuspended in RPMI complete. 2 × 105 T cells were cultured together with 105 of the sorted CD11chigh subsets and 0.2 μg/ml OVA peptide in 96-well plates for 4 d. Cultures were supplemented with fresh medium containing recombinant human 10 U/ml IL-2 and incubated for a further 3 d. T cells were then restimulated with platebound anti-CD3 mAb and assessed for intracytoplasmic cytokines as described above.
Statistical analysis.
Colitis scores were compared using the Mann-Whitney U test, and differences were considered statistically significant when P < 0.05.
Online supplemental material
Fig. S1 shows similar incidence and severity of CD4+CD45RBhi T cell–mediated colitis in Rag−/− and CD103−/− Rag−/− recipients. Mice received 4 × 105 CD4+CD45RBhi T cells, and colitis was assessed as described in Materials and methods. Fig. S2 depicts the effect of chimeric anti-CD103 mAbs on development of colitis in recipients of WT CD4+CD45RBhi T cells. Animals were treated with anti-CD103 mAbs or isotype control mAbs throughout the experiment. Colitis was assessed as described in Materials and methods. In Fig. S3, CD103 is not required for T reg cell–mediated suppression in vitro. CD103−/− CD4+CD25− T cells were cultured for 3 d with MACS-sorted CD103−/−CD4+CD25+ T cells and irradiated splenocytes from WT or CD103−/− animals. In Fig. S4, CD103+ DCs are present in the LP and isolated leukocytic clusters of protected and colitic mice. Representative staining for CD103 and MHC class II is shown. Double-positive cells are CD103+ DCs. Single-positive cells for CD103 are transferred T cells. Fig. S5 depicts similar DC subset composition, distribution, and function in Balb/c Rag−/− and CD103−/−Rag−/− mice. In A, Cell suspensions were prepared from spleen, MLN, and colon as described in Materials and methods and analyzed by FACS. Data are shown as the mean ± SD for six mice analyzed (pooled into three groups). In B, sections of colon from Balb/c Rag−/− and CD103−/−Rag−/− mice were stained with anti-CD11c and DAPI. In C, MACS-sorted, CFSE-labeled CD4+ T cells from D011.10 SCID donors were incubated with MACS-sorted CD11c+ cells from the MLN of Balb/c or CD103−/− mice and OVA peptide. T cell proliferation was analyzed after 4 d in culture.
Acknowledgments
We would like to thank Peter Kilshaw for helpful comments and suggestions, Ulf Yrlid and Kevin Maloy for critical readings of the manuscript, Viv Perkins, Anu Arya, and Ingrid Dodge, Liz Darley for the histology, Nigel Rust for cell sorting, and all staff involved in animal care.
O. Annacker and F. Powrie are supported by the Wellcome Trust. J.L. Coombes is supported by the Medical Research Council.
The authors have no conflicting financial interests.
Abbreviations used: CFSE, carboxyfluorescein diacetate succinimidyl ester; GALT, gut-associated lymphoid tissue; IBD, inflammatory bowel disease; LP, lamina propria; MLN, mesenteric LN.
C.M. Parker's present address is Harvard Medical School, Office of Research, Boston, MA 02459.
References
- 1.Morrissey, P.J., K. Charrier, S. Braddy, D. Liggitt, and J.D. Watson. 1993. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 178:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powrie, F., M.W. Leach, S. Mauze, L.B. Caddle, and R.L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461–1471. [DOI] [PubMed] [Google Scholar]
- 3.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 5.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 6.Gavin, M., and A. Rudensky. 2003. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr. Opin. Immunol. 15:690–696. [DOI] [PubMed] [Google Scholar]
- 7.Mottet, C., H.H. Uhlig, and F. Powrie. 2003. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170:3939–3943. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 9.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T.C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25(+) CD4(+) T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166:3008–3018. [DOI] [PubMed] [Google Scholar]
- 12.Fahlen, L., S. Read, L. Gorelik, S.D. Hurst, R.L. Coffman, R.A. Flavell, and F. Powrie. 2005. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 201:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavin, M.A., S.R. Clarke, E. Negrou, A. Gallegos, and A. Rudensky. 2002. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat. Immunol. 3:33–41. [DOI] [PubMed] [Google Scholar]
- 14.McHugh, R.S., M.J. Whitters, C.A. Piccirillo, D.A. Young, E.M. Shevach, M. Collins, and M.C. Byrne. 2002. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323. [DOI] [PubMed] [Google Scholar]
- 15.Andrew, D.P., L.S. Rott, P.J. Kilshaw, and E.C. Butcher. 1996. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur. J. Immunol. 26:897–905. [DOI] [PubMed] [Google Scholar]
- 16.Kilshaw, P.J. 1993. Expression of the mucosal T cell integrin alpha M290 beta 7 by a major subpopulation of dendritic cells in mice. Eur. J. Immunol. 23:3365–3368. [DOI] [PubMed] [Google Scholar]
- 17.McLellan, A.D., M. Kapp, A. Eggert, C. Linden, U. Bommhardt, E.B. Brocker, U. Kammerer, and E. Kampgen. 2002. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 99:2084–2093. [DOI] [PubMed] [Google Scholar]
- 18.Pribila, J.T., A.A. Itano, K.L. Mueller, and Y. Shimizu. 2004. The alpha1beta1 and alphaEbeta7 integrins define a subset of dendritic cells in peripheral lymph nodes with unique adhesive and antigen uptake properties. J. Immunol. 172:282–291. [DOI] [PubMed] [Google Scholar]
- 19.Edwards, A.D., D. Chaussabel, S. Tomlinson, O. Schulz, A. Sher, and E.S.C. Reis. 2003. Relationships among murine CD11c(high) dendritic cell subsets as revealed by baseline gene expression patterns. J. Immunol. 171:47–60. [DOI] [PubMed] [Google Scholar]
- 20.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 21.Cepek, K.L., S.K. Shaw, C.M. Parker, G.J. Russell, J.S. Morrow, D.L. Rimm, and M.B. Brenner. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 372:190–193. [DOI] [PubMed] [Google Scholar]
- 22.Geiger, B., and O. Ayalon. 1992. Cadherins. Annu. Rev. Cell Biol. 8:307–332. [DOI] [PubMed] [Google Scholar]
- 23.Tang, A., M. Amagai, L.G. Granger, J.R. Stanley, and M.C. Udey. 1993. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 361:82–85. [DOI] [PubMed] [Google Scholar]
- 24.Hermiston, M.L., and J.I. Gordon. 1995. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol. 129:489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown, D.W., J. Furness, P.M. Speight, G.J. Thomas, J. Li, M.H. Thornhill, and P.M. Farthing. 1999. Mechanisms of binding of cutaneous lymphocyte-associated antigen-positive and alphaebeta7-positive lymphocytes to oral and skin keratinocytes. Immunology. 98:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauch, U.G., R.C. Mueller, X.Y. Li, M. Cernadas, J.M. Higgins, D.G. Binion, and C.M. Parker. 2001. Integrin alpha E(CD103)beta 7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J. Immunol. 166:3506–3514. [DOI] [PubMed] [Google Scholar]
- 27.Lefrancois, L., C.M. Parker, S. Olson, W. Muller, N. Wagner, M.P. Schon, and L. Puddington. 1999. The role of β7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludviksson, B.R., W. Strober, R. Nishikomori, S.K. Hasan, and R.O. Ehrhardt. 1999. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2−/− mice. J. Immunol. 162:4975–4982. [PubMed] [Google Scholar]
- 29.Lehmann, J., J. Huehn, M. de la Rosa, F. Maszyna, U. Kretschmer, V. Krenn, M. Brunner, A. Scheffold, and A. Hamann. 2002. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc. Natl. Acad. Sci. USA. 99:13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banz, A., A. Peixoto, C. Pontoux, C. Cordier, B. Rocha, and M. Papiernik. 2003. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur. J. Immunol. 33:2419–2428. [DOI] [PubMed] [Google Scholar]
- 31.Suffia, I., S.K. Reckling, G. Salay, and Y. Belkaid. 2005. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 174:5444–5455. [DOI] [PubMed] [Google Scholar]
- 32.Asseman, C., S. Read, and F. Powrie. 2003. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J. Immunol. 171:971–978. [DOI] [PubMed] [Google Scholar]
- 33.Annacker, O., O. Burlen-Defranoux, R. Pimenta-Araujo, A. Cumano, and A. Bandeira. 2000. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J. Immunol. 164:3573–3580. [DOI] [PubMed] [Google Scholar]
- 34.Kilshaw, P.J., and J.M.G. Higgins. 2003. Integrin αEβ7: molecular features and functional significance in the immune system. I Domains in Integrins. D. Gullberg, editor. Landes Bioscience/Eurekah.com, Georgetown, TX.
- 35.Johansson-Lindbom, B., M. Svensson, M.A. Wurbel, B. Malissen, G. Marquez, and W. Agace. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mora, J.R., M.R. Bono, N. Manjunath, W. Weninger, L.L. Cavanagh, M. Rosemblatt, and U.H. von Andrian. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 424:88–93. [DOI] [PubMed] [Google Scholar]
- 37.Stagg, A.J., M.A. Kamm, and S.C. Knight. 2002. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur. J. Immunol. 32:1445–1454. [DOI] [PubMed] [Google Scholar]
- 38.Iwata, M., A. Hirakiyama, Y. Eshima, H. Kagechika, C. Kato, and S.Y. Song. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21:527–538. [DOI] [PubMed] [Google Scholar]
- 39.Kilshaw, P.J., and K.C. Baker. 1988. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol. Lett. 18:149–154. [DOI] [PubMed] [Google Scholar]
- 40.Cerf-Bensussan, N., A. Jarry, N. Brousse, B. Lisowska-Grospierre, D. Guy-Grand, and C. Griscelli. 1987. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur. J. Immunol. 17:1279–1285. [DOI] [PubMed] [Google Scholar]
- 41.Kilshaw, P.J., and S.J. Murant. 1990. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur. J. Immunol. 20:2201–2207. [DOI] [PubMed] [Google Scholar]
- 42.Cerf-Bensussan, N., D. Guy-Grand, B. Lisowska-Grospierre, C. Griscelli, and A.K. Bhan. 1986. A monoclonal antibody specific for rat intestinal lymphocytes. J. Immunol. 136:76–82. [PubMed] [Google Scholar]
- 43.Feng, Y., D. Wang, R. Yuan, C.M. Parker, D.L. Farber, and G.A. Hadley. 2002. CD103 expression is required for destruction of pancreatic islet allografts by CD8+ T cells. J. Exp. Med. 196:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Asady, R., R. Yuan, K. Liu, D. Wang, R.E. Gress, P.J. Lucas, C.B. Drachenberg, and G.A. Hadley. 2005. TGF-β–dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J. Exp. Med. 201:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang, F.P., N. Platt, M. Wykes, J.R. Major, T.J. Powell, C.D. Jenkins, and G.G. MacPherson. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mora, J.R., G. Cheng, D. Picarella, M. Briskin, N. Buchanan, and U.H. von Andrian. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudda, J.C., A. Lembo, E. Bachtanian, J. Huehn, C. Siewert, A. Hamann, E. Kremmer, R. Forster, and S.F. Martin. 2005. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 35:1056–1065. [DOI] [PubMed] [Google Scholar]
- 48.Malmstrom, V., D. Shipton, B. Singh, A. Al-Shamkhani, M. Puklavec, A.N. Barclay, and F. Powrie. 2001. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell restored SCID mice. J. Immunol. 166:6972–6981. [DOI] [PubMed] [Google Scholar]
- 49.Robinson, P.W., S.J. Green, C. Carter, J. Coadwell, and P.J. Kilshaw. 2001. Studies on transcriptional regulation of the mucosal T-cell integrin alphaEbeta7 (CD103). Immunology. 103:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilsborough, J., and J.L. Viney. 2004. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology. 127:300–309. [DOI] [PubMed] [Google Scholar]
- 51.Iwasaki, A., and B.L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamazaki, S., T. Iyoda, K. Tarbell, K. Olson, K. Velinzon, K. Inaba, and R.M. Steinman. 2003. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein, L., K. Khazaie, and H. von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker, L.S., A. Chodos, M. Eggena, H. Dooms, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedl, E., J. Stockl, O. Majdic, C. Scheinecker, W. Knapp, and H. Strobl. 2000. Ligation of E-cadherin on in vitro-generated immature Langerhans-type dendritic cells inhibits their maturation. Blood. 96:4276–4284. [PubMed] [Google Scholar]
- 56.Maloy, K.J., L. Salaun, R. Cahill, G. Dougan, N.J. Saunders, and F. Powrie. 2003. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serra, P., A. Amrani, J. Yamanouchi, B. Han, S. Thiessen, T. Utsugi, J. Verdaguer, and P. Santamaria. 2003. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 19:877–889. [DOI] [PubMed] [Google Scholar]
- 58.Carramolino, L., A. Zaballos, L. Kremer, R. Villares, P. Martin, C. Ardavin, A.C. Martinez, and G. Marquez. 2001. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood. 97:850–857. [DOI] [PubMed] [Google Scholar]
- 59.Powrie, F., M.W. Leach, S. Mauze, S. Menon, L.B. Caddle, and R.L. Coffman. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1:553–562. [DOI] [PubMed] [Google Scholar]
- 60.Openshaw, P., E.E. Murphy, N.A. Hosken, V. Maino, K. Davis, K. Murphy, and A. O'Garra. 1995. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 182:1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]