Abstract
Allergic diseases mediated by T helper type (Th) 2 cell immune responses are rising dramatically in most developed countries. Exaggerated Th2 cell reactivity could result, for example, from diminished exposure to Th1 cell–inducing microbial infections. Epidemiological studies, however, indicate that Th2 cell–stimulating helminth parasites may also counteract allergies, possibly by generating regulatory T cells which suppress both Th1 and Th2 arms of immunity. We therefore tested the ability of the Th2 cell–inducing gastrointestinal nematode Heligmosomoides polygyrus to influence experimentally induced airway allergy to ovalbumin and the house dust mite allergen Der p 1. Inflammatory cell infiltrates in the lung were suppressed in infected mice compared with uninfected controls. Suppression was reversed in mice treated with antibodies to CD25. Most notably, suppression was transferable with mesenteric lymph node cells (MLNC) from infected animals to uninfected sensitized mice, demonstrating that the effector phase was targeted. MLNC from infected animals contained elevated numbers of CD4+CD25+Foxp3+ T cells, higher TGF-β expression, and produced strong interleukin (IL)-10 responses to parasite antigen. However, MLNC from IL-10–deficient animals transferred suppression to sensitized hosts, indicating that IL-10 is not the primary modulator of the allergic response. Suppression was associated with CD4+ T cells from MLNC, with the CD4+CD25+ marker defining the most active population. These data support the contention that helminth infections elicit a regulatory T cell population able to down-regulate allergen induced lung pathology in vivo.
Introduction
The prevalence of allergic diseases, such as asthma and allergic rhinitis, continues to rise in developed countries (1–4). Asthma is a multifarious polygenic disease, in which allergens elicit early- and late-phase airway inflammatory responses culminating in broncho-constriction and airway remodeling. However, sensitization to allergens and disease manifestation is pliant to environmental influences (5–9).
Allergies have traditionally been considered to be Th2 cell–derived immunopathologies, and earlier thinking suggested that declining microbial exposure in Western populations resulted in a weaker Th1 cell responsiveness, and a propensity to develop Th2 cell responses to innocuous allergens (10). However, it is increasingly clear that an imbalance between immunoregulatory and Th2 effector mechanisms can modulate allergy in a critical fashion (2, 3, 11–15). This conclusion has been supported by studies of Th2 cell–inducing human helminth infections, in which both observational and post-therapy data show an inverse association between chronic infection and overt allergic responsiveness (16, 17). Interestingly, infection primarily regulates late-stage effector phase mechanisms, as proallergic IgE responses remain intact in infected patients (18–23).
Evidence for immune suppression during helminth infections is strong (24–27), and recent studies identified inhibitory mechanisms that dampen allergic and/or autoimmune pathologies (28, 29). Further data now support a role for regulatory T cells (T reg cells) in helminth infection. In human studies, peripheral T cells from infected patients are nonresponsive to parasite antigens, but responses can be restored by antibodies to IL-10 and TGF-β (26). Moreover, T cell clones from Onchocerca volvulus–infected patients show antigen-specific IL-10 production and release of TGF-β characteristic of T reg cells (25, 30). Our most recent studies have demonstrated the expansion of regulatory phenotype T cells in the mouse model filarial infection Litomosoides sigmodontis, and the killing of parasites in animals given antibodies to T reg cell surface marker proteins (31).
New approaches to allergy intervention has focused on restoring regulation. Notably, there is a case building for T reg cells controlling allergic airway inflammation (13, 15, 32–35). For example, T cells that were transfected with IL-10 or TGF-β to confer regulatory function (36, 37) prevent allergic airway inflammation (38). Therefore, the emergence of pathogen-induced T cell regulation together with new concepts of allergy control by T cells provides a feasible model for helminth suppression of allergy.
The data from human studies provide an enticing scenario in which helminth infections keep allergies at bay, but such epidemiological inferences must be tested experimentally if we are to distinguish cause from consequence in these complex immunological settings. Here, we investigate the host immune response at the convergence of these two immunological challenges, which as we show, interact in a dramatic manner. We test allergic airway inflammation, induced by either OVA in the BALB/c strain of mouse or Der p 1 in the C57BL/6 mouse, in mice infected with the intestinal nematode Heligmosomoides polygyrus that elicits a strongly Th2 cell–biased systemic response (39, 40). This parasite has been reported to down-regulate allergies to dietary antigens (28), as well as other intestinal pathologies (41, 42). By studying airway allergy in our experiments, we can exploit the fact that H. polygyrus remains entirely within the gastrointestinal tract, and test the immunological intersection between helminth infection and allergic reactivity in two different locales.
Our data show helminth-mediated protection from airway inflammatory responses in both OVA and Der p 1 models. Helminth-driven suppression of effector functions, downstream of allergen sensitization, is responsible for protection from airway inflammation, as down-regulation can be transferred from infected mice to uninfected, presensitized animals by mesenteric LN cells (MLNC). Furthermore, protection was most strongly associated with CD4+CD25+ T cells, which is consistent with the hypothesis that parasite-induced regulatory T cells can down-modulate Th2 allergic inflammation.
Results
Significantly reduced airway inflammation in H. polygyrus–infected animals
We first tested allergic reactivity in the airways of animals harboring chronic H. polygyrus infections, having established that no changes in airway cell composition or bronchoalveolar lavage fluid (BALF) cytokine secretion in mice occurred as a result of parasite infection per se (unpublished data). Mice were sensitized twice with allergen, at day 28 and 42 of infection, before airway challenge at day 56 and 58. Recovery of airway cellular infiltrates in BALF was performed on day 59, at which time infection status was also evaluated by detection of intestinal adult worms.
Infected mice were found to have significantly reduced airway cellular infiltrates after challenge with OVA (BALB/c, P < 0.001) or Der p 1 (C57BL/6, P < 0.003) (Fig. 1 A). Differential counting of cells recovered revealed a profound reduction of airway eosinophilia (Fig. 1 B, P < 0.0005) and neutrophilia (Table I). Infected BALB/c mice showed 81.6 and 66.7% decreases in airway eosinophils and neutrophils, respectively, 24 h after final OVA challenge (Table I). Similarly, C57BL/6 mice had reduced airway eosinophils (89.0%) and neutrophils (29.0%) 24 h after final challenge with Der p 1, compared with uninfected controls. Macrophage and lymphocyte numbers were also reduced in infected mice after airway challenge (Table I), although the changes did not reach statistical significance.
Figure 1.
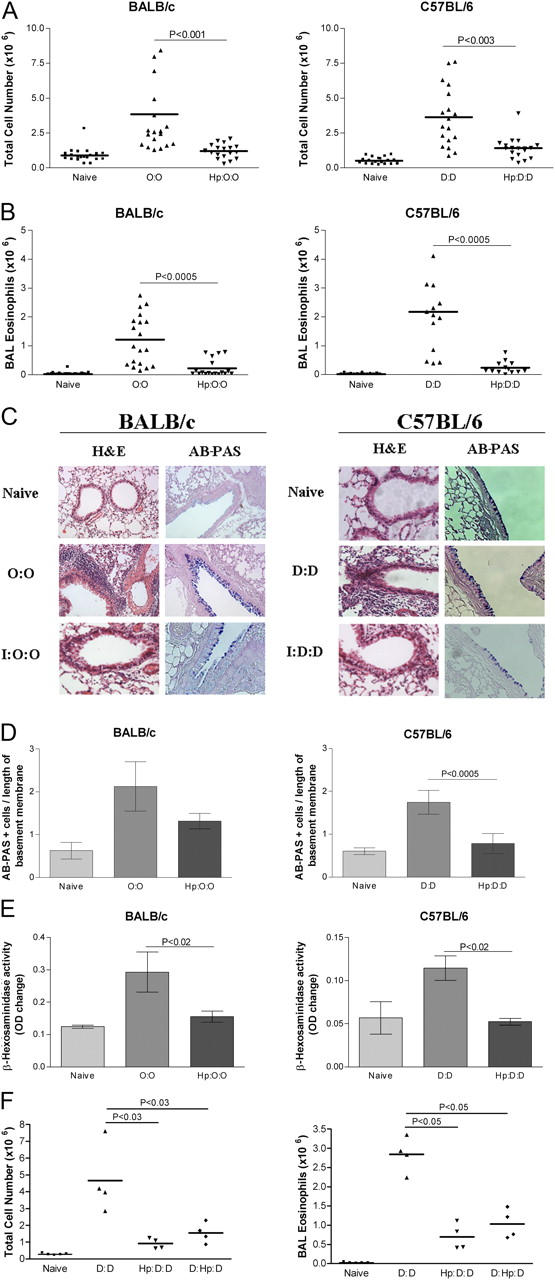
Airway cell infiltration and tissue pathology are inhibited in H. polygyrus-infected mice. BALB/c or C57BL/6 mice were sensitized to OVA or Der p 1, respectively, by two i.p. injections of 10 μg of allergen adsorbed to Alum; 14 and 17 d after the second sensitization, mice were given soluble allergen by direct tracheal inoculation. Lavage cells were recovered 24 h after final airway challenge (day 31), and histological sections made of lung tissues. O:O denotes OVA sensitized (day 0 and 14), and challenged (day 28 and 30). D:D denotes Der p 1 sensitized (day 0 and 14), and challenged (day 28 and 30). Cells were harvested at day 31. Hp:O:O and Hp:D:D are mice infected with H. polygyrus 28 d before allergen sensitization; D:Hp:D are mice infected 14 d after sensitization, but before challenge. (A) Total lavage cell numbers. Data represent means and individual data from four experiments (18–20 mice per group) using the same protocol. Horizontal bars denote paired experimental groups for which statistical significance is displayed in the figure. (B) Eosinophil numbers. All four experiments displayed significant reductions in airway eosinophilia in infected-allergen challenged animals compared with uninfected allergen challenged littermates. (C) Formalin-fixed lung 5-μm sections from naive, allergic, and infected-allergic mice stained with hematoxylin and eosin (H&E) for nuclear staining of infiltrating cells or stained with AB-PAS for mucin-producing goblet cell numbers. (D) Enumeration of goblet cells, stained with AB-PAS in the three groups of mice. Data represent mean and SEM of five mice, with a mean score from three representative lung sections per mouse. (E) β-Hexosaminidase in BALF of allergic and infected mice. (F) Total cells and eosinophils in mice infected before or after allergen sensitization.
Table I.
Total cell counts in BALF
| Cell Type (×106 ± SD) |
Eosinophils | Neutrophils | Macrophages | Lymphocytes | |
|---|---|---|---|---|---|
| BALB/c | Naive | 0.028 ± 0.014 | 0.089 ± 0.011 | 0.646 ± 0.089 | 0.104 ± 0.025 |
| O:O | 1.054 ± 0.175 | 1.265 ± 0.349 | 0.909 ± 0.275 | 0.236 ± 0.042 | |
| Hp:O:O | 0.224 ± 0.062 | 0.421 ± 0.050 | 0.410 ± 0.053 | 0.144 ± 0.027 | |
| C57BL/6 | Naive | 0.018 ± 0.005 | 0.073 ± 0.016 | 0.460 ± 0.026 | 0.064 ± 0.011 |
| D:D | 1.639 ± 0.312 | 1.591 ± 0.343 | 0.394 ± 0.058 | 0.339 ± 0.059 | |
| Hp:D:D | 0.239 ± 0.045 | 1.129 ± 0.236 | 0.371 ± 0.041 | 0.188 ± 0.024 | |
O:O denotes OVA sensitized (day 0 and 14) and challenged (day 28 and 30). D:D denotes Der p 1 sensitized (day 0 and14) and challenged (day 28 and 30). Cells were harvested at day 31. Hp:O:O and Hp:D:D are mice infected with H. polygyrus 28 d before allergen sensitization.
Reduced tissue pathology in infected mice
To determine whether suppressed airway cellular infiltration represented a more general down-modulation of pathology, lung histological sections were compared in allergic and infected-allergic animals. Hematoxylin and eosin staining was used to characterize cellular infiltrates, and Alcian blue–periodic acid schiff (AB-PAS) to identify mucus-producing goblet cells in the epithelial border. In addition, mast cell degranulation was estimated by measuring levels of β-hexosaminidase, in BALF.
In uninfected, allergen-sensitized mice of both strains, airway challenge leads to a dense peribronchiolar inflammatory infiltrate of lymphocytes, and mononuclear and polymorphonuclear cells with epithelial shedding and extended columnal cells (Fig. 1 C). Furthermore, an accumulation of mucin-containing goblet cells line the connecting airways, underpinning the overall increase in mucus production. In infected mice, however, tissue inflammation after OVA or Der p 1 challenge was greatly reduced (Fig. 1 C) with significantly less peribronchial and perivascular cellular infiltration and mucin staining. Goblet cell numbers showed a substantial reduction in infected mice (Fig. 1 D, P < 0.0005), whereas the allergen-induced increase in BALF β–hexosaminidase, indicating mast cell mediator release, was also attenuated in infected mice (Fig. 1 E).
To distinguish whether infection interfered with allergen priming or suppressed overt allergic reactions, mice were infected with H. polygyrus 14 d after the second sensitization with allergen. As shown in Fig. 1 F, animals infected subsequent to sensitization showed a similar suppression of airway allergy to mice infected at the time of first allergen exposure.
Thus, chronic H. polygyrus infection protects mice against a range of allergic airway inflammatory pathologies, including fluid and tissue infiltration, mast cell degranulation, and goblet cell proliferation. We selected BAL infiltration as the keynote parameter to dissect the immunological mechanisms leading to this broader diminution of pathology.
Suppression of local type 2 effector cytokines
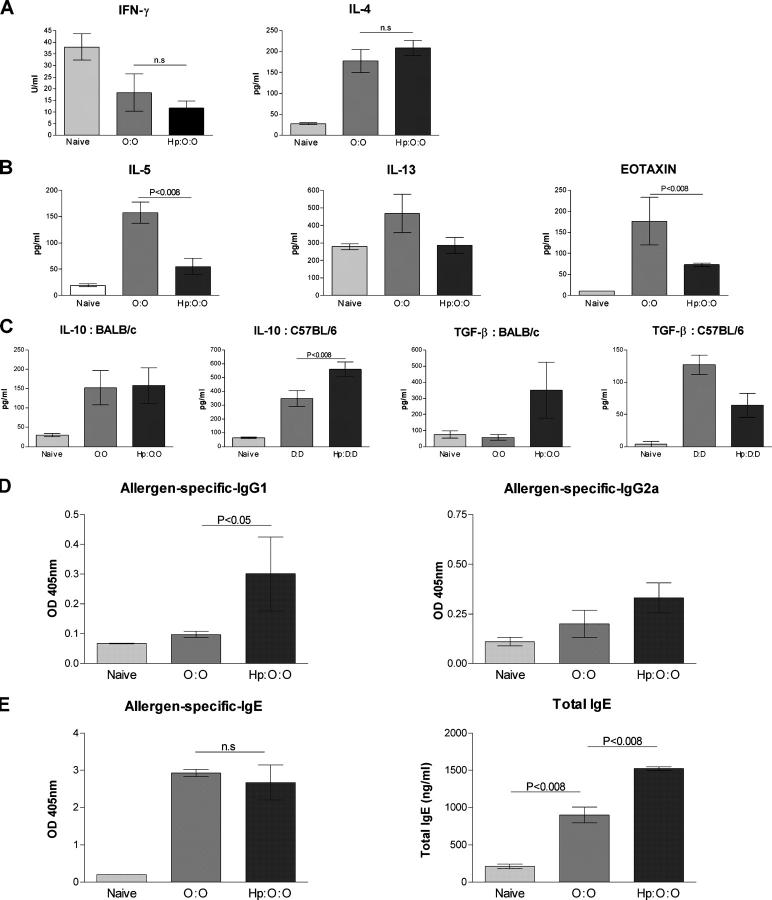
Local cytokine and chemokine levels were measured in the BALF, 24 h after the final allergen challenge in vivo. Infected mice did not show diminished IL-4 responses, while the Th1 cell signature cytokine IFN-γ was undetectable in both infected and uninfected mice (Fig. 2 A). The dominance of Th2 cytokines over Th1 was also observed at equivalent levels in allergen-challenged thoracic LN (TLN) cells from both groups (unpublished data). These data indicate that infection does not alter the highly polarized type 2 cytokine environment that is associated with airway allergy.
Figure 2.
Bronchio-alveolar lavage cytokine responses. BALF from naive, allergic, and infected-allergic mice assayed for the indicated cytokines and eotaxin. Data are from individual mice, with arithmetic mean points shown in histograms. In cases where responses are similar in BALB/c and C57BL/6 mice, data are shown only from the BALB/c response to OVA. (A) IFN-γ and IL-4, in BALB/c mice; (B) IL-5, IL-13, and eotaxin in BALB/c mice; (C) IL-10 and TGF-β in BALB/c and C57BL/6 mice. (D) Allergen- specific serum IgG1 and IgG2a titers 1 d after the final airway challenge in BALB/c mice. (E) Allergen-specific IgE and total IgE levels 1 d after final airway challenge in BALB/c mice.
The type 2 effector cytokine IL-5, and the chemokine eotaxin, were both elevated in the BALF of uninfected BALB/c mice after airway challenge (Fig. 2 B). However, IL-5 and eotaxin were significantly diminished by infection (P < 0.008). Similar data were obtained for C57BL/6 mice (IL-5 reduced from 803.6 ± 201.6 to 80.5 ± 29.4 pg/ml, and eotaxin from 434.4 ± 131.1 to 92.1 ± 13.7 pg/ml). Reductions in these two key agents in the mobilization and extravasation of eosinophils provides a mechanistic explanation for the dramatically reduced airway eosinophilia in infected mice. Although IL-13 levels altered less, they were maximal in uninfected allergic mice with the most marked goblet cell hyperplasia.
We also examined the regulatory cytokines IL-10 and TGF-β (Fig. 2 C). The trend for higher IL-10 in infected animals, only reached statistical significance in C57BL/6 mice (P < 0.08). In some individual BALB/c mice, the levels of active TGF-β in BALF were elevated, suggesting that this mediator may play a major part in immune regulation during infection. Overall, the two strains appear to differ in prominence of IL-10 (in C57BL/6) versus TGF-β (in BALB/c), and studies are now under way to evaluate the roles of each cytokine in the two genetic backgrounds for infection and allergy.
Antibody isotype responses in infection and allergy
Helminth infections can stimulate a type 2 antibody response (IgG1 and IgE) both to the parasite and to bystander antigens (43, 44). We therefore measured allergen-specific IgG1 and IgG2a isotype responses in BALB/c mice to OVA allergen. It was found that IgG1 increased significantly in infected mice (Fig. 2 D, P < 0.05), which is consistent with the reported high IL-4 environment (45). Less expectedly, IgG2a also rose, although without reaching statistical significance.
We next established that allergen-driven IgE responses were not compromised in helminth-infected mice (Fig. 2 E). This observation, together with the finding that allergy is suppressed in animals infected after sensitization (Fig. 1 F), confirms that Th2 cell priming is not ablated by the parasite infection. In fact, infected animals had greatly elevated polyclonal IgE titers compared with uninfected animals after airway challenge. Elevated polyclonal IgE is symptomatic of most helminth infections, and has been posed as a mechanism of escape from allergy, out-competing parasite-specific IgE for binding sites on mast cell IgE receptors (19). We show, using a transfer experiment (see “Transfer of protection…”), that this hypothesis is not supported in this model system.
Anti-CD25 antibodies block suppression
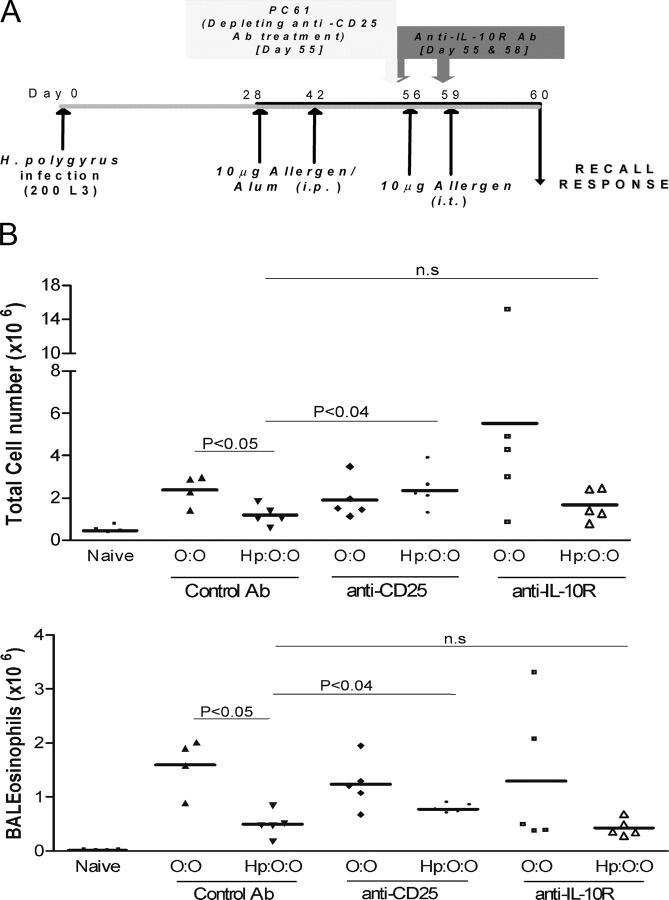
Allergic responses can be down-modulated by the action of T reg cells, through suppressive mediators such as TGF-β (46) or IL-10 (47). Many T reg cell populations constitutively express the IL-2Rα chain, CD25, and such cells can be depleted in vivo using anti-CD25 (PC61) monoclonal antibody (48), with the caveat that activated CD25+ effector cells may also be affected. Thus, enhanced responsiveness after CD25+ cell depletion is likely to reflect loss of T reg cell activity, whereas diminished responses may result from effector cell depletion. In addition, IL-10 is considered to exert a major influence on airway allergy, and its overexpression can suppress airway inflammation (36). The action of IL-10 family cytokines can be ablated in vivo by injection of antibody to the IL-10R (49).
We administered anti-CD25 antibody to infected and uninfected OVA–sensitized mice 1 d before airway challenge (Fig. 3 A), and found that airway infiltration was restored in infected mice (Fig. 3 B). However, recipients of anti–IL-10R antibody showed unchanged levels of airway infiltration and eosinophilia (Fig. 3 B). Importantly, antibodies to CD25 or IL-10R in uninfected-allergic mice had no significant effects on airway infiltrates. These studies suggest that a cellular population expressing CD25 may be responsible for the suppression of airway infiltration, but that IL-10 signaling during airway provocation is not responsible for the observed protection from allergy.
Figure 3.
Anti-CD25 and anti–IL-10R antibody intervention in infected and allergic mice. (A) Protocol for treatment with anti-CD25 and anti–IL-10R antibodies. Naive or chronically infected mice were sensitized as previously described in “Significantly reduced airway inflammation…”; mice received 1 mg of isotype control, PC61 (anti-CD25), or 1B1.3a (anti–IL-10R) i.p. 1 d before airway challenge on day 56. Anti–IL-10R was also administered on day 58, 1 d before a final airway challenge on day 59. The experiment was terminated on day 60. (B) Total cell counts and eosinophil numbers in BALB/c mice treated with isotype control, and anti-CD25 or anti–IL-10R mAbs. In control Ab-treated animals, both total (P < 0.05) and eosinophil infiltration (P < 0.05) in H. polygyrus–infected mice were significantly reduced, using Student's t test. In PC61 treated mice, uninfected and infected groups were not statistically different.
Transfer of protection against allergy with MLNC from infected mice
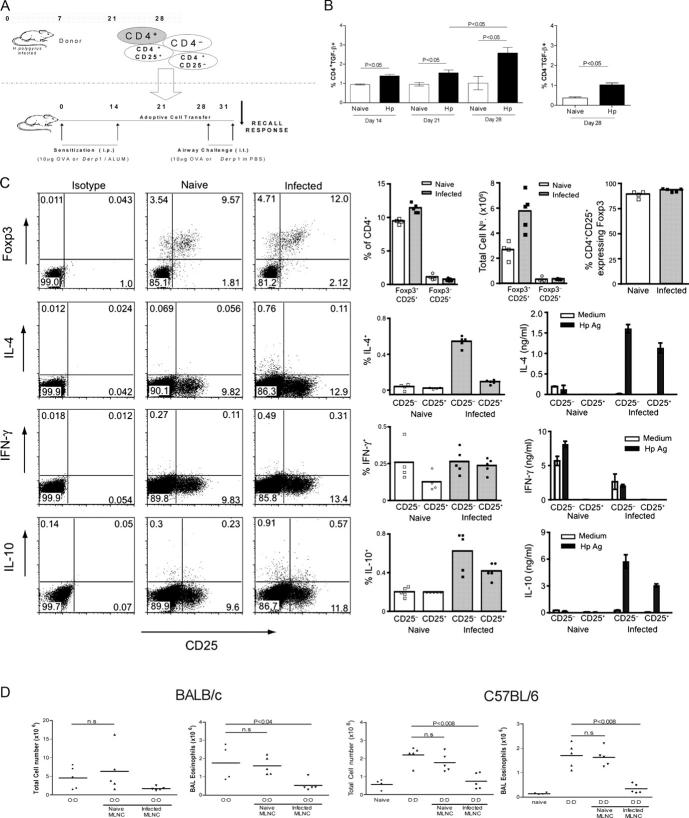
To investigate whether a defined cellular population that was generated during chronic infection is capable of suppressing airway inflammation, we adoptively transferred MLNC taken 28 d after H. polygyrus infection (Fig. 4 A). MLNC at d 28 were found to express elevated levels of the T reg cell–associated cytokine TGF-β (Fig. 4 B) as well as the transcription factor, Foxp3, and the proportion of CD4+CD25+ MLN T cells from infected mice that were Foxp3+ was consistently >85% (Fig. 4 C). Foxp3 expression was also determined by RT-PCR: CD4+ MLNC from 28-d infected mice contained more than fivefold higher levels of Foxp3 mRNA than similar cells from naive animals. Moreover, within the CD4+ T cell population, levels were maximal in the CD25+ cell fraction (0.88 relative to β-actin), whereas Foxp3 mRNA was below threshold values in the CD25− population. CD4+CD25+ cells contained relatively little IL-4 or IFN-γ by cytoplasmic staining. Both CD25+ and CD25− subsets responded to parasite antigen challenge with IL-10 release, with the CD25− population making the larger contribution (Fig. 4 C). Day 28 MLNC were therefore transferred into the tail veins of uninfected, allergen-sensitized recipient mice. After a further 7 d, recipient mice were given the first of two airway challenges, and airway responses were measured 1 d after the final challenge (Fig. 4 A).
Figure 4.
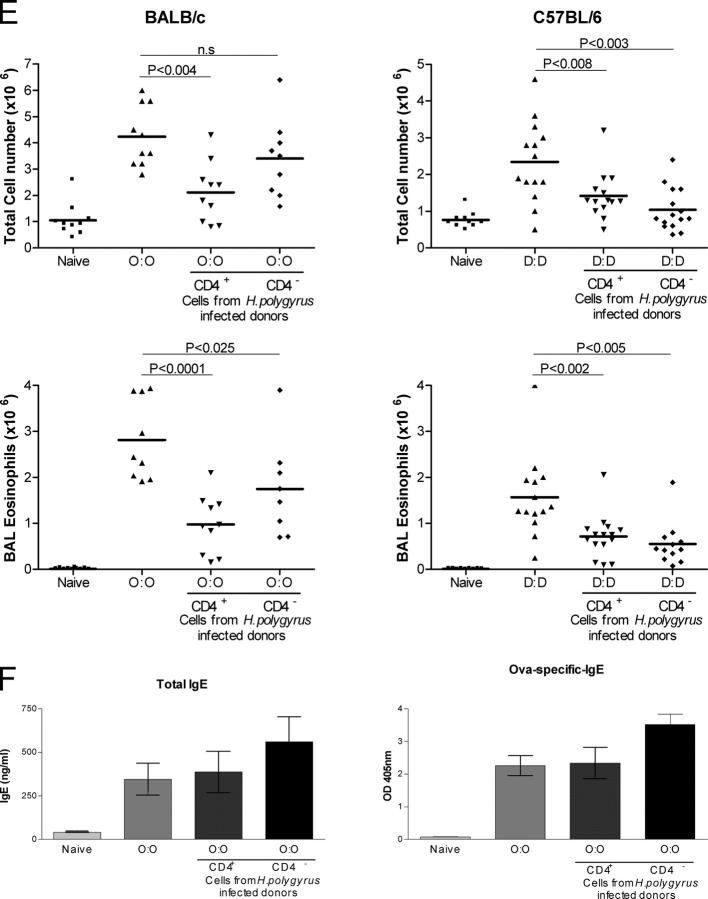
Transfer of protective effect with MLNC. (A) Protocol for transfer. All MLNC populations were harvested from donor mice 28 d after infection with H. polygyrus and transferred to recipients 7 d before airway challenge. (B) TGF-β expression in MLNC from naive and infected mice assayed by flow cytometry at day 14, 21, and 28 after infection with monoclonal anti–TGF-β1. Error bars show the mean ± SEM. (C) Expression of Foxp3 transcription factor and IL-4, IFN-γ, and IL-10 by CD25+ and CD25− MLNC from naive and 28-d-infected mice. For intracellular staining of Foxp3 and cytokines, representative individual FACS plots are shown against CD25 staining, together with a summary of percentage of positive cells taken from groups of five mice. The total number of CD25+ MLNC expressing Foxp3 in naive and infected mice is presented, together with the frequency of these cells within the whole CD4+ T cell population and the proportion of CD25+ cells that express Foxp3. Antigen-specific cytokine release was measured by ELISA from supernatants of 72-h cultured CD25-depleted or -enriched MLNC stimulated with medium or antigen extract from H. polygyrus adult parasites. (D) Total cell counts and eosinophil numbers in mice receiving 5 × 107 MLNC from naive or infected donors. In the experiment with BALB/c mice, 100 μg of soluble adult parasite antigen was given 1 d after administration of either naive or infected donor cells. (E) Total cell counts and eosinophil numbers in mice receiving 4 × 106 CD4+ or CD4− MLNC from infected donors. Cell fractions were 92.4% (CD4+) and 94.9% (CD4−) pure for BALB/c and 93.1% (CD4+) and 98.1% (CD4−) pure for C57BL/6. Group sizes were 8–10 for BALB/c and 15 for C57BL/6 mice. (F) Total IgE and allergen-specific IgE, measured in IgG-depleted serum, measured in BALB/c mice receiving 4 × 106 CD4+ or CD4− MLNC from infected donors. Serum samples were collected from blood 1 d after final airway challenge. (E) Total cell counts and eosinophil numbers in mice receiving 4 × 106 CD4+ or CD4− MLNC from infected donors. Cell fractions were 92.4% (CD4+) and 94.9% (CD4−) pure for BALB/c and 93.1% (CD4+) and 98.1% (CD4−) pure for C57BL/6. Group sizes were 8–10 for BALB/c and 15 for C57BL/6 mice. (F) Total IgE and allergen-specific IgE, measured in IgG- depleted serum, measured in BALB/c mice receiving 4 × 106 CD4+ or CD4− MLNC from infected donors. Serum samples were collected from blood 1 d after final airway challenge.
In the first instance, 5 × 107 MLNC from infected or naive donor mice were transferred i.v. into allergen-sensitized BALB/c or C57BL/6 mice. MLNC from infected donors could transfer the protective effect to uninfected sensitized recipients, but naive MLNC induced no marked changes in response. Substantial declines in eosinophil infiltration were observed in mice receiving infected MLNC, compared with those receiving naive MLNC, after allergen challenge. Naive MLNC had no effect on total airway infiltrates or eosinophilia after allergen challenge. Suppression could be observed with as few as 107 MLNC from chronically infected donors (data not shown). Transfer of MLNC from chronically infected C57BL/6 mice into Der p 1–sensitized recipients obtained similar results, with significantly reduced total (Fig. 4 D, P < 0.008) and eosinophil (P < 0.02) infiltrates. Der p 1-sensitized C57BL/6 mice showed alleviation of airway infiltration when given MLNC from infected donors, whether or not recipient animals were given 100 μg H. polygyrus antigen 1 d later (unpublished data). From these experiments, MLNC were shown not to require antigen reexposure to transfer the protective effect to allergen-sensitized recipients.
Transfer of protection with CD4+ T cells
CD4+ and CD4− cells were isolated from MLNC of d 28 infected animals and transferred into uninfected sensitized recipients. OVA-sensitized BALB/c mice displayed a significant reduction in total airway infiltrates after CD4+ cell transfer and airway challenge (Fig. 4 D). Suppression of airway eosinophilia was observed after the transfer of CD4+ (P < 0.0001) or CD4− (P < 0.025) cells, respectively. These results from the BALB/c model demonstrate that CD4+ T reg cells, engendered during a chronic H. polygyrus infection, can transfer protection against allergy to an allergen-sensitized animal if given before the time of airway challenge.
The regulatory activity may not, however, be limited to a single cellular population. In C57BL/6 mice sensitized to Der p1, transfer of either CD4+ or CD4− cells resulted in reduced airway infiltrates and eosinophilia, and in some cases, (Fig. 4 E) the CD4− population showed greater potency. Because the two regulatory populations evident in infected C57BL/6 mice develop in the absence of allergen sensitization, it is likely that the prominence of CD4− activity in this strain relative to BALB/c reflects a genetic difference rather than the choice of allergen used in the two systems.
Unaltered IgE responses accompany suppressed allergy
The transfer of CD4+ cells from infected animals to uninfected, sensitized mice (Fig. 4 F) did not alter total or allergen-specific IgE levels in recipient mice. This finding supports the thesis that a cellular population that was engendered during helminth infection is the major contributor to overall protection from allergic reactivity, and although increased levels of total IgE may play a role in diluting allergen-specific IgE and/or occupy FcɛR on mast cells and basophils, it is not the major component of the protective effect observed.
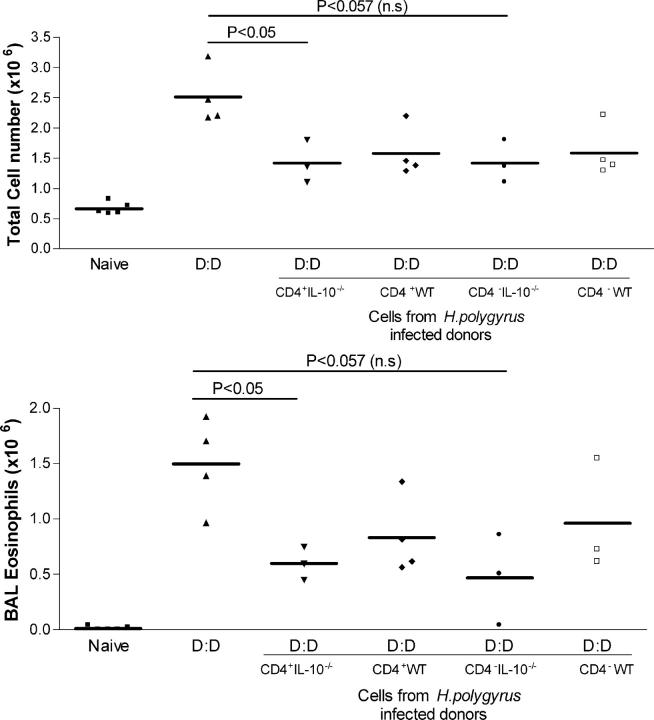
IL-10−/− MLNC transfer protection
CD4+ and CD4− cells were isolated from MLN of chronically infected IL-10–/– mice and transferred into uninfected sensitized wild-type recipients. As shown in Fig. 5, the lack of IL-10 did not compromise the ability of transferred CD4+ cells to suppress allergic reactions. Recipients of IL-10–/– CD4+ cells showed significantly reduced airway infiltration and eosinophilia (P < 0.05) compared with animals receiving wild-type CD4+ cells. Transfer of CD4− cells from either IL-10–/– or wild type showed a diminution of allergic outcome that narrowly failed to show statistical significance. Hence, IL-10 is not an essential component of the pathway by which helminth-induced cells can suppress host allergic reactivity.
Figure 5.
Suppression of allergy by MLNC transfer is intact in IL-10–deficient donors. Total cell counts and eosinophil numbers in Der p 1-sensitized WT C57BL/6 mice receiving 4 × 106 CD4+ or CD4− MLNC from WT or IL-10–deficient infected donors. At day 7 and 9 after cell transfer, airway challenges were given, and BALF harvested 24 h after final challenge.
CD4+CD25+ T cells transfer protection
To test directly whether infection induces CD25+ T reg cells, day 28 MLNC from infected mice were separated into CD4+CD25+– and CD4+CD25−–enriched populations, and transferred into allergen-sensitized recipients 7 d before airway challenge (Fig. 4 A). In both strains of mice, the most puissant reduction of airway inflammation occurred with the transfer of CD4+CD25+ cells. CD4+CD25+ cell transfer reduced not only total airway infiltrates (Fig. 6) but also airway eosinophilia (BALB/c, P < 0.02; C57BL/6, P < 0.02), and goblet cell proliferation (BALB/c, P < 0.05). Evidence was also found that the CD4+CD25− subset also contributes to an abatement of allergic reactivity, but not as consistently as CD4+CD25+ cells. Overall, our data suggest that the CD4+CD25+ phenotype T cell population, at least, can mediate suppression of airway allergy in both strains of mice.
Figure 6.
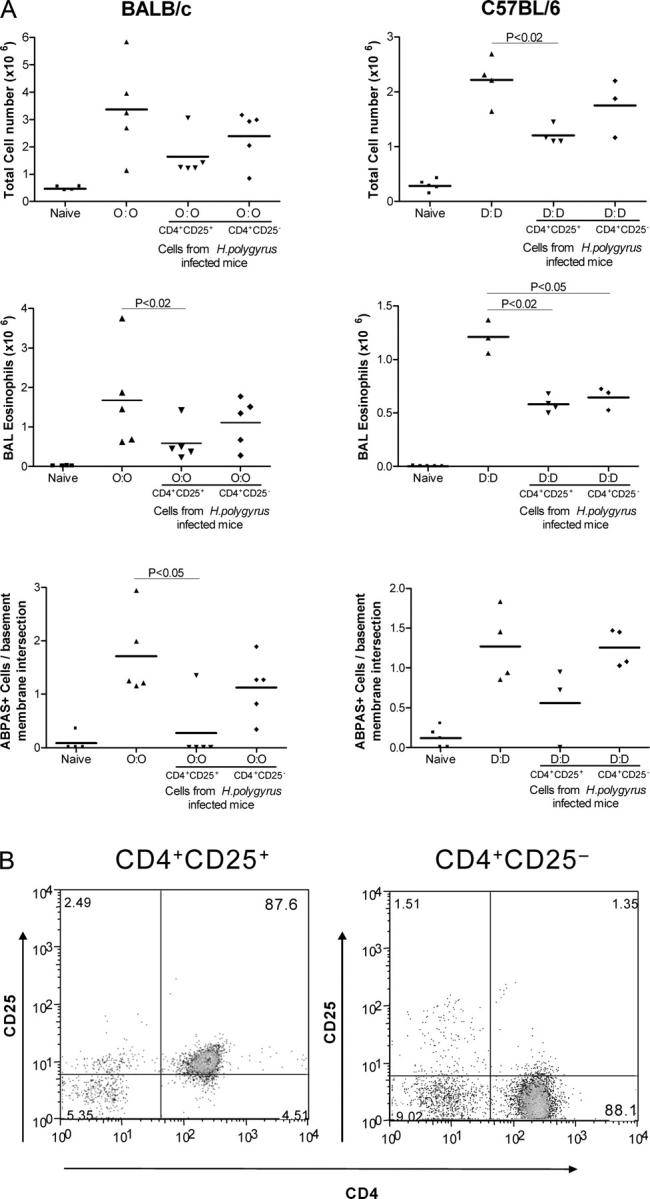
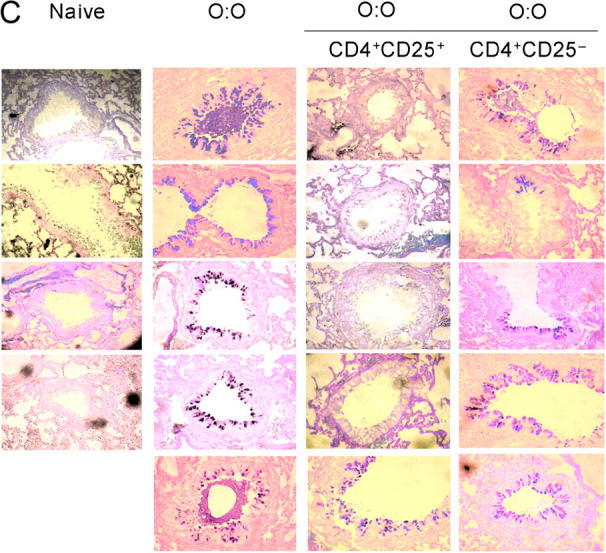
Transfer of protective effect with CD4+CD25+ MLNC. (A) Total cell, eosinophil, and goblet cell numbers in mice receiving 3 × 105 CD4+CD25+ or CD4+CD25− MLNC from infected donors. (B) Purity of CD4+CD25+ and CD4+CD25− populations assayed by flow cytometry. C57BL/6 MLNC fractions, which were 87.6% (CD4+CD25+) and 88.1% (CD4+CD25−) pure, are shown. Enriched cell fractions from BALB/c were 60.6% (CD4+CD25+) and 79.6% (CD4+CD25−) pure. (C) AB-PAS staining of formalin-fixed lung tissue, staining mucin- producing goblet cells, in BALB/c mice receiving 3 × 105 CD4+CD25+ or CD4+CD25− MLNC from infected donors.
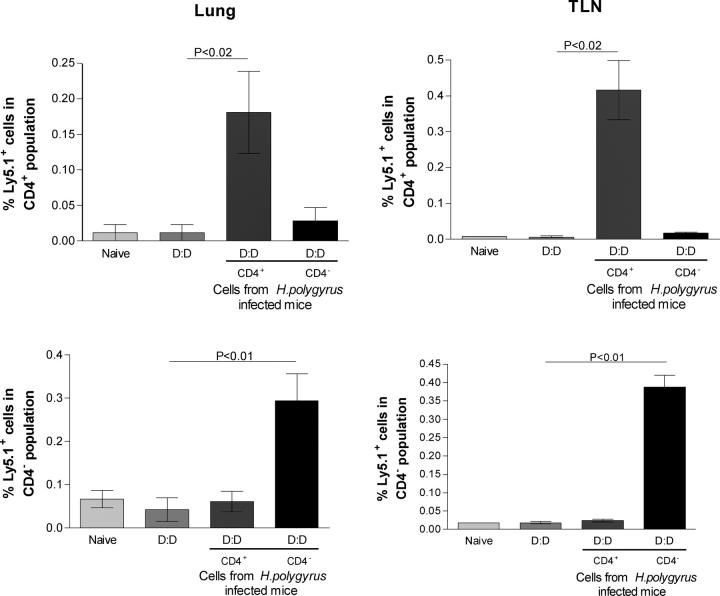
Migration of donor MLNC to the lungs and TLNs
To investigate whether transferred MLNC are capable of relocating to airway-associated tissues in recipient sensitized mice, we followed the fate of transferred cells bearing the Ly5.1 allotypic marker, which permits donor cell identification after transfer into C57BL/6 (Ly5.2) mice. CD4+ and CD4− Ly5.1 MLNC transferred i.v. into sensitized C57BL/6 mice 7 d before airway challenge could be found in the local draining LNs, composing 0.42 ± 0.18% and 0.39 ± 0.07% of the CD4+ and CD4− population, respectively (Fig. 7). Similarly, 0.18 ± 0.10% and 0.30 ± 0.14% of lung CD4+ and CD4− cells were Ly5.1+. These data suggest that donor cells from chronically infected mice migrate to sites of inflammation, either to directly suppress host responses, or to conscript resident cells into a regulatory phenotype.
Figure 7.
Tracking of donor lymphocytes in recipient mice. 4 × 106 CD4+ or CD4− MLNC from infected mice were transferred i.v. into C57BL/6 (Ly5.2) recipients, 7 d before airway challenge. After the second airway challenge, lungs and thoracic lymph node (TLN) cells were recovered and stained for Ly5.1 and CD4 expression. Error bars show the mean ± SEM.
Discussion
Allergies are Th2 cytokine–mediated pathologies, involving IL-4–, IL-5– and IL-13–dependent amplification of innate effector cell populations acting together with antibodies and inflammatory mediators (50). As Th1 and Th2 cell responses are mutually antagonistic, it has been argued that declining microbial stimulation of Th1 cell responses in the developed world has led to over-vigorous allergic Th2 cell reactions (51). However, allergen-specific Th1 cells can in fact exacerbate airway inflammation (52). Moreover, epidemiological reports increasingly link Th2 cell–inducing helminth parasite infections with reduced allergic disease in humans (53), whereas nematode-infected mice display attenuated allergic responses (28, 54, 55). Current theories postulate that pathogen-induced T reg cells control both Th1 and Th2 effector populations (2, 3, 11, 16, 27, 53). The possibility that T reg cells inhibit allergic disease has received growing support from both animal (13, 56) and human (14, 15, 57) studies.
We now provide direct experimental evidence to support the hypothesis that helminth infection down-regulates allergic reactions through the action of regulatory T cells rather than by altering the Th1–Th2 balance. To do this, we selected the murine intestinal nematode H. polygyrus as a model of helminthiasis (28, 58), because it follows a purely enteric infective cycle and establishment; has the ability to establish stable, chronic infections; and evokes a Th2 cell–dominated immune response similar to that observed in general with gastrointestinal nematodes. We chose an allergic airway inflammation model that permited us to observe modifications in cellular recruitment, cytokine and chemokine expression, and tissue pathology. We also opted to study two allergens in parallel, which induce airway inflammation in different strains of mice: the environmental house dust mite allergen Der p 1, in C57BL/6 mice; and the experimental allergen, OVA in BALB/c mice.
Mice carrying an established helminth infection are shown here to be less prone to allergic airway inflammation, whether measured by the inflammatory infiltrate in BALF or by peribronchial and perivascular inflammation. Our results extend earlier work with H. polygyrus that demonstrate a reduced allergy to food antigens in mice infected with this parasite (28). More broadly, these findings can be translated to the human situation, in which individuals carrying active helminth infections appear, in epidemiological studies, to be less responsive to allergen provocation than coresidents free of infection (17, 20, 21, 59, 60).
To address the mechanisms involved in helminth-induced suppression, we first established that the Th2-driving cytokine IL-4 was unaffected, as was the overall Th1–Th2 balance. However, the Th2 effector cytokines IL-5 and IL-13 were diminished, as was the inflammatory phase within the lung. Thus, Th2 cell–associated pathological reactions were suppressed within the context of a Th2 cell–inducing helminth infection. The fact that Th2 cell priming occurs normally is shown by the similar allergen-specific IgE responses in infected and uninfected mice, and the ability of the suppressive mechanism to target the effector phase is further demonstrated by the impact of infection on a previously sensitized allergy-prone host (Fig. 1 F). The hypothesis that T reg cell activity may be responsible for this phenomenon was supported by our finding that anti-CD25 antibody treatment reversed suppression. However, allergic inflammation was not restored by the administration of anti–IL-10R antibody, indicating that T reg cell activity in this setting is not IL-10 dependent.
We then used the adoptive transfer system of MLNC from infected mice into sensitized, but uninfected, hosts to demonstrate that down-regulation is mediated by T cells, primarily those with the CD4+CD25+ phenotype associated with T reg cells (61, 62). Several distinct regulatory phenotypes of T reg cell cells have been proposed, including “Th3” cells, primarily acting through TGF-β, which are most closely associated with the gut mucosal environment, and “Tr1” cells, which are capable of suppressing airway allergy through the action of IL-10 (13, 56). Interestingly, the H. polygyrus-elicited T reg cells are generated in the Th3 environment, but manifest their function in the Tr1 environment.
Although the CD4+CD25+ subset was the most potent at suppressing allergic inflammation, some activity could also be observed in the CD4− population, particularly in the C57BL/6 system. Although this may represent a CD8+ regulatory T cell (63), we also have evidence that CD19+ B cell populations are able, on transfer, to exert a dampening effect on immunopathology. Perhaps, in the down-regulatory milieu of a chronic infection, additional non–T reg cell types are recruited to curtail pathology more completely.
The cells transferred from infected mice express elevated levels of IL-10 and TGF-β, the two principal T reg cell–associated down-regulatory cytokines, and contain a significantly higher number of CD4+CD25+Foxp3+ cells. However, despite the prominence of IL-10 production by H. polygyrus-exposed T cells, their ability to suppress airway allergy is not mediated by IL-10, as MLNC from infected IL-10–/– mice can mediate suppression, which is consistent with the failure of anti–IL-10R antibodies to reverse suppression in infected animals. In contrast, TGF-β remains a strong candidate for immune suppression by T reg cells from helminth-infected mice, particularly as this cytokine is known to alleviate experimental airway allergy (37) and has the capacity to instruct peripheral T cells to develop regulatory capacity (46, 64).
CD4+CD25+ T cells are generally associated with self-reactive regulatory cells that prevent autoimmune reactivities (61, 62). Hence, the expansion of CD4+CD25+ cell–mediated regulatory activity in infection raises the issue of the origin and antigen specificity of these cells (65, 66). Are there preexisting “natural” T reg phenotype cells that expand in response to infection, or can parasite antigen-specific regulatory cells arise from naive Th0 precursors? In this respect, the fact that transfer of suppression did not require renewed antigen stimulation was striking. One possibility is that the T reg cell population is self-reactive rather than parasite specific, and is restimulated by ligands in the recipient host. However, it has been shown that T reg cells specific for exogenous antigen (alloantigen or HGG), reactivated in a donor immediately before transfer, suppressed bystander allograft responses in a recipient without the need for antigen restimulation in the new host (67). Thus, it is possible that chronic parasite infection maintains a high level of activation in T reg cells, sufficient for their function in our short-term experiments. As with other instances of T reg cell responses to pathogens (66), these issues are currently under active investigation.
The transfer model also allows us to exclude changes in antibody production as a major mechanism for abatement of allergy. For example, the production of allergen-specific-IgE is comparable in mice who have received naive or infected MLNC, which show respectively normal and suppressed allergic reactions. There is also no rise in polyclonal IgE in recipients of infected CD4+ T cells, arguing that changes in either absolute allergen-specific IgE, or in the ratio of nonspecific to specific IgE, are not responsible for the diminution of allergic responses in infected animals. A similar conclusion was drawn from measurements of specific and total IgE in atopic and nonatopic humans harboring chronic schistosome infections (21).
A final intriguing question is why helminth parasites such as H. polygyrus and Schistosoma mansoni (68) induce T reg cells. As argued elsewhere (27), parasites that can exploit host down-regulatory networks are likely to gain advantage in the battle for long-term survival in the host. Much of the pathology encountered in helminth infection is immune mediated (69), and a dampening of responsiveness would not necessarily compromise the host. However, the immune system may have evolved to operate optimally in the regulated environment of infection, and in our more hygienic environment, we are prone to overzealous reactions to innocuous targets, generating the rapidly increasing levels of allergy and autoimmunity being experienced in the developed world.
Materials and Methods
Animals.
Female BALB/c, C57BL/6, and C57BL/6-Ly5.1 mice, 6–8 wk old, were housed in individually ventilated cages licensed under UK Home Office guidelines. At least five mice were used per experimental group. IL-10–deficient mice were a gift from S. Anderton (University of Edinburgh, Edinburgh, UK).
Parasites.
Mice were infected with 200 H. polygyrus L3 larvae (provided in the first instance by J. Behnke, The University of Nottingham, Nottingham, UK) using a gavage tube. Infections were verified by detection of eggs in fecal samples.
Antigens and allergens.
For H. polygyrus antigen, a PBS homogenate of adult worms was centrifuged (13,000 g for 10 min), and the supernatant was passed through a 0.2-μm filter (Millipore). Der p 1, was affinity purified from spent mite medium using 4C1 mAb as previously published (70). Grade V OVA (A5503) was purchased from Sigma-Aldrich.
Allergen-induced airway inflammation.
Mice were sensitized i.p. with 10 μg OVA (BALB/c) or Der p 1 (C57BL/6) adsorbed to 9% potassium alum (Sigma A7167), and boosted with the same antigen 14 d later. On day 28 and 31, mice were challenged with 10 μg OVA or Der p 1 in PBS by the intratracheal route. Mice were killed 24 h after final airway challenge to assess airway inflammation. For histopathology, formalin-fixed lungs were embedded in paraffin and sectioned. Hematoxylin and eosin stained sections were analyzed for airway inflammation and pathological changes. Mucus-containing goblet cells were stained with AB-PAS, and the histological mucus index was quantified for goblet cell hyperplasia (71).
BALF cell counts.
At 24 h after final challenge, mice were terminally anesthetized, the tracheas were cannulated, and internal airspaces were lavaged with 500 μl PBS, followed by two 350-μl washes. Fluids were centrifuged at 1,200 g, and pellets recovered for cellular analysis. Initial 500-μl BALF samples were stored at −80°C for biochemical analyses. Cytospins were prepared by spinning 5 × 105 cells onto poly-l-lysine–coated slides (BDH) followed by Diff Quick (Boehringer) staining. Differential cell counts were performed on a minimum of 200 cells at magnification of 100.
Allergen-specific antibodies and total IgE.
Allergen-specific responses were determined by ELISA. Multisorp (Nunc) plates were coated with 4 μg/ml OVA or Der p 1 in 0.06 M of carbonate buffer, overnight at 4°C. Plates were blocked with 5% BSA (fraction V; GIBCO BRL) for 2 h at 37°C. Sera were diluted in TBS, 0.05% Tween (TBS-T) and added to wells overnight at 4°C. Allergen-specific IgG isotypes were detected with HRP-conjugated goat anti–mouse IgG1 (Southern Biotechnology Associates, Inc.) and anti-IgG2a and ABTS peroxidase substrate (KPL). For allergen-specific IgE assays, protein G–Sepharose beads were used to remove IgG from sera; biotinylated anti–mouse IgE (BD Biosciences), ExtrAvidin–alkaline phosphatase conjugate (Sigma-Aldrich) and pNPP Substrate (Sigma-Aldrich) were then used. Total IgE was measured with anti–mouse IgE capture (BD Biosciences) and biotinylated anti–mouse IgE detection, using a monoclonal IgE standard curve.
Cytokines and chemokines.
Cytokines were measured by ELISA according to suppliers' guidelines. Capture antibodies for IL-4 (11B11, 4 μg/ml), IL-5 (2 μg/ml), IL-10 (4 μg/ml) and IFN-γ (R46A2, 3 μg/ml) were produced in-house or purchased from BD Biosciences. Capture antibodies for IL-13 (2 μg/ml) and eotaxin (0.4 μg/ml) were obtained from R&D Systems. Biotinylated detection antibodies were purchased from BD Biosciences (5 μg/ml IL-4, 2 μg/ml IL-5, 2 μg/ml IL-10, and 0.5 μg/ml IFN-γ) or R&D Systems (0.1 μg/ml IL-13 and 0.4 μg/ml eotaxin. TGF-β was measured with transfected mink lung epithelial cells expressing luciferase under the plasminogen activator inhibitor 1 promoter (72). To assay β-hexosaminidase activity, samples were incubated with 80 μl of substrate solution (1.3 mg/ml p-nitrophenyl-β-d-2-acetamido-2-eoxyglucopyranozide [Sigma-Aldrich] in 0.1 M citrate, pH 4.5). The reaction was stopped by the addition of 200 μl of 0.2 M glycine, pH 10.7, and ODs were read at 405 nm.
Antibodies to CD25 and IL-10R.
Anti-CD25 mAb was PC61 rat anti–mouse IL-2Rα monoclonal, produced in-house from cells provided by F. Powrie (University of Oxford, Oxford, UK), and grown in serum-free media in a cell growth bag (Bio-Vectra). Antibody was purified on protein G–sepharose, dialyzed against PBS, and given i.p. at 10 mg/ml. Rat anti–mouse CD210 (IL-10R) antibody was IgG1 monoclonal 1B1.3a (BD Biosciences).
Adoptive cell transfer.
MLNs were removed from mice infected 28 d earlier with H. polygyrus. Single-cell suspensions in RPMI–0.5% normal mouse serum were made using a cell strainer. For whole MLNC transfers, 1–5 × 107 cells were injected i.v. in PBS. Mice received MLNC 7 d after a second allergen sensitization, which was 7 d before airway challenge.
CD4+ and CD25+ cell enrichment.
For CD4+ cell purification, cell suspensions were incubated with CD4 (L3T4) microbeads (Miltenyi Biotech) and separated on MACS LS columns with preseparation filters; 4 × 106 CD4+ or CD4− cells were injected i.v. into recipient mice. For CD4+CD25+ cell enrichment, CD4+ cells were first negatively isolated using streptavidin microbeads (Miltenyi Biotec) and biotinylated anti-CD11b (BD Biosciences), anti-CD8α (BD Biosciences), anti-MHC class II (M5114) and anti-Igκ (187.1). Antibody-bound beads and cell solutions were separated on MACS LS columns. CD25+ cells were then positively selected with biotinylated anti-CD25 (BD Biosciences) and streptavidin microbeads. Uninfected, allergen-sensitized mice received 3 × 105 CD4+CD25+ or CD4+CD25− cells from infected donors 7 d before the first airway challenge.
Flow cytometry.
Antibodies were diluted in PBS, 0.5% BSA (Sigma-Aldrich), 0.05% sodium azide. Cells were stained for 20 min at 4°C. For detection of CD4+CD25+ and CD4+CD25− cells, rat anti–mouse CD4 (L3T4, clone RM4-5, IgG2a) and anti-CD25 (clone PC61, IgG1; CALTAG) mAbs were used. For detection of donor C57BL/6-Ly5.1 (CD45.1) cells in recipient mice, anti-CD45.1 (clone A20, mouse IgG2a) was used. For staining intracellular cytokines, cells were permeabilized in cytofix/cytoperm, washed in perm/wash buffer (BD Biosciences), and stained with anti–mouse IL-4 (11B11), IFN-γ (XMG1,2), or IL-10 (JES5-16E3) for 30 min. For Foxp3 staining, a kit from eBioscience using mAb FKJ-16s was used according to the manufacturer's instructions. Surface bound TGF-β was detected using rat anti–mouse TGF-β1 (A75-3, IgG2a). The expression of surface markers and intracellular IL-10 was analyzed on a FACSCalibur flow cytometer using FlowJo software (Tree Star). All fluorochrome-labeled antibodies were obtained from BD Biosciences, unless otherwise stated.
Statistics.
Student's t test was used for all statistical comparisons; p-values <0.05 were considered significant.
Acknowledgments
We thank Judith Allen for critical comments and J. Tweedie for meticulous assistance; M. Leech, A. Jeske, and W. Thomas for providing Der p 1 allergen; and G. Grant and F. Rae for help with histology.
M.S. Wilson and R.M. Maizels thank the Wellcome Trust for a Prize Studentship and a Programme Grant. We thank Medical Research Council (MRC) for salary support for M.D. Taylor and a Postgraduate Studentship for C.A.M. Finney. J.R. Lamb thanks MRC and Wellcome Trust for funding.
The authors have no conflicting financial interests.
Abbreviations used in this paper: AB-PAS, Alcian blue–periodic acid Schiff; BALF, bronchoalveolar lavage fluid; MLNC, mesenteric LN cells; TLN, thoracic LN; T reg cell, regulatory T cell.
J.R. Lamb's present address is Translational Medicine and Genetics, Clinical Pharmacology and Discovery Medicine, GlaxoSmithKline, Greenford UB6 0HE, UK.
References
- 1.Sears, M.R. 1997. Epidemiology of childhood asthma. Lancet. 350:1015–1020. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp, M., J. Santeliz, and C.L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69–75. [DOI] [PubMed] [Google Scholar]
- 3.Umetsu, D.T., J.J. McIntire, O. Akbari, C. Macaubas, and R.H. DeKruyff. 2002. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 3:715–720. [DOI] [PubMed] [Google Scholar]
- 4.Geha, R.S. 2003. Nature versus nurture in allergy and hypersensitivity. Curr. Opin. Immunol. 15:603–608. [DOI] [PubMed] [Google Scholar]
- 5.Matricardi, P.M., F. Franzinelli, A. Franco, G. Caprio, F. Murru, D. Cioffi, L. Ferrigno, A. Palermo, N. Ciccarelli, and F. Rosmini. 1998. Sibship size, birth order, and atopy in 11,371 Italian young men. J. Allergy Clin. Immunol. 101:439–444. [DOI] [PubMed] [Google Scholar]
- 6.Kramer, U., J. Heinrich, M. Wjst, and H.E. Wichmann. 1999. Age of entry to day nursery and allergy in later childhood. Lancet. 353:450–454. [DOI] [PubMed] [Google Scholar]
- 7.Nafstad, P., P. Magnus, and J.J. Jaakkola. 2000. Early respiratory infections and childhood asthma. Pediatrics. 106:E38; 10.1542/peds.106. 3.e38. [DOI] [PubMed]
- 8.Illi, S., E. von Mutius, S. Lau, R. Bergmann, B. Niggemann, C. Sommerfeld, and U. Wahn. 2001. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ. 322:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gale, E.A.M. 2002. A missing link in the hygiene hypothesis? Diabetologia. 45:588–594. [DOI] [PubMed] [Google Scholar]
- 10.Strachan, D.P. 1989. Hay fever, hygiene, and household size. BMJ. 299:1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazdanbakhsh, M., A. van den Biggelaar, and R.M. Maizels. 2001. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 22:372–377. [DOI] [PubMed] [Google Scholar]
- 12.Rook, G.A., and L.R. Brunet. 2002. Give us this day our daily germs. Biologist (London). 49:145–149. [PubMed] [Google Scholar]
- 13.Akbari, O., P. Stock, R.H. DeKruyff, and D.T. Umetsu. 2003. Role of regulatory T cells in allergy and asthma. Curr. Opin. Immunol. 15:627–633. [DOI] [PubMed] [Google Scholar]
- 14.Umetsu, D.T., O. Akbari, and R.H. Dekruyff. 2003. Regulatory T cells control the development of allergic disease and asthma. J. Allergy Clin. Immunol. 112:480–487. [PubMed] [Google Scholar]
- 15.Akdis, M., J. Verhagen, A. Taylor, F. Karamloo, C. Karagiannidis, R. Crameri, S. Thunberg, G. Deniz, R. Valenta, H. Fiebig, et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdanbakhsh, M., P.G. Kremsner, and R.S. van Ree. 2002. Allergy, parasites, and the hygiene hypothesis. Science. 296:490–494. [DOI] [PubMed] [Google Scholar]
- 17.Araujo, M.I., B. Hoppe, M. Medeiros Jr., L. Alcantara, M.C. Almeida, A. Schriefer, R.R. Oliveira, R. Kruschewsky, J.P. Figueiredo, A.A. Cruz, and E.M. Carvalho. 2004. Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J. Infect. Dis. 190:1797–1803. [DOI] [PubMed] [Google Scholar]
- 18.Hagel, I., N.R. Lynch, M.C. DiPrisco, R.I. Lopez, and N.M. Garcia. 1993. Allergic reactivity of children of different socioeconomic levels in tropical populations. Int. Arch. Allergy Immunol. 101:209–214. [DOI] [PubMed] [Google Scholar]
- 19.Lynch, N.R., J. Goldblatt, and P.N.S. Le Souef. 1999. Parasite infections and the risk of asthma and atopy. Thorax. 54:659–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selassie, F.G., R.H. Stevens, P. Cullinan, D. Pritchard, M. Jones, J. Harris, J.G. Ayres, and A.J. Newman Taylor. 2000. Total and specific IgE (house dust mite and intestinal helminths) in asthmatics and controls from Gondar, Ethiopia. Clin. Exp. Allergy. 30:356–358. [DOI] [PubMed] [Google Scholar]
- 21.van den Biggelaar, A.H., R. van Ree, L.C. Rodrigues, B. Lell, A.M. Deelder, P.G. Kremsner, and M. Yazdanbakhsh. 2000. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 356:1723–1727. [DOI] [PubMed] [Google Scholar]
- 22.Nyan, O.A., G.E. Walraven, W.A. Banya, P. Milligan, M. Van Der Sande, S.M. Ceesay, G. Del Prete, and K.P.S. McAdam. 2001. Atopy, intestinal helminth infection and total serum IgE in rural and urban adult Gambian communities. Clin. Exp. Allergy. 31:1672–1678. [DOI] [PubMed] [Google Scholar]
- 23.van den Biggelaar, A.H., C. Lopuhaa, R. van Ree, J.S. van der Zee, J. Jans, A. Hoek, B. Migombet, S. Borrmann, D. Luckner, P.G. Kremsner, and M.S. Yazdanbakhsh. 2001. The prevalence of parasite infestation and house dust mite sensitization in Gabonese schoolchildren. Int. Arch. Allergy Immunol. 126:231–238. [DOI] [PubMed] [Google Scholar]
- 24.Maizels, R.M., D.A.P. Bundy, M.E. Selkirk, D.F. Smith, and R.M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature. 365:797–805. [DOI] [PubMed] [Google Scholar]
- 25.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Loliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-β but not by a Th1 to Th2 shift. Int. Immunol. 12:623–630. [DOI] [PubMed] [Google Scholar]
- 26.Maizels, R.M., and M. Yazdanbakhsh. 2003. Regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733–743. [DOI] [PubMed] [Google Scholar]
- 27.Maizels, R.M., A. Balic, N. Gomez-Escobar, M. Nair, M. Taylor, and J.E. Allen. 2004. Helminth parasites: masters of regulation. Immunol. Rev. 201:89–116. [DOI] [PubMed] [Google Scholar]
- 28.Bashir, M.E., P. Andersen, I.J. Fuss, H.N. Shi, and C. Nagler-Anderson. 2002. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 169:3284–3292. [DOI] [PubMed] [Google Scholar]
- 29.Chiaramonte, M.G., M. Mentink-Kane, B.A. Jacobson, A.W. Cheever, M.J. Whitters, M.E. Goad, A. Wong, M. Collins, D.D. Donaldson, M.J. Grusby, and T.A. Wynn. 2003. Regulation and function of the interleukin 13 receptor α2 during a T helper cell type 2-dominant immune response. J. Exp. Med. 197:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoerauf, A., and N. Brattig. 2002. Resistance and susceptibility in human onchocerciasis–beyond Th1 vs. Th2. Trends Parasitol. 18:25–31. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, M., L. Le Goff, A. Harris, E. Malone, J.E. Allen, and R.M. Maizels. 2005. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174:4924–4933. [DOI] [PubMed] [Google Scholar]
- 32.Tsitoura, D.C., R.L. Blumenthal, G. Berry, R.H. Dekruyff, and D.T. Umetsu. 2000. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J. Allergy Clin. Immunol. 106:239–246. [DOI] [PubMed] [Google Scholar]
- 33.Hadeiba, H., and R.M. Locksley. 2003. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J. Immunol. 170:5502–5510. [DOI] [PubMed] [Google Scholar]
- 34.Zuany-Amorim, C., E. Sawicka, C. Manlius, A. Le Moine, L.R. Brunet, D.M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625–629. [DOI] [PubMed] [Google Scholar]
- 35.Jutel, M., M. Akdis, F. Budak, C. Aebischer-Casaulta, M. Wrzyszcz, K. Blaser, and C.A. Akdis. 2003. IL-10 and TGF-β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 33:1205–1214. [DOI] [PubMed] [Google Scholar]
- 36.Oh, J.W., C.M. Seroogy, E.H. Meyer, O. Akbari, G. Berry, C.G. Fathman, R.H. Dekruyff, and D.T. Umetsu. 2002. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J. Allergy Clin. Immunol. 110:460–468. [DOI] [PubMed] [Google Scholar]
- 37.Hansen, G., J.J. McIntire, V.P. Yeung, G. Berry, G.J. Thorbecke, L. Chen, R.H. DeKruyff, and D.T. Umetsu. 2000. CD4+ T helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. J. Clin. Invest. 105:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohm, A.P., P.A. Carpentier, H.A. Anger, and S.D. Miller. 2002. CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712–4716. [DOI] [PubMed] [Google Scholar]
- 39.Urban, J.F., Jr., I.M. Katona, W.E. Paul, and F.D. Finkelman. 1991. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA. 88:5513–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahid, F.N., J.M. Behnke, R.K. Grencis, K.J. Else, and A.W. Ben-Smith. 1994. Immunological relationships during primary infection with Heligmosomoides polygyrus: Th2 cytokines and primary response phenotype. Parasitology. 108:461–471. [DOI] [PubMed] [Google Scholar]
- 41.Fox, J.G., P. Beck, C.A. Dangler, M.T. Whary, T.C. Wang, H.N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536–542. [DOI] [PubMed] [Google Scholar]
- 42.Elliott, D.E., T. Setiawan, A. Metwali, A. Blum, J.F. Urban Jr., and J.V. Weinstock. 2004. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 34:2690–2698. [DOI] [PubMed] [Google Scholar]
- 43.Jarrett, E., and H. Bazin. 1974. Elevation of total serum IgE in rats following helminth parasite infection. Nature. 251:613–614. [DOI] [PubMed] [Google Scholar]
- 44.Finkelman, F.D., J. Holmes, I.M. Katona, J.F. Urban Jr., M.P. Beckmann, L.S. Park, K.A. Schooley, R.L. Coffman, T.R. Mosmann, and W.E. Paul. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8:303–333. [DOI] [PubMed] [Google Scholar]
- 45.Finkelman, F.D., T. Shea-Donohue, J. Goldhill, C.A. Sullivan, S.C. Morris, K.B. Madden, W.C. Gause, and J.F. Urban Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505–533. [DOI] [PubMed] [Google Scholar]
- 46.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira, P.L., J.R. Christensen, S. Minaee, E.J. O'Neill, F.J. Barrat, A. Boonstra, T. Barthlott, B. Stockinger, D.C. Wraith, and A. O'Garra. 2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 172:5986–5993. [DOI] [PubMed] [Google Scholar]
- 48.McHugh, R.S., and E.M. Shevach. 2002. Depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 168:5979–5983. [DOI] [PubMed] [Google Scholar]
- 49.O'Farrell, A.M., Y. Liu, K.W. Moore, and A.L. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17:1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holt, P.G., C. Macaubas, P.A. Stumbles, and P.D. Sly. 1999. The role of allergy in the development of asthma. Nature. 402(Suppl.):B12–B17. [DOI] [PubMed] [Google Scholar]
- 51.Martinez, F.D., and P.G. Holt. 1999. Role of microbial burden in aetiology of allergy and asthma. Lancet. 354(Suppl 2):SII12–SII15. [DOI] [PubMed] [Google Scholar]
- 52.Hansen, G., G. Berry, R.H. DeKruyff, and D.T. Umetsu. 1999. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson, M.S., and R.M. Maizels. 2004. Regulation of allergy and autoimmunity in helminth infection. Clin. Rev. Allergy Immunol. 26:35–49. [DOI] [PubMed] [Google Scholar]
- 54.Wang, C.C., T.J. Nolan, G.A. Schad, and D. Abraham. 2001. Infection of mice with the helminth Strongyloides stercoralis suppresses pulmonary allergic responses to ovalbumin. Clin. Exp. Allergy. 31:495–503. [DOI] [PubMed] [Google Scholar]
- 55.Wohlleben, G., C. Trujillo, J. Muller, Y. Ritze, S. Grunewald, U. Tatsch, and K.J. Erb. 2004. Helminth infection modulates the development of allergen-induced airway inflammation. Int. Immunol. 16:585–596. [DOI] [PubMed] [Google Scholar]
- 56.Weiner, H.L. 2001. The mucosal milieu creates tolerogenic dendritic cells and TR1 and TH3 regulatory cells. Nat. Immunol. 2:671–672. [DOI] [PubMed] [Google Scholar]
- 57.Ling, E.M., T. Smith, X.D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V.A. Carr, and D.S. Robinson. 2004. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363:608–615. [DOI] [PubMed] [Google Scholar]
- 58.Monroy, F.G., and F.J. Enriquez. 1992. Heligmosomoides polygyrus: a model for chronic gastrointestinal helminthiasis. Parasitol. Today. 8:49–54. [DOI] [PubMed] [Google Scholar]
- 59.Lynch, N.R., I. Hagel, M. Perez, M.C. Di Prisco, R. Lopez, and N. Alvarez. 1993. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J. Allergy Clin. Immunol. 92:404–411. [DOI] [PubMed] [Google Scholar]
- 60.Araujo, M.I., A.A. Lopes, M. Medeiros, A.A. Cruz, L. Sousa-Atta, D. Solé, and E.M. Carvalho. 2000. Inverse association between skin response to aeroallergen and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123:145–148. [DOI] [PubMed] [Google Scholar]
- 61.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 62.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 63.Zheng, S.G., J.H. Wang, M.N. Koss, F. Quismorio Jr., J.D. Gray, and D.A. Horwitz. 2004. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-β suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 172:1531–1539. [DOI] [PubMed] [Google Scholar]
- 64.Fantini, M.C., C. Becker, G. Monteleone, F. Pallone, P.R. Galle, and M.F. Neurath. 2004. TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 65.Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. [DOI] [PubMed] [Google Scholar]
- 66.Belkaid, Y., and B.T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360. [DOI] [PubMed] [Google Scholar]
- 67.Karim, M., G. Feng, K.J. Wood, and A.R. Bushell. 2005. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific re-activation in vivo is critical for bystander regulation. Blood. In press. [DOI] [PubMed] [Google Scholar]
- 68.McKee, A.S., and E.J. Pearce. 2004. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J. Immunol. 173:1224–1231. [DOI] [PubMed] [Google Scholar]
- 69.Hoffmann, K.F., T.A. Wynn, and D.W. Dunne. 2002. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv. Parasitol. 52:265–307. [DOI] [PubMed] [Google Scholar]
- 70.Hoyne, G.F., R.E. O'Hehir, D.C. Wraith, W.R. Thomas, and J.R. Lamb. 1993. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J. Exp. Med. 178:1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, X., Y. Fan, S. Wang, X. Han, J. Yang, L. Bilenki, and L.S. Chen. 2002. Mycobacterial infection inhibits established allergic inflammatory responses via alteration of cytokine production and vascular cell adhesion molecule-1 expression. Immunology. 105:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Escobar, N., W.F. Gregory, and R.M. Maizels. 2000. Identification of Bm-tgh-2, a filarial nematode homolog of C. elegans daf-7 and human TGF-β, expressed in microfilarial and adult stages of Brugia malayi. Infect. Immun. 68:6402–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]