Abstract
A better understanding of the role of CD4+CD25+ regulatory T cells in disease pathogenesis should follow from the discovery of reliable markers capable of discriminating regulatory from activated T cells. We report that the CD4+CD25+ population in synovial fluid of juvenile idiopathic arthritis (JIA) patients comprises both regulatory and effector T cells that can be distinguished by expression of CD27. CD4+CD25+CD27+ cells expressed high amounts of FoxP3 (43% of them being FoxP3+), did not produce interleukin (IL)-2, interferon-γ, or tumor necrosis factor, and suppressed T cell proliferation in vitro, being, on a per cell basis, fourfold more potent than the corresponding peripheral blood population. In contrast, CD4+CD25+CD27− cells expressed low amounts of FoxP3, produced effector cytokines and did not suppress T cell proliferation. After in vitro activation and expansion, regulatory but not conventional T cells maintained high expression of CD27. IL-7 and IL-15 were found to be present in synovial fluid of JIA patients and, when added in vitro, abrogated the suppressive activity of regulatory T cells. Together, these results demonstrate that, when used in conjunction with CD25, CD27 is a useful marker to distinguish regulatory from effector T cells in inflamed tissues and suggest that at these sites IL-7 and IL-15 may interfere with regulatory T cell function.
There is now clear evidence that a distinct population of naturally occurring regulatory T cells, which can be identified by the constitutive expression of CD4 and CD25, plays an essential role in controlling autoimmunity (1). Regulatory T cells are generated in the thymus or in periphery (2, 3) and, once activated, suppress other T cells by an as yet uncharacterized contact-dependent, cytokine-independent mechanism (4). A functional result of suppression is impaired production of IL-2 (4), although evidence has been provided that an initial IL-2 production by responder cells is necessary for expansion of CD4+CD25+ T cells and induction of their suppressor function (5). The suppressor function of regulatory T cells can be relieved by exogenous IL-2 that acts on both regulatory and responder T cells and by IL-6 that blocks suppression at the level of responder cells (6, 7).
The development and function of regulatory T cells is critically dependent on the transcriptional repressor FoxP3 (8–10). Mice and humans that lack Foxp3 die from severe autoimmune diseases (11–14), whereas transduction of Foxp3 in naive CD4+ T cells is sufficient to convert these cells into regulatory T cells (8, 9). Because Foxp3 is the master control gene, it is in principle the most specific marker for regulatory T cells. However, the facts that FoxP3 is expressed exclusively intracellularly and that reliable reagents for staining are not yet available prevent its use for the identification and isolation of regulatory T cells.
CD25 is the hallmark antigen of regulatory T cells in mice and humans (15–19). In normal conditions, CD25 appears to identify a relatively homogeneous population of anergic regulatory T cells, although some heterogeneity may exist. For instance, it has been reported that among CD4+CD25+ T cells those expressing CD103 or CD62L are more suppressive than their negative counterparts (20–22). Other useful markers of regulatory T cells under normal conditions include GITR, CTLA-4, and, in mice, neuropilin-1 (23–25).
There is growing interest in the identification of regulatory T cells in various pathological conditions and recent studies indicate that CD4+CD25+ cells with regulatory function can be indeed detected in inflamed tissues (26–28). However, the identification of regulatory T cells in an ongoing immune response or in inflamed tissues is complicated by the fact that all the aforementioned markers, including CD25, are also expressed on activated T cells (29). A possible heterogeneity of the CD4+CD25+ subset in inflamed tissues has not been addressed so far and the problem, therefore, remains how to discriminate in an ongoing immune response regulatory from activated effector T cells.
Here, we report that CD27 is stably expressed on regulatory T cells and can be used in conjunction with CD25 expression to discriminate in inflamed synovia regulatory T cells, expressing high amounts of FoxP3 and endowed with potent suppressive activity, from FoxP3− effector T cells devoid of suppressor activity. We also show that Il-7 and IL-15 are present in synovial fluid and in vitro abrogate the suppressive function of regulatory T cells.
Results
CD4+CD25+ T cells from synovial fluid of juvenile idiopathic arthritis (JIA) patients express high amounts of FoxP3 and show suppressor activity in vitro
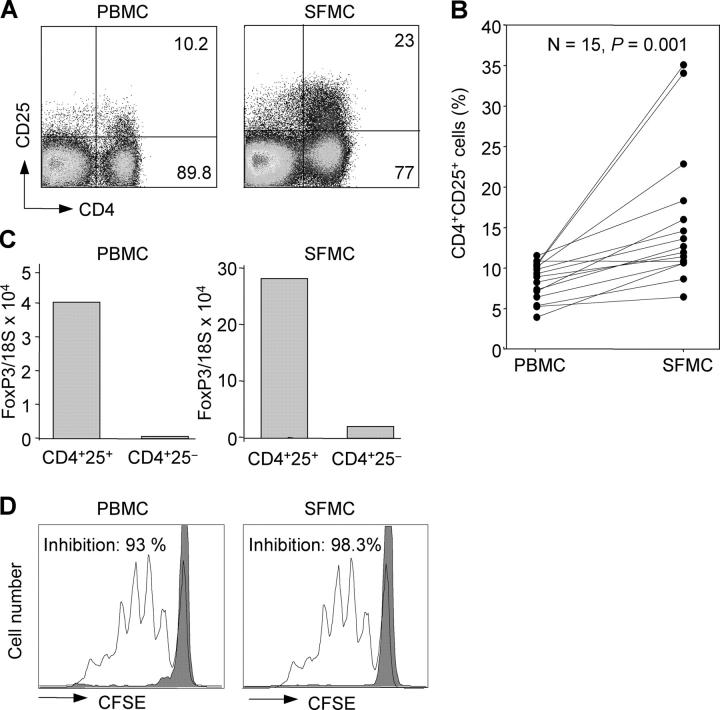
Mononuclear cells were isolated from both synovial fluid and peripheral blood of 15 JIA patients (7 with polyarticular and 8 with oligoarticular disease course) and analyzed for the expression of CD4 and CD25 (Fig. 1, A and B). The percentage of CD25+ cells within the CD4+ population ranged from 4 to 11.6 (median, 8.6) in peripheral blood, a value comparable to that found in healthy donors (not depicted), whereas in synovial fluid it was significantly higher ranging from 6.5 to 35.2 (median, 12.3; Fig. 1 B).
Figure 1.
CD4+CD25+ T cells are enriched in synovial fluid of JIA patients, express high amounts of FoxP3 mRNA, and efficiently suppress proliferation of CD4+CD25− autologous T cells. (A) Mononuclear cells from peripheral blood (PBMC) and synovial fluid (SFMC) were obtained from the same JIA patient and stained with antibodies to CD4 and CD25. Shown is the percentage of CD25+ and CD25− cells within the CD4+ population. (B) Percentages of CD4+CD25+ in PBMCs and SFMCs in 15 JIA patients. p-value determined by Wilcoxon rank test. (C) PBMCs and SFMCs were sorted based on expression of CD4 and CD25, and analyzed for expression of FoxP3 mRNA relative to 18S rRNA by quantitative real-time PCR. 1 representative experiment out of 10 is shown. (D) Proliferation of 1.5 × 104 CFSE-labeled CD4+CD25− peripheral blood T cells stimulated by anti-CD3 and DCs in the presence of equal numbers of autologous CD4+CD25− T cells (unshaded histograms) or CD4+CD25+ T cells (shaded histograms) isolated from PBMCs or SFMCs. The total number of responder T cells that had performed one or more cell divisions was determined by CFSE dilution analysis. Responder T cells proliferated to a similar extent in the absence of CD4+CD25− control T cells (not depicted). The percentage inhibition was calculated from the number of dividing responder cells in presence of CD4+CD25+ T cells as compared with their number in presence of CD4+CD25− control cells. One representative experiment out of five is shown.
CD4+CD25+ and CD4+CD25− T cells were isolated by cell sorting from blood and synovial fluid of the same individual and analyzed for expression of the transcription factor FoxP3 using quantitative real-time PCR. FoxP3 mRNA was higher in CD4+CD25+ as compared with CD4+CD25− cells in both peripheral blood and synovial fluid (Fig. 1 C). However, the amount of FoxP3 mRNA was much higher in the two populations isolated from synovial fluid than in those isolated from peripheral blood (Fig. 1 C). These findings suggest that synovial CD4+CD25+ T cells may be activated in vivo and that some regulatory T cells may be present within the CD4+CD25− subset. Indeed, upon in vitro stimulation peripheral blood CD4+CD25+ regulatory T cells, and to a lower extent CD4+CD25− T cells (30), rapidly up-regulated FoxP3 mRNA (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20050085/DC1).
To examine the suppressive activity in vitro, synovial and peripheral blood CD4+CD25+ T cells were added at a 1:1 ratio to cultures of CFSE-labeled autologous peripheral blood CD4+CD25− responder T cells stimulated by DCs and anti-CD3. As control, responder T cells were cultured in the absence or presence of unlabeled CD4+CD25− T cells. 4 d later, cultures were analyzed by FACS and the total number of responder T cells that had performed one or more cell divisions was determined by CFSE dilution analysis. The percentage of suppression was calculated from the number of dividing responder cells in presence of CD4+CD25+ T cells compared with their number in presence of CD4+CD25− control cells. As shown in Fig. 1 D, CD4+CD25+ T cells isolated from blood or synovial fluid potently suppressed proliferation of CD4+CD25− T cells (93 and 98.3% inhibition, respectively). We conclude that synovial fluid contains a large population of CD4+CD25+ T cells characterized by high expression of FoxP3 and suppressor activity in vitro.
CD27 discriminates between regulatory and activated T cells in synovial fluid
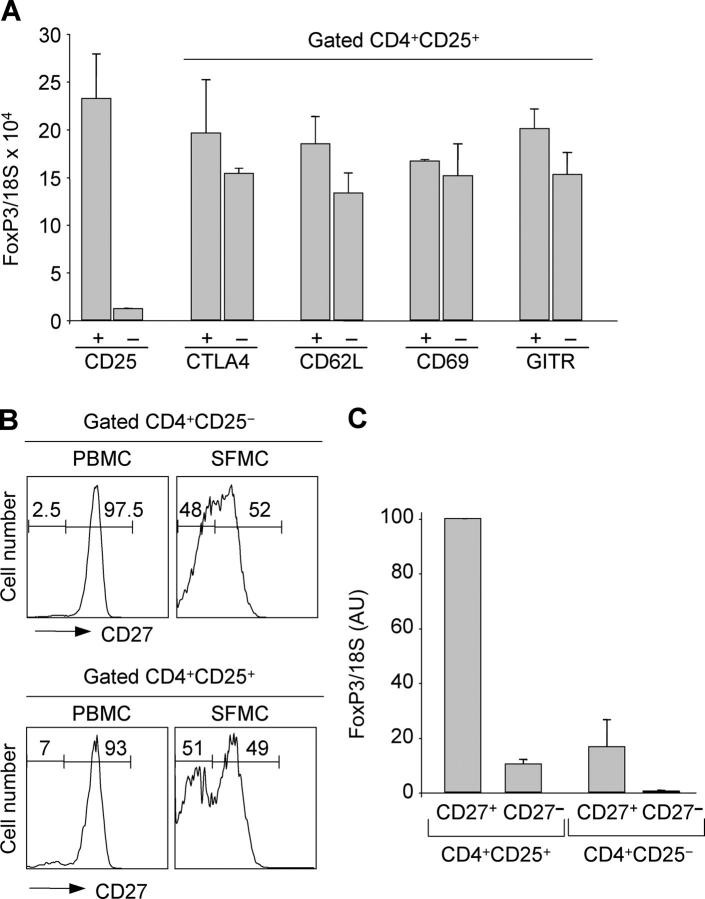
Because CD25 is an activation marker, the pool of CD4+ CD25+ cells in synovial fluid may contain not only regulatory T cells but also activated T cells. To dissect a possible heterogeneity, we separated synovial CD4+CD25+ T cells according to the expression of several cell surface markers and used FoxP3 mRNA to monitor the presence of regulatory T cells. Subsets defined by presence or absence of CTLA-4, CD62L, CD69, and GITR contained comparable amounts of FoxP3 mRNA (Fig. 2 A). Similar data were obtained using CCR4, VLA-4, and CD103 (unpublished data). Thus, these markers, known to be expressed on circulating regulatory T cells (16–19, 31–33), did not segregate with FoxP3 expression in cells of inflamed synovia.
Figure 2.
CD27 identifies FoxP3-expressing cells within CD25+ synovial CD4+ T cells. (A) CD4+CD25+ SFMCs were stained with antibodies to CTLA-4, CD62L, CD69, and GITR. Positive and negative subsets were sorted and analyzed for FoxP3 mRNA. Mean ± SD of three separate experiments. (B) PBMCs and SFMCs from patient 12 were stained with antibodies to CD4, CD25, and CD27. The histograms show the expression of CD27 on gated CD4+CD25− or CD4+CD25+ T cells. (C) SFMCs were sorted according to the expression of CD4, CD25, and CD27 and analyzed for expression of FoxP3 mRNA. For comparison, the value in CD25+CD27+ was set to 100. Mean ± SD of three separate experiments.
CD27 is a TNFR-family member that is expressed on naive and subsets of memory T cells and is lost on terminally differentiated effector T cells (34, 35). Because the latter are highly enriched in synovial fluid of adult rheumatoid arthritis and JIA patients (36, 37), we asked whether CD27 may discriminate regulatory from activated effector T cells in inflamed joints. Indeed, in some synovial samples, we noted that a high proportion (up to 50%) of CD4+CD25+ T cells were CD27− (Fig. 2 B and see Table II). CD4+CD25+ CD27− cells were also found in peripheral blood of patients and healthy adults although at lower frequency (mean 15.6%, range 8.3–33 in 14 patients and 7.9%, range 2.6–11.3 in 5 controls).
Table II.
Distribution of CD27 in synovial CD4+CD25+ and CD4+CD25− T cells subsets in JIA patients with oligoarticular and polyarticular disease course
| CD4+
|
Percentage of CD27− in:
|
||||||
|---|---|---|---|---|---|---|---|
| Patient | Disease course | CD25+CD27+ | CD25+CD27− | CD25−CD27+ | CD25−CD27− | CD4+CD25+ | CD4+CD25− |
| 1 | polyarticular | 16.8a | 9.7a | 48a | 24.9a | 36.5b | 34.1c |
| 2 | polyarticular | 6.8 | 4.3 | 42.7 | 46.2 | 38.7 | 52.0 |
| 3 | polyarticular | 11.2 | 5.8 | 23.3 | 59.7 | 34.1 | 41.1 |
| 4 | polyarticular | 6.3 | 3.4 | 43 | 47.6 | 35.1 | 52.5 |
| 5 | polyarticular | 8.7 | 4.2 | 42 | 45 | 32.6 | 51.7 |
| 6 | polyarticular | 8.1 | 5.6 | 57.2 | 29.0 | 40.9 | 33.6 |
| 7 | polyarticular | 7.5 | 1.4 | 39.7 | 51.4 | 15.7 | 56.4 |
| 8 | polyarticular | 5.3 | 1.2 | 76 | 17.3 | 18.4 | 18.5 |
| 9 | polyarticular | 32.3 | 2.9 | 19.7 | 43 | 8.2 | 68.5 |
| 10 | polyarticular | 8.3 | 2.4 | 27.7 | 61 | 22.4 | 68.8 |
| 11 | polyarticular | 10.5 | 2.2 | 68.2 | 19 | 17.3 | 27.0 |
| 12 | polyarticular | 7.7 | 8.4 | 39 | 44.2 | 52.2 | 53.1 |
| 13 | oligoarticular | 9.1 | 2.7 | 32 | 55 | 22.9 | 63.2 |
| 14 | oligoarticular | 10.7 | 1 | 85 | 4.9 | 8.5 | 5.4 |
| 15 | oligoarticular | 16 | 1.2 | 73 | 8 | 7.0 | 9.9 |
| 16 | oligoarticular | 33 | 1.2 | 54 | 11.5 | 3.5 | 17.5 |
| 17 | oligoarticular | 6.8 | 3 | 36 | 54 | 30.6 | 60.0 |
| 18 | oligoarticular | 7.3 | 3.6 | 50 | 38 | 33.0 | 43.2 |
| 19 | oligoarticular | 16.9 | 1.5 | 56 | 25 | 8.1 | 30.9 |
| 20 | oligoarticular | 9.9 | 1.2 | 68.8 | 20 | 10.8 | 22.5 |
| 21 | oligoarticular | 9.0 | 1.7 | 55 | 34 | 15.9 | 43.0 |
| 22 | oligoarticular | 10.2 | 1.8 | 56 | 31 | 15.0 | 35.6 |
| 23 | oligoarticular | 11.0 | 0.5 | 71 | 17 | 4.3 | 19.3 |
| 24 | oligoarticular | 7.6 | 1.1 | 62 | 28 | 12.6 | 31.1 |
| 25 | oligoarticular | 13.6 | 1.1 | 63 | 23 | 11.1 | 26.7 |
Percentages within CD4+ synovial cells.
Percentages within CD4+CD25+ synovial cells.
Percentages within CD4+CD25− synovial cells.
The four subsets of CD4+ cells identified according to the expression of CD25 and CD27 were isolated from the synovia and blood of JIA patients and from the blood of adult healthy controls and tested for FoxP3 mRNA expression. FoxP3 mRNA was at least 10-fold higher in CD4+ CD25+CD27+ cells as compared with CD4+CD25+CD27− cells (Fig. 2 C and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20050085/DC1). Low amounts of FoxP3 mRNA were also found in CD4+CD25−CD27+ T cells, whereas FoxP3 was not detected in CD4+CD25− CD27− cells.
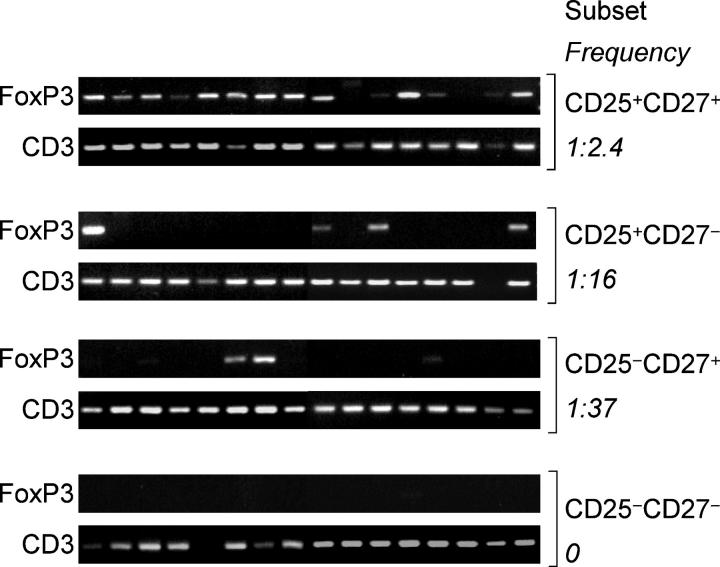
To estimate the frequency of FoxP3-expressing synovial T cells within the four subsets, we used a sensitive PCR method to detect FoxP3 in replicate samples containing limiting numbers of T cells (five cells per sample). The highest frequency of FoxP3+ cells (41.6%) was found in the CD4+CD25+CD27+ subset, whereas the CD4+CD25+ CD27− and the CD4+CD25−CD27+ subsets showed much lower frequencies (6.2% and 2.7%, respectively) and the CD4+CD25−CD27− subset was negative (Fig. 3). Considering that frequencies are underestimated, these results suggest that CD27 marks a rather homogeneous population of FoxP3 expressing cells in inflamed synovia.
Figure 3.
FoxP3 mRNA in CD4+CD25+ T cell subsets in samples containing limiting cell numbers. CD4+ T cell subsets were sorted according to the expression of CD25 and CD27 and resorted, collecting 16 replicates of five cells each that were subsequently analyzed for expression of FoxP3 by PCR. Amplification of CD3 was used as control.
GITR and CTLA-4 were expressed at comparable levels on CD4+CD25+CD27+ and CD4+CD25+CD27− cells, whereas CD62L and CCR4 were expressed on a higher proportion of CD27+ cells (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20050085/DC1). In addition, upon TCR triggering CD4+CD25+CD27+ T cells did not express IL-2, TNF, or IFN-γ mRNAs, whereas CD4+CD25+CD27− cells expressed high amounts of cytokine mRNAs (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20050085/DC1). Together, these findings are consistent with the notion that CD4+CD25+CD27+ cells are regulatory cells, whereas CD4+CD25+CD27− represent activated effector cells.
To directly test the suppressive function, the four subsets identified by the expression of CD25 and CD27 were isolated by cell sorting from the synovial fluid and added to cultures of CFSE-labeled peripheral blood CD4+CD25− T cells. As shown in Fig. 4 A, suppressive activity was restricted to the CD4+CD25+CD27+ subset. The other three subsets either did not interfere or even enhanced T cell proliferation (Fig. 4 A). Synovial CD4+CD25+CD27+ T cells were, on a per cell basis, fourfold more potent as compared with CD4+CD25+CD27+ T cells isolated from peripheral blood of the same patient (Fig. 4 B). We conclude that based on four different criteria (expression of FoxP3 mRNA, expression of surface markers, lack of cytokine production, and suppression of T cell proliferation) CD27 expression in the context of CD25 expression allows discrimination of regulatory from activated/effector T cells in inflamed joints.
Figure 4.
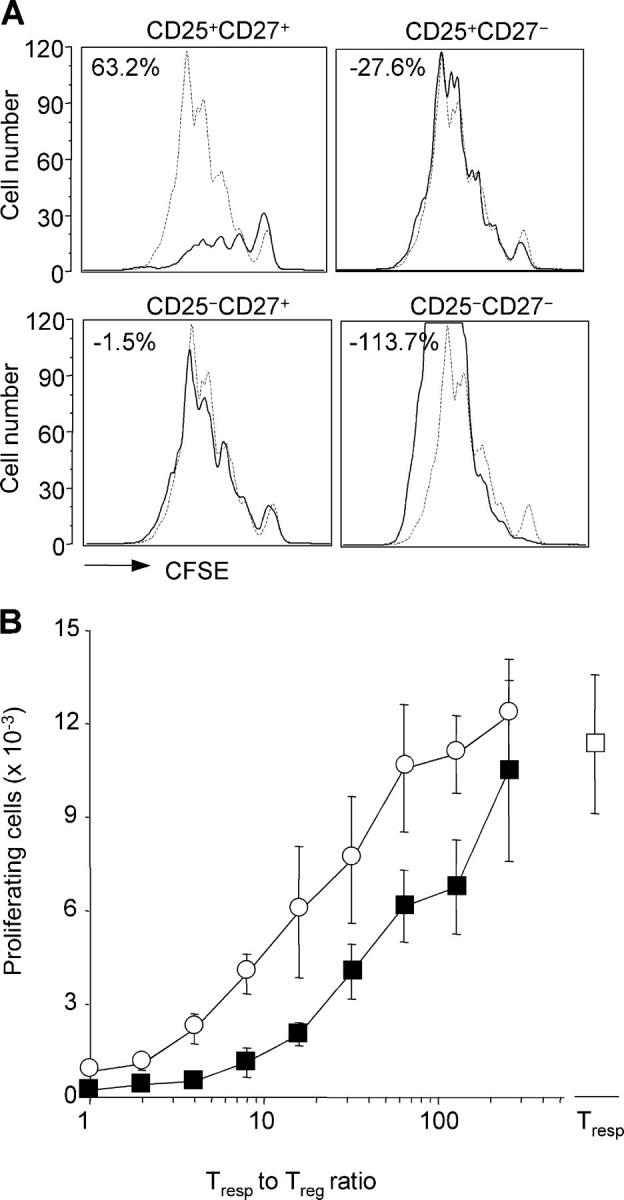
CD27 identifies potent suppressor cells within CD4+CD25+ synovial T cells. (A) Proliferation of CFSE-labeled CD4+CD25− peripheral blood T cells stimulated by anti-CD3 and DCs in the absence (dashed lines) or presence (solid line) of equal numbers of the indicated autologous CD4+ T cells isolated from synovial fluid. The percentage inhibition was calculated as in Fig. 1. Comparable results were obtained using synovial T cells isolated from patient no. 14 (depicted) and nos. 2, 5, and 7. (B) Proliferation of CFSE-labeled CD4+CD25− peripheral blood T cells in the presence of serial twofold dilutions of autologous CD4+CD25+CD27+ T cells from peripheral blood (open circle) or synovial fluid (closed square). Shown is the total number of responder T cells that had undergone more than one cell division. Mean ± SD of triplicate cultures. Comparable results were obtained with samples from patient no. 15 (depicted) and nos. 5 and 7.
CD27 is retained on regulatory T cells after activation and expansion
It is known that CD27 is lost upon terminal differentiation of conventional CD4+ T cells (34). To investigate whether regulatory T cells may lose or retain CD27 expression upon activation, we isolated CD4+CD25+CD27+ regulatory T cells and CD4+CD25−CD27+ naive and memory T cells from peripheral blood (Fig. 5 A). Cells were labeled with CFSE and stimulated with anti-CD3 in the absence or presence of exogenous IL-2 or anti-CD28. As shown in Fig. 5 (B and C), CD4+CD25− T cells proliferated extensively and down-regulated CD27 expression as a function of cell division. In contrast, CD4+CD25+ regulatory T cells proliferated poorly in the absence of IL-2, whereas they performed several cell divisions when exogenous IL-2 or costimulation was provided. Remarkably, in both conditions, proliferating regulatory T cells retained high expression of CD27. We conclude that CD27 is a stable marker of regulatory T cells that is retained after activation and expansion.
Figure 5.
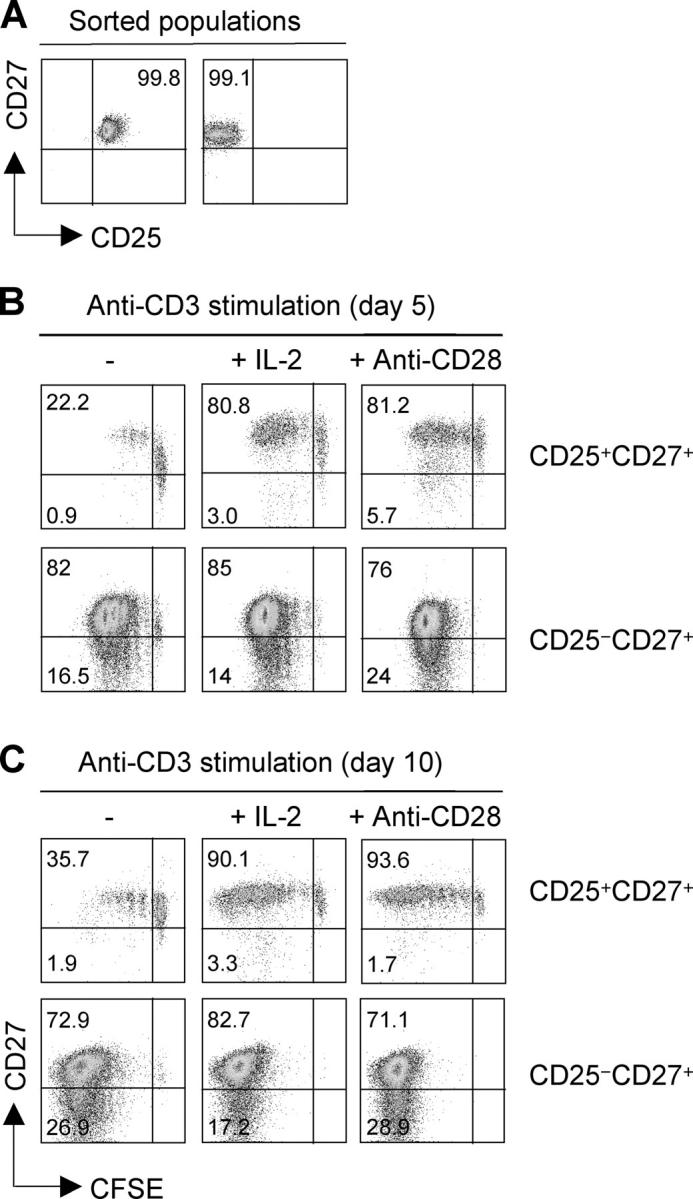
CD27 is stably expressed in proliferating regulatory T cells, whereas it is down-regulated on activated conventional T cells. (A) CD4+CD25+CD27+ regulatory T cells and CD4+CD25−CD27+T cells (comprising naive and memory cells) were sorted from peripheral blood to >99% purity. (B and C) Cells were labeled with CFSE and stimulated with plastic-bound CD3 antibodies in the absence or presence of IL-2 or CD28 antibodies. CD27 expression was measured as a function of cell division on days 5 (B) and 10 (C). One representative experiment out of three performed is shown.
Different proportions of CD4+CD25+ subsets in JIA patients with polyarticular or oligoarticular disease
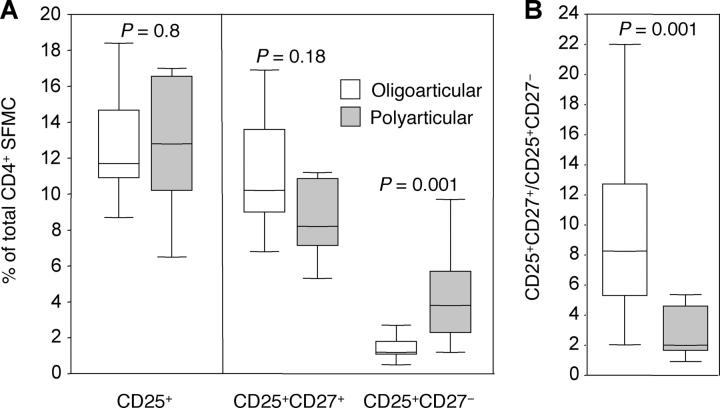
Some of the patients in this study had the disease limited to a few joints (persistent oligoarticular), whereas others showed a more aggressive polyarticular involvement (extended oligoarticular, polyarticular, and systemic forms; Tables I and II). Although the percentage of total CD4+CD25+ cells did not differ significantly between the two groups, the relative proportion of CD27+ and CD27− cells within the CD4+CD25+ subset in affected joints was significantly different (Fig. 6 A). Patients with polyarticular disease showed higher proportion of CD27− activated/effector cells and slightly lower proportion of CD27+ regulatory T cells as compared with patients with oligoarticular disease that showed a significantly higher ratio of regulatory to activated cells (Fig. 6 B).
Table I.
Clinical characteristics of JIA patients included in the study
| Patient | Sex | JIA form | Age | Disease duration | No. active jointsa | ESR | Treatment |
|---|---|---|---|---|---|---|---|
| yr | yr | mm/first hour | |||||
| 1 | F | polyarticular RF- | 6.2 | 3.5 | 25 | 20 | MTX, CyA |
| 2 | F | extended oligoarticular | 2.8 | 0.9 | 20 | 36 | NSAID |
| 3 | F | extended oligoarticular | 9.5 | 7.1 | 2 | 96 | MTX |
| 4 | F | polyarticular RF- | 5.4 | 0.8 | 27 | 53 | NSAID |
| 5 | F | extended oligoarticular | 10.4 | 9.3 | 7 | 34 | MTX |
| 6 | M | systemic | 4.3 | 1.7 | 4 | 97 | MTX, CS |
| 7 | F | extended oligoarticular | 5.1 | 3.2 | 3 | 25 | MTX |
| 8 | F | extended oligoarticular | 6.9 | 5.0 | 2 | 24 | MTX |
| 9 | F | polyarticular RF- | 5.2 | 1.9 | 8 | 20 | MTX, NSAID |
| 10 | F | extended oligoarticular | 6.3 | 5.2 | 6 | 60 | MTX |
| 11 | F | polyarticular RF- | 9.9 | 8.5 | 3 | 34 | CyA, NSAID |
| 12 | F | systemic | 10.3 | 0.8 | 16 | 96 | MTX, NSAID |
| 13 | F | persistent oligoarticular | 8.5 | 7.1 | 4 | 12 | - |
| 14 | M | persistent oligoarticular | 4.2 | 2.1 | 1 | 11 | NSAID |
| 15 | F | persistent oligoarticular | 12.9 | 4.7 | 1 | 11 | NSAID |
| 16 | F | persistent oligoarticular | 4.0 | 1.3 | 2 | 13 | NSAID |
| 17 | F | persistent oligoarticular | 2.5 | 1.1 | 1 | 11 | NSAID |
| 18 | F | persistent oligoarticular | 9.3 | 6.2 | 3 | 14 | NSAID |
| 19 | F | persistent oligoarticular | 2.9 | 1.6 | 2 | 25 | - |
| 20 | F | persistent oligoarticular | 11.9 | 3.1 | 1 | 26 | NSAID |
| 21 | F | persistent oligoarticular | 6.0 | 1.6 | 2 | 36 | - |
| 22 | F | persistent oligoarticular | 3.6 | 1.9 | 2 | 14 | NSAID |
| 23 | F | persistent oligoarticular | 6.7 | 5.8 | 1 | 18 | NSAID |
| 24 | F | persistent oligoarticular | 8.1 | 3.6 | 2 | 26 | NSAID |
| 25 | M | persistent oligoarticular | 14.2 | 9.3 | 1 | 12 | NSAID |
Defined as presence of swelling and/or limitation of motion with tenderness.
CS, corticosteroids, CyA, cyclosporin A; ESR, erythrocyte sedimentation rate; MTX, methotrexate; NSAID, nonsteroidal antiinflammatory drugs; RF, rheumatoid factor.
Figure 6.
Differential distribution of CD25+CD27+ regulatory and CD25+CD27− activated effector cells within synovial CD4+ T cells of JIA patients with oligoarticular or polyarticular disease course. (A) Percentages of total CD4+CD25+ T cells and CD27+ and CD27− subsets in 13 JIA patients with oligoarticular disease course (white bars) and 12 JIA patients with polyarticular disease course (gray bars). (B) Ratio between regulatory and activated effector CD4+ cells as defined by CD25 and CD27 expression in oligoarticular (white bars) or polyarticular (gray bars) JIA patients. Boxes contain values falling between the 25th and 75th percentiles. Lines that extend from the boxes represent the highest and the lowest values from each subgroup. The lines within the boxes represent median values. p-values were determined by Mann-Whitney U test. Also, see Table II for data from individual patients.
IL-7 and IL-15 are present in synovial fluid and limit the suppressor activity of regulatory T cells
Biopsies of synovial tissues were obtained from three JIA patients. When analyzed by immunohistochemistry, regulatory T cells coexpressing CD4, CD25, and CD27 were found in lymphoid aggregates (Fig. 7) consistent with the possibility that they may participate in the ongoing immune response in the inflamed tissue. To investigate the possibility that cytokines that are present in the inflamed tissue may interfere with regulatory T cell function, we tested a large panel of recombinant cytokines for their capacity to block the suppressive function of regulatory T cells in vitro. The activity of regulatory T cells was strongly reduced in the presence of IL-2, IL-7, and IL-15 and virtually abolished when IL-7 and IL-15 were added together (Fig. 8 A). In contrast, the proinflammatory cytokines IL-6, IL-12, TNF, and IFN-γ, as well as another γ-common–dependent cytokine, IL-4, were ineffective, whereas TGF-β and IL-10 showed direct suppressive activity even in the absence of regulatory T cells (Fig. 8 A). Remarkably, IL-7 and IL-15 were detected in the synovial fluid of JIA patients, IL-7 being significantly higher in patients with polyarticular as compared with patients with oligoarticular disease (Fig. 8 B). Together, these results suggest that in inflamed tissues IL-7 and Il-15 may substantially limit the suppressor function of regulatory T cells.
Figure 7.
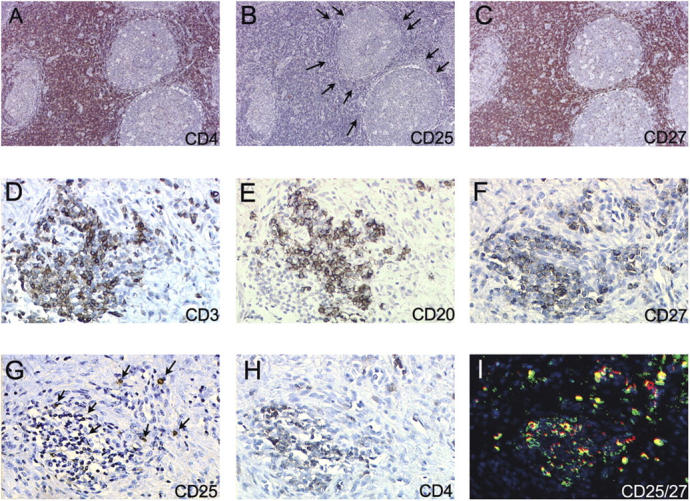
Distribution of CD4+CD25+CD27+ regulatory T cells in lymph nodes and synovial tissues. (A–C) Serial sections of a reactive lymph node stained with antibodies to CD4 (A), CD25 (B), and CD27 (C). (D–H) Serial sections of synovial biopsy from a JIA patient showing a T and B lymphoid aggregate stained with antibodies to CD3 (D), CD20 (E), CD27 (F), CD25 (G), and CD4 (H). (I) A consecutive section stained with CD25 (red) and CD27 (green) by two-color fluorescence.
Figure 8.
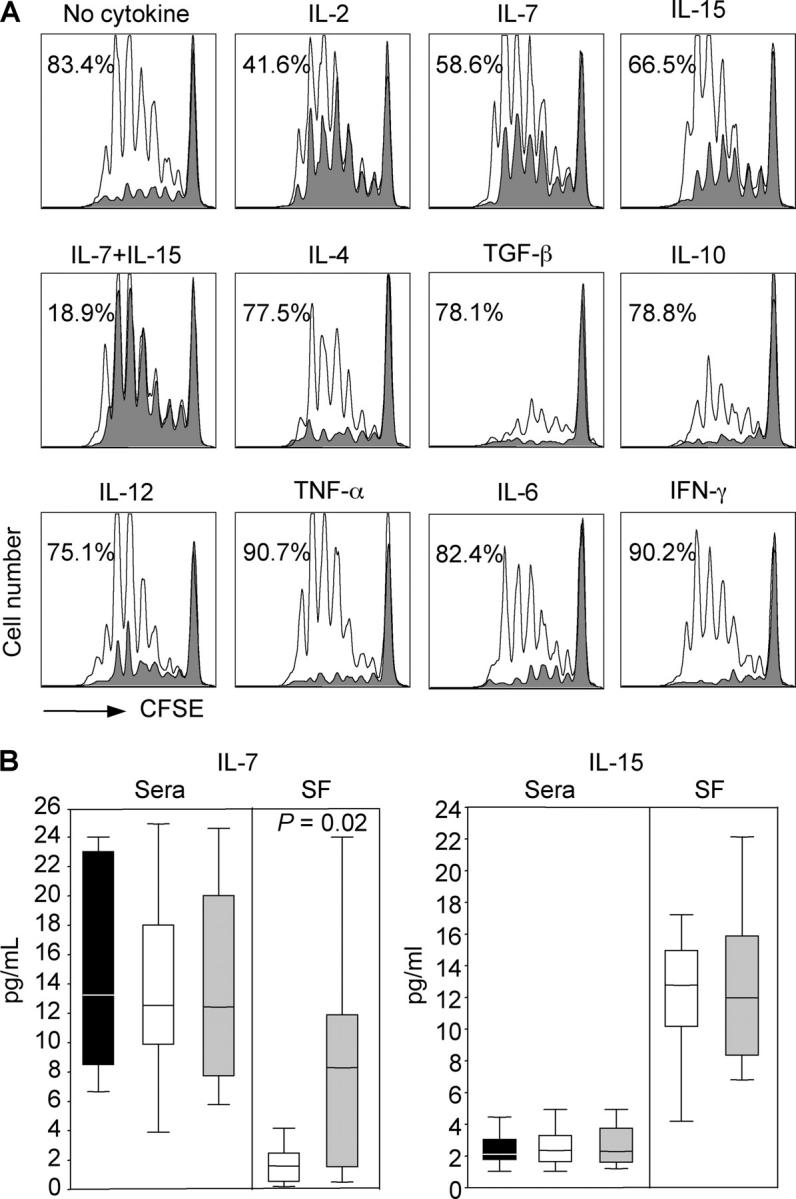
IL-7 and IL-15 counteract the suppressor activity of regulatory T cells and are present in synovial fluid of JIA patients. (A) Proliferation of CFSE-labeled CD4+CD25− peripheral blood T cells stimulated by TSST-pulsed DCs in the absence (unshaded histograms) or presence (shaded histograms) of equal numbers of the CD4+CD25+ T cells. The cultures were set up in medium alone (no cytokines) or in medium supplemented with the indicated cytokines. (B) IL-7 and IL-15 were quantified by ELISA in sera and synovial fluid (SF) from 15 oligoarticular (white bars) and 15 polyarticular (gray bars) JIA patients and in sera from 12 age-matched healthy controls (black bars). Boxes contain values falling between the 25th and 75th percentiles. Lines that extend from the boxes represent the highest and the lowest values from each subgroup. The lines within the boxes represent median values. p-values were determined by Mann-Whitney U test.
Discussion
In this study, we addressed the problem of identifying naturally occurring regulatory T cells in inflamed tissues. We found that in synovial fluid of JIA patients, CD27 expression can be used to discriminate, within the CD4+CD25+ subset, regulatory T cells from activated effector T cells. CD27 is expressed on regulatory T cells in both peripheral blood and synovial tissues and is retained by these cells after activation and clonal expansion in vitro, whereas CD27 is absent on effector T cells and is rapidly lost on CD27+ naive and memory T cells after activation (38, 39). Thus, although CD27 is not a specific marker for regulatory T cells, the differential regulation of expression in regulatory and conventional T cells makes it a suitable marker for the identification of regulatory T cells in inflamed tissues.
The intensity of CD25 expression is considered a reliable marker for regulatory T cells in peripheral blood (16). However, this may not be sufficient to identify regulatory T cells in inflamed tissues. Indeed, a recent study demonstrated that CD25dim cells from synovial fluid express FoxP3 and have suppressive activity (40). In this regard, we found that CD27 is expressed not only on all CD25bright cells, but also on a sizeable proportion of CD25dim cells and that CD25dim CD27+ and CD25brightCD27+ T cells express comparable amounts of FoxP3 mRNA (unpublished data). We conclude that the combination of CD25 and CD27 allows identification of most of regulatory T cells, while effectively excluding effector T cells.
Growing evidence over the past few years indicates that CD27, as well as other members of the TNFR family, such as OX40 (CD134) and 4-1BB (CD137), plays an important role for the effective generation of many types of T cell responses (41). It remains to be established what role CD27 may play in regulatory T cell function and whether sustained expression of CD27 on regulatory T cells contribute to their maintenance in vivo.
Using limiting dilution analysis, we could estimate that 41% of CD4+CD25+CD27+ T cells isolated from inflamed joints express FoxP3. The possibility of isolating a rather homogeneous population of regulatory T cells from inflamed tissues was instrumental to establish their relative potency as compared with regulatory T cells from peripheral blood. A direct comparison of regulatory T cells from synovial fluid and peripheral blood of the same patient revealed that synovial regulatory T cells expressed much higher levels of FoxP3 and were on a per cell basis fourfold more potent in suppressing T cell proliferation than peripheral blood regulatory T cells. As activation by anti-CD3 and IL-2 increases both FoxP3 expression and suppressor function of peripheral blood regulatory T cells (42), these findings suggest that the increased potency of synovial regulatory T cells is a consequence of their activated state in vivo.
The relevance of discriminating regulatory from effector T cells in inflamed tissues is underlined by our finding that, in the joints of JIA patients, up to 50% of CD4+CD25+ T cells were CD27− effector T cells and that the ratio of regulatory to activated T cells was higher in patients with oligoarticular disease course than in those with severe polyarticular disease course. Because the contamination of activated T cells in the CD25+ population can be substantial and varies with disease state, it will be important to reassess the presence of regulatory T cells in other pathological tissues (26, 28) by using a combination of markers as those described in the present study.
Although regulatory T cells can be found in tissues undergoing chronic inflammation it remains to be established whether they exert their function in vivo. Besides possible intrinsic defects in regulatory T cells (43–45), there may be several mechanisms that limit the efficacy of regulatory T cells in peripheral inflamed tissues. For instance, in vitro preactivated T cells become resistant to suppression and this resistance is dependent on the strength and duration of the stimulus (28, 42). We also found that naive T cells become completely refractory to suppression 24 h after TCR stimulation (unpublished data). In addition, exogenous signals such as those provided by GITR-L and IL-6 render responsive T cells resistant to suppression mediated by CD4+CD25+ regulatory T cells (7, 46). We screened a large panel of cytokines for their capacity to relieve suppression in vitro and found that IL-7 and IL-15, and more effectively a combination of the two, can counteract the suppressor function of regulatory T cells. Based on these results and on the finding that both IL-7 and IL-15 can be detected in the joint fluid of JIA patients, we suggest that in target tissues the function of regulatory T cells may be substantially limited by these cytokines and that therapies that aim at neutralizing such cytokines may not only decrease bystander T cell activation but also reconstitute the suppressor function of regulatory T cells.
Materials and methods
Patients.
25 consecutive JIA patients diagnosed according to ILAR Durban's criteria (47) were included in the study. All patients had active disease and underwent synovial fluid aspiration for steroid injection. To be included in the study, patients with persistent oligoarticular course should have presented a disease duration >1 yr. In all cases, a steroid injection in the same joint in the previous 6 mo was considered as an exclusion criterion. The patients tested in the functional studies were under NSAID and/or methotrexate treatment. The main clinical and laboratory features and the ongoing treatment at the moment of the study are reported in Table I. Patients' samples were taken after parents' permission according to the informed consent approved by the ethical committee of the G. Gaslini Institute.
Media and reagents.
The medium used throughout the experiments was RPMI 1640 supplemented with 10% fetal calf serum, 1% Glutamax, 1% nonessential amino acids, 1% pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin (all obtained from Invitrogen), and 5 × 10−5 M 2-mercaptoethanol (Merck). Recombinant human IL-6, IL-7, IL-10, IL-12, IL-15, IFN-γ, TNF, and TGF-β were purchased from BD Biosciences. IL-2 and IL-4 were produced in our laboratory using the myeloma-based expression system.
FACS analysis
The following monoclonal antibodies were used: mouse CD4-FITC, CD4-APC, CD27-FITC, CD27-PE, CD62L-PE, CD69-PE, CTLA4-PE, CCR4-PE (all obtained from Becton Dickinson), CD25-FITC (DakoCytomation), CD25-PE (Miltenyi Biotec), and GITR-PE (R&D Systems).
Cell isolation
PBMCs and synovial fluid mononuclear cells (SFMCs) were isolated by Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation. After staining for various surface markers, subpopulations of CD4+ T cells were sorted by FACSVantage (Becton Dickinson). Purity of cell preparations was typically >97%. Monocytes were isolated from PBMCs by positive selection using CD14 microbeads (Miltenyi Biotec). The purified monocytes were cultured for 3–5 d in RPMI 1640 10% FCS containing 50 ng/ml GM-CSF (Leukomax; Novartis) and 1,000 U/ml IL-4.
Proliferation assay
Peripheral blood CD4+CD25− T cells were labeled with 0.5 μM CFSE (Molecular Probes) for 8 min at room temperature. After quenching of the labeling reaction by addition of RPMI 1640 10% FCS, cells were washed extensively. 1.5 × 104 cells were cultured alone or together with different numbers of unlabeled regulatory or control cells in the presence of 103 immature DCs and either 2 ng/ml TSST (Toxin Technology) or 0.25 ng/ml CD3 antibodies (supernatant from clone OKT3). Proliferation was measured on day 4 on a FACSCalibur (Becton Dickinson) using propidium iodide (Sigma-Aldrich) to exclude dead cells. In some experiments, T cells were stimulated with plate-bound CD3 antibodies (2 μg/ml, from clone TR66) in the absence or presence of 100 U/ml of recombinant IL-2 or 2 μg/ml plate-bound CD28 antibodies (BD Biosciences).
Real-time PCR
For quantitative assessment of relative mRNA levels, total RNA was prepared from sorted subpopulations using TRIzol LS reagent (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed using M-MLV RT reverse transcription kit with random hexamer primers (Invitrogen). The relative level of FoxP3 mRNA in each subset was determined by real-time PCR on an ABI PRISM 7700 sequence detector (Applied Biosystems) using the Assay-On-Demand product for FoxP3 detection (Hs00203958_m1) and universal PCR master mix (both obtained from Applied Biosystems). The obtained values were normalized to the amount of 18S rRNA (4310893E; Applied Biosystems) present in each sample. For detection of relative cytokine mRNA levels, sorted T cell subsets were stimulated for 6 h with 50 nM phorbol 12,13-dibutyrate (PdBu; Sigma-Aldrich) and 1 μg/ml anti-CD3 (clone TR66) before extraction of total RNA. Cytokine mRNA levels were determined using Applied Biosystems products for IL-2 (4309882P), TNF (Hs00174128_m1), and IFN-γ (4327052F).
Cytokine detection assays
IL-7 and IL-15 were measured using commercial ELISA (R&D Systems) in the sera and synovial fluid of 30 JIA patients (15 with oligoarticular course and 15 with polyarticular course) and in sera of 12 age-matched controls that were obtained for routinely preoperative examination before minor surgery. The assay detection limit was 0.1 pg/ml for IL-7 and 2 pg/ml for IL-15.
Five-cell PCR
For determination of FoxP3 mRNA at a five-cell level, the four CD4+ T cell subsets were first sorted by flow cytometry. From each of the purified subpopulations, five-cell aliquots were resorted directly into wells of a 96-well conic plate. The subsequent procedures for cDNA preparation and nonspecific cDNA amplification were performed as described by Bigouret et al. (48). 1 μl of the nonspecifically amplified cDNA was used to amplify FoxP3 cDNA with 0.5 μM of the specific primers FoxP3-F (5′-CACCTACGCCACGCTCATC-3′) and FoxP3-R (5′-ACTCAGGTTGTGGCGGATG-3′) (both obtained from Microsynth) in presence of 1.5 mM MgCl2. As a control, the expression of CD3 was assessed using the primers CD3 S1 (5′-CGTTCAGTTCCCTCCTTTTCTT-3′) and CD3 AS1 (5′-GATTAGGGGGTTGGTAGGGAGTG-3′) (Microsynth). The program used for amplification was 3 min at 94°C; 30 s at 94°C, 30 s at 58°C, 30 s at 72°C, 40 cycles.
Immunohistochemistry
Tissue specimens were prepared for immunohistochemistry according to standard technique. In brief, specimens were fixed in 10% formalin for 4 h, dehydrated, and embedded in paraffin. Paraffin serial sections were stained for 30 min at room temperature with mouse antibodies to CD4 (4B12), CD25 (25C04), CD27 (137B4; obtained from Neomarkers), CD20 (L26), and CD3 (polyclonal antisera; obtained from DakoCytomation) followed by anti–mouse Ig antibody conjugated to peroxidase-labeled dextran polymer (EnVision; DakoCytomation) and chromogenic diaminobenzidine substrate (DakoCytomation). Slides were counterstained with Mayer's hematoxylin. For double immunofluorescence, secondary labeling was performed for 30 min at room temperature with Alexa Fluor 594 goat anti–mouse IgG2b (Molecular Probes) and, subsequently, with Alexa Fluor 488 goat anti–mouse IgG1 (Molecular Probes) to label CD25 and CD27, respectively.
Statistical analysis
Differences in the percentages of matched peripheral blood and synovial fluid CD4+CD25+ T cells were analyzed by the Wilcoxon matched pairs signed rank test. Differences in the percentages of CD4+CD25+CD27+ and CD4+CD25+CD27− and in the amounts of IL-7 and IL-15 in oligoarticular and polyarticular JIA patients were analyzed by Mann-Whitney U test.
Online supplemental material
Fig. S1 shows the kinetics of FoxP3 mRNA accumulation in peripheral blood CD4+CD25+regulatory T cells and CD4+CD25− naive and memory T cells upon TCR stimulation. Fig. S2 depicts the amount of FoxP3 mRNA in peripheral blood CD4+ T cells sorted from JIA patients and adult healthy donors according to the expression of CD25 and CD27. Fig. S3 shows the expression of markers associated with regulatory T cells on the synovial CD4+CD25+CD27+ and CD4+CD25+CD27− subset. Fig. S4 depicts the amount of cytokine mRNA produced by synovial CD4+ T cell subsets isolated according to the expression of CD25 and CD27 upon TCR stimulation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050085/DC1.
Acknowledgments
We thank D. Jarrossay for cell sorting, M. Uguccioni for help with immunohistochemistry, and J. Geginat for critical reading of the text.
This work was supported by grants from the National Institute of Health (U19 AI057266-01) and the Swiss National Science Foundation (grant no. 3100-101962).
The authors have no conflicting financial interests.
Abbreviations used: JIA, juvenile idiopathic arthritis; SFMC, synovial fluid mononuclear cell.
C.R. Ruprecht and M. Gattorno contributed equally to this work.
References
- 1.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 2.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. [PubMed] [Google Scholar]
- 3.Apostolou, I., and H. von Boehmer. 2004. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton, A.M., E.E. Donovan, C.A. Piccirillo, and E.M. Shevach. 2004. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J. Immunol. 172:6519–6523. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 7.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 8.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 10.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 11.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 12.Chatila, T.A., F. Blaeser, N. Ho, H.M. Lederman, C. Voulgaropoulos, C. Helms, and A.M. Bowcock. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildin, R.S., F. Ramsdell, J. Peake, F. Faravelli, J.L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20. [DOI] [PubMed] [Google Scholar]
- 14.Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 16.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 17.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann, J., J. Huehn, M. de la Rosa, F. Maszyna, U. Kretschmer, V. Krenn, M. Brunner, A. Scheffold, and A. Hamann. 2002. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc. Natl. Acad. Sci. USA. 99:13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor, P.A., A. Panoskaltsis-Mortari, J.M. Swedin, P.J. Lucas, R.E. Gress, B.L. Levine, C.H. June, J.S. Serody, and B.R. Blazar. 2004. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 104:3804–3812. [DOI] [PubMed] [Google Scholar]
- 22.Ermann, J., P. Hoffmann, M. Edinger, S. Dutt, F.G. Blankenberg, J.P. Higgins, R.S. Negrin, C.G. Fathman, and S. Strober. 2005. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 105:2220–2226. [DOI] [PubMed] [Google Scholar]
- 23.McHugh, R.S., M.J. Whitters, C.A. Piccirillo, D.A. Young, E.M. Shevach, M. Collins, and M.C. Byrne. 2002. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 16:311–323. [DOI] [PubMed] [Google Scholar]
- 24.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruder, D., M. Probst-Kepper, A.M. Westendorf, R. Geffers, S. Beissert, K. Loser, H. von Boehmer, J. Buer, and W. Hansen. 2004. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 34:623–630. [DOI] [PubMed] [Google Scholar]
- 26.Cao, D., V. Malmstrom, C. Baecher-Allan, D. Hafler, L. Klareskog, and C. Trollmo. 2003. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33:215–223. [DOI] [PubMed] [Google Scholar]
- 27.Cao, D., R. van Vollenhoven, L. Klareskog, C. Trollmo, and V. Malmstrom. 2004. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res. Ther. 6:R335–R346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Amelsfort, J.M., K.M. Jacobs, J.W. Bijlsma, F.P. Lafeber, and L.S. Taams. 2004. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 50:2775–2785. [DOI] [PubMed] [Google Scholar]
- 29.Shevach, E.M. 2004. Regulatory/suppressor T cells in health and disease. Arthritis Rheum. 50:2721–2724. [DOI] [PubMed] [Google Scholar]
- 30.Walker, M.R., D.J. Kasprowicz, V.H. Gersuk, A. Benard, M. Van Landeghen, J.H. Buckner, and S.F. Ziegler. 2003. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ CD25- T cells. J. Clin. Invest. 112:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stassen, M., S. Fondel, T. Bopp, C. Richter, C. Muller, J. Kubach, C. Becker, J. Knop, A.H. Enk, S. Schmitt, et al. 2004. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur. J. Immunol. 34:1303–1311. [DOI] [PubMed] [Google Scholar]
- 33.Wang, H.Y., D.A. Lee, G. Peng, Z. Guo, Y. Li, Y. Kiniwa, E.M. Shevach, and R.F. Wang. 2004. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 20:107–118. [DOI] [PubMed] [Google Scholar]
- 34.De Jong, R., M. Brouwer, B. Hooibrink, T. Van der Pouw-Kraan, F. Miedema, and R.A. Van Lier. 1992. The CD27- subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur. J. Immunol. 22:993–999. [DOI] [PubMed] [Google Scholar]
- 35.Hamann, D., P.A. Baars, M.H. Rep, B. Hooibrink, S.R. Kerkhof-Garde, M.R. Klein, and R.A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohem, C.L., R.I. Brezinschek, H. Wisbey, C. Tortorella, P.E. Lipsky, and N. Oppenheimer-Marks. 1996. Enrichment of differentiated CD45RBdim,CD27- memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 39:844–854. [DOI] [PubMed] [Google Scholar]
- 37.Gattorno, M., I. Prigione, S. Vignola, F. Falcini, S. Chiesa, F. Morandi, P. Picco, A. Buoncompagni, A. Martini, and V. Pistoia. 2002. Levels of soluble CD27 in sera and synovial fluid and its expression on memory T cells in patients with juvenile idiopathic arthritides. Clin. Exp. Rheumatol. 20:863–866. [PubMed] [Google Scholar]
- 38.Hintzen, R.Q., R. de Jong, S.M. Lens, M. Brouwer, P. Baars, and R.A. van Lier. 1993. Regulation of CD27 expression on subsets of mature T-lymphocytes. J. Immunol. 151:2426–2435. [PubMed] [Google Scholar]
- 39.Godfrey, W.R., D.J. Spoden, Y.G. Ge, S.R. Baker, B. Liu, B.L. Levine, C.H. June, B.R. Blazar, and S.B. Porter. 2004. Cord blood CD4+CD25+ derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 105:750–758. [DOI] [PubMed] [Google Scholar]
- 40.de Kleer, I.M., L.R. Wedderburn, L.S. Taams, A. Patel, H. Varsani, M. Klein, W. de Jager, G. Pugayung, F. Giannoni, G. Rijkers, et al. 2004. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J. Immunol. 172:6435–6443. [DOI] [PubMed] [Google Scholar]
- 41.Croft, M. 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3:609–620. [DOI] [PubMed] [Google Scholar]
- 42.Baecher-Allan, C., V. Viglietta, and D.A. Hafler. 2002. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J. Immunol. 169:6210–6217. [DOI] [PubMed] [Google Scholar]
- 43.Ling, E.M., T. Smith, X.D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V.A. Carr, and D.S. Robinson. 2004. Relation of CD4+ CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363:608–615. [DOI] [PubMed] [Google Scholar]
- 44.Viglietta, V., C. Baecher-Allan, H.L. Weiner, and D.A. Hafler. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrenstein, M.R., J.G. Evans, A. Singh, S. Moore, G. Warnes, D.A. Isenberg, and C. Mauri. 2004. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens, G.L., R.S. McHugh, M.J. Whitters, D.A. Young, D. Luxenberg, B.M. Carreno, M. Collins, and E.M. Shevach. 2004. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 173:5008–5020. [DOI] [PubMed] [Google Scholar]
- 47.Petty, R.E., T.R. Southwood, J. Baum, E. Bhettay, D.N. Glass, P. Manners, J. Maldonado-Cocco, M. Suarez-Almazor, J. Orozco-Alcala, and A.M. Prieur. 1998. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J. Rheumatol. 25:1991–1994. [PubMed] [Google Scholar]
- 48.Bigouret, V., T. Hoffmann, L. Arlettaz, J. Villard, M. Colonna, A. Ticheli, A. Gratwohl, K. Samii, B. Chapuis, N. Rufer, and E. Roosnek. 2003. Monoclonal T-cell expansions in asymptomatic individuals and in patients with large granular leukemia consist of cytotoxic effector T cells expressing the activating CD94:NKG2C/E and NKD2D killer cell receptors. Blood. 101:3198–3204. [DOI] [PubMed] [Google Scholar]