Abstract
Although emotional responses to stimuli may be automatic, explicit evaluation of emotion is a voluntary act. These bottom-up and top-down processes may be supported by distinct neural systems. Previous studies reported bottom-up responses in the amygdala, top-down responses in the orbital and ventromedial prefrontal cortices, and top-down modulation of the amygdalar response. The current study used event-related fMRI on fifteen healthy males to examine these responses in the absence of stimulus anticipation or task repetition. Factorial analysis distinguished bottom-up responses in the amygdala from top-down responses in the orbitofrontal cortex. Activation of ventromedial prefrontal cortex and modulation of amygdalar response were not observed, and future studies may investigate whether these effects are contingent upon anticipation or cognitive set.
Keywords: Affect, fMRI, event-related, factorial, anticipation
The ability to evaluate our feelings is essential to normal emotional function. Although emotional responses to stimuli may be automatic, explicit evaluation of emotion is a voluntary act. Accordingly, the bottom-up1, or stimulus-driven, and top-down, or task-driven, components of emotional evaluation may be mediated by different neural systems. Studies of emotional evaluation using fMRI have associated bottom-up processing with the amygdala and top-down processing with the orbital and ventromedial prefrontal cortices. Furthermore, some studies reported modulation of the amygdala response under different task conditions, representing top-down modulation of bottom-up processing. However, technical issues with many of these studies (use of blocked designs, which confound responses to stimuli with anticipation, and varying baseline controls tasks) render interpretation somewhat ambiguous. Therefore, the current study uses an optimized event-related emotional evaluation paradigm to attempt to locate distinct and interacting neural responses to the bottom-up and top-down components of emotional evaluation.
Bottom-up responses during emotional evaluation are those associated with the emotional content of the stimuli. The amygdala has been implicated in responses to emotional stimuli by animal studies and by human lesion and imaging studies (Aggleton and Saunders, 2000; Adolphs, 1994; LeDoux, 2000). Activation of the amygdala in imaging studies is commonly associated with perception of visual emotional stimuli such as facial expressions (Breiter et al. 1996; Morris et al. 1996) or unpleasant pictures (Irwin et al., 1996; Reiman et al. 1997), and a meta-analysis of imaging studies found more frequent amygdala responses to visual emotional stimuli than to emotion induced intentionally by recall (Phan et al., 2002). Although early work found responses in the amygdala to unconsciously perceived (backwards masked) emotional faces (Whalen et al., 1998), the extent to which amygdalar responses are automatic or modulatable is a topic of current debate (Pessoa, 2005; Vuilleumier and Portois, 2007). It is proposed that emotional responses may be modulated by a range of strategies, from attentional distraction to cognitive transformation (Ochsner and Gross, 2005). The effects of attentional distraction on neural responses to emotional stimuli are variable, with studies reporting either sparing or reduction of the amygdalar response to emotional faces during visuo-spatial distraction. These differences may be due to varying levels of attentional demands of the distractor task, and varying sensitivity between individuals’ amygdalae to unattended stimuli (Pessoa, 2005). In other studies, cognitive modulation of amygdalar responses was investigated by comparing explicit evaluation of emotional stimuli with passive viewing or non-emotional judgments. Amygdalar responses to unpleasant pictures during explicit emotional evaluation were larger than responses during picture recognition (Liberzon et al., 2000) and self-relevance rating (Phan et al., 2004), and smaller than responses during passive viewing (Taylor et al., 2003). The relationship between amygdalar responses and the subjective experience of emotion was supported by parallel changes in skin conductance responses (Liberzon et al., 2000) and ratings of sadness (Taylor et al., 2003). Although attentional distraction may modulate responses in the amygdala indirectly by restricting its access to bottom-up visual information, explicit evaluation of emotional stimuli may involve direct, top-down modulation of the amygdalar response.
Top-down responses during emotional evaluation are those associated with the knowledge or intention of the participant, for example responses that correlate with instructions to evaluate an emotional stimulus. A region frequently implicated in top-down processing of emotion is the orbitofrontal cortex (OFC). Although the amygdala responds relatively inflexibly to emotional stimuli, responses in the OFC are context-dependent, being modified by changes in satiety state and stimulus-reward association (Rolls, 1999; Zald & Kim, 2001). In functional imaging studies on humans, responses in the OFC were larger during emotional evaluation tasks than during recognition of previously presented pictures (Liberzon et al., 2000), gender judgment (Gorno-Tempini et al., 2001) and passive smelling of pleasant and unpleasant odors (Royet et al., 2003). The OFC is selectively implicated in tasks involving a context-dependent choice between emotional stimuli (Arana et al., 2003; O’Doherty, 2004). Other regions implicated in top-down processing include the ventromedial prefrontal cortex (vmPFC) and anterior cingulate cortex (ACC) (Lane et al., 1997; Taylor et al., 2003; Ochsner et al., 2004).
Functional imaging studies of emotional evaluation have localized distinct and interacting neural responses to the bottom-up and top-down components of emotional evaluation; however, these studies’ designs prevented unambiguous conclusions. In most studies using emotional pictures, stimuli were presented in blocks with the same emotional valence, allowing participants to anticipate the emotional content of prospective images. Thus responses obtained by contrasting blocks of stimuli may have been driven in part by a top-down effect. Although it is clear that the amygdala responds consistently to visually-presented emotional stimuli (Phan et al., 2002), the amygdala may also respond during the anticipation of unpleasant pictures (Ueda et al., 2003). Furthermore, instructing individuals to regulate their emotional responses has been shown to modulate amygdalar activity during anticipation but not during perception of emotion (Erk et al., 2006).
In two exceptional studies, emotional pictures of differing valence were presented randomly, preventing anticipation (Phan et al., 2004, Grimm et al., 2006). In the first study, self-relevance judgment was used as a control task (Phan et al., 2004) Because emotional evaluation is thought to include an assessment of self-relevance (Eysenck, 2000), this study cannot be compared directly with those using a non-emotional judgment as a control task. Furthermore task instructions were varied on the block level rather than the event level, making the detection power of task- and stimulus-related responses unequal. In the second event-related study, both task and stimulus were varied at the event level (Grimm et al., 2006). Arbitrary button presses were used as a control task, which do not require a judgment and may therefore facilitate amygdala activation by general release of attentional resources rather than specific changes in top-down regulation of emotion.
In the current study, therefore, participants performed an emotion rating task in which pleasant and unpleasant pictures were presented in random order, allowing for observation of amygdala activity in the absence of anticipation (a top-down process). Furthermore, to maximize the comparability across conditions designed to test for top-down effects, we compared emotional rating of pictures with non-emotional rating (frequency of appearance of television). These two conditions permitted a contrast in which pictures are attended, numerical judgments generated, and motor responses made in both cases, but only one condition required emotional evaluation. Both stimulus valence and task instructions were randomized between trials, equalizing the temporal variance of the two factors, and consequently the detectability of the resulting hemodynamic responses (Liu et al., 2001). The neural responses to the two factors were mapped using ANOVA, and to fully account for non-linear relationships between task and brain activity, both the main effects and the interaction were modeled (Friston et al., 1996). Thus distinct bottom-up and top-down responses would be detected as main effects of stimulus valence and task instructions, and brain regions in which bottom-up responses were modulated by top-down effects (e.g. the amygdala) would be detected as an interaction. This approach extends previous work by using a fully-balanced paradigm to investigate the bottom-up and top-down components of emotional evaluation. In accordance with previous studies, we hypothesized that top-down responses would be observed in the orbital and ventromedial prefrontal cortices and that bottom-up responses would be observed in the amygdala. We also tested whether bottom-up responses in the amygdala would be modulated by top-down effects.
Methods
Subjects
Sixteen healthy male participants gave informed consent as approved by the University of Florida’s Institutional Review Board. The participants’ ages ranged from 18 to 24 (M = 19.67, SD = 1.63). The participants had no history of psychiatric or neurological illness, and were taking no psychotropic medication at the time of the study. One participant was excluded due to discrete head movements greater than 1mm during scanning.
Picture Rating Task Paradigm
Participants viewed pictures from the International Affective Picture System (IAPS) (Center for the Study of Emotion and Attention [CSEA-NIMH], 2001) in two categories: pleasant or unpleasant. IAPS pictures are rated in two dimensions, valence (pleasant/unpleasant) and arousal (exciting/calm) (Lang et al., 2001). Pictures in the pleasant set received high pleasure scores (6.7 +/− 0.9, mean +/− standard deviation, scale from 1–9) and pictures in the unpleasant set received low pleasure scores (3.7 +/− 1.1); both categories received similar arousal scores (pleasant: 4.7 +/− 1.0, unpleasant: 4.8 +/− 1.3). IAPS picture codes are listed in supplementary data. Each picture appeared above one of two task instructions, either “How pleasant do you find the content of this image?” or “How frequent do images with similar content appear on television?” Participants were instructed to indicate their response to each picture by pressing one of four buttons, indicating in the emotion rating task, “very unpleasant”, “moderately unpleasant”, “moderately pleasant”, or “very pleasant”, and in the frequency rating task, “weekly”, “daily”, “hourly”, or “continuously”. We designated the four trial types emotion rating pleasant (EP), emotion rating unpleasant (EU), frequency rating pleasant (FP), and frequency rating unpleasant (FU).
Prior to performing the task, each participant was familiarized with the scanner environment and response system by completing a training run consisting only of emotion ratings. Different sets of pictures were used for the training and task runs. The effects of training are reported elsewhere, in a study of the effects of training upon emotional and non-emotional ratings (Li, H., Albarracin, D., Wright, P., Brown, R. D., & Liu, Y. (2007). Evaluation Proceduralization and its Neural Correlates. In preparation).
The rating tasks were presented using a rapid event-related design. Fifteen trials of each type and 30 null trials were presented in random order. Pictures to be rated and rating instructions were presented simultaneously in contiguous three-second trials, and the entire run lasted 4 min 30 sec. Including 30, three-second null trials randomized the stimulus onset asynchrony in a geometric distribution with a mean of 4.5 sec. Jittering trial timing in this way increases the detectability of task-related responses in event-related fMRI (Burock et al., 1998), and is most efficient using a geometric distribution (Serences, 2004). For emotional stimuli, a stimulus onset asynchrony with a mean of 4.5 sec maximizes trial presentation rate while minimizing response attenuation due to stimulus repetition (Soon et al., 2003).
The stimuli were presented using an Integrated Functional Imaging System (IFIS, MRI Devices, Inc., Waukesha, WI). Images were generated by a PC running E-Prime (Psychology Software Tools, Pittsburgh, PA) in synchronization with the first RF pulse of each scan. Participants viewed images at 640 × 480 pixel resolution on a 7” LCD screen that subtended approximately 14° × 11° of the visual field, via a mirror mounted on the head coil. Responses were collected with a MRI-compatible button glove attached to the participant’s right hand.
Functional Imaging Data Acquisition
Participants were scanned using a 3 Tesla Siemens Allegra scanner with a standard head coil (Siemens, Munich, Germany). Anatomic images were acquired using an MPRAGE sequence with TR = 1500 ms, TE = 4.38 ms, and flip angle = 8°. In the axial plane, 160 slices were acquired (thickness 1.0–1.2 mm, according to the height of the brain) with in-plane field of view 240 mm × 180 mm and matrix size 256 × 192. Functional images covering the whole brain were acquired using echo-planar imaging sensitive to blood-oxygenation level dependent (BOLD) effects, with TR = 3000 ms, TE = 30 ms, flip angle = 90°. In the axial plane, 38 slices with a thickness of 3.8 mm were acquired in the plane of the intercommissural line with an in-plane field of view 240 × 240 mm and matrix size 64 × 64. The first two volumes of each functional run were discarded to allow for T1 equilibration. These settings have previously been shown to provide reasonable coverage of the amygdala while allowing coverage of the whole brain, and without sacrificing BOLD sensitivity (Wright and Liu, 2005). We inspected the functional images using an outline of the amygdala drawn on the average anatomic image according to the guidelines of Brierley et al. (Brierley et al., 2002), and found full coverage in ten out of fifteen participants, with partial coverage in the remaining five. Because our hypotheses predicted responses in the vmPFC, coverage of this region was also inspected. Susceptibility artifact was seen, but due to the large extent of this region, coverage was determined post-hoc within clusters of activation, by comparing mean signal for each participant within the vmPFC and other activated regions.
Functional Imaging Data Analysis
Data were analyzed using BrainVoyager QX version 1.7.6 (Brain Innovations, Maastricht, Holland). The functional images were coregistered with anatomic images and normalized to Talairach space for each participant. Functional data underwent 3D motion correction, linear trend removal, slice scan time correction, and Gaussian spatial smoothing using a kernel of 5.7 mm (1.5 voxels) full-width half-maximum (FWHM).
Task-related activity was mapped using a voxel-wise general linear modeling analysis. The BOLD responses to each trial type were estimated by convolving the stimulus time course with a canonical hemodynamic model (Friston et al., 1998). The estimated responses were combined in a multiple regression model of the MR signal at each voxel, generating beta weights reflecting the magnitude of the contribution of each trial type to the overall model. Second-level comparisons of the beta weights at each voxel generated random-effects statistical maps. Two-way repeated-measures ANOVA was used to estimate separately the main effects of task instructions and stimulus valence, and their interaction. This approach avoids the assumption of pure insertion inherent in linear contrasts by accounting for non-linear neural responses to different combinations of cognitive factors (Friston et al., 1996). For whole-brain analysis, thresholds were set to exclude clusters smaller than 100 mm3 (after functional data were resampled to 1 mm resolution), and statistical scores below F (1,14) = 12, p < 0.005. In order to identify the most reliable responses, we calculated the minimum cluster size necessary to achieve a false activation probability α = 0.05, using the cluster threshold estimator plugin for BrainVoyager QX (Forman et al., 1995). This procedure excluded clusters smaller than 662 mm3. Clusters between 100 and 661 mm3 are reported to facilitate comparison with other studies (Poline et al., 2006). Because activation was hypothesized a priori in the amygdala, the statistical threshold in this region was lowered to F(1,14) = 5, p < 0.05. At each cluster of activation, mean signal for all cluster voxels was entered into post-hoc contrasts to produce t-scores indicating the direction of the effect.
Mean BOLD responses were plotted for selected clusters of activation. Percentage signal change was calculated relative to the signal at the time of stimulus onset. Percentage values were then averaged by stimulus type across trials and participants in a time window from −3 to 18 seconds relative to stimulus onset. Contamination from subsequent stimuli occurring within the 18-second window was eliminated in the overall average due to the jittered SOA (Dale and Buckner, 1997).
Results
Behavioral Data
Participants rated pleasant and unpleasant stimuli appropriately. Pleasure ratings, adjusted from the obtained four-button responses to the standard scale used in the IAPS of 1–9 were significantly higher for pleasant pictures than for unpleasant pictures (6.6 +/− 0.9 vs. 3.6 +/− 0.8, p < 0.001). Participants made use of all four buttons during the emotion rating task, rather than simplifying the task by using only the index finger for unpleasant and the little finger for pleasant pictures (Table 1). In the frequency rating task, participants judged pictures as shown “weekly”, “daily”, or “hourly” more frequently than “continuously”. Response times were slower during frequency rating than emotional evaluation, regardless of stimulus valence, implying that the control task was more difficult (Table 1). Because this difference in response time may confound the effect of task instructions with increased performance effort, we included response time as a confound in the general linear model. For each participant, the time series of response times for individual trials was z-normalized and convolved with the canonical hemodynamic response function (responses times < 500 ms were excluded). The resulting predictor was included in the general linear model to reduce the influence factors associated with reaction time, such as performance effort, on the estimated response to each trial type, and in particular on the resulting contrast between emotional and non-emotional evaluation.
Table 1.
Behavioral responses
| Responses per trial (% (SD))
|
|||||
|---|---|---|---|---|---|
| Trial type | Button 1 | Button 2 | Button 3 | Button 4 | RT (ms) |
| EP | 46 (21) | 38 (23) | 9 (7) | 1 (2) | 1902 (295) |
| EU | 6 (7) | 19 (10) | 44 (16) | 26 (18) | 1899 (265) |
| FP | 24 (16) | 43 (17) | 20 (19) | 4 (9) | 2088 (263) |
| FU | 16 (14) | 43 (16) | 24 (14) | 9 (9) | 2087 (295) |
EU: emotion rating on unpleasant pictures, EP: emotion rating on pleasant pictures, FU: frequency rating on unpleasant pictures, FP: frequency rating on pleasant pictures. Responses < 500 ms were excluded from RT analysis as they most likely reflected carried-over late responses to previous trials. Two-way repeated measures ANOVA of reaction time using the factors task and valence revealed a significant main effect of task (F (1, 14) = 36.5, p < 0.001).
fMRI Data
We hypothesized that top-down responses would be observed in the orbital and ventromedial prefrontal cortices and that bottom-up responses would be observed in the amygdala. We localized these responses using two-way repeated measures ANOVA to create statistical maps showing main effects of stimulus valence and task instructions. We also tested whether bottom-up effects in the amygdala was modulated by top-down effects by mapping interaction effects.
A main effect of stimulus valence was observed in the left amygdala (Figure 1, Table 2). Post-hoc statistics indicated a larger response to unpleasant than pleasant pictures, and examination of BOLD responses indicated that this effect was independent of task instructions. MR signal values in this region for all participants were within one standard deviation of mean signal values from all other regions showing a main effect of stimulus valence (Table 2), indicating that susceptibility effects did not affect signal within the activated cluster. A main effect of rating task was observed in the left OFC (Figure 2, Table 3). Post-hoc statistics indicated a larger response to emotional evaluation than to frequency rating, and BOLD responses indicated that this effect was independent of stimulus content (Figure 2C). In order to test whether the responses in the amygdala and OFC were truly independent, post-hoc region-of-interest ANOVA was performed to assess the orthogonal effect and interaction. In neither region were these significant. In the amygdala, main effect of task and interaction reached F(1,14) = 0.2 & 0.3, p = 0.6, 0.5 respectively. In the orbitofrontal cortex main effect of valence and interaction reached F(1,14) = 0.1 & 0.6, p = 0.7 & 0.5 respectively.
Figure 1.
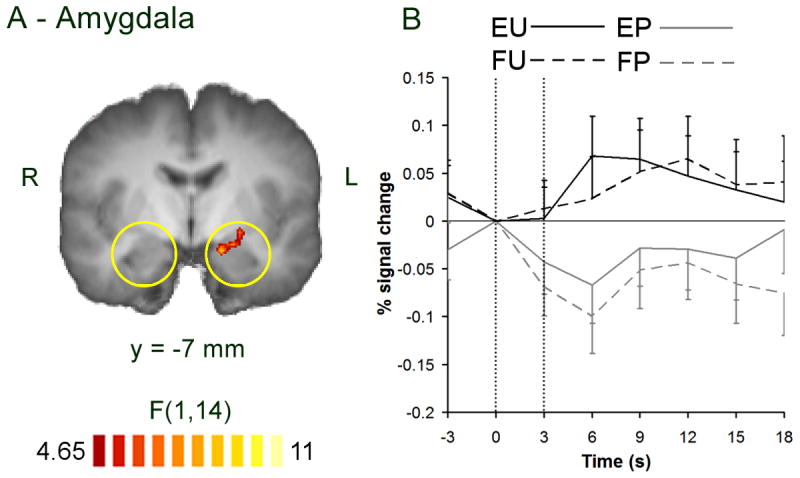
Main effect of stimulus valence. A) reduced-threshold statistical map showing a main effect of valence at the left amygdala. Responses are only shown inside the a priori regions of interest (yellow circles). Color scale indicates F score on two-way ANOVA, threshold: p < 0.05. L: left. R: right. Y: position of coronal slice in Talairach space. B) BOLD response in the left amygdala. Error bars show standard error. Vertical dotted lines show beginning and end of trial. EP: emotion rating on pleasant pictures, EU: emotion rating on unpleasant pictures, FP: frequency rating on pleasant pictures, FU: frequency rating on unpleasant pictures.
Table 2.
Main effects of stimulus valence and interaction effect.
| Region | Side | BA | x | y | z | Size | F(1,14) | t(14) |
|---|---|---|---|---|---|---|---|---|
| Pleasant > Unpleasant | ||||||||
| Posterior cingulate cortex | R | 30 | 9 | −52 | 12 | 1151 | 38.8 | 5.11 |
| Posterior cingulate cortex | L | 30 | −9 | −54 | 10 | 404 | 18.5 | 4.20 |
| Declive | L | −6 | −85 | −20 | 170 | 22.7 | 4.34 | |
| Unpleasant > Pleasant | ||||||||
| Middle frontal gyrus | L | 6 | −35 | 3 | 62 | 546 | 38.4 | −6.35 |
| Amygdala | L | −19 | −7 | −10 | 164 | 10.0 | −3.57 | |
| Inferior parietal lobule | L | 40 | −56 | −39 | 53 | 104 | 16.4 | −4.38 |
| Inferior occipital gyrus | L | 19 | −40 | −74 | −8 | 622 | 36.9 | −5.76 |
| Interaction effect | ||||||||
| Middle frontal gyrus | R | 9 | 36 | 20 | 36 | 421 | 27.9 | 5.12 |
Only clusters > 100 mm3 shown. Bold text: cluster meets criteria for false positive rate α = 0.05 (cluster size > 662). Italic text: cluster identified at reduced threshold (p < 0.05) in a priori region (amygdala). MCC: midcingulate cortex. L: left, R: right. BA: Brodmann Area. X, Y & Z: Talairach co-ordinates (mm). Size: number of 1 mm3 voxels. F: result of whole-brain ANOVA (degrees of freedom); score is taken from the peak voxel of the cluster. t (degrees of freedom): post-hoc contrast of main effect of valence [(EP + FP) − (EU + FU)] or interaction effect [(EP − EU) − (FP − FU)]; score is taken from mean signal of all cluster voxels. EU: emotion rating on unpleasant pictures, EP: emotion rating on pleasant pictures, FU: frequency rating on unpleasant pictures, FP: frequency rating on pleasant pictures.
Figure 2.

Main effect of task instructions. A & C: statistical maps showing a main effect of task in the left orbitofrontal cortex (A) and left fusiform gyrus (C). Color scale indicates F score on two-way ANOVA, threshold: p < 0.005, minimum cluster size = 662 (estimated false positive rate α = 0.05). L: left. R: right. Z: position of axial slice in Talairach space. B & D: BOLD responses in the left orbitofrontal cortex (B) and left fusiform gyrus (D). Error bars show standard error. Vertical dotted lines show beginning and end of trial. EP: emotion rating on pleasant pictures, EU: emotion rating on unpleasant pictures, FP: frequency rating on pleasant pictures, FU: frequency rating on unpleasant pictures.
Table 3.
Main effects of task instructions.
| Region | Side | BA | X | Y | Z | Size | F(1,14) | t(14) |
|---|---|---|---|---|---|---|---|---|
| Emotion > Frequency | ||||||||
| Orbitofrontal cortex | L | 47 | −26 | 34 | −8 | 873 | 46.9 | 7.47 |
| Precentral gyrus | L | 6 | −59 | −1 | 11 | 138 | 14.1 | 4.07 |
| Superior temporal gyrus | L | 42 | −63 | −12 | 7 | 287 | 17.1 | 4.35 |
| Fusiform gyrus | R | 37 | 27 | −45 | −10 | 135 | 17.6 | 4.08 |
| Fusiform gyrus | L | 37 | −30 | −53 | −13 | 1023 | 35.8 | 5.52 |
| Precuneus | L | 7 | −13 | −53 | 51 | 294 | 25.0 | 4.88 |
| Posterior cingulate cortex | R | 30 | 18 | −53 | 13 | 211 | 18.2 | 4.20 |
| Superior occipital gyrus | R | 18 | 25 | −79 | 20 | 302 | 18.0 | 4.10 |
| Superior occipital gyrus | L | 19 | −26 | −82 | 19 | 618 | 22.1 | 4.86 |
| Frequency > Emotion | ||||||||
| Subgenual ACC | L | 25 | −1 | 20 | −8 | 134 | 31.8 | −5.21 |
| Middle frontal gyrus | L | 9 | −42 | 16 | 28 | 655 | 28.5 | −5.97 |
| Middle frontal gyrus | L | 6 | −35 | 3 | 52 | 991 | 31.2 | −5.06 |
| Posterior cingulate cortex | L | 31 | −3 | −37 | 35 | 256 | 19.2 | −4.06 |
| Middle temporal gyrus | L | 21 | −61 | −40 | −1 | 1244 | 40.7 | −6.31 |
| Posterior cingulate cortex | R | 30 | 5 | −47 | 18 | 249 | 20.2 | −4.27 |
| Intraparietal Sulcus | R | 19 | 42 | −66 | 40 | 1806 | 20.0 | −4.82 |
| Intraparietal Sulcus | L | 19 | −41 | −69 | 36 | 1292 | 11.1 | −5.20 |
| Cerebellum (uvula) | R | 11 | −82 | −36 | 523 | 22.9 | −4.31 | |
Only clusters > 100 mm3 shown. Bold text: cluster meets criteria for false positive rate α = 0.05 (cluster size > 662). ACC: anterior cingulate cortex. L: left, R: right. BA: Brodmann Area. X, Y & Z: Talairach co-ordinates (mm). Size: number of 1mm3 voxels. F: result of whole-brain ANOVA (degrees of freedom); score is taken from the peak voxel of the cluster. t (degrees of freedom): post-hoc contrast of main effect of valence [(EP + FP) − (EU + FU)] or interaction effect [(EP − EU) − (FP − FU)]; score is taken from mean signal of all cluster voxels. EU: emotion rating on unpleasant pictures, EP: emotion rating on pleasant pictures, FU: frequency rating on unpleasant pictures, FP: frequency rating on pleasant pictures.
A main effect of task was not observed in the vmPFC, but a small region in the subgenual anterior cingulate cortex (BA 25) showed larger responses to frequency rating than emotional evaluation. BOLD responses in the region did not, however, show a clear effect of task. Furthermore, MR signal values in this region were affected by susceptibility artifact, being reduced by more than two standard deviations in five participants, and between one and two standard deviations in seven participants, relative to mean signal values for all other regions showing a main effect of task (Table 3).
Several regions other than those predicted showed a main effect of valence, notably the bilateral posterior cingulate cortices, left inferior occipital gyrus, and middle frontal gyrus (Table 2). In the posterior cingulate cortices, post-hoc statistics indicated a greater response to pleasant than unpleasant pictures, and BOLD responses indicated that this effect was due to selective deactivation during emotional evaluation of unpleasant pictures. In the left inferior occipital gyrus, post-hoc statistics indicated a larger response to unpleasant than pleasant pictures, and BOLD responses indicated a response to all four stimulus types, with a slight increase in magnitude for unpleasant pictures. In the middle frontal gyrus, post-hoc statistics indicated a larger response to unpleasant than pleasant pictures, but BOLD responses indicated a larger response to both picture types during frequency rating than during emotional evaluation.
A main effect of task was seen in several regions other than those predicted (Table 3). Post-hoc statistics indicated larger responses to emotional evaluation than frequency rating in the bilateral fusiform gyri and superior occipital gyri. BOLD responses in these regions indicated responses to all four stimulus types, with small increases during emotional evaluation. Figure 2 (B & D) illustrates the largest of these response in the left fusiform gyrus. Post-hoc statistics indicated a larger response to frequency than emotion rating in the bilateral intraparietal sulci, left middle temporal gyrus, and two regions within the left middle frontal gyrus. In the intraparietal sulci and middle temporal gyrus, BOLD responses indicated a negative response to emotional evaluation, with a small positive response to frequency rating. In the middle frontal gyri, BOLD responses indicated a positive response to frequency rating, regardless of stimulus valence.
Discussion
In this study, we confirmed that distinct neural networks responded to top-down and bottom-up components of emotional evaluation. As hypothesized, top-down effects were observed in the orbitofrontal cortex (OFC), and bottom-up effects in the amygdala. Contrary to previous studies, we did not observe top-down responses in the ventromedial prefrontal cortex (vmPFC), and we observed no modulation of the amygdala. Using an event-related paradigm to investigate emotional evaluation, we replicated some, but not all, of the neural responses obtained using block-design paradigms. These differences and possible future directions are discussed.
A bottom-up response was observed in the left amygdala: responses in this region were larger for unpleasant than pleasant pictures, regardless of task instructions (Figure 1). Unlike several previous studies, this response was not modulated by explicit evaluation of emotion (Liberzon et al., 2000; Taylor et al., 2003; Phan et al., 2004). It is possible that modulation of responses in the amygdala reflects modulation of anticipated emotion that occurs during blocked designs. Interestingly, in the studies using a blocked design, the modulated response occurred in the right amygdala (Liberzon et al., 2000; Taylor et al., 2003), consistent with a previously reported right-sided amygdalar response to anticipated emotion (Ueda et al., 2003). It is also possible that the amygdalar response in the current study was altered following the training period (see Methods). A previous study reported a shifting response from right to left amygdala during repeated viewing of emotional faces (Gur et al., 2002), which the authors suggested reflected a shift towards more cognitive processing of stimuli. Furthermore, training in emotional evaluation increases the likelihood that an individual will spontaneously evaluate stimuli (Li et al., 2007, in preparation). Therefore it is possible that the observed responses in the left amygdala reflect automatic cognitive or associative processing of stimulus features that occurred even when individuals were instructed to make frequency ratings. It should be noted, however, that the lateralization of amygdala responses is a topic of current debate: clear associations between laterality and processing style have yet to be confirmed (Baas et al., 2004). Finally, the unpleasant stimuli used in the current study may have elicited a weaker amygdalar response than those used in previous studies. The current unpleasant stimuli were less negatively valenced than those used in some other studies (3.7 vs. approximately 2.0), and we compared unpleasant and pleasant pictures to obtain a full range of ratings, whereas previous studies almost all compared unpleasant and neutral pictures. Although contrasting unpleasant and pleasant pictures results in a larger difference in valence than contrasting unpleasant and neutral pictures, the difference in amygdalar response may be smaller, since pleasant pictures have been shown to activate the amygdala relative to uninteresting neutral pictures (Hamann et al., 2002). However, the negative BOLD responses to pleasant pictures indicate that this was not the case in the current study (Figure 1).
Contrary to the current findings, two previous studies found responses in the amygdala to randomly-presented emotional pictures that were modulated by explicit evaluation (Phan et al., 2004, Grimm et al., 2006). This modulation effect cannot be explained in terms of stimulus anticipation, but these studies differed from the current study in several respects. First, the contrasts used to detect responses in the amygdala were not comparable between the current study and either of the two previous studies: the study by Phan et al. identified amygdalar responses that correlated with emotional intensity, which is high for both pleasant and unpleasant pictures and low for neutral pictures, and the study of Grimm et al. focused on contrasts between task conditions (emotional judgment vs. arbitrary button press) and not between stimuli with different emotional content. A future study may include pleasant, unpleasant, and neutral pictures to test whether amygdalar responses driven by emotional intensity or by emotional valence are differentially susceptible to top-down modulation. Second, all three studies differed in the timing of task presentation. In the current study task instructions were varied from trial to trial, whereas in Phan et al., the two tasks were presented in two contiguous epochs. It is therefore possible that the modulating effect of emotional evaluation requires the establishment of a cognitive set over repeated trials of the same task. Grimm et al. varied task instructions on a trial-by-trial level, but the stimulus onset asynchrony was longer than in the current study (10–12 sec versus mean 4.5 sec in the current study). This may have increased sensitivity to task-related modulation of the amygdalar response by reducing overlap of the hemodynamic responses or by reducing carry-over effects between trials. It is interesting to note that in Grimm et al., the response in the left amygdala differed between emotional evaluation and simple viewing only when task instructions were unanticipated, inviting further study of the effect of anticipation upon top-down processing of emotion. Finally, because the control task used by Grimm et al. did not involve a judgment, differences in the amygdalar response may be attributed to the greater availability of attentional resources during the less demanding control task, rather than to a specific release from top-down inhibition.
Top-down responses were observed in the left orbitofrontal cortex (OFC): responses in these regions were larger during emotional evaluation than during frequency rating (Figure 2). This OFC response accords with those reported in previous studies (Liberzon et al., 2000; Gorno-Tempini et al., 2001; Royet et al., 2003) and supports the notion that the OFC is crucial for the selection of responses based on the emotional value of a stimulus (Zald & Kim, 2001). Post-hoc testing confirmed that the responses in the OFC and amygdala were orthogonal: there was no significant main effect of valence in the OFC, and no significant main effect of task in the amygdala, and neither region showed a significant interaction. These results suggest that top-down and bottom-up components of emotional evaluation are dissociated between these two regions.
Top-down effects were not observed in the ventromedial prefrontal cortex (vmPFC), as reported in some (Lane et al., 1997; Taylor et al., 2003; Phan et al., 2004), but not all previous studies of emotional evaluation (Liberzon et al., 2000; Gorno-Tempini et al., 2001). The vmPFC and OFC have been implicated in the selection of behavioral responses on the respective bases of internal feelings and external stimuli (Damasio, 1994; Bechara et al., 2000; Zald & Kim, 2001). In the current study, the speed of stimulus and task variation may have biased participants toward an external feature-based strategy for emotional evaluation, rather than a strategy involving attention to internal feelings. It is interesting to note that in macaque experiments, neurons in the lateral OFC responded more readily to visual stimuli (which may vary rapidly) but neurons in the medial OFC responded more readily to olfactory stimuli (which typically vary slowly) (Zald and Kim, 2001). The vmPFC is also implicated in the extinction of responses in the amygdala (Sotres-Bayon et al., 2004) and intentional regulation of emotion (Ochsner & Gross, 2005). It is possible, therefore, that the absence of interaction effects in the current study is related to a lack of regulatory input from the vmPFC to the amygdala.
These findings suggest a number of methodological issues that may be investigated in future studies. First, responses to emotional stimuli are described in two dimensions: valence and arousal (Lang et al., 2001). To test whether bottom-up responses related to these two factors are differentially susceptible to modulation by emotional evaluation, future studies may include pleasant, unpleasant, and neutral pictures. Second, including a training period may result in changes in the neural responses to emotional stimuli. Future studies may include both emotional and non-emotional evaluation in the training period, and investigate how the neural correlates of each task evolve over the training period. Third, the current task design did not elicit activation of the vmPFC or modulation of amygdala responses. This result may have been due to the random presentation of emotional stimuli, which prohibited anticipation, or to the rapid variation in task instructions. Although the stimulus onset asynchrony was chosen to allow detection of responses to visual emotional stimuli (Serences, 2004), it is possible that task-related responses evolve more slowly than stimulus-related responses. Alternatively, the regulatory effects associated with emotional evaluation may occur only when evaluative and control tasks are alternated in blocks, allowing establishment of a cognitive set associated with evaluation. Future studies may compare anticipated and unanticipated emotional stimuli side-by-side, and may also investigate the optimum timing for the presentation of task instructions.
Both bottom-up and top-down responses were observed in regions additional to those hypothesized. Visual activation in the fusiform and occipital gyri was modulated both by stimulus content and task instructions. This finding is consistent with previous work demonstrating the effects of emotion and attention on visual responses (Vuilleumier and Driver, 2007). The effect of emotional stimulus content on visual activation is likely to be mediated by feedback from the amygdala to visual cortex (Morris et al., 1998; Sabatinelli et al., 2005). An apparent response to pleasant pictures was observed in the bilateral posterior cingulate cortices, although BOLD responses in this region indicated deactiviation in response to unpleasant pictures only during emotional evaluation. The posterior cingulate cortex is part of a network of regions implicated in task-induced deactivation, and is posited to mediate attention-dependent processing during the conscious resting state (McKiernan et al., 2003). Deactivation of this region during rating of unpleasant pictures suggests that this task condition more than the others disrupts resting cognition.
Top-down responses were observed in the bilateral parieto-occipital sulci: responses in these regions were larger during frequency rating than emotional evalution, regardless of stimulus valence. Similar responses were reported in previous emotion rating studies during the spatial control task (indoor/outdoor judgment) consistent with the role of the parietal lobe in visuo-spatial attention (Lane et al., 1997; Ochsner et al., 2004). In the current study, the control task did not involve an explicit spatial judgment, however, the parietal cortex is an associative region, receiving multimodal inputs, and being activated by a wide range of cognitive tasks (Culham and Kanwisher, 2001). A recent meta-analysis of mathematical studies associated the region of the intraparietal sulcus activated in the current study with attentional orientation along a mental “number line” (Dehaene et al., 2004). Thus, the activation in the current study may reflect the numerical approximation involved in the frequency judgment task.
In conclusion, the current study showed that bottom-up responses in the amygdala and top-down responses in the OFC may be dissociated using an event-related emotional evaluation paradigm. Responses in the amygdala were not modulated by varying task instructions, as in some previous studies, warranting future investigation into the role of anticipation and task timing in the potential regulatory effects of explicit emotional evaluation.
Supplementary Material
Acknowledgments
This research was facilitated by grants R03 MH072776, R01 NR08325 and K02 MH075616 from the National Institutes of Health.
We thank Profs. Christiana Leonard, Dawn Bowers, and Russell Bauer for helpful suggestions.
Footnotes
Generation of emotional responses to stimuli may require associative processing determined by the individual’s experience and therefore may include “top-down” components, but for brevity, we refer to neural responses that correlate with changes in stimulus content as “bottom-up”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The amygdala. Oxford: Oxford University Press; 2000. [Google Scholar]
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable Contributions of the Human Amygdala and Orbitofrontal Cortex to Incentive Motivation and Goal Selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev. 2002;39:84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention [CSEA-NIMH] The international affective picture system: Digitized photographs. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective Averaging of Rapidly Presented Individual Trials Using fMRI. Hum Brain Map. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Reason, and the human brain. New York: GP Putnam’s Sons; 1994. [Google Scholar]
- Dehaene S, Molko N, Cohen L, Wilson AJ. Arithmetic and the brain. Curr Opin Neurobiol. 2004;14:218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Erk S, Abler B, Walter H. Cognitive modulation of emotion anticipation. European J Neurosci. 2006;24:1227–1236. doi: 10.1111/j.1460-9568.2006.04976.x. [DOI] [PubMed] [Google Scholar]
- Eysenck W, Keane MT. Cognitive psychology. Philadelphia: Taylor and Francis; 2000. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ. The trouble with cognitive subtraction. Neuroimage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Pradelli S, Serafín M, Pagnoni G, Baraldi P, Porro C, Nicoletti R, Umita C, Nichelli P. Explicit and incidental facial expression processing: an fMRI study. Neuroimage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, Hell D, Boesinger P, Boeker H, Northoff G. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex–an fMRI study. Neuroimage. 2006;30:325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstacy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport. 1996;7:1765–1769. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. Technical report A-5. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S. Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology. 2000;23:508–516. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficienct, and predictability in event-related fMRI. NeuroImage. 2001;13:759–773. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41:585–96. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- O’Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Poline JB, Strother SC, Dehaene-Lambertz G, Egan GF, Lancaster JL. Motivation and synthesis of the FIAC experiment: Reproducibility of fMRI results across expert analyses. Hum Brain Map. 2006;27:351–359. doi: 10.1002/hbm.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The brain and emotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20:713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Serences JT. A comparison of methods for characterizing the event-related BOLD timeseries in rapid fMRI. Neuroimage. 2004;21:1690–1700. doi: 10.1016/j.neuroimage.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Soon CS, Venkatraman V, Chee MW. Stimulus repetition and hemodynamic response refractoriness in event-related fMRI. Hum Brain Map. 2003;20:1–12. doi: 10.1002/hbm.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional Perseveration: An Update on Prefrontal-Amygdala Interactions in Fear Extinction. Learning and Memory. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Ueda K, Okamoto Y, Okada G, Yamashita H, Hori T, Yamawaki S. Brain activity during expectancy of emotional stimuli: an fMRI study. Neuroreport. 2003;14:51–55. doi: 10.1097/00001756-200301200-00010. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007 doi: 10.109/rstb.2007.2092. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Liu Y. Neutral faces activate the amygdala during identity matching. Neuroimage. 2005;29:628–636. doi: 10.1016/j.neuroimage.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. The Orbitofrontal Cortex. In: Salloway SP, Malloy PF, Duffy JD, editors. The Frontal Lobes and Neuropsychiatric Illness. Washington, DC: American Psychiatry Publishing, Inc; 2001. pp. 33–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


