Abstract
Objective
To investigate the movement and muscle activation strategies during walking of individuals with medial knee osteoarthritis (OA) to determine the influence of quadriceps strength, medial knee laxity, limb alignment, and self-reported knee instability on knee motion.
Methods
Twenty-eight persons with medial knee OA and 26 control subjects participated. Quadriceps strength, medial knee laxity, and limb alignment were measured. Knee instability (IKOS score) was assessed with the Activities of Daily Living Scale of the Knee Outcome Survey. Knee motion and muscle activation patterns were measured with motion analysis. Group differences were detected with independent samples t-tests and predictive relationships were determined with linear and hierarchical regression analyses.
Results
Individuals with OA were weaker, had greater medial knee laxity, and had more varus alignment. The OA group used less knee motion and higher muscle co-contraction during weight acceptance and single-limb support. Quadriceps strength and IKOS score significantly strengthened the prediction of knee motion during weight acceptance and single-limb support, whereas limb alignment and medial laxity did not.
Conclusion
The knee stiffening and higher muscle co-contraction used by the OA group may be detrimental to joint integrity. IKOS scores predicted knee motion after accounting for quadriceps strength, underscoring the importance of addressing knee instability with appropriate rehabilitation strategies in persons with medial knee OA in order to promote long-term joint integrity.
Keywords: Knee stability, Muscle activity, Osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is one of the most common and disabling medical conditions in the US and worldwide (1-4). The tibiofemoral joint is commonly involved and osteoarthritic changes are more prevalent in the medial compartment than the lateral compartment (5,6). The role of joint mechanics in the development and progression of OA is becoming better understood as many studies have investigated the walking patterns of persons with knee OA. Hallmark gait deviations of patients with medial knee OA include high knee adduction moments and reduced knee flexion that are associated with varus alignment and quadriceps weakness, respectively (7-12). Much less information is known about the corresponding muscle activation patterns or about the impairments that influence movement patterns. Further insight into the movement and muscle activation strategies of individuals with knee OA will facilitate the development of effective rehabilitation interventions.
During walking, knee flexion during weight acceptance is controlled by eccentric action of the quadriceps (13) and weak muscles are associated with limited knee motion (14). Reduced knee flexion can have important consequences by increasing the impact load borne by the articular cartilage (15). Such may be the case for individuals with knee OA who commonly show knee stiffening during weight acceptance (8,10,11). The presence of this knee stiffening strategy in persons with knee OA is not surprising given the prevalence of weakness in this population (11,16-19); however, the muscle activation strategies associated with the knee stiffening strategy are not well defined. Stiffening the knee is typically accompanied by increased co-contraction of muscles around the knee (8,10,14,20,21), which can increase joint contact pressures (22). Higher joint contact forces along with higher impact loads may act together to put the joint at risk for progressive cartilage destruction.
Stiffening the knee during walking is frequently noted in persons who experience knee instability (buckling, shifting, or giving way of the knee), such as those with anterior cruciate ligament (ACL) injury (20,21). Our recent work (Schmitt et al.: unpublished observations) and that of others (23) established that knee instability is commonly reported by patients with knee OA. It is known that quadriceps strength largely impacts function (24-26), but our work and that of others (23) demonstrate that self-reported knee instability predicts task-oriented function beyond the influence of quadriceps strength. We have identified self-reported knee instability as a distinct impairment that is not related to the amount of knee laxity and as a major predictor of knee function and quality of life. It is critical to determine if instability influences movement patterns during typical daily activities, such as walking, in ways that could accelerate joint destruction.
Another important characteristic of individuals with knee OA is excessive frontal plane laxity. Passive structures around the knee protect articular cartilage by controlling fine joint motion and limiting excessive shear forces (27,28). Work by Sharma et al (29) indicates that excessive frontal plane laxity is associated with a higher likelihood of progression of knee OA, but only in persons with relatively strong quadriceps muscles. This suggests that the manner in which knee muscles are activated in the presence of excessive joint laxity may be an important factor in long-term joint integrity.
The high incidence of quadriceps weakness (11,16-19), excessive frontal plane laxity (10,30,31), and joint instability (23) in persons with knee OA along with the recent findings suggesting that laxity and muscle activity around the knee influence joint integrity (29) prompt the need to investigate how movement and muscle activation patterns are influenced by these characteristics. The purpose of this study was to investigate the movement and muscle activation strategies during walking in individuals with medial knee OA to determine the influence of factors related to medial knee OA on movement patterns. We hypothesized that compared with control subjects, individuals with knee OA would show knee stiffening with increased muscle co-contraction during weight acceptance and that knee flexion during weight acceptance would be predicted by quadriceps strength, knee instability, varus alignment, and medial laxity.
SUBJECTS AND METHODS
Subjects
Twenty-eight individuals with medial knee OA (OA group) and 26 individuals without knee OA (control group) were recruited from the community or were referred from local physicians to participate (Table 1). All subjects provided written consent approved by the institutional review board. Control subjects were included if they reported no history of knee pain or previous lower extremity injury and showed no radiographic evidence of knee OA (Kellgren/Lawrence [K/L] grade 0 or 1 [32]). Subjects with knee OA were included if they had K/L grade 2 or greater radiographic changes in the medial tibiofemoral compartment. Subjects with OA were excluded if they 1) had a K/L grade 2 or greater in the lateral tibiofemoral and patellofemoral compartments, 2) had a history of other orthopedic injury in the lower extremities or spine, 3) had a history of neurologic injury, 4) had a history of rheumatoid arthritis, 5) had undergone joint arthroplasty or skeletal realignment in either lower extremity, 6) used an assistive device, or 7) were pregnant. If a participant had bilateral knee OA fitting the criteria, the more involved knee was identified by the individual and was used in the analysis.
Table 1.
Group characteristics and average radiograph and strength data*
| Characteristic | OA group (n = 28) | Control group (n = 26) | P |
|---|---|---|---|
| Age, mean (range) years | 60.4 (39–78) | 58.5 (38–76) | 0.556 |
| Sex, no. (%) | |||
| Female | 14 (50) | 13 (50) | – |
| Male | 14 (50) | 13 (50) | |
| Height, mean ± SD meters | 1.70 ± 0.11 | 1.68 ± 0.11 | 0.414 |
| Body mass, mean ± SD kg | 92.01 ± 16.16 | 83.93 ± 18.85 | 0.096 |
| Radiographic K/L grade, no. (%) | |||
| 0 or 1 | 0 (0) | 26 (100) | – |
| 2 | 17 (61) | 0 (0) | |
| 3 | 8 (28) | 0 (0) | |
| 4 | 3 (11) | 0 (0) | |
| Knee alignment, mean (95% CI) degrees | 174.82 (173.58, 176.07) | 179.24 (178.35, 180.13) | < 0.001† |
| Medial laxity, mean (95% CI) mm | 4.23 (3.57, 4.89) | 2.76 (2.38, 3.14) | < 0.001† |
| Lateral laxity, mean (95% CI) mm | 2.77 (2.28, 3.27) | 3.52 (3.04, 4.00) | 0.032† |
| Quadriceps strength/weight (NMVIC), mean (95% CI) N/kg | 7.8 (6.84, 8.80) | 10.14 (8.75, 11.54) | 0.007† |
OA = osteoarthritis; K/L grade = Kellgren/Lawrence grade of severity (32); 95% CI = 95% confidence interval; NMVIC = normalized maximal voluntary isometric contraction of the quadriceps muscles.
Difference is significant.
Radiographs
Limb alignment
Tibiofemoral joint alignment was measured using standing anteroposterior (AP) radiographs. Subjects stood barefoot and bearing weight equally on both lower extremities. Subjects were positioned with both knees extended and with the tibial tubercles facing forward. The radiograph tube was positioned 2 meters above the center of the knee. Alignment was measured as the angle of the intersection of the mechanical axes of the femur and tibia (33-35). An angle <180° was defined as varus and >180° was defined as valgus. Measurements were made by one author (LCS) with high test–retest reliability (intraclass correlation coefficient [ICC] 0.978).
Frontal plane laxity
Medial and lateral knee laxities were measured with the open space technique (36) using AP stress radiographs as described by Lewek et al (10). Subjects were supine with the involved limb positioned in a TELOS stress device (Austin & Associates, Fallston, MD) with the knee flexed to 20° and the patella facing anteriorly. The radiograph tube was centered ~91 cm above the knee joint and a 150N force was applied at the joint line in a varus and valgus direction. Open joint space was measured at the most narrow location in the medial and lateral compartments. Laxities were calculated by subtracting open joint space during joint closing from open joint space during joint opening following force application. Laxity measurements were adjusted for magnification using a known measurement visible in every radiograph to scale the joint space measurements. Using this technique, laxity was assessed by one investigator (LCS) with high test–retest reliability for medial laxity (ICC 0.96) and lateral laxity (ICC 0.97).
Quadriceps strength assessment
Quadriceps strength was measured with an isokinetic dynamometer (KinCom, Chattanooga, TN) during a maximal voluntary isometric contraction (MVIC). Subjects sat with the hip and knee flexed to 90°, the knee joint line aligned with the dynamometer axis, and the trunk, pelvis, and thigh stabilized. Visual and verbal feedback were given and a supramaximal burst of electrical current (100 pulses/second, 600-μsec pulse duration, 10 pulse titanic train, 130 volts) was applied during the MVIC using the Grass S48 stimulator (Grass Instrument, Quincy, MA) (37). Subjects were given up to 3 attempts and the highest volitional force was used for analysis. MVIC was normalized by body weight (NMVIC).
Self-assessment of knee instability
Knee instability (IKOS) was assessed from the Activities of Daily Living Scale of the Knee Outcome Survey (38). Subjects rated their instability on a 6-point scale in response to the question, “To what degree does giving way, buckling, or shifting of the knee affect your daily activity?” with scores as follows: 5 = no instability, 4 = instability not affecting activity, 3 = instability affecting activity slightly, 2 = instability affecting activity moderately, 1 = instability affecting activity severely, and 0 = instability preventing all activity. IKOS score is a reliable means of assessing knee instability in individuals with knee OA (23).
Motion analysis and electromyographic data
Knee motion during walking was tracked by a 6-camera, passive, 3-dimensional motion analysis system (Vicon 512; Oxford Metrics, London, UK) at 120 Hz. The cameras tracked the motion of 15.5-mm retroreflective markers secured over the posterior aspects of the thigh and shank, and individually placed over the bilateral greater trochanters, the medial and lateral knee joint, and the medial and lateral malleoli. Kinetic and electromyogram (EMG) data were collected simultaneously and sampled at 1,080 Hz. Ground reaction force data were sampled from a force platform (Bertec, Worthington, OH). Preamplified surface electrodes (Motion Lab Systems, Baton Rouge, LA) with an 18-mm interelectrode distance were positioned over the lateral quadriceps (LQ) and medial quadriceps (MQ), lateral hamstrings (LH) and medial hamstrings (MH), lateral gastrocnemius (LG) and medial gastrocnemius (MG), and soleus muscles (39).
Testing procedures
Subjects walked along a 13-meter walkway at their self-selected walking speed. Walking velocity was monitored with 2 photoelectric beams to ensure that velocity did not vary more than 5% from the self-selected pace. Ten usable trials were collected.
Data management
Data were processed using Visual3D software (C-Motion, Rockville, MD). Stance phase was identified from the force platform and data during stance were filtered with a second-order, phase-corrected Butter-worth filter with a cutoff frequency of 6 Hz for video data and 40 Hz for force platform data (40). Sagittal and frontal plane knee motion and moments were calculated with Euler angles and inverse dynamics, respectively (C-Motion). Joint moments were normalized by body mass times height. Knee kinematic and kinetic data were analyzed during 2 intervals: weight acceptance (defined from initial contact [IC] through peak knee flexion angle) and midstance (defined from peak knee flexion angle through peak knee extension angle). Each interval was time normalized to 100 data points. Data from the 10 trials were averaged and the average was used for analysis.
EMG data were band-pass filtered from 20–350 Hz and a linear envelope was created with full-wave rectification and filtering with a 10-Hz low-pass Butterworth filter (eighth order, phase-corrected). The linear envelope of each muscle was normalized to the maximum activation for that muscle during walking. Magnitude of muscle activity and co-contraction between opposing muscles were analyzed over 3 intervals: preparation (100 msec prior to IC through IC), weight acceptance, and midstance. Magnitude of individual muscle activity is expressed as the average activity across each interval (average rectified value). Co-contraction was calculated using equation 1 developed in our laboratory (41):
Co-contraction value
in which i is the sample number and division by 100 takes the average across the normalized interval. This method accounts for both the timing and magnitude of antagonist muscle activity. Co-contraction was calculated between the LQ and LH (LQH), LQ and LG (LQG), MQ and MH (MQH), and MQ and MG (MQG).
Statistical analysis
Group means and standard deviations or 95% confidence intervals were calculated for variables of interest (SPSS 13.0 for Windows; SPSS, Chicago, IL). Group differences were detected with independent t-tests. Significance was established when P ≤ 0.050 and because of the exploratory nature of this study, adjustments for multiple comparisons were not made.
To investigate potential factors related to the gait deviations shown by individuals with medial knee OA, several regression analyses were performed. Separate forward regression analyses were used to investigate relationships between sagittal plane knee motion and muscle activation patterns during weight acceptance and midstance (Table 2). Individual muscle activities or co-contraction values that were found to be significantly different between the groups were entered into the model as independent variables. Knee motion was entered as the dependent variable. Separate regressions were performed for the OA and control groups.
Table 2.
Results of forward regressions to predict knee motion*
| OA group
|
Control group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | R | R2 | F | df | P | R | R2 | F | df | P |
| Dependent variable: knee flexion during WA | ||||||||||
| Independent variable | ||||||||||
| MQ (WA), MH (WA), LG (WA) | 0.432 | 0.187 | 1.682 | 3, 22 | 0.200 | 0.441 | 0.195 | 1.531 | 3, 19 | 0.239 |
| MQH (P), LQG (P), MQH (WA), LQG (WA) | 0.609 | 0.371 | 3.097 | 4, 21 | 0.038† | 0.685 | 0.469 | 3.979 | 4, 18 | 0.017† |
| Dependent variable: knee extension during MS | ||||||||||
| Independent variable | ||||||||||
| MQ (MS), LQ (MS), LH (MS) | 0.669 | 0.447 | 5.926 | 3, 22 | 0.004† | 0.146 | 0.021 | 0.145 | 3, 20 | 0.932 |
| MQH (MS), LQH (MS), MQG (MS), LQG (MS) | 0.713 | 0.509 | 4.397 | 4, 17 | 0.013† | 0.173 | 0.030 | 0.132 | 4, 17 | 0.969 |
Separate forward regressions were performed for the OA and control groups to predict knee motion from individual muscle magnitudes and muscle co-contractions that were found to be different between the groups. OA = osteoarthritis; WA = muscle activity during weight acceptance phase; MQ = muscle activity of the medial quadriceps; MH = muscle activity of the medial hamstrings; LG = muscle activity of the lateral gastrocnemius; MQH = co-contraction between MQ and MH; P = muscle activity during preparation phase; LQG = co-contraction between lateral quadriceps and LG; MS = muscle activity during midstance phase; LQ = muscle activity of the lateral quadriceps; LH = muscle activity of the lateral hamstrings; LQH = co-contraction between LQ and LH; MQG = co-contraction between MQ and medial gastrocnemius.
Independent variable significantly changed the R2 value at P ≤ 0.050.
For the OA group only, hierarchical regressions were used to evaluate the influence of NMVIC, IKOS score, alignment, and medial laxity on sagittal plane knee motion. Separate hierarchical regressions were performed to predict knee motion during weight acceptance and midstance (Table 3). Hierarchical regression is an incremental approach to multiple regression and the investigators determine the order of variables entered into the model. Research in persons with ACL deficiency suggests that quadriceps strength and self-reported knee instability influence knee motion during walking (14,20,21). We therefore expected these variables to be important in predicting the knee motion of persons with knee OA. We have identified self-reported knee instability as a distinct factor in this population and we wanted to determine the influence of IKOS score on knee motion after accounting for the influence of quadriceps NMVIC. Therefore, quadriceps NMVIC was entered into the model first, followed by IKOS score, then alignment, and finally medial laxity. This method allowed for assessment of the unique contribution of each variable in the prediction.
Table 3.
Results of hierarchical regression to predict knee motion (osteoarthritis group only)*
| Variable | R2 | R2 change | F change | df | P |
|---|---|---|---|---|---|
| Dependent: knee flexion during WA | |||||
| Independent | |||||
| NMVIC† | 0.554 | 0.554 | 29.866 | 1, 24 | < 0.001† |
| IKOS† | 0.628 | 0.074 | 4.581 | 1, 23 | 0.043† |
| Alignment | 0.638 | 0.009 | 0.553 | 1, 22 | 0.465 |
| Med lax | 0.638 | 0.000 | 0.013 | 1, 21 | 0.911 |
| Dependent: knee extension during MS | |||||
| Independent | |||||
| MVIC† | 0.416 | 0.416 | 17.110 | 1, 24 | < 0.001† |
| IKOS† | 0.604 | 0.188 | 10.924 | 1, 23 | 0.003† |
| Alignment | 0.626 | 0.022 | 1.287 | 1, 22 | 0.269 |
| Med lax | 0.628 | 0.002 | 0.090 | 1, 21 | 0.768 |
Relative contribution (R2 value) of quadriceps NMVIC, IKOS score, alignment, and medial laxity in predicting knee motion during weight acceptance and midstance. WA = weight acceptance phase; NMVIC = normalized maximal voluntary isometric contraction of the quadriceps muscles; IKOS = instability score; med lax = medial knee laxity; MS = midstance phase.
Indicates the addition of the independent variable to the model significantly changed the R2 value at P ≤ 0.050.
RESULTS
Radiographs and quadriceps strength
The OA subjects had significantly more varus alignment (P < 0.001), had greater medial knee laxity (P < 0.001), had less lateral knee laxity (P = 0.032), and were weaker (P = 0.007) compared with the control group (Table 1).
Gait characteristics
Walking speed was no different between the groups (OA group: 1.50 meters/second, control group: 1.51 meters/second; P = 0.846).
Weight acceptance
Knee flexion excursion was less in the OA group (P = 0.046), but peak flexion moments were not different between the groups (Table 4). In the frontal plane, subjects with OA maintained a greater knee adduction angle (P ≤ 0.047), but there were no differences in adduction excursion (Table 4). The OA group showed greater first knee adduction moment (P = 0.001) (Table 4).
Table 4.
Average data during walking trials*
| OA group | Control group | P | |
|---|---|---|---|
| Sagittal plane | |||
| Flexion excursion (degrees) | 13.6 (11.6, 15.6) | 16.2 (14.6, 17.7) | 0.046† |
| Extension excursion (degrees) | 14.6 (12.3, 16.9) | 20.1 (18.3, 21.8) | < 0.001† |
| Peak external flexion moment (Nm/kg × meter) | 0.29 (0.23, 0.34) | 0.29 (0.24, 0.35) | 0.859 |
| Peak external extension moment (Nm/kg × meter) | −0.18 (−0.27, −0.21) | −0.24 (−0.22, −0.14) | 0.011† |
| Frontal plane | |||
| Frontal plane angle at initial contact (degrees)‡ | 1.26 (−0.37, 2.88) | −0.95 (−2.46, 0.56) | 0.047† |
| Peak frontal plane angle (degrees)‡ | 5.66 (3.81, 7.50) | 2.68 (0.93, 4.42) | 0.020† |
| Adduction excursion (degrees) | 4.40 (3.48, 5.31) | 3.63 (2.81, 4.45) | 0.207 |
| Peak external adduction moment (first) (Nm/kg × meter) | −0.37 (−0.41, −0.33) | −0.28 (−0.31, −0.25) | 0.001† |
| Peak external adduction moment (second) (Nm/kg × meter) | −0.28 (−0.31, −0.25) | −0.21 (−0.25, −0.18) | 0.006† |
Values are the mean (95% confidence interval) unless otherwise indicated. OA = osteoarthritis.
Difference is significant.
Positive values indicate adduction.
As they prepared to accept body weight, the OA group demonstrated trends of higher activity in the LH (P = 0.067) and LG (P = 0.057) muscles and higher co-contraction in LQG and MQH muscle pairs (P ≤ 0.050) (Figure 1). During weight acceptance, the OA group used higher MQ, MH, and LG activity (P ≤ 0.050) and greater co-contraction of the LQG and MQH (P ≤ 0.050) (Figure 1).
Figure 1.
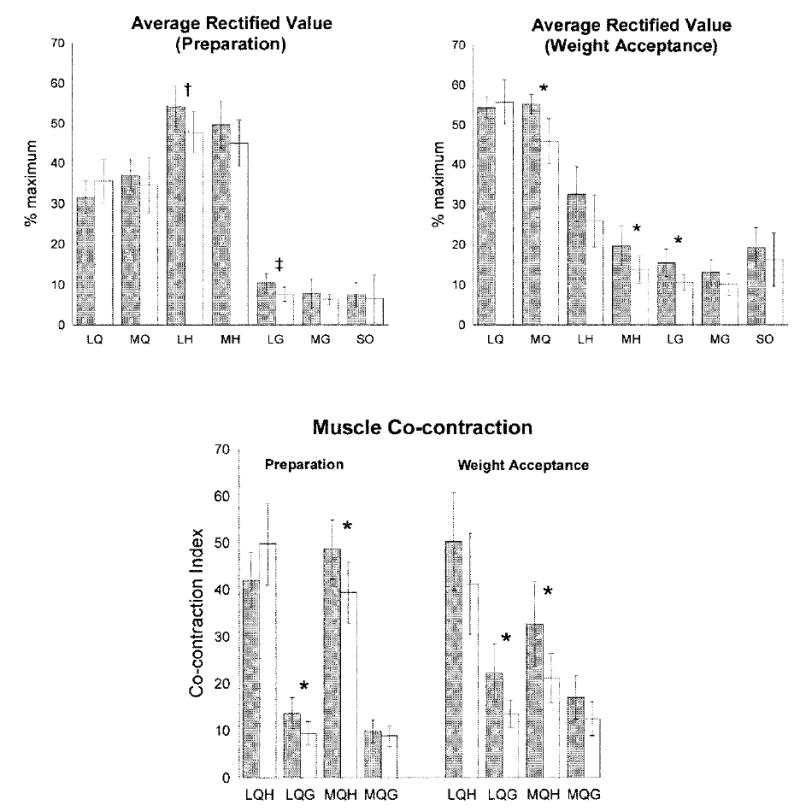
Individual muscle activity and co-contraction during preparation and weight acceptance intervals in the osteoarthritis group (shaded bar) and controls (open bars). Average data, error bars represent 95% confidence intervals. * P ≤ 0.050. † Trend (P = 0.067). ‡ Trend (P = 0.057). LQ = lateral quadriceps; MQ = medial quadriceps; LH = lateral hamstrings; MH = medial hamstrings; LG = lateral gastrocnemius; MG = medial gastrocnemius; SO = soleus; LQH = co-contraction between LQ and LH; MQH = co-contraction between MQ and MH; LQG = co-contraction between LQ and LG; MQG = co-contraction between MQ and MG.
Knee flexion excursion was predicted by muscle co-contraction during preparation (MQH and LQG) and weight acceptance intervals (MQH and LQG) in both OA subjects (R2 = 0.371, P = 0.038) and control subjects (R2 = 0.469, P = 0.017). Individual muscle activity during weight acceptance (MQ, MH, and LG) did not predict flexion excursion in either group (Table 2).
In the OA group, variables uniquely contributing to knee flexion excursion were NMVIC (change in R2 = 0.554, P < 0.001) and IKOS score (change in R2 = 0.074, P = 0.043). The addition of alignment or medial laxity to the regression model did not strengthen the prediction (Table 3).
Midstance
Pronounced group differences were observed in movement patterns as the knee moved from a flexed to a more extended position and entered single-limb support. The OA group extended their knees less (P < 0.001) and showed lower knee extension moments (P = 0.011) (Table 4). In the frontal plane, the OA group continued to show higher peak knee adduction moments (P = 0.006) (Table 4).
The muscle activation patterns were also different between the groups. The OA group used higher LQ, MQ, and LH activity and higher co-contraction of all muscle pairs (P ≤ 0.050) (Figure 2).
Figure 2.
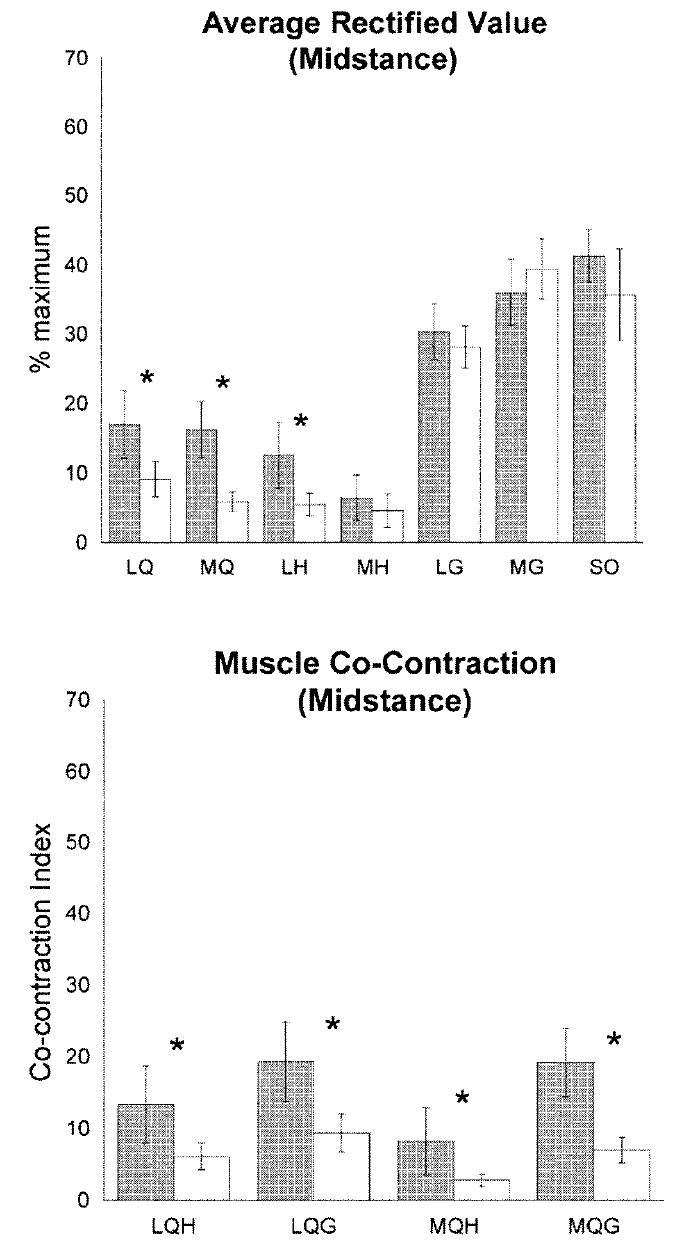
Individual muscle activity and co-contraction during midstance interval in the osteoarthritis group (shaded bar) and controls (open bars). Average data, error bars represent 95% confidence intervals. * P ≤ 0.050. LQ = lateral quadriceps; MQ = medial quadriceps; LH = lateral hamstrings; MH = medial hamstrings; LG =lateral gastrocnemius; MG = medial gastrocnemius; SO = soleus; LQH = co-contraction between LQ and LH; MQH = co-contraction between MQ and MH; LQG = co-contraction between LQ and LG; MQG = co-contraction between MQ and MG.
Forward regression analysis predicted knee extension motion from muscle activation in the OA group only. For those with OA, activation of the LQ, MQ, and LH muscles significantly predicted knee extension motion (R2 = 0.447, P = 0.004) but that relationship was not present in the control group (R2 = 0.021, P = 0.932). Similarly, co-contraction of all muscle pairs (LQH, LQG, MQH, MQG) predicted knee motion in the OA group (R2 = 0.509, P = 0.013) but not in the control group (R2 = 0.030, P = 0.969) (Table 2). In the OA group, NMVIC (change in R2 = 0.416, P < 0.001) and IKOS score (change in R2 =0.188, P = 0.003) significantly strengthened the prediction of knee motion, whereas alignment and medial laxity did not (Table 3).
DISCUSSION
Investigations of walking patterns of persons with medial knee OA typically focus on the early stance phase when the knee is accepting body weight and the articular cartilage is subjected to high loads. This study is one of the first to investigate factors potentially contributing to the altered walking patterns, and as such is exploratory in nature. Although the sample size is relatively small, we maintain that the observed movement and muscle activation patterns provoke interesting questions and warrant further investigation. As we hypothesized, individuals with knee OA stiffen their knees during weight acceptance, and as part of the stiffening strategy use higher co-contraction of the knee flexors and extensors. This study is one of the first to report marked alterations in knee motion and muscle activation patterns during single-limb support as the knee extends and the stiffening strategy used by the OA group may influence long-term joint integrity.
During weight acceptance, the OA group used a knee stiffening strategy involving higher muscle co-contraction, which is consistent with other work (8,10). The relationship between muscle co-contraction and knee flexion observed in both the OA group and control group suggests that some level of co-contraction is necessary to stabilize the knee; however, the co-contraction used by the OA group was accompanied by reduced knee motion. Decreased knee motion can result in greater impact load on the knee (15) and higher co-contraction can increase joint contact pressures (22). The cumulative effect of higher impact loads and greater contact pressures may put articular cartilage at greater risk for damage.
Interestingly, the OA group also used higher muscle activation during the preparation phase, which may represent an attempt to influence muscle activation later in the cycle. In studies of reflex muscle activation subjects commonly generate baseline muscle activity prior to a perturbation, resulting in greater responses to the perturbation (42). It is possible that the OA group used higher muscle activity during late swing phase to enhance muscle function as the body accepted weight.
We expected the OA group to use different movement and muscle activation strategies during weight acceptance, but we did not expect to see group differences during single-limb support as the knee was extending. Subjects with OA extended their knees less than the control group and used greater muscle activity and co-contraction. Furthermore, the influence of muscle activation on movement patterns was only noted in the subjects with OA. The OA group used reduced knee motion and accompanying higher muscle co-contraction, similar to the knee stiffening strategy used during weight acceptance. A stiffening strategy during the midstance phase can be important to joint integrity because of the transverse plane knee rotation occurring as the knee approaches full extension. During the last 20° of closed chain knee extension, the femur internally rotates on the tibia, which is commonly referred to as the screw-home mechanism (43). The collateral knee ligaments contribute to the control of this rotation, and ligament laxity may disrupt the normal motion and lead to joint degeneration. Wilson et al (44) found that disruption of the medial collateral ligament increases normal anterior translation that occurs with knee motion. Abnormal joint geometry, such as that seen in joints with erosion, could also reduce the constraints to motion (44). Wilson et al (44) speculated that the increased number of degrees of freedom could appear clinically as joint instability, which was found to be predictive of knee motion in our study. The findings of Wilson et al (44), along with our results, suggest that the presence of instability may explain the high rate of OA development in persons with lax ligaments and abnormal joint geometry, underscoring the need to address knee instability in research and treatment of individuals with knee OA.
The muscle activation patterns used by the subjects with OA during single-limb support demonstrate interesting differences from the control group. After the ground reaction force moves anterior to the knee center, knee extension normally occurs passively requiring little quadriceps activity (13). However, the OA group used higher quadriceps activity and higher muscle co-contraction, which predicted knee motion and is consistent with the lower external extension moment observed during this phase of gait. Higher muscle co-contraction and higher LH activity may be an attempt to control rotation directly or indirectly because less rotation would necessarily occur with less knee extension. Although higher muscle activation may help to limit the number of degrees of freedom available in the joint, it could also result in high joint compression (22), particularly when only one limb is supporting the entire weight of the body. Higher muscle activity and reduced knee motion may put the knee at risk for damage, but further investigation is needed to clarify the impact of these alterations on OA progression.
Reduced knee motion during walking is typically associated with quadriceps weakness (14), but our results demonstrate that knee instability is also an important predictor of knee movement strategies in persons with knee OA. Recently, Sharma et al (29) reported that stronger quadriceps muscles, in the presence of excessive frontal plane knee laxity or malalignment, increased the likelihood of OA progression. Our results suggest that the manner in which individuals activate their muscles may be important for joint integrity. If an individual with stronger quadriceps used activation of the quadriceps and antagonist muscles in a global co-contraction strategy such as shown by the individuals with OA in our study, this could result in higher contact forces than if the muscles were activated in a more selective manner (22). Although higher muscle co-contraction may enhance knee stability, a more selective muscle activation strategy could result in better knee stability along with lower co-contraction. Studies show that neuromuscular training can result in greater knee stability in the presence of lower muscle co-contraction in persons with ACL deficiency (45). Improved knee stability (that might lessen joint shear forces) and lower co-contraction (that mitigates joint contact pressures) would likely be desirable in persons with knee OA.
This study was the first to investigate potential contributors to the deviant gait patterns used by individuals with medial knee OA and we acknowledge that there are other factors associated with knee OA that may also influence walking patterns. Nonetheless, the results of this preliminary investigation suggest that persons with medial knee OA use different muscle activation strategies, and suggest that the observed walking patterns are influenced by the altered muscle activation patterns, by quadriceps strength, and by knee instability. We have demonstrated that the same impairments, quadriceps strength and knee instability, predict physical function in this patient population. The influence of knee instability and quadriceps strength on both knee function and walking patterns underscores the importance of addressing knee instability in the care of individuals with knee OA. We speculate that the manner in which persons with knee OA activate their muscles could hasten joint destruction. It is possible that training strategies that encourage greater knee motion during walking, with or without increased co-contraction, might improve knee function and slow OA progression over time. Further research is needed to clarify how quadriceps weakness, knee instability, and associated muscle co-contraction strategies affect joint degeneration in order to develop rehabilitation strategies targeting those specific impairments associated with disease progression.
Acknowledgments
We acknowledge the staff at Papastavros Medical Imaging for their assistance in obtaining radiographs, Drs. Michael Axe and Andrew Reisman for their support of the project, and the efforts of Sarah Trager.
Supported by grants from the NIH (P20-RR016458 and T32-HR7490), and by the Foundation for Physical Therapy Promotion of Doctoral Studies Program.
References
- 1.Carmona L, Ballina J, Gabriel R, Laffon A EPISER Study Group. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60:1040–5. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 3.Kannus P, Jarvinen M, Kontiala H, Bergius L, Hyssy E, Salminen E, et al. Occurrence of symptomatic knee osteoarthrosis in rural Finland: a prospective follow up study. Ann Rheum Dis. 1987;46:804–8. doi: 10.1136/ard.46.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106:151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 5.Thomas RH, Resnick D, Alazraki NP, Daniel D, Greenfield R. Compartmental evaluation of osteoarthritis of the knee: a comparative study of available diagnostic modalities. Radiology. 1975;116:585–94. doi: 10.1148/116.3.585. [DOI] [PubMed] [Google Scholar]
- 6.Dearborn JT, Eakin CL, Skinner HB. Medial compartment arthrosis of the knee. Am J Orthop. 1996;25:18–26. [PubMed] [Google Scholar]
- 7.Baliunas AJ, Hurwitz DE, Ryals AB, Karrar A, Case JP, Block JA, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573–9. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 8.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19:44–9. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–7. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 10.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:745–51. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messier SP, Loeser RF, Hoover JL, Semble EL, Wise CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility [published erratum appears in Arch Phys Med Rehabil 1992;73:252] Arch Phys Med Rehabil. 1992;73:29–36. [PubMed] [Google Scholar]
- 12.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67:1188–94. [PubMed] [Google Scholar]
- 13.Perry J. Gait analysis: normal and pathological function. New York: McGraw-Hill; 1992. [Google Scholar]
- 14.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 15.Lafortune MA, Hennig EM, Lake MJ. Dominant role of interface over knee angle for cushioning impact loading and regulating initial leg stiffness. J Biomech. 1996;29:1523–9. [PubMed] [Google Scholar]
- 16.Fisher NM, Pendergast DR. Reduced muscle function in patients with osteoarthritis. Scand J Rehabil Med. 1997;29:213–21. [PubMed] [Google Scholar]
- 17.Hassan BS, Mockett S, Doherty M. Static postural sway, proprioception, and maximal voluntary quadriceps contraction in patients with knee osteoarthritis and normal control subjects. Ann Rheum Dis. 2001;60:612–8. doi: 10.1136/ard.60.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley MV, Newham DJ. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. Br J Rheumatol. 1993;32:127–31. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- 19.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9:62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award Paper: movement patterns after anterior cruciate ligament injury: a comparison of patients who compensate well for the injury and those who require operative stabilization. J Electromyogr Kinesiol. 1998;8:349–62. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 22.Hodge WA, Fijan RS, Carlson KL, Burgess RG, Harris WH, Mann RW. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986;83:2879–83. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941–6. doi: 10.1002/art.20825. [DOI] [PubMed] [Google Scholar]
- 24.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–62. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller ME, Rejeski WJ, Messier SP, Loeser RF. Modifiers of change in physical functioning in older adults with knee pain: the Observational Arthritis Study in Seniors (OASIS) Arthritis Rheum. 2001;45:331–9. doi: 10.1002/1529-0131(200108)45:4<331::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Herzog W, Adams M, Matyas J, Brooks J. Hindlimb loading, morphology and biochemistry of articular cartilage in the ACL-deficient cat knee. Osteoarthritis Cartilage. 1993;1:243–51. doi: 10.1016/s1063-4584(05)80330-9. [DOI] [PubMed] [Google Scholar]
- 28.Wu JZ, Herzog W, Epstein M. Joint contact mechanics in the early stages of osteoarthritis. Med Eng Phys. 2000;22:1–12. doi: 10.1016/s1350-4533(00)00012-6. [DOI] [PubMed] [Google Scholar]
- 29.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003;138:613–9. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Sharma L, Lou C, Felson DT, Dunlop DD, Kirwan-Mellis G, Hayes KW, et al. Laxity in healthy and osteoarthritic knees. Arthritis Rheum. 1999;42:861–70. doi: 10.1002/1529-0131(199905)42:5<861::AID-ANR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Wada M, Imura S, Baba H, Shimada S. Knee laxity in patients with osteoarthritis and rheumatoid arthritis. Br J Rheumatol. 1996;35:560–3. doi: 10.1093/rheumatology/35.6.560. [DOI] [PubMed] [Google Scholar]
- 32.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao EY, Neluheni EV, Hsu RW, Paley D. Biomechanics of malalignment. Orthop Clin North Am. 1994;25:379–86. [PubMed] [Google Scholar]
- 34.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990;255:215–27. [PubMed] [Google Scholar]
- 35.Tetsworth K, Paley D. Malalignment and degenerative arthropathy. Orthop Clin North Am. 1994;25:367–77. [PubMed] [Google Scholar]
- 36.Moore TM, Meyers MH, Harvey JP., Jr Collateral ligament laxity of the knee: long-term comparison between plateau fractures and normal. J Bone Joint Surg Am. 1976;58:594–8. [PubMed] [Google Scholar]
- 37.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–9. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–45. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Delagi E, Iazzetti J, Perotto A, Morrison D. Anatomic guide for the electromyographer: the limbs. In: Charles C, editor. Springfield (IL) Thomas Publisher; 1981. [Google Scholar]
- 40.Winter DA. Biomechanics and motor control of human movement. 2. New York: Wiley and Sons; 1990. [Google Scholar]
- 41.Rudolph KS, Axe MJ, Snyder-Mackler L. Dynamic stability after ACL injury: who can hop? Knee Surg Sports Traumatol Arthrosc. 2000;8:262–9. doi: 10.1007/s001670000130. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan TS, Kim AW, Lloyd DG. Selective muscle activation following rapid varus/valgus perturbations at the knee. Med Sci Sports Exerc. 1996;28:870–6. doi: 10.1097/00005768-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Hallen LG, Lindahl O. The “screw-home” movement in the knee-joint. Acta Orthop Scand. 1966;37:97–106. doi: 10.3109/17453676608989407. [DOI] [PubMed] [Google Scholar]
- 44.Wilson DR, Feikes JD, O’Connor JJ. Ligaments and articular contact guide passive knee flexion. J Biomech. 1998;31:1127–36. doi: 10.1016/s0021-9290(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 45.Chmielewski TL, Hurd WJ, Snyder-Mackler L. Elucidation of a potentially destabilizing control strategy in ACL deficient non-copers. J Electromyogr Kinesiol. 2005;15:83–92. doi: 10.1016/j.jelekin.2004.07.003. [DOI] [PubMed] [Google Scholar]


