Abstract
Several major areas of work by the author and his international collaborators are reviewed. 1) The ciliary muscle in the nonhuman primate eye was disinserted at the scleral spur. Pilocarpine was then ineffective in increasing outflow facility, indicating that ciliary muscle contraction mediated the IOP-lowering effect of muscarinic cholinergics. 2) Compounds such as cytochalasins, H-7 and latrunculin A/B, which alter the actin cytoskeleton, cellular contractility and cellular adhesions in cultured trabecular meshwork cells, relaxed trabecular pathway cells and consequently the meshwork itself so as to decrease IOP and enhance trabecular outflow facility in nonhuman primates. Gene transfer approaches utilizing C3 and caldesmon over-expression by viral vectors to target specific steps in the cellular contractility/cytoskeleton/cell adhesion cascades characteristically altered trabecular meshwork cell morphology and increased outflow facility in organ-cultured anterior segments. 3) Prostaglandin F2α analogues enhanced matrix metalloproteinase production by ciliary muscle cells and scleral fibroblasts, leading to remodeling of the extracellular matrix of the ciliary muscle and sclera and consequently to increased uveoslceral outflow and decreased IOP in primates. 4) The rhesus monkey was an excellent model for human presbyopia, losing the accommodative response to cholinergic stimulation in the same timeframe relative to lifespan. No changes were found in ciliary muscle enzymes involved in acetylcholine biosynthesis or degradation or in muscarinic receptor numbers or affinity. Contractility of isolated ciliary muscle did not diminish with age, but posterior ciliary muscle attachments stiffened, suggesting a possible role in restricting muscle and consequently lens movement during accommodation. A model to reproducibly stimulate accommodation through central stimulation of the Edinger-Westphal nucleus was developed. Goniovideography and ultrasound biomicroscopic techniques allowed real-time recording and analysis of the accommodation-relevant structures. Surgical ablation of the intraocular structures involved in the accommodation response has led to further understanding of their roles and changes with age related to presbyopia. 5) Global collaborations such as those involved in these studies will be essential in the future, as science becomes “bigger”.
Keywords: ciliary muscle, cytoskeleton, gene therapy, intraocular pressure, outflow facility, presbyopia, trabecular meshwork, uveoscleral outflow
Introductory Comments and Background
I am honored and grateful to receive the Ernst H. Bárány prize from ISER. It is especially meaningful as I was Professor Bárány’s fellow for over two years. My time with him shaped my entire career and many important professional and personal relationships that last to this day.
The story I will tell is embodied in the title. It is about recognizing questions, asking how and why things happen, recognizing signs along the way, and about focus yet with openness to surprises. Most importantly, it is about realizing the need for and joy in collaborating with others in your group, your institution and globally. Science is now far too textured and complex for the single investigator working alone in his/her own laboratory to accomplish nearly as much as can be achieved by the ability and willingness to interact globally – for both input and output. This is crucial to advancing any discipline, for science is now truly global and we are all travelers every day.
My journey began as an ophthalmology resident at Washington University in St. Louis, where I was mentored by the legendary Bernard Becker, a brilliant physician-scientist and one of the 20th century’s giants in glaucoma. He shaped my interests in glaucoma and ocular physiology and pharmacology and suggested that, since my clinical residency experience in glaucoma had been so strong, I consider spending fellowship time delving deeper into the how’s and why’s of the anterior segment as they related to ocular hydrodynamics and intraocular pressure (IOP) regulation.
Dr. Becker said there was only one place to go, and that was to the laboratory of Dr. Ernst Bárány, at the University of Uppsala in Sweden. Trained as an ophthalmologist, Dr. Bárány was Professor and Chair of the Department of Medical Pharmacology there. He was the first to appreciate the role played by the ciliary muscle and the potential importance of the cytoskeleton, the shape of trabecular cells, and the extracellular matrix of the trabecular meshwork (TM) in regulating aqueous outflow; and a pioneer in identifying and characterizing the outward-directed transport systems of the ciliary body.
Dr. Bárány had trained a younger associate, who after several years on his faculty, had recently been made Chair of Physiology at Uppsala with laboratories just down the hall -Professor Anders Bill, the discoverer of uveoscleral outflow. It was a dream for a young physician-scientist to be able to go back and forth between the two laboratories. They were also working, in various groupings, with a renowned anatomist and fine structuralist from Germany, Professor Johannes Rohen, who was assuming the Chair of Anatomy at the University of Erlangen-Nürnberg. Professor Rohen has written many anatomy textbooks that are standards used all over the world. At the time he became Chairman at Erlangen-Nürnberg, Professor Rohen brought with him from Marburg to Erlangen, his most brilliant student, Elke Lütjen-Drecoll, who, subsequently has been recipient of many awards. She is perhaps the most distinguished anterior segment ultrastructuralist in the world today. Together in various combinations, and independently, Professors Bárány, Bill, Rohen and Lütjen-Drecoll unraveled many structure-function correlates of the primate anterior segment with regard to age-related changes and how drugs work.
I was privileged to join this partnership that has made important basic scientific and translational contributions and forged deep personal relationships. Just as our mentors collaborated like brothers, Elke and I became like brother and sister, and began to work together under their tutelage and subsequently as our own collaborating team.
Role of the Ciliary Muscle in Outlfow Facility
Earlier work by these collaborators and others demonstrated that the ciliary muscle inserts at the scleral spur and well into the TM, inserting into the subendothelial region adjacent to the inner wall of Schlemm’s canal (Rohen et al., 1967; Rohen et al., 1981). Outflow facility increased following topical, intracameral, or systemically administered pilocarpine (Bárány, 1962, 1967) presumably primarily by causing contraction of the ciliary muscle increasing tension on the TM and decreasing outflow resistance. However, not all the experimental evidence supported a strictly mechanical view of cholinergic effects on TM function. In monkeys, intravenous atropine rapidly reversed some, but not all of the pilocarpine-induced resistance decrease (Bárány, 1966). Topical pilocarpine caused a much greater resistance decrease per diopter of induced accommodation than did systemic pilocarpine (Bárány, 1966).
Thus, my post-doctoral research project while at Uppsala, was to prove definitively how pilocarpine increases outflow facility across the TM in the nonhuman primate eye. The hypothesis was that ciliary muscle traction on the TM is essential for the pilocarpine effect on outflow.
The overall strategy was to disinsert the ciliary muscle so that effects of pilocarpine on the TM, independent of the ciliary muscle, could be determined. This surgery was itself quite challenging. First the iris had to be removed. This required development of the technique for total iris removal (Kaufman and Lütjen-Drecoll, 1975). Fortunately, the eye healed perfectly from this surgery with no effect on IOP, baseline outflow facility or outflow facility responsiveness to intracameral or intravenous pilocarpine (Kaufman, 1979), proving definitively that miosis was not needed for the outflow effect of cholinomimetics. The surgical adventure of ciliary muscle disinsertion surgery was the next step. An ab-interno goniotomy-like approach was used in which a coronal cut was made lightly into the sclera just behind the apex of the scleral spur at the insertion of the ciliary muscle, over the entire 360-degree circumference. The muscle immediately fell away. Gonioscopic and anatomic studies demonstrated that the TM remained undamaged and that the CM had been retrodisplaced and had healed/reattached to the inner scleral wall well posterior to the scleral spur (Kaufman and Bárány, 1976; Lütjen-Drecoll et al., 1977). In this preparation outflow facility and IOP were mildly reduced. The outflow facility increasing effect of intravenous and intracameral pilocarpine were nearly completely eliminated despite the muscle’s ability to contract (Figure 1) (Kaufman and Bárány, 1976). and the TM’s ability to respond with a facility change to epinephrine and various cytoskeletal agents (which will be discussed in detail later). We concluded that the effect of pilocarpine on outflow facility depended entirely on TM deformation by ciliary muscle contraction and that this preparation could be used to distinguish facility responses due to ciliary muscle contraction from those due to direct effects of a drug on the TM.
Figure 1.
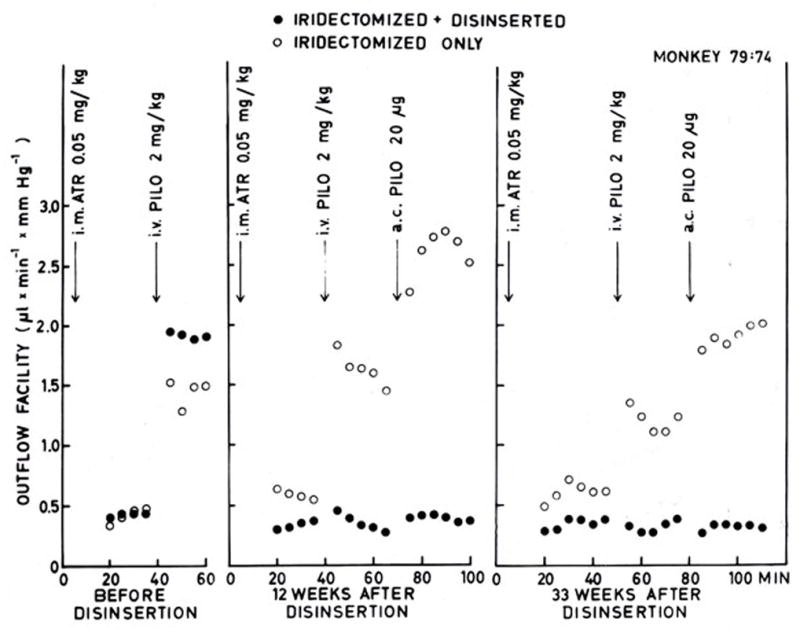
Outflow facility and facility responses to intravenous and intracameral pilocarpine-HCl (i.v. PILO; a.c. PILO) before and after unilateral ciliary muscle disinsertion in a bilaterally iridectomized monkey. Intramuscular atropine sulfate (i.m. ATR) was given before each perfusion to minimize systemic effects of intravenous pilocarpine. Note absence of facility increase following intravenous and intracameral pilocapine in the “disinserted” eye as opposed to the large facility increase in the opposite eye. Note also the difference in the resting (pre-pilocarpine) facilities between the “disinserted” and opposite eye following the initial perfusion and subsequent disinsertion operation. From (Kaufman and Bárány, 1976) with permission
Role of the Trabecular Meshwork Cytoskeleton in Outlfow Facility
We were interested in how the TM cytoskeleton affects outflow. We had the idea that epinephrine, as first suggested by Dr. Bárány (Bárány, 1968), by interacting with the cytoskeleton, could change the contractile properties of the TM. Bill and Bárány had shown that epinephrine increased outflow facility (Bárány, 1968; Bill, 1969). This resulted from a β2 adrenergic receptor mediated effect on the TM and an increase in cAMP (Neufeld et al., 1972; Neufeld et al., 1973; Neufeld and Sears, 1975; Neufeld and Bartels, 1982; Sears, 1966; Sears and Neufeld, 1975). Our disinsertion model showed that the iris and ciliary muscle were not involved in this outflow facility effect (Kaufman and Bárány, 1981; Kaufman and Rentzhog, 1981).
What was the physical mechanism? Old work in German anatomic literature (from 1938, citation unavailable) showed that epinephrine could change the shape of cat omentum cells. If epinephrine worked on facility by contracting the TM, could actin microfilament disruption relax the TM and block the effect?
Cytochalasins thus became interesting compounds to probe the question. Cytochalasins, which are fungal metabolites, interfere with the process by which globular cytoplasmic actin aggregates into actin microfilaments (Brown and Spudich, 1981). First “control” experiments tested the hypothesis that cytochalasins had no effect on, or even reduced, outflow facility. Surprisingly, cytochalasins dramatically increased outflow facility, albeit briefly, following intracameral bolus injection in intact as well as ciliary muscle-disinserted eyes (Kaufman and Bárány, 1977; Kaufman et al., 1977). This result started our long hunt. The effect was associated in time with a very expanded TM (Figure 2) as a result of the fluid pressure being higher in the anterior chamber than in Schlemm’s canal and thus pushing its way through the juxtacanalicular region where the weakened cytoskeleton and cell attachments allowed tremendous expansion of intercellular spaces and washout of extracellular material (Svedbergh et al., 1978). Also present were breaks in the inner wall of Schlemm’s canal. Aggregations of platelets were present trying to close the ruptures at the inner wall epithelium, as they would for any damaged blood vessel. Clearly cytochalasins were not agents one was going to use clinically but it did leave us in a conundrum as to what was going on.
Figure 2.
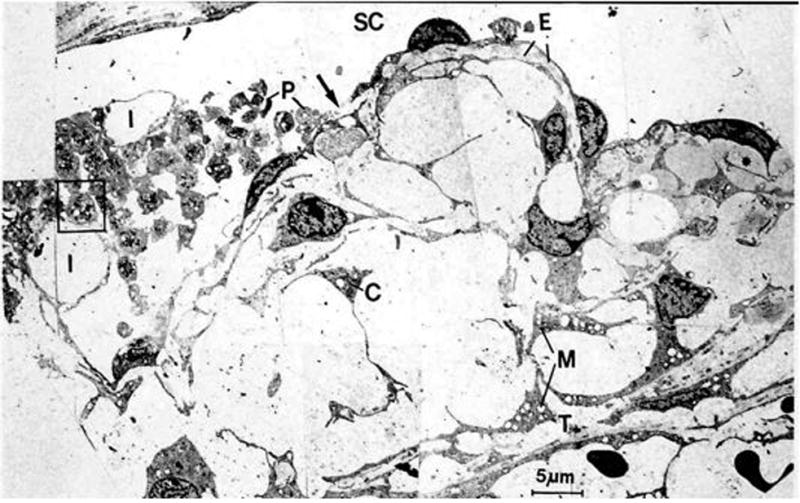
Schlemm’s canal and cribriform meshwork approximately 30 min after intracameral infusion of 5μg of cytochalasin B. Inner wall endothelium (SC) demonstrates ruptures (arrow) and abnormally large invaginations (I). Extracellular material (E) has been lost from some areas between inner wall endothelium and the first subendothelial cell layer (and replaced by plasma (asterisk)) and is completely absent from most parts of the cribriform meshwork. P, Degranulated platelets; C, cells of cribriform meshwork; M, swollen mitochondria; T, first corneoscleral trabeculum. From (Svedbergh et al., 1978) with permission
Meanwhile human TM (HTM) cells were isolated and cultured by Jon Polansky (Polansky et al., 1979). Treatment of HTM and monkey TM cells in culture with cytochalasin B resulted in marked changes in cell shape associated with organizational changes in actin filaments (Ryder et al., 1988; Weinreb et al., 1986). Treatment of HTM cells grown on filters with cytochalasins resulted in increased hydraulic conductivity (Perkins et al., 1988).
Cytochalasins also potentiated the outflow facility increasing effect of epinephrine (Robinson and Kaufman, 1991). Intracameral doses of cytochalasin B and epinephrine, which were subthreshold for increasing outflow facility when given singly, produced significant outflow facility increases when given concurrently. Subthreshold doses of cytochalasin B given concurrently with a maximal dose of epinephrine, produced significantly larger outflow facility increases than a maximal dose of epinephrine alone.
More definitive proof that epinephrine and cytochalasin B both were acting through disruption of actin microfilaments was provided with additional experiments using phalloidin, which antagonizes cytochalsin B’s effects on actin in other biologic systems (Low et al., 1975). Phalloidin itself did not affect outflow facility but it inhibited up to 50% of the outflow facility increasing effect of cytochalasin B and of epinephrine (Robinson and Kaufman, 1994). Thus it was clear that actin filaments were involved in regulating aqueous outflow.
Already at this point the collaboration involved myself, Dr. Lütjen-Drecoll, Dr. Bill, Dr. Bárány, and Dr. Rohen, and thus was quite multinational.
The search for better cytoskeletal compounds and better mechanistic understanding began. Cell adhesions, unlike what you see under the microscope, are not static structures but rather are dynamic – they come and go, vary in tightness, location, adhesivity – depending on what is going on in the environment of the cell. They are very complex with many structural proteins and many housekeeping signal transduction proteins that govern the assembly and disassembly of the junctions and the interactions of the structural proteins. The complexes are tied to the actin cytoskeleton and functionally to acto-myosin contractility (Geiger et al., 2001). In HTM cells this is visualized as long actin filaments, connected to vinculin providing structural anchoring to the plasma membrane at the junctional complexes, in turn linked to the matrix molecules on the other side (Tian et al., 2000). One can manipulate these adhesions with hormones, drugs, or mediators, but the system also is governed by the amount of force applied to and by various components, by stretch, shear stress and other physical factors. For instance, stretch induced external force (e.g., intraocular pressure) may be counteracted by actin-myosin contractility force (Figure 3) (Geiger and Bershadsky, 2002). This may be analogous to a reflex, and act as part of a complex regulatory system in a delicate balance depending on what the tissue is trying to do.
Figure 3.
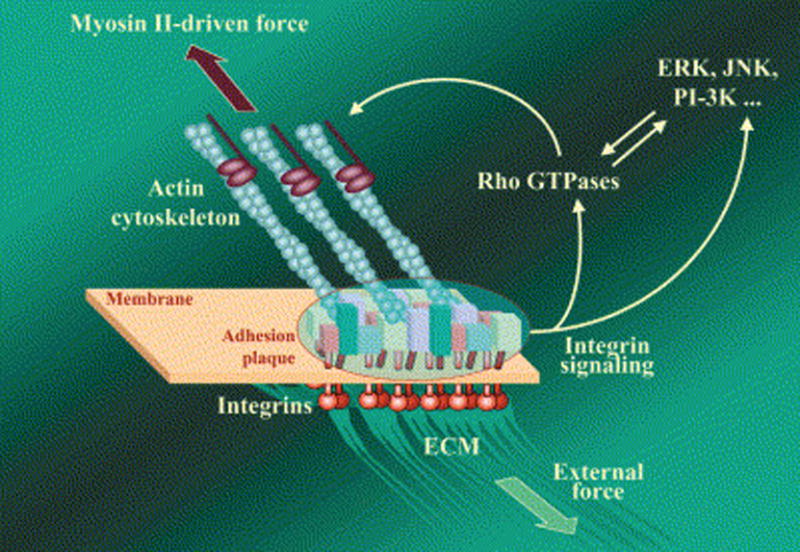
Focal Adhesion (FA) as a Mechanosensor. Focal adhesion is a multi-molecular complex connecting the extracellular matrix with the actin cytoskeleton. Heterodimeric transmembrane integrin receptors (red) bind matrix proteins via their extracellular domains, while their cytoplasmic domains are associated with a dense submembrane plaque containing more than 50 different proteins (“boxes” enclosed in the oval area) including structural elements as well as signal transduction proteins such as FAK, Src, ILK, etc. The plaque, in turn, is connected to the termini of actin filament bundles. The assembly and maintenance of FA depend on local mechanical forces. This force may be generated by myosin II-driven isometric contraction of the actin cytoskeleton, or by extracellular perturbations such as matrix stretching or fluid shear stress. Force-induced assembly of the adhesion plaque leads to the activation of a variety of signaling pathways that control cell proliferation, differentiation, and survival (e.g., MAP kinase and PI 3-kinase pathways) as well as the organization of the cytoskeleton (e.g., Rho family GTPase pathways). Rho, in particular, is an indispensable regulator of FA assembly affecting, via its immediate targets Dia1 and ROCK, actin polymerization and myosin II-driven contractility. From (Geiger and Bershadsky, 2002) with permission
About this time, at the TM symposium in Chatham, MA in 1993. an encounter with Benny Geiger, the discoverer of vinculin and perhaps “the king of the cytoskeleton,” led to my sabbatical in his laboratory at the Weizmann Institute of Science in Rehovot, Israel in 1995–1996. He was working in all these areas, but not in the eye. I spent a year there amongst people of many different cultures and disciplines.
We began to understand the biochemical pathways involved that would allow us to regulate assembly and disassembly of the actin microfilaments. Myosin II-driven contractility plays a crucial role in the assembly and maintenance of two domains of the actin cytoskeleton, stress fibers and associated focal adhesions that link the actin cytoskeleton to the extracellular matrix. Formation of stress fibers and focal adhesions in the cell is triggered by the small GTPase Rho, which activates members of the Rho-associated kinase family (Burridge and Wennerberg, 2004). Stimulating the Rho pathway and enhancing phosphorylation of the myosin light chain increases contractility. Conversely, inhibiting the Rho pathway and myosin light chain kinase with a variety of small molecules (H-7, ML-7, Y-27632) (Epstein et al., 1999; Rao et al., 2001; Tian et al., 1998) or with bacterial toxins such as C3 (Liu et al., 2005), will uncouple actin from myosin, resulting in relaxation of the cells and disassembly of the actin cytoskeleton. In addition to small molecules and toxins, there are other proteins, such as caldesmon, that uncouple the linkage of actin and myosin II resulting in focal adhesion disassembly and loss of actomyosin contractility (Helfman et al., 1999).
We investigated in cells, how these substances worked and then were able to convey results, plans, course corrections, etc., within the same day, via email, phone calls and fax, back to my laboratory in Wisconsin where experiments were conducted in living nonhuman primates within days. It turned out that the dosages in cells were very predictive of what was going to happen to outflow facility in the live monkey.
One molecule that we examined originated from Latrunculia (now Negombata) magnifica, a sponge that lives in the bottom of the Red Sea. Latrunculins disrupt actin filaments in a much more gentle and perhaps physiologic way than cytochalasins; they inhibit the assembly of actin by binding free actin in the cell. Consequently, the actin microfilament degrades, resulting in loosening of cell-cell junctions, rounding of cells and cell separation. Figure 4 shows a bovine aortic endothelial (BAEC) cell stained for actin following treatment with 0.2μM latrunculin-A for 5 hours, resulting in attenuation of the actin filaments.
Figure 4.
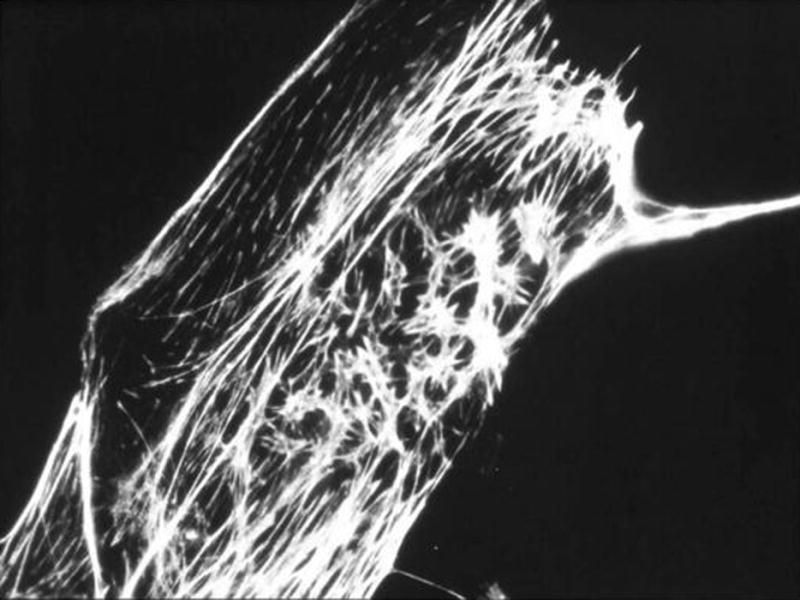
Bovine aortic endothelial (BAEC) cells stained for actin following treatment with 0.2μM latrunculin-A for 5 hours, resulting in attenuation of the actin filaments. [original]
Administration of latrunculin B into the anterior chamber of a monkey eye causes a dose-dependent increase in outflow facility (Peterson et al., 2000). Further, infusing the drug into the anterior chamber, allowing the facility effect to develop, then washing the drug out with drug-free solution, and stopping the infusion for an hour reveals no loss of the facility effect when the drug-free infusion is restarted. However, leaving the system off for 4 hrs after the washout reveals that the outflow facility has returned to normal, but when the drug-free infusion is resumed the facility increases as it did during the initial drug infusion (Peterson et al., 1999). Treating the normotensive monkey eye topically with one of these compounds as would be done for glaucoma therapy, causes a substantial reduction in IOP (Figure 5) (Okka et al., 2004; Peterson et al., 2000). The outflow facility effect following topical treatment does not start right away but only develops with continued perfusion even though the drug concentration in the anterior chamber is decreasing with time (Okka et al., 2004; Peterson et al., 1999). Thus the system has been weakened such that the pressure gradient and fluid flow across the system can now collapse the “house of cards” that the drug initially created. This is a potentially wonderful situation for glaucoma therapy where an even greater pressure gradient is present.
Figure 5.
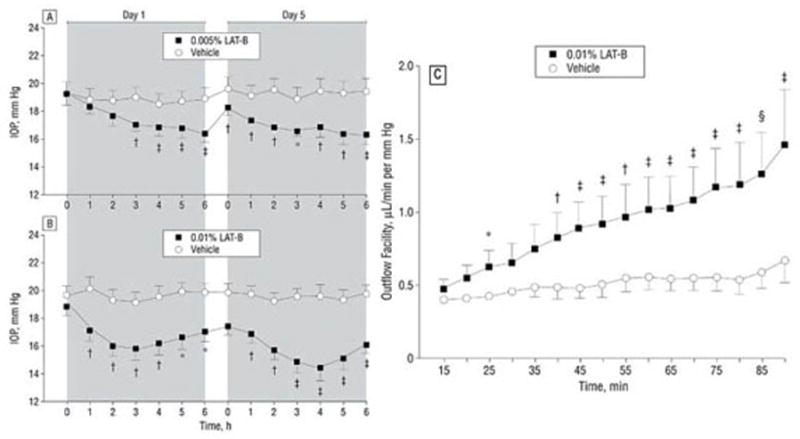
Effects of Latrunculin (LAT) B on IOP and outflow facility in monkeys. A,B. 0.005/0.01% Lat-B and vehicle (4×5μl or 2×10μl) were administered to opposite eyes topically twice daily for 4.5 days. IOP was measured before and after the 1st (on Day 1) and 9th (on Day 5) treatment. C. Outflow facility was measured by 2-level constant pressure perfusion for 90 minutes on day 9 (2 hours after the 15th treatment with 0.01% LAT-B once or twice daily). Data are expressed as mean±s.e.m. N=8 (IOP); n=7 (outflow facility). IOP difference between eyes corrected for baseline was tested for differences vs 0.0 by the 2-tailed paired t-test: *p<0.01; †p<0.005; ‡p<0.001. Outflow facility difference between eyes was tested vs 0.0 by the 2-tailed paired t-test: *p<0.05; †p<0.03; ‡p<0.05; §p<0.01. From (Okka et al., 2004) with permission
On the kinase side, H-7, which is available off the shelf, is a rather nonselective Rho kinase/myosin light chain kinase inhibitor, and a protein kinase C inhibitor (Citi et al., 1994). Y-27632 is a more selectively a Rho kinase inhibitor. Both reduce actomyosin-driven contractility resulting in the deterioration of actin microfilament bundles, perturbation of membrane anchorage of the microfilament system and loosening or weakening of cell-extracellular matrix junctions. Intracameral exchange with either of these compounds results in a dramatic several fold increase in outflow facility in the nonhuman primate eye after an initial delay. Similarly topical H-7 produced about the same doubling of outflow facility (Tian et al., 1998; Tian et al., 2004).
To examine the structural effects, we are working with Dr. Geiger’s group. Following intracameral exchange of monkey eyes with latrunculin B (0.5μM) there is expansion of the juxtacanalicular area, separation of inner wall endothelium along with the first subendothelial cell layer such that it lifts away from the juxtacanalicular meshwork resulting in a ballooning of the juxtacanalicular region (Figure 6). The entire meshwork is expanded allowing fluid to get through more easily as shown with tracer studies. However there are no breaks in the inner wall itself (Sabanay et al., 2006).
Figure 6.
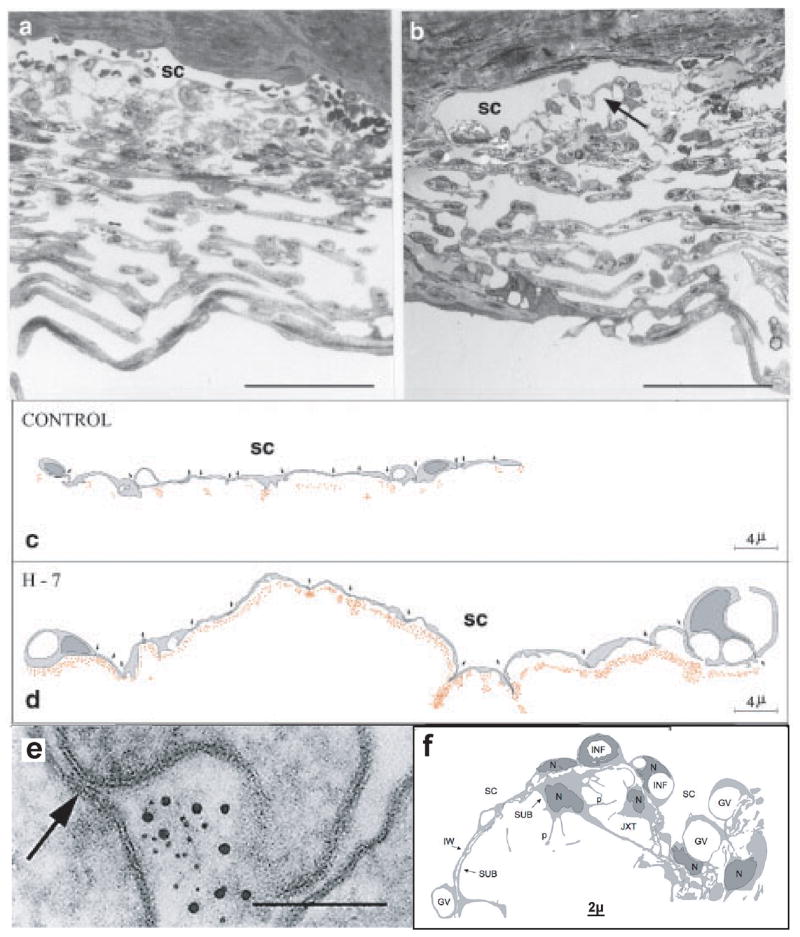
Morphology after perfusion of monkey eyes in vivo with H-7 or LAT-B. Light micrographs of vehicle (A) and H7 (B) treated eyes showing expanded intercellular spaces (arrow), extended IW cells, and maintained cell-cell junctions after H-7. (bar = 50μm) Schematic drawings depicting 15-cell stretches (cell-cell junctions marked by arrows) along the SC of control (C) and H-7-treated (D) eyes. The location of individual gold particles is represented by red dots. (E). Extracellular subcanalicular gold reaches cell-cell junctions (arrow in E) but apparently does not penetrate through them. (bar = 0.1μm) From (Sabanay et al., 2000) with permission. (F). Schematic figure showing a long “montage” of transmission EM images, depicting the inner wall IW–JXT regions of the TM following LAT-B. Massive “ballooning” of the JXT region is shown along with retention of close contact between IW and SUB, the irregular diameter of P of IW cells and the prominent GV. It is difficult to state whether LAT-B increased GV prominence due to the apparent variability in the prominence of GV in the vehicle-treated eye, as well as their non-homogeneous distribution along the canal’s wall. Original (created by Nili Dezorella, unpublished) based on Figure 3 in (Sabanay et al., 2006) with permission.
GV, giant vacuoles; INF, membrane infoldings; IW, inner wall; JXT, juxtacanalicular region; OW, outer wall; P, cellular processes; SC, Schlemm’s canal; SUB, sub-canalicular cells; TM, trabecular meshwork
H-7 treatment also produces dilation of Schlemm’s canal and expansion of the juxtacanalicular meshwork as the endothelial cells are relaxed, as shown in Figure 6, which is a schematic 15 cell stretch of Schlemm’s canal inner wall endothelium where the cells are more relaxed and much longer after H-7 treatment (Sabanay et al., 2000). Tracer injected into the anterior chamber is spread evenly along the inner wall as opposed to the untreated eye (Sabanay et al., 2000) in which there is a funneling of fluid flow through preferential channels (Johnson et al., 1992; Overby et al., 2002), again without inner wall breaks. Thus with these cytoskeletal compounds, the entire wall becomes available for filtration.
The effects of H-7 (Sabanay et al., 2004) and Lat-A (Peterson et al., 1999) are reversible, indicating they are due to alterations in cellular contractility and cytoskeletal organization rather than irreversible toxicity. In addition, intravitreal administration of H-7 or latrunculin-B at doses that increase outflow facility and lower IOP when given intracamerally, had no effect on retinal vascular permeability, retinal electrophysiology, or the clinical or angiographic appearance of the retina (Kiland et al., 2006).
Interestingly the cornea is not affected at concentrations of these compounds that affect the TM and increase outflow facility (Okka et al., 2004; Sabanay et al., 2006). Perhaps there is a differential sensitivity to different cell types. Or, since the corneal endothelium sits on a hard backing, Descemet’s membrane, whereas the TM is basically suspended like a hammock between two fluid compartments at different pressures, the corneal endothelium is not comparably exposed to the concomitant assault by pressure and shear stress.
Derivatives of all these compounds are now being tested in clinical trials. For the latrunculins, it is the first time such molecules have ever been in man. Thus the science has evolved from hard-core cell biology, through organ culture and in vivo animal physiology, and into the clinical arena, involving multinational collaborations and friendships along the way.
Gene Therapy to Increase Outflow Facility
This leads us to the concept of gene therapy. Rather than using small molecules to inhibit a kinase or “dry up” the free actin pool, one can over-express proteins that either regulate actin-myosin interaction (caldesmon) or inhibit the Rho cascade (C3 exotoxin). The principle here is to get an exogenous gene into the cell to make it produce more or less of something to get the desired effect. It is not a question of fixing a defective gene that is causing a problem, but rather producing a product that is therapeutically useful (Liu et al., 2007).
There are many ways to put genes into cells, but viral vectors are Mother Nature’s own delivery system. The idea here is to cripple the virus so it doesn’t cause problems in the cell you are dealing with and then to put in the gene you want with the appropriate promoter. Figure 7 is an example of the effect of caldesmon over-expression in TM cells (Grosheva et al., 2006). HTM cells were exposed to an adenoviral vector expressing rat non-muscle caldesmon fused to green fluorescent protein (AdCaldGFP), resulting in morphological changes within 24–48 hrs. Cells expressing moderate levels of caldesmon exhibit short bundles containing actin and myosin II that can form arrays of triangular actin structures with small vinculin-positive focal adhesions at their vertices. The fraction of cells displaying large lamellipodia is also increased. In cells expressing high levels of caldesmon, severe changes in the actin cytoskeleton occur manifested by the disappearance of stress fibers and the formation of curved actin- and myosin-containing bundles that form dynamic pulsating loops filling the cytoplasm.
Figure 7.
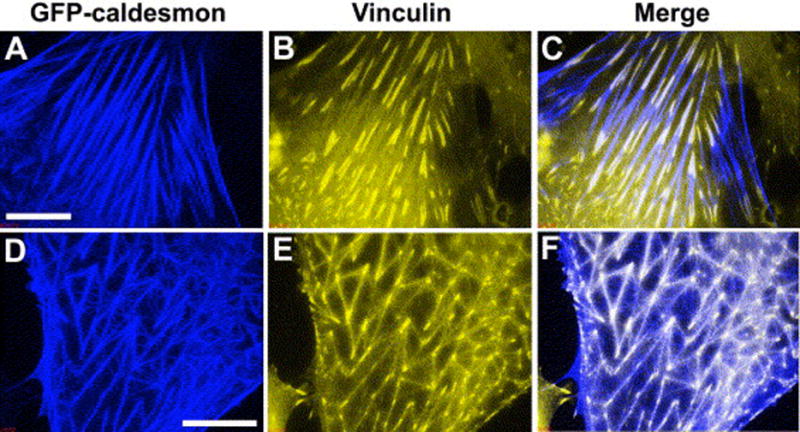
Organization of the stress fibers and focal adhesions in HTM cells mildly affected by GFP-caldesmon expression. Two cells expressing GFP-caldesmon and stained with vinculin antibody are shown. GFP-caldesmon is shown in blue in A and D; distributions of vinculin in the same cells are shown in yellow (B and E), and merged images are shown in C and F, respectively. Regions of overlap between blue and yellow are seen as white in the merged images. In cells, which preserve apparently intact stress-fibers (A), vinculin-containing FAs are also well preserved (B). Superimpostition of GFP-caldesmon and vinculin images (C) reveals that GFP-caldesmon often partially or entirely co-localize with vinculin at FAs. In many GFP-caldesmon expressing cells, stress fibers are short and form triangular networks (D). Vinculin is concentrated in the vertices and shows dim fluorescence along the stress fibers (E). GFP-caldesmon localizes prominently to both stress fibers and vertices (D,F). Thin curved fibers enriched in caldesmon do not contain vinculin (F). Scale bars: 10μm. From (Grosheva et al., 2006) with permission
Taking this into the real world, Terete Borrás, a Spaniard by birth now living in the United States, injected the AdCaldGFP into the human organ cultured anterior segment, and demonstrated GFP-caldesmon expression in the TM associated with a 50% increase in outflow facility albeit with moderate variation (Figure 8) (Gabelt et al., 2006). Of course, human autopsy eyes are not really fresh, and this may contribute the smallish response and to the variability. When we inject an adenoviral vector expressing GFP into a live monkey eye, the TM lights up as viewed gonioscopically (Figure 8) (Borrás et al., 2001). The TM is a brilliant green streak that can be seen through the gonioscopy lens without even looking through a slit lamp. However, the adeno viral vector causes a transient inflammatory response and, since expression with the adeno vector is also transient, one really cannot do good in vivo physiology. In organ cultured monkey eyes (Hu et al., 2006) the tissue really is fresh and there is no inflammatory response since there is no immune system. With the appropriate controls for baseline, washout of the contralateral segment, and viral vector control, one sees about a 70% increase in outflow facility following caldesmon over-expression (Gabelt et al., 2006). The same thing happens with Clostridium exotoxin C3, blocking the top of the Rho cascade and doubling outflow facility (Liu et al., 2005). This response following C3 (using 1.2×108 viral particles) is about the same as one gets with small molecule treatments. In the caldesmon studies it is unknown whether the maximum outflow facility response was achieved since a dose-response study was not conducted and different units of measure of the vector were used for the injection. Thus one can “play the system” with both gene therapy and with small molecules.
Figure 8.
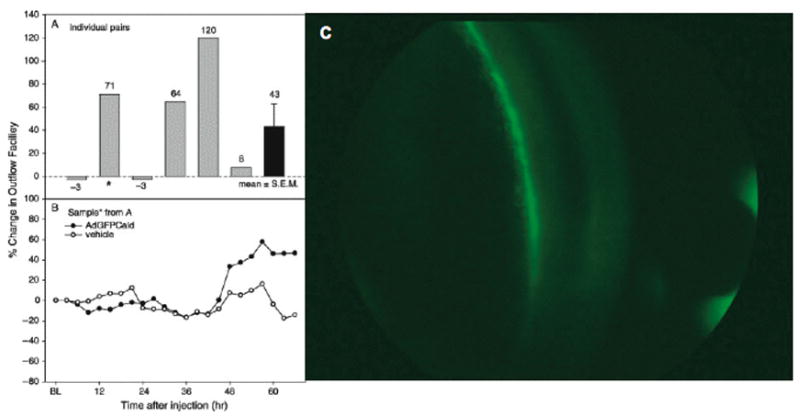
Adeno viral vector delivery of genes to the trabecular meshwork. A,B. Outflow facility in human organ-cultured anterior segments after injection of AdGFPCald or vehicle. (A) Percent change in outflow facility at 66 h post-injection in pairs of segments: AdGFPCald/baseline compared to vehicle/baseline. (B) Percent change in outflow facility compared to baseline for each segment of the pair indicated by * in A: AdGFPCald/baseline and vehicle/baseline. From (Gabelt et al., 2006) with permission. C. Adeno vector delivery of GFP reporter gene to the trabecular meshwork in a living cynomolgus monkey. Gonio images of GFP fluorescence 6 days post intracameral injection of 5×107 pfu AdGFP. Slit lamp examination revealed the presence of 3–4+ cells, 3+ flare.[original]
The challenges with gene therapy include gene-targeting technology, tissue specific promoters, long-term gene expression, vector-associated side effects, repeated injections, and restriction factors (Liu et al., 2007). As vectors improve, lentiviral vectors may be utilized for long-term expression. Following GFP delivery by lentiviral vectors into the anterior segment of monkey eyes, we, in collaboration with Eric Poeschla at Mayo Clinic in Rochester, MN, have achieved long-term expression for over a year in quiet eyes (Poeschla et al., 2006). The next step is long-term over-expression of cytoskeletal disrupting genes in the TM in vivo.
Prostaglandins and Enhancement of Uveoscleral Outflow
Another collaboration that originated during my time in Sweden and then developed further as I was setting up my own laboratory in Madison, WI was with Laszlo Bito, who was then a professor at Columbia University in New York. He had an interest in prostaglandins. Prostaglandins had long been thought to only be involved in ocular irritation. However, Laszlo’s experiments on transport processes and ocular and systemic pharmacokinetics led him to conclude that prostaglandins function like local hormones and are uniquely suited to achieve localized effects after topical application, without systemic effects (Bito, 1989). Together with Carl Camras, they produced a seminal paper demonstrating that low doses of prostaglandins reduce IOP in uncannulated rabbit eyes (Camras et al., 1977). We all thought that the monkey would be a much better model in terms of similarity to the human for studying physiologic and pharmacologic responses (Kaufman and Barany, 1977). The complete prostaglandin story and development of latanoprost for glaucoma therapy is summarized in the Proctor Lectures by Laszlo Bito (Bito, 2001) and Johan Stjernchantz (Stjernschantz, 2001).
My own initial studies on the mechanism of IOP reduction by prostaglandins in monkeys suggested there was no effect on outflow facility shortly after a single intracameral bolus injection of PGE1, E2, or F2α (Kaufman, 1986). We next investigated the possibility that uveoscleral outflow might be involved by using pilocarpine to obliterate the spaces between the ciliary muscle bundles to block uveoscleral outflow, an effect demonstrated years earlier by Bárány and Rohen (Bárány and Rohen, 1965) and by Bill (Bill, 1967). We found that topical pilocarpine administered prior to the 7th dose of twice daily application of PGF2α blocked the IOP lowering effect of PGF2α (Figure 9) (Crawford et al., 1987). Subsequently, studies in my laboratory directly measuring uveoscleral outflow using fluoresceinated dextran and isotope accumulation techniques (Gabelt and Kaufman, 1989) and in Dr. Bill’s laboratory indirectly measuring uveoscleral outflow using isotope dilution and accumulation techniques (Nilsson et al., 1989) showed that uveoscleral outflow in monkeys was increased 2–3 fold after multiple topical treatments (Gabelt and Kaufman, 1989) or by 60% after a single topical dose (Nilsson et al., 1989) of PGF2α-isopropyl ester.
Figure 9.
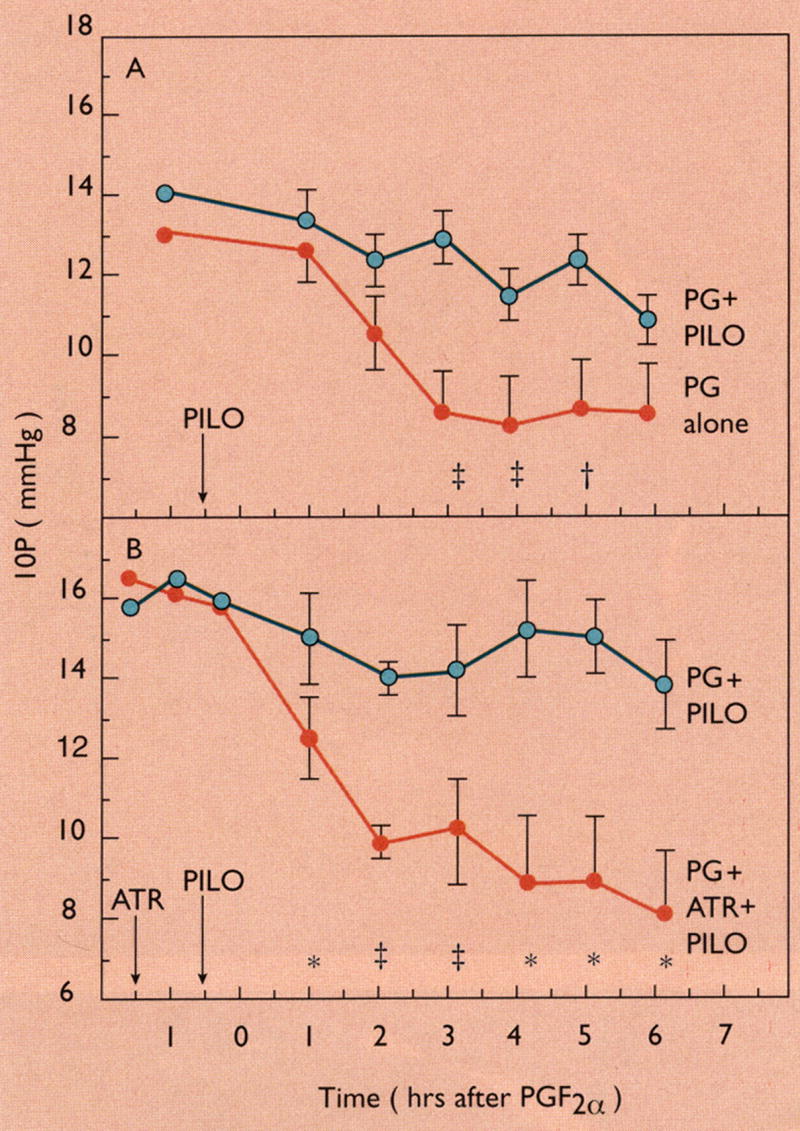
Ciliary muscle contraction with pilocarpine blocks uveoscleral outflow and the IOP lowering response to PGF2α. A: Pilocarpine HCl (PILO) 1000 μg administered to one eye before the seventh bilateral 50-μg dose of PGF2α tromethamine (day 4 of twice-daily treatment). PILO partially blocked the hypotensive effect of PGF2α, presumably by causing ciliary muscle contraction, which obstructs uveoscleral outflow. Data are mean ± SEM IOP for 11 cynomolgus monkeys, each contributing one eye treated with PGF2α + PILO and one eye treated with PGF2α only. B: Atropine sulfate (ATR) 100 μg was administered unilaterally approximately 45 min before bilateral PILO 1000 μg, and approximately 85 min before the seventh bilateral dose of PGF2α tromethamine (day 4 of twice-daily treatment). ATR prevents the PILO-induced contraction of the ciliary muscle so that the hypotensive effect of PGF2α is unaffected. Data are mean ± SEM IOP for four cynomolgus monkeys, each contributing one eye treated with PGF2α + PILO and one eye treated with PGF2α + PILO + ATR. Significantly different from opposite eye by the two-tailed paired t-test: *p < 0.05; †p < 0.02; ‡p < 0.01; §p < 0.001. From (Crawford and Kaufman, 1987) with permission.
Morphologic studies conducted by our collaborators in Germany (Tamm et al., 1990) showed that topical treatment of monkeys with PGF2α-isopropy ester induces a reduction in extracellular matrix in the spaces between ciliary smooth muscle fiber bundles. We also examined the effects of other drugs on anterior segment morphology during my sabbatical in Germany (1985–86).
With Max Cynader at the University of British Columbia, during a 1995 sabbatical, we began to investigate the possibility of using gene therapy to deliver the PGF synthase gene to the anterior segment as an alternative to topical prostaglandin therapy.
Another collaboration evolved with Robert Weinreb and James Lindsey at the University of California, San Diego to determine whether and how PGF2α could induce the degradation of the extracellular matrix to reduce the hydraulic resistance around the muscle bundles, thus facilitating aqueous flow through the muscle. Their earlier work had shown that following treatment of human ciliary muscle cells in culture with PGF2α, collagens type I and III were reduced while matrix metalloproteinases 1, 2, 3 and 9 were increased (Lindsey et al., 1996; Lindsey et al., 1997). In vivo, following topical treatment of monkey eyes for 5 days with PGF2α, collagen types I, III, and IV immunoreactivity in the ciliary muscle and adjacent sclera was reduced (Sagara et al., 1999) while matrix metalloproteinases 1, 2, and 3 were increased(Table 1) (Gaton et al., 2001; Weinreb et al., 2002). Thus the reduction of ciliary muscle extracellular matrix within the interbundle spaces reduces hydraulic resistance to aqueous movement and contributes to the PG-mediated increase of uveoscleral outflow.
Table 1.
Increased MMPs and Reduced Collagens in the Ciliary Muscle of Monkey Eyes Treated With PGF2α-Isopropyl Ester
| Percentage Change | |
|---|---|
| MMP-1 (interstitial collagenase) | + 61±8 |
| MMP-2 (gelatinase A) | + 82±27 |
| MMP-3 (stromelysin-1) | + 83±49 |
| Collagen type I (interstitial fibrillar) | −52±7 |
| Collagen type III (interstitial fibrillar) | −45±7 |
| Collagen type IV (basement membrane) | − 34 ± 11 |
From (Weinreb et al., 2002) with permission
Presbyopia and the Dynamics of Accommodation
Another area of collaboration developed with Laszlo who knew that I measured accommodation in monkeys. He was interested in ocular aging and had the idea that the monkey would be a good model to study human presbyopia. Initial studies were conducted at the Wisconsin Regional Primate Research Center in Madison, where there was a large colony of caged rhesus monkeys of known ages. Indeed, the age related decline in accommodation in monkeys (Figure 10) (Bito et al., 1982; Bito and Miranda, 1987) adjusted for their lifespan, could be superimposed on the classic accommodation vs age curve generated by Duane in humans (Duane, 1922). Other age-related ocular changes in monkeys had already been described (Kaufman and Bito, 1982).
Figure 10.
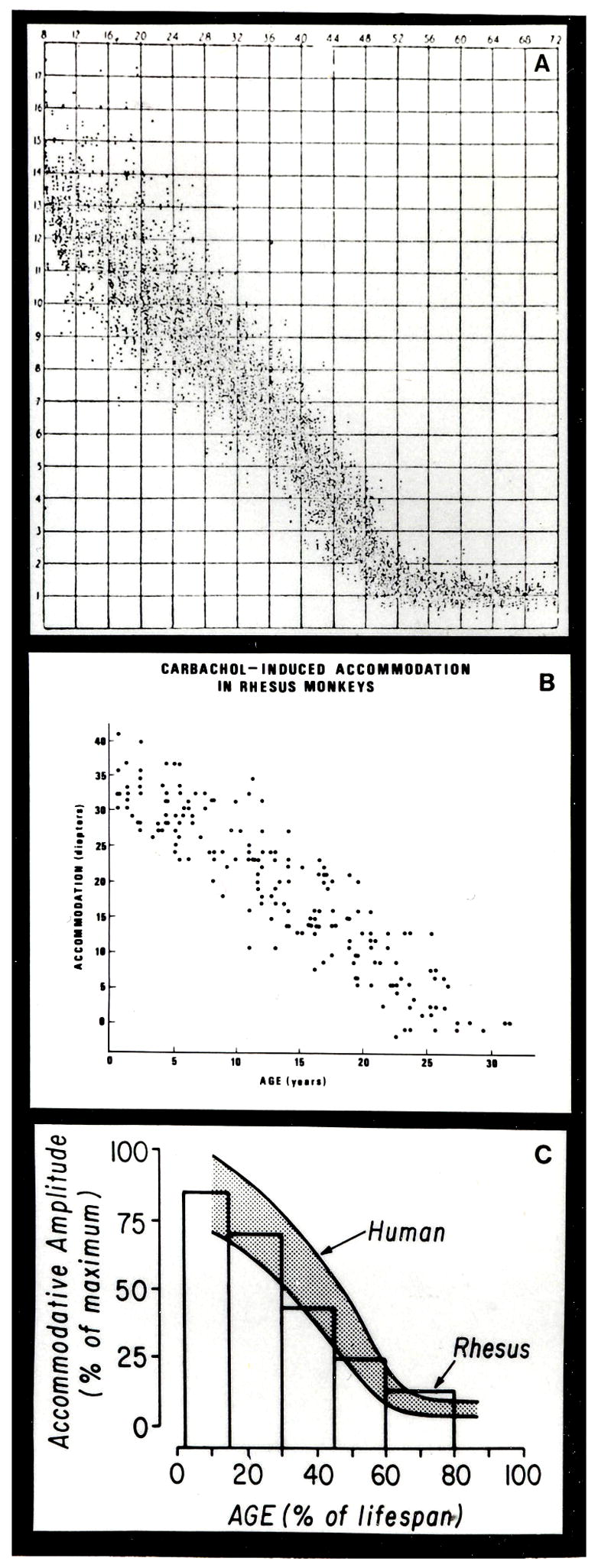
Human and rhesus patterns of age-dependent loss in accommodative amplitude. Human data (A) From (Duane, 1922) with permission Carbachol-induced accommodation (B) in rhesus monkeys From (Kaufman and Gabelt, 1993) with permission. Composite (C) showing the mean accommodative amplitude for each age group of rhesus monkeys (bars) superimposed on the time course of decrease in accommodative amplitude in humans, shown by the shaded area that includes most normal human cases. It is assumed that the lifespans of rhesus monkeys and humans are 35 and 80 years, respectively, and that their maximum accommodative amplitudes are 40 and 16 diopters, respectively. From (Bito and Miranda, 1987) with permission.
Subsequently our adventures took us to the Carribean Primate Research Center in Puerto Rico where similar studies were conducted on free ranging rhesus monkeys. Each morning we traveled by motor boat to the small island where the monkeys roamed free. All of our supplies and equipment had been transported there from the US and housed in shacks. We had to take our own generator to supply electricity. The monkeys would often run into the hut and steal food while we ate lunch. Most of the time the workers would bring the monkeys to us after netting them in the feeding corals where they came to eat each day. We were there at the time the monkeys were having annual check ups and blood draws so the monkeys were brought to us anesthetized. However, some older monkeys were very smart and did not go into the corals since they realized something was going on. Of course, these were the most interesting to study. Therefore the workers on the island and even Laszlo himself would try to dart them with blow-guns as the monkeys observed us from the trees. Ultimately, similar results were obtained in the accommodation vs age responses in caged and free-ranging rhesus monkeys (Miranda et al., 1986).
During this time, including my 1985–1986 sabbatical in Germany, we also collaborated with Profs Lütjen-Drecoll and Rohen, and one of their rising stars, Ernst Tamm (now Professor and Chairman of Anatomy at the University of Regensberg, Germany), to examine the morphologic changes in the monkey eye with age, and the age-related response of the ciliary muscle to cholinergic stimulation. The anatomy of the rhesus monkey accommodation apparatus was found to be very similar to that of the human (Lütjen-Drecoll et al., 1988). In the intact rhesus monkey eye, movement of the ciliary muscle in response to pilocarpine declined with age (Lütjen-Drecoll et al., 1988). However, if the attachments of the ciliary muscle were severed, the old ciliary muscle was capable of contracting as much as the ciliary muscle in a young eye. This was due to an age-related loss of elasticity of the posterior attachments of the ciliary muscle (Figure 11) (Tamm et al., 1992). Therefore, we hypothesized that perhaps presbyopia might in part be due to lack of mobility of the ciliary muscle in addition to changes that occurred in the lens.
Figure 11.
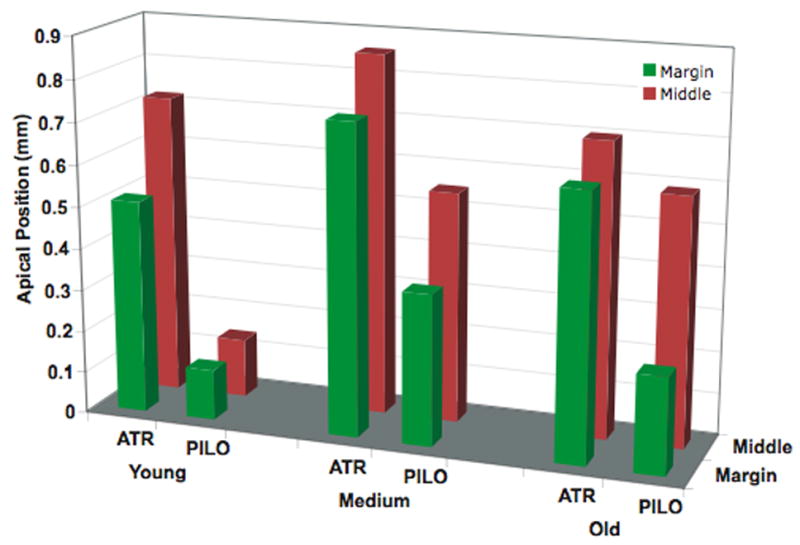
Loss of ciliary muscle mobility with age. Data plotted denote mean apical position of the ciliary muscle grouped according to age, drug, and section location. Red bars represent sections obtained from the middle (farthest from the original meridional cut), where the ciliary muscle is intact. Green bars represent sections taken from the margin (closest to the original meridional cut), where some of the posterior and outer attachments of the ciliary muscle have been severed. (Modified from (Tamm et al., 1992) with permission.)
Consistent with these findings were studies conducted in my laboratory showing that the isolated rhesus monkey ciliary muscle did not lose its ability to contract with age in response to cholinergic stimulation (Poyer et al., 1993). Also, there was no change in the ciliary muscle activity levels for the biosynthetic and biodegradative enzymes for the cholinergic neurotransmitter acetylcholine and no change in the affinity or number of muscarinic receptor binding sites (Gabelt et al., 1990). However, in vivo, where the muscle still has its posterior attachments, there was an age-related loss in the outflow facility response to pilocarpine in rhesus monkeys (Gabelt et al., 1991) but not in humans (Croft et al., 1996).
In order to advance our understanding of presbyopia in nonhuman primates, we developed a technique, in collaboration with Ei Terasawa at the Wisconsin Regional Primate Center, to implant an electrode into the Edinger Westphal nucleus so that we could reproducibly stimulate accommodation (Crawford et al., 1989). In the iridectomized eye, Scheimplug (Figure 12) (Neider et al., 1990) and gonioscopic imaging (Table 2) (Croft et al., 2006) showed an age-related loss of lens and perhaps centripetal ciliary body accommodative movement response during central stimulation similar to the age-related loss in accommodative response we had already shown with cholinergic stimulation (Bito et al., 1982). Ongoing studies are investigating the age-related dynamics of the lens, ciliary body and zonules during accommodation. The age-dependent decline in amplitude and velocity of ciliary body movements during accommodation suggests that ciliary body dysfunction plays a role in presbyopia. The age-related dampening of lens movement could be a consequence of increasing inelasticity or hardening of the lens, or of age-related changes in ciliary body mobility (Croft et al., 1998). In any case, the decreased ciliary muscle mobility needs to be considered when developing accommodating IOLs. The addition of ultrasound biomicroscopic imaging and surgical manipulation or ablation of accommodation relevant structures can provide clues as to their roles in accommodation and presbyopia. Thus far the width of the circumlenticular space and the magnitude of forward ciliary body movement (Figure 13 and Table 2) have been found to decrease with age. The latter may contribute to decreased lens movement with age (Croft et al., 2006; Croft et al., 2006). Morphologic analysis of these eyes may provide new insights about zonular anatomy.(unpublished data).
Figure 12.
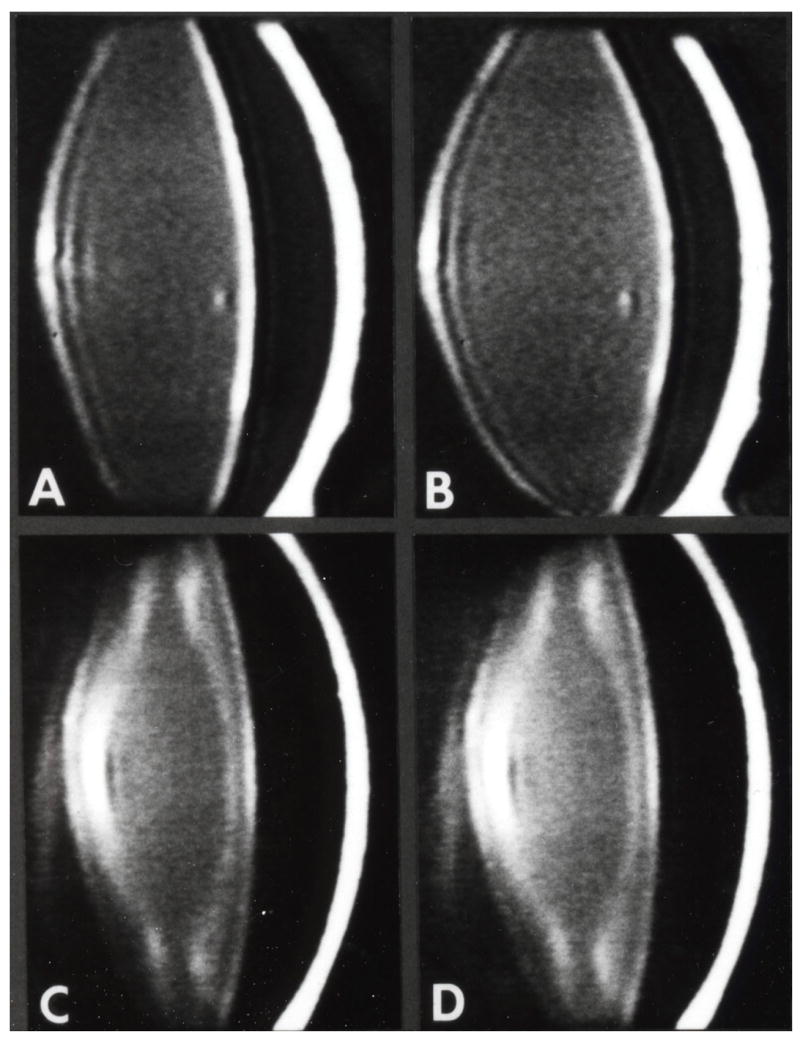
Scheimpflug imaging to show an age-related loss of accommodation following central stimulation. A-D. Scheimpflug videography of rhesus monkey anterior segment 1 to 3 years following total iridectomy and midbrain electrode implantation. A and B, 4 years; C and D, 23 years. A and C, nonaccommodating; B and D accommodating maximally (14.0 and 1.5 diopters, respectively) in response to central electrical stimulation. Note: (1) decreasing overall transparency and increasing prominence of discontinuity zones of the lens with increasing age (A vs C); (2) lens thickening and anterior chamber shallowing during accommodation (A vs B, C vs D); and (3) loss of dissimilarity between nonaccommodating and accommodating eye with increasing age (A and B vs C and D). Modified from (Neider et al., 1990) with permission.
Table 2.
Decline in Ciliary Body(CB)-Cornea Angle and Temporal Centripetal Ciliary Ciliary Process(CP) Movement with Age
| A. Temporal CB-Cornea Angle Measurements (degrees) | B. Smax Temporal Centripetal Movement (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Accommodative Angle-Narrowing | ||||||||
| Resting (°) | Smax (°) | Resting – Smax (°) | n | Accommodation (Diopters) | CP | Lens | n | |
| Young | ||||||||
| Mean | 157.5 | 84.1 | 73.0 | 6 | 15.2 | 0.41 | 0.30 | 5 |
| SEM | 3.8 | 2.8 | 6.4 | 1.1 | 0.02 | 0.01 | ||
| Older | ||||||||
| Mean | 147.5 | 123.5 | 24.0 | 11 | 2.4 | 0.32 | 0.15 | 11 |
| SEM | 2.5 | 3.1 | 3.0 | 0.5 | 0.05 | 0.03 | ||
| Young vs older p= | 0.065 | 0.001 | 0.001 | 0.001 | 0.109 | 0.001 | ||
| Decline Older vs Young (%) | 67.1 | 84.1 | 22.5 | 49.3 | ||||
(A) Data are the mean ± SEM angle between the anterior aspect of CB and the inner aspect of the cornea (CB-cornea angle) in the temporal quadrant measured in degrees. Measurements were taken from UBM images in the unaccommodated (resting) eye and during supramaximal (Smax) stimulation in 5 young rhesus and 1 young cynomolgus (age range, 5.8–9.5 years), and in 11 older rhesus monkey eyes (age range, 17–26 years). The farther the CB moved forward during accommodation, the more narrow the CB-cornea angle. (B) Data are the mean ± SEM centripetal CP and lens movement amplitude (mm) in the unaccommodated eye and during supramaximal stimulation as measured from goniovideography images taken in the same eyes as in (A). P≤0.05 denotes a significant difference between young and older monkey eye by the two-sample t-test. Percentage of decline older versus young is calculated as [(older/young) −1] *100. Loss of forward CB movement as represented by accommodative CB-cornea angle change was more pronounced than loss of centripetal movement in the temporal quadrant. From (Croft et al., 2006), with permission
Figure 13.
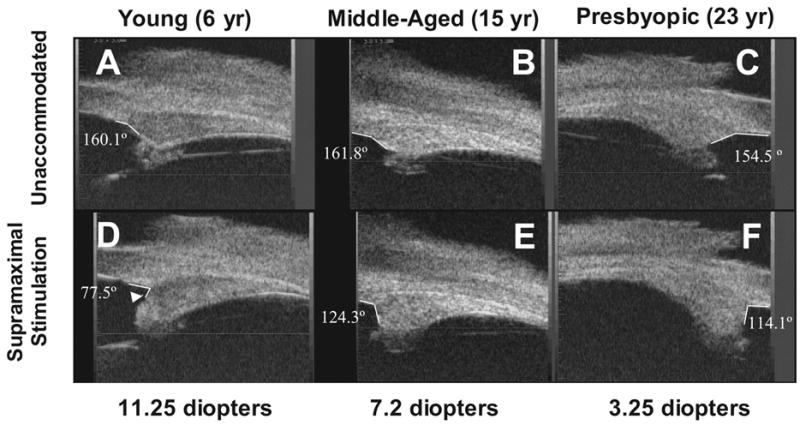
UBM images of the temporal quadrants in three normal iridectomized monkey eyes ages 6 (A, D), 15 (B, E) and 23 (C, F) years in the unaccommodated and accommodated states. The degrees represent the angle between the anterior aspect of the ciliary body (CB) and the inner aspect of the cornea (CB-cornea angle). During centrally stimulated accommodation, the CB moved forward and inward, and its anterior aspect (D, white arrowhead) moved past the scleral spur at higher accommodative amplitudes in the young eye but not in the older eye. The anterior aspect of the CB did not move past the scleral spur at any stimulus current in the older presbyopic eye and did not form an acute angle with the inner aspect of the cornea. The young eye accommodated 11.25 D and the older presbyopic eye accommodated 3.25 D. From (Croft et al., 2006) with permission.
Final comments
I hope this summary illustrates that global science involves complex coordination between many universities/laboratories, colleagues and countries, and convergence of thought processes toward major insights. One never truly reaches the “end-game”, but collectively the hunt generates important advances and relationships that last a lifetime. The collaborative interrogation of Mother Nature has resulted in many dozens of papers and patents. Such global collaboration will be essential in the future, as science becomes “bigger”.
I am grateful to ISER for awarding me the Ernst H. Bárány Prize. It is especially meaningful to me that it honors Dr. Bárány and by extension the people he helped father professionally, among them Dr. Bill, Dr. Lütjen-Drecoll, Dr. Rohen, Dr. Sears, Dr. Becker, Dr. Tamm and myself, who have cherished these relationships for decades.
Acknowledgments
NEI R01 EY02698, NEI P30 EY016665 (Core Grant for Vision Research); Research to Prevent Blindness, Inc, New York, NY; unrestricted departmental, Senior Scientific Investigator and Physician-Scientist awards; Ocular Physiology Research and Education Foundation; Walter Helmerich Chair from the Retina Research Foundation; P51 RR000167 (Wisconsin National Primate Research Center grant from the NCRR)
Carol Rasmussen assisted with preparation of slides for the award presentation. B’Ann Gabelt assisted with drafting the manuscript text.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bárány EH. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus ethiops) Invest Ophthalmol. 1962;1:712–727. [PubMed] [Google Scholar]
- Bárány EH, Rohen JW. Localized contraction and relaxation within the ciliary muscle of the vervet monkey (Cercopithecus ethiops) In: Rohen JW, editor. The Structure of the Eye, Second Symposium. FK Schattauer Verlag; Stuttgart: 1965. pp. 287–311. [Google Scholar]
- Bárány EH. The mode of action of miotics on outflow resistance. A study of pilocarpine in the vervet monkey (Cercopithecus ethiops) Trans Ophthalmol Soc UK. 1966;86:539–578. [PubMed] [Google Scholar]
- Bárány EH. The immediate effect on outflow resistance of intravenous pilocarpine in the vervet monkey. Invest Ophthalmol. 1967;6:373–380. [Google Scholar]
- Bárány EH. Topical epinephrine effects on true outflow resistance and pseudofacility in vervet monkeys studied by a new anterior chamber perfusion technique. Invest Ophthalmol. 1968;7:88–104. [PubMed] [Google Scholar]
- Bill A. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (macaca irus) Exp Eye Res. 1967;6:120–125. doi: 10.1016/s0014-4835(67)80062-9. [DOI] [PubMed] [Google Scholar]
- Bill A. Early effects of epinephrine on aqueous humor dynamics in vervet monkeys (Cercopithecus ethiops) Exp Eye Res. 1969;8:35–43. doi: 10.1016/s0014-4835(69)80078-3. [DOI] [PubMed] [Google Scholar]
- Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- Bito LZ, Miranda OC. Presbyopia: the need for a closer look. In: Stark L, Obrech G, editors. Presbyopia: recent research and reviews from the third international symposium. Churchill/Professional Press; New York: 1987. pp. 411–429. [Google Scholar]
- Bito LZ. A physiological approach to glaucoma management: The use of local hormones and the pharmacokinetics of prostaglandin esters. In: Bito LZ, Stjernschantz J, editors. The Ocular Effects of Prostaglandins and Other Eicosanoids. Progress in Clinical and Biological Research vol 312. Alan R. Liss; New York: 1989. pp. 329–347. [PubMed] [Google Scholar]
- Bito LZ. A new approach to the medical management of glaucoma, from the bench to the clinic, and beyond (Proctor Lecture) Invest Ophthalmol Vis Sci. 2001;42:1126–1133. [PubMed] [Google Scholar]
- Borrás T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3:437–449. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- Brown SS, Spudich JA. Mechanism of action of cytochalasin: evidence that it binds to actin filament ends. J Cell Biol. 1981;88:487–491. doi: 10.1083/jcb.88.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Camras CB, Bito LZ, Eakins KE. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Invest Ophthalmol Vis Sci. 1977;16:1125–1134. [PubMed] [Google Scholar]
- Citi S, Volberg TAD, Bershadsky A, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell-cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107:683–692. [PubMed] [Google Scholar]
- Crawford K, Kaufman PL. Pilocarpine antagonizes PGF2a-induced ocular hypotension: Evidence for enhancement of uveoscleral outflow by PGF2a. Arch Ophthalmol. 1987;105:1112–1116. doi: 10.1001/archopht.1987.01060080114039. [DOI] [PubMed] [Google Scholar]
- Crawford K, Kaufman PL, Gabelt BT. Effects of topical PGF2a on aqueous humor dynamics in cynomolgus monkeys. Curr Eye Res. 1987;6:1035–1044. doi: 10.3109/02713688709034874. [DOI] [PubMed] [Google Scholar]
- Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Research. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- Croft MA, Oyen MJ, Gange SJ, Fisher MR, Kaufman PL. Aging effects on accommodation and outflow facility responses to pilocarpine in humans. Arch Ophthalmol. 1996;114:586–592. doi: 10.1001/archopht.1996.01100130578015. [DOI] [PubMed] [Google Scholar]
- Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. Am J Physiol. 1998;275:R1885–97. doi: 10.1152/ajpregu.1998.275.6.R1885. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Dahl DB, Nadkarni NV, Kaufman PL. Accommodative ciliary body and lens function in rhesus monkeys. 1. Normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006;47:1076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Nadkarni NV, Kaufman PL. The zonula, lens and circumlental space in the normal iridectomized rhesus monkey eye. Invest Ophthalmol Vis Sci. 2006;47:1087–1095. doi: 10.1167/iovs.04-1524. [DOI] [PubMed] [Google Scholar]
- Duane A. Studies in monocular and binocular accommodation with their clinical applications. Am J Ophthalmol. 1922;5:867–877. [PMC free article] [PubMed] [Google Scholar]
- Epstein DL, Rowlette L-L, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81. [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Prostaglandin F2a increases uveoscleral outflow in the cynomolgus monkey. Exp Eye Res. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL, Polansky JR. Ciliary muscle muscarinic binding sites, choline acetyltransferase and acetylcholinesterase in aging rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:2431–2436. [PubMed] [Google Scholar]
- Gabelt BT, Crawford K, Kaufman PL. Outflow facility and its response to pilocarpine decline in aging rhesus monkeys. Arch Ophthalmol. 1991;109:879–882. doi: 10.1001/archopht.1991.01080060143044. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Hu Y, Vittitow JL, Rasmussen CA, Grosheva I, Bershadsky AD, Geiger B, Borras T, Kaufman PL. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. 2006;82:935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gaton DD, Sagara T, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway following topical prostaglandin F(2 alpha)-isopropyl ester treatment. Arch Ophthalmol. 2001;119:1165–1170. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nature Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Vittitow JL, Goichberg P, Gabelt BT, Kaufman PL, Borras T, Geiger B, Bershadsky AD. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006;82:945–958. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Helfman DM, Lemy ET, Berthier C, Shtutman M, Riveline D, Grosheva I, Lachish-Zalait A, Elbaum M, Bershadsky AD. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell. 1999;10:3097–3112. doi: 10.1091/mbc.10.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Gabelt BT, Kaufman PL. Monkey organ-cultured anterior segments; technique and response to H-7. Exp Eye Res. 2006;82:1100–1108. doi: 10.1016/j.exer.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol. 1975;14:766–771. [PubMed] [Google Scholar]
- Kaufman PL, Bárány EH. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol. 1976;15:793–807. [PubMed] [Google Scholar]
- Kaufman PL, Barany EH. Recent observation concerning the effect of cholinergic drugs on outflow facility in monkeys. In: Bito LZ, Davson H, Fenstermacher JD, editors. The Ocular and Cerebrospinal Fluids. Academic Press; London: 1977. pp. 415–418. [Google Scholar]
- Kaufman PL, Bárány EH. Cytochalasin B reversibly increases outflow facility in the eye of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1977;16:47–53. [PubMed] [Google Scholar]
- Kaufman PL, Bill A, Bárány EH. Effect of cytochalasin B on conventional drainage of aqueous humor in the cynomolgus monkey. In: Bito LZ, Davson H, Fenstermacher JD, editors. The Ocular and Cerebrospinal Fluids. Fogarty International Center Symposium. Exp Eye Res. Suppl. Vol. 25. 1977. pp. 411–414. [DOI] [PubMed] [Google Scholar]
- Kaufman PL. Aqueous humor dynamics following total iridectomy in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1979;18:870–875. [PubMed] [Google Scholar]
- Kaufman PL, Bárány EH. Adrenergic drug effects on aqueous outflow facility following ciliary muscle retrodisplacement in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1981;20:644–651. [PubMed] [Google Scholar]
- Kaufman PL, Rentzhog L. Effect of total iridectomy on outflow facility responses to adrenergic drugs in cynomolgus monkeys. Exp Eye Res. 1981;33:65–74. doi: 10.1016/s0014-4835(81)80082-6. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Bito LZ. The occurrence of senile cataracts, ocular hypertension and glaucoma in rhesus monkeys. Exp Eye Res. 1982;34:287–291. doi: 10.1016/0014-4835(82)90061-6. [DOI] [PubMed] [Google Scholar]
- Kaufman PL. Effects of Intracamerally Infused prostaglandins on outflow facility in cynomolgus monkey eyes with intact or retrodisplaced ciliary muscle. Exp Eye Res. 1986;43:819–827. doi: 10.1016/s0014-4835(86)80012-4. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Gabelt BT. Aging, accommodation and outflow facility. In: Lütjen-Drecoll E, editor. Basic Aspects of Glaucoma Research III. Schattauer; Stuttgart: 1993. pp. 257–274. [Google Scholar]
- Kiland JA, Miller CL, Kim CBY, Ver Hoeve JN, Gabelt BT, Peterson J, Nork TM, Kaufman PL. Effect of H-7 and Lat-B on retinal physiology. Curr Eye Res. 2006;31:441–455. doi: 10.1080/02713680600672185. [DOI] [PubMed] [Google Scholar]
- Lindsey JD, Kashiwagi K, Boyle D, Kashiwagi F, Firestein GS, Weinreb RN. Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr Eye Res. 1996;15:869–875. doi: 10.3109/02713689609017628. [DOI] [PubMed] [Google Scholar]
- Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN. Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest Ophthalmol Vis Sci. 1997;38:2214–2223. [PubMed] [Google Scholar]
- Liu X, Hu Y, Filla MS, Gabelt BT, Peters DM, Brandt CR, Kaufman PL. The effects of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- Liu X, Brandt CR, Rasmussen CA, Kaufman PL. Glauocma gene therapy. Expert Rev Ophthalmol. 2007;2:227–236. [Google Scholar]
- Low I, Dancker P, Wieland T. Stabilization of F-actin by phalloidin. Reversal of the destabilizing effect of cytochalasin B. FEBS Lett. 1975;54:263–265. doi: 10.1016/0014-5793(75)80088-3. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Kaufman PL, Bárány EH. Light and electron microscopy of the anterior chamber angle structures following surgical disinsertion of the ciliary muscle in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1977;16:218–225. [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Tamm E, Kaufman PL. Age changes in rhesus monkey ciliary muscle: Light and electron microscopy. Exp Eye Res. 1988;47:885–899. doi: 10.1016/0014-4835(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Tamm E, Kaufman PL. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch Ophthalmol. 1988;106:1591–1598. doi: 10.1001/archopht.1988.01060140759051. [DOI] [PubMed] [Google Scholar]
- Miranda OC, Bito LZ, Kaufman PL, DeRousseau CJ, Raley SE. Ocular development and aging. 2. Different basic patterns of lenticular growth in cats and rhesus monkeys (RhMs) and the lack of iscernible effect of sunlight on lneticular aging in RhMs. Invest Ophthalmol Vis Sci. 1986;27 (ARVO abstracts), Abs nr 215. [Google Scholar]
- Neider MW, Crawford K, Kaufman PL, Bito LZ. In vivo videography of the rhesus monkey accommodative apparatus. Age-related loss of ciliary muscle response to central stimulation. Arch Ophthalmol. 1990;108:69–74. doi: 10.1001/archopht.1990.01070030075032. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Jampol LM, Sears ML. Cyclic-AMP in the aqueous humor: the effects of adrenergic agonists. Exp Eye Res. 1972;14:242–250. doi: 10.1016/0014-4835(72)90009-7. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Chavis RM, Sears ML. Cyclic-AMP in the aqueous humor: the effects of repeated topical epinephrine administration and sympathetic denervation. Exp Eye Res. 1973;16:265–272. doi: 10.1016/0014-4835(73)90092-4. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Sears ML. Adenosine 3′,5′-monophosphate analogue increases the outflow facility of the primate eye. Invest Ophthalmol. 1975;14:688–689. [PubMed] [Google Scholar]
- Neufeld AH, Bartels SP. Receptor mechanisms for epinephrine and timolol. In: Lütjen-Drecoll E, editor. Basic Aspects of Glaucoma Research. Schattauer-Verlag; Stuttgart: 1982. pp. 113–122. [Google Scholar]
- Nilsson SFE, Samuelsson M, Bill A, Stjernschantz J. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2a-1-isopropylester in the cynomolgus monkey. Exp Eye Res. 1989;48:707–716. doi: 10.1016/0014-4835(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Okka M, Tian B, Kaufman PL. Effect of low-dose latrunculin B on anterior segment physiologic features in the monkey eye. Arch Ophthalmol. 2004;122:1482–1488. doi: 10.1001/archopht.122.10.1482. [DOI] [PubMed] [Google Scholar]
- Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002;42:3455–3464. [PubMed] [Google Scholar]
- Perkins TW, Alvarado JA, Polansky JR, Stilwell L, Maglio M, Luster R. Trabecular meshwork cells grown on filters: conductivity and cytochalasin effects. Invest Ophthalmol Vis Sci. 1988;29:1836–1846. [PubMed] [Google Scholar]
- Peterson JA, Tian B, Bershadsky AD, Volberg T, Gangnon RE, Spector I, Geiger B, Kaufman PL. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40:931–941. [PubMed] [Google Scholar]
- Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculin’s effects on intraocular pressure, aqueous humor flow and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:1749–1758. [PubMed] [Google Scholar]
- Poeschla EM, Loewen N, Rasmussen C, Teo W, Khare P, Kaufman PL. Transduction of nonhuman primate trabecular meshwork with lentiviral vectors. Invest Ophthalmol Vis Sci. 2006;47 (ARVO abstracts), Abs nr 2696. [Google Scholar]
- Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells: I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–1049. [PubMed] [Google Scholar]
- Poyer JF, Kaufman PL, Flügel C. Age does not affect contractile responses of the isolated rhesus monkey ciliary muscle to muscarinic agonists. Curr Eye Res. 1993;12:413–422. doi: 10.3109/02713689309024623. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Robinson JC, Kaufman PL. Cytochalasin B potentiates epinephrine’s outflow facility increasing effect. Invest Ophthalmol Vis Sci. 1991;32:1614–1618. [PubMed] [Google Scholar]
- Robinson JC, Kaufman PL. Phalloidin inhibits epinephrine’s and cytochalsin B’s facilitation of aqueous outflow. Arch Ophthalmol. 1994;112:1610–1613. doi: 10.1001/archopht.1994.01090240116035. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Lütjen E, Bárány E. The relation between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1967;172:23–47. doi: 10.1007/BF00577152. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Futa R, Lütjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–585. [PubMed] [Google Scholar]
- Ryder MI, Weinreb RN, Alvarado J, Polansky J. The cytoskeleton of the cultured human trabecular cell. Characterization and drug responses. Invest Ophthalmol Vis Sci. 1988;29:251–260. [PubMed] [Google Scholar]
- Sabanay I, Gabelt BT, Tian B, Kaufman PL, Geiger B. H-7 effects on structure and fluid conductance of monkey trabecular meshwork. Arch Ophthalmol. 2000;118:955–962. [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Topical prostaglandin F2a treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol. 1999;117:794–801. doi: 10.1001/archopht.117.6.794. [DOI] [PubMed] [Google Scholar]
- Sears ML. The mechanism of action of adrenergic drugs in glaucoma. Invest Ophthalmol. 1966;5:115–119. [Google Scholar]
- Sears ML, Neufeld AH. Adrenergic modulation of the outflow of aqueous humor. Invest Ophthalmol. 1975;14:83–86. [PubMed] [Google Scholar]
- Stjernschantz JW. From PGF2a-isopropyl ester to latanoprost: a review of the development of Xalatan. The Proctor Lecture. Invest Ophthalmol Vis Sci. 2001;42:1134–1145. [PubMed] [Google Scholar]
- Svedbergh B, Lütjen-Drecoll E, Ober M, Kaufman PL. Cytochalasin B-induced structural changes in the anterior ocular segment of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1978;17:718–734. [PubMed] [Google Scholar]
- Tamm E, Lütjen-Drecoll E, Rohen JW. Age-related changes of the ciliary muscle in comparison with changes induced by treatment with prostaglandin F2a. An ultrastructural study in rhesus and cynomolgus monkeys. Mechanisms of Ageing and Development. 1990;51:101–120. doi: 10.1016/0047-6374(90)90093-u. [DOI] [PubMed] [Google Scholar]
- Tamm E, Croft MA, Jungkunz W, Lütjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey: role of the choroid. Arch Ophthalmol. 1992;110:871–876. doi: 10.1001/archopht.1992.01080180143043. [DOI] [PubMed] [Google Scholar]
- Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Opthalmol. 1998;116:633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tian B, Wang RF, Podos SM, Kaufman PL. Effects of topical H-7 on outflow facility, intraocular pressure and corneal thickness in monkeys. Arch Ophthalmol. 2004;122:1171–1177. doi: 10.1001/archopht.122.8.1171. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Ryder MI, Polansky JR. The cytoskeleton of the cynomolgus monkey trabecular cell II. Influence of cytoskeleton-active drugs. Invest Ophthalmol Vis Sci. 1986;27:1312–1317. [PubMed] [Google Scholar]
- Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor pathways. Surv Ophthalmol. 2002;47:S53–S64. doi: 10.1016/s0039-6257(02)00306-5. [DOI] [PubMed] [Google Scholar]


