Abstract
The high affinity interleukin (IL)-15 receptor, IL-15Rα, is essential for supporting lymphoid homeostasis. To assess whether IL-15Rα's role in vivo is to trans present IL-15, we generated mixed bone marrow chimera from IL-15Rα– and IL-2/15Rβ–deficient mice. We find that IL-15Rα–competent, IL-2/15Rβ–deficient cells are able to support IL-15Rα–deficient natural killer (NK) and memory CD8+ T cells, thus ruling out secondary signals on these cells and demonstrating that IL-15Rα–mediated presentation of IL-15 in trans is the primary mechanism by which IL-15Rα functions in vivo. Surprisingly, using IL-15– and IL-15Rα–deficient mixed chimera, we also find that IL-15 and IL-15Rα must be expressed by the same cells to present IL-15 in trans, indicating that IL-15Rα is required on a cellular level for the elaboration of IL-15. These studies indicate that IL-15Rα defines homeostatic niches for NK and memory CD8+ T cells by controlling both the production and the presentation of IL-15 in trans to NK and CD8+ memory T cells.
Keywords: intracellular cytokine receptor, IL-15/IL-15Rα preassociation, mixed chimera, IL-2Rβ
Introduction
NK cells and memory CD8+ T cells both play vital roles in protecting the host from intracellular pathogens. Understanding how the survival and maintenance of these populations is regulated in vivo has recently been a field of intensive investigation. Memory CD8+ T cell maintenance is a dynamic process that is critically dependent upon two common γ chain (γc)-dependent cytokines, IL-7 and IL-15. Whereas IL-7 promotes the survival of both naive and memory CD8+ T cells, IL-15 uniquely supports basal memory CD8+ T cell proliferation (1–3). Thus, in the absence of proliferative IL-15 signals, memory CD8+ T cells undergo a slow atrophy in number, until they become essentially undetectable (2–4). In addition to maintaining memory CD8+ T cells, IL-15 and IL-15Rα are also critical for the maintenance of peripheral NK cells (5, 6). Whereas the maintenance of memory CD8+ T cells by IL-15 is mediated by both proliferation and survival, NK cell numbers are primarily maintained by regulating survival (5, 6). Thus, IL-15 plays important, nonredundant roles in maintaining the numbers of both memory CD8+ T cells and NK cells in the periphery.
Earlier studies suggested that IL-15 mediates its biological effects by binding to a high affinity, heterotrimeric receptor complex comprised of IL-15Rα, IL-2/15Rβ, and γc. IL-15Rα, which uniquely binds IL-15, is widely expressed by both hematopoietic and parenchymal cell types and has a high affinity for IL-15 (K d ∼10−11 M; reference 7). Although IL-15Rα may play a role in intracellular signal transduction in certain cell types, other studies suggest that the cytoplasmic tail of IL-15Rα is not critically necessary for enhancing IL-15–induced proliferation (8–11). By contrast, IL-2/15Rβ and γc heterodimers exhibit lower affinity binding for IL-15 (K d ∼10−9 M) in the absence of IL-15Rα, but are clearly essential for transducing IL-15–induced intracellular signals (12, 13). Gene targeting experiments have further demonstrated that all three receptor chains are required to support IL-15–dependent cell populations in vivo (14–16). Importantly, the phenotypes of IL-15– (IL-15−/−) and IL-15Rα– (IL-15Rα−/−) deficient mice are indistinguishable, suggesting that physiologically relevant IL-15 signals require IL-15Rα (16, 17).
Although the studies above are consistent with the idea that soluble IL-15 binds to heterotrimeric IL-15Rα, IL-2/15Rβ, and γc receptors on responsive lymphocytes (e.g., NK cells and memory CD8+ T cells) and stimulates their survival and proliferation, more recent studies have demonstrated that IL-15Rα is required in a non-cell–autonomous manner, i.e., not on responding lymphocytes, but rather on a variety of accessory cell types in the mouse (4, 5, 18). IL-15Rα expression on hematopoietic cells other than the CD8+ T cell is required for CD8+ T cell bystander proliferation and preferentially supports the basal maintenance of memory CD8+ T cells (4, 18). Moreover, IL-15Rα expression by both radiation-sensitive and radiation-resistant cells, but not by responding NK cells, is required for the peripheral survival of NK cells (5 and unpublished data). Finally, IL-15Rα expression by radiation-resistant cells, likely intestinal epithelial cells, is critical for the development of TCR-γ/δ intraepithelial lymphocytes (19). Therefore, as far as the development and subsequent support of several distinct IL-15–dependent cell types is concerned, the critical in vivo functions of IL-15Rα do not appear to be mediated by IL-15Rα expression on IL-15–dependent cell types.
IL-15Rα's non-cell–autonomous role in supporting NK and memory CD8+ T cells is consistent with multiple indirect mechanisms by which IL-15 might signal through IL-15Rα on accessory cells to induce the production of proteins that subsequently support NK and memory CD8+ T cells. It is also consistent with a novel mechanism described in vitro by which IL-15Rα on accessory cells can present IL-15 in trans to IL-2/15Rβ– and γc-bearing lymphocytes (20). Which of these multiple mechanisms is physiologically relevant has not been addressed in vivo, and the molecular mechanisms underlying this novel cellular physiology have not been investigated. Accordingly, we have used a variety of mixed radiation chimera to examine the in vivo roles of IL-15 and its various receptor chains in supporting NK and memory CD8+ T cell homeostasis.
Materials and Methods
Mice, Adoptive Transfers, and Immunization.
C57BL/6J IL-15Rα and congenic Ly5.2+ C57BL/6J/SJL IL-15Rα mice, and OT-1 RAG-1 and IL-15Rα OT-1 RAG-1 mice were generated and interbred as described previously (4, 5, 16). All strains were backbred to a C57Bl/6J background for at least nine generations. IL-2/15Rβ−/− and Ly5.2+ C57BL/6J/SJL mice on a C57BL/6J background were purchased from The Jackson Laboratory. IL-15−/− mice on a C57BL/6J background were purchased from Taconic Laboratories. Radiation bone marrow chimeras were produced as described previously (4), except that mixed radiation chimera were generated using mixtures of bone marrow cells from congenic donors of distinct genotypes of mice. All mice were housed and bred in specific pathogen-free facilities according to University of Chicago and University of California, San Francisco Institutional and Animal Care Use Committee guidelines. Adoptive transfers of naive OT-1+ CD8+ T cells and subsequent immunizations were performed as described previously (4). NK cells were isolated and adoptively transferred as described previously, except that NK cells were purified from RAG-1−/− mice (5).
Cellular Analyses by Flow Cytometry.
Single cell suspensions from peripheral blood or tissues were prepared, incubated with monoclonal antibodies or dimers of H-2Kb–OVA, and analyzed by flow cytometry using a FACSCalibur and CELLQuest software as described previously (4). Antibodies specific for CD3, CD4, CD8, Ly5.1, Ly5.2, CD44, CD122 (IL-2/15Rβ), NK1.1, IFN-γ (BD Biosciences), and IL-15Rα (R&D Systems) were used at 5 μg/ml. Dimers of H-2Kb (BD Biosciences) were incubated with SIINFEKL peptide, and then used to detect OT-1+ cells as described previously (4).
Results
IL-15Rα Expression by Hematopoietic Cells, But Not NK Cells, Supports Development and Maintenance of NK Cells in a Non-cell–autonomous Manner.
Our previous studies indicated that IL-15Rα–competent, radiation-sensitive cells appear to play a greater role than radiation-resistant cells in supporting the development and maintenance of NK cells. Therefore, we examined the ability of IL-15Rα–competent hematopoietic cells to rescue NK cell development in chimeric IL-15Rα−/− mice. Consistent with previous studies, significant numbers of NK cells were observed in the peripheral blood of lethally irradiated IL-15Rα−/− mice reconstituted with IL-15Rα+/−, but not IL-15Rα−/−, bone marrow stem cells (Fig. 1 A, top). Although recent studies indicated that IL-15Rα expression on NK cells was not absolutely required for their development (5, 21), it was unclear from these experiments whether IL-15Rα expression on NK cells might partly contribute to their development and survival. To examine this question, IL-15Rα+/− and IL-15Rα−/− bone marrow stem cells from congenic backgrounds were mixed and coinjected into irradiated IL-15Rα−/− mice. Analyses of the resulting mixed chimera revealed that significant numbers of NK cells were obtained from these chimera. Moreover, the ratio of NK cells derived from each genotype was virtually identical to the ratio of non–IL-15Rα–dependent B and CD4+ T lymphocytes (Fig. 1 A, bottom left). Thus, IL-15Rα−/− NK cells differentiate as well as IL-15Rα+/− NK cells in the presence of other IL-15Rα+/− hematopoietic cells, suggesting that IL-15Rα expression on NK cells does not play an essential role in their differentiation or peripheral maintenance.
Figure 1.
IL-15Rα expression by RAG-1–independent hematopoietic cells is sufficient to maintain NK and memory CD8+ T cells. IL-15Rα−/− mice were lethally irradiated and reconstituted with bone marrow from either IL-15Rα+/− (Wt) or IL-15Rα−/− (RαKO) mice, or a 1:1 mixture of IL-15Rα+/− and IL-15Rα−/− bone marrow (Wt/RαKO), or a 1:1 mixture of RAG-1−/− and IL-15Rα−/− bone marrow (RAG/RαKO). (A) Flow cytometric analyses of NK cell reconstitution in chimeric mice. The percentage of total lymphocytes (defined by forward and side scatter as the R1 gate) that are NK1.1+ CD3− cells is indicated in the top plots. The bottom plots are gated on NK1.1+ CD3− cells. The percentage of total lymphocytes expressing either Ly5.1 or Ly5.2 is indicated in the top right corner of the bottom plots. Note that RAG-1−/− hematopoietic cells support peripheral NK cell development. (B) Graphic representation of percentages of H2Kb-OVA+ CD8+ T cells in immunized chimeric mice. 8 wk after irradiation and reconstitution, OT-1+ CD8+ T cells were adoptively transferred into the indicated chimeric mice, after which mice were immunized with OVA and poly I:C. The percentage of total lymphocytes that are H2Kb-OVA+ CD8+ T cells after immunization was tracked via serial peripheral blood analyses. Note that RAG-1−/− hematopoietic cells support memory CD8+ T cell homeostasis. Data represent mean ± SEM of at least three mice per group.
IL-15Rα Expression by RAG-1–independent Hematopoietic Cells Supports NK Cell Development.
As IL-15Rα is expressed by many types of hematopoietic cells, including macrophages and dendritic cells, we investigated whether IL-15Rα expression by RAG-1–independent cell types could support NK cell development. Therefore, radiation chimera were generated by reconstituting lethally irradiated IL-15Rα mice with a mixture of congenic bone marrow stem cells from RAG-1−/− and IL-15Rα2/− mice (RAG/RαKO→RαKO), or WT and IL-15Rα mice (WT/RαKO→RαKO). After 8 wk, analyses of these mixed chimera revealed that similar numbers of NK cells were present in WT/RαKO→RαKO compared with RAG/RαKO→RαKO chimera (Fig. 1 A, top). Moreover, NK cells in RAG/RαKO→RαKO chimera were derived from both RAG-1−/− and IL-15Rα−/− bone marrow progenitors in approximately equal proportions (Fig. 1 A, bottom right). Thus, RAG-1–independent hematopoietic cells support NK cell development as well as WT hematopoietic cells.
IL-15Rα Expression by RAG-1–independent Cells Supports Development and Maintenance of Memory CD8+ T Cells.
Recent studies indicated that non-cell–autonomous expression of IL-15Rα is important for the maintenance of memory CD8+ T cells (4). To further define the hematopoietic cells that perform this function, we assessed whether IL-15Rα expression by RAG-1–independent cells is sufficient to support memory CD8+ T cells. At least 8 wk after irradiation and reconstitution, we adoptively transferred transgenic OT-1+ RAG-1−/− CD8+ T cells into WT→RαKO, RαKO→RαKO, WT/RαKO→RαKO, and RAG/RαKO→RαKO chimera. 2 d after adoptive transfer of OT-1+ CD8+ T cells, these chimera were immunized with OVA and poly I:C, and the initial expansion, memory generation, and maintenance of OT-1+ CD8+ T cells were quantitated by analyzing the numbers of H2Kb-OVA+–reactive CD8+ T cells in serial peripheral blood samples. At all time points examined after immunization, WT→RαKO, WT/RαKO→RαKO, and RAG/RαKO→RαKO chimera possessed similar frequencies of H2Kb-OVA+ CD8+ T cells (Fig. 1 B). In contrast, despite similar primary responses (i.e., day 4 after immunization), the population of H-2Kb-OVA+ CD8+ T cells declined progressively after 30 d in RαKO→RαKO chimera (Fig. 1 B). Moreover, normal frequencies of functional memory H-2Kb-OVA+ CD8+ T cells were observed in the spleens and lymph nodes of RAG/RαKO→RαKO, but not RαKO→RαKO, chimera up to 90 d after immunization (not depicted). Therefore, IL-15Rα expression on RAG-1–independent hematopoietic cells supports memory CD8+ T cell generation and maintenance.
IL-2/15Rβ−/− Hematopoietic Cells Support IL-15–dependent Cell Types in Trans; In Vivo Evidence for Trans Presentation as the Exclusive Mechanism of IL-15Rα–mediated Lymphoid Homeostasis.
Homeostatic maintenance of NK cells and memory CD8+ T cells requires IL-15Rα expression in a non-cell–autonomous fashion, and in vitro studies suggest that IL-15Rα may function by presenting IL-15 in trans to these cells (4, 5, 19, 20). Taken together, these studies suggest that trans presentation of IL-15 may support lymphocytes in vivo. However, no direct evidence exists to rule out the possibility that IL-15Rα–dependent secondary signals on accessory cells lead to the production of unidentified proteins that in turn support NK and memory CD8 T cells in vivo. To distinguish IL-15Rα–mediated trans presentation of IL-15 from IL-15Rα–mediated signaling on accessory cells, we used IL-2/15Rβ−/− bone marrow stem cells to generate chimeric mice in which hematopoietic cells expressed IL-15Rα, but were unable to signal through IL-15R complexes (Fig. 2 A). Because IL-2/15Rβ−/− bone marrow cells are unable to generate regulatory T cells, leading to spontaneous autoimmunity and inflammation, we coinjected IL-15Rα−/− bone marrow stem cells with IL-2/15Rβ−/− bone marrow cells to generate mixed chimera. Intact IL-15Rα−/− mice and chimera derived from IL-15Rα−/− bone marrow stem cells possess normal numbers of CD4+ CD25+ regulatory T cells and do not develop spontaneous autoimmunity (not depicted). Mixed chimera generated from IL-15Rα−/− and IL-2/15Rβ−/− bone marrow stem cells (RαKO/RβKO) were then examined for their ability to support NK and CD8+ T cell homeostasis.
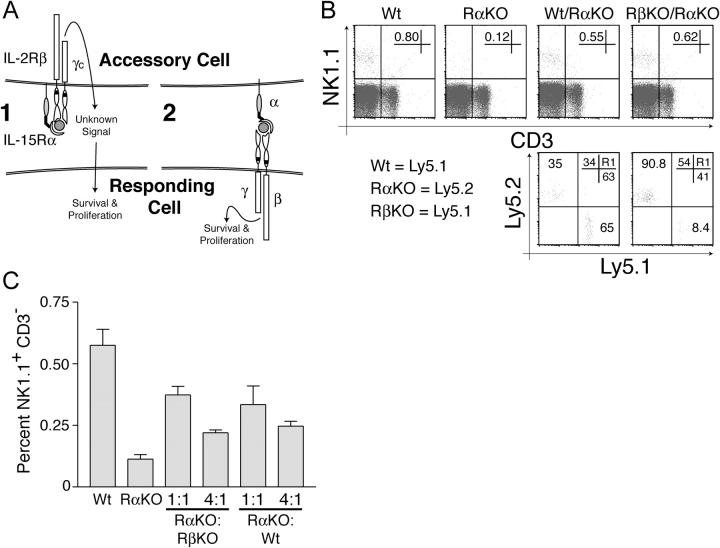
Figure 2.
IL-15Rα, but not IL-2/15Rβ, expression is required by hematopoietic cells to support NK cell development and survival. (A) Model illustrating two distinct mechanisms by which non-cell–autonomous IL-15Rα expression is required to support IL-15–dependent cell types. Mechanism 1 requires IL-2Rβ expression on accessory cells to mediate signal transduction, whereas mechanism 2 (trans presentation) does not. (B) Flow cytometric analysis of NK cell reconstitution in indicated chimeric mice depends upon IL-15Rα–, but not IL-2/15Rβ–, competent cells. IL-15Rα−/− mice were lethally irradiated and reconstituted with either IL-15Rα+/− (Wt) or IL-15Rα−/− (RαKO) bone marrow cells, or a mixture of IL-15Rα+/− and IL-15Rα−/− bone marrow cells (Wt/RαKO), or a mixture of IL-2/15Rβ−/− and IL15Rα−/− bone marrow cells (RβKO/RαKO). NK cell populations in chimeric mice were assessed 8 wk after reconstitution. The percentages of total lymphocytes that are NK cells (NK1.1+ CD3− cells) are indicated in each of the top panels. The bottom plots are gated on NK1.1+ CD3− cells in WT/RαKO and RβKO/RαKO chimera. The percentages of total lymphocytes expressing either Ly5.1 or Ly5.2 in WT/RαKO and RβKO/RαKO chimera are shown in the top right corner of the bottom panels. Note that NK cell reconstitution in chimeric mice depends upon IL-15Rα–, but not IL-2/15Rβ–, competent cells. (C) The frequency of IL-15Rα–competent cells controls the frequency of peripheral NK cells. IL-15Rα−/− mice were lethally irradiated and reconstituted with bone marrow from either IL-15Rα+/− or IL-15Rα−/− mice, or with various ratios of IL-15Rα+/− and IL-15Rα−/− bone marrow, or assorted ratios of IL-2/15Rβ−/− and IL-15Rα−/− bone marrow. Mice were bled and NK cell populations in chimeric mice were assessed 8 wk after reconstitution. The percentage of total lymphocytes that are NK1.1+ CD3− cells is shown. Plots are representative of at least two mice per condition, and all experiments were performed three times with similar results.
As previously observed, significant numbers of NK cells were observed in the periphery of WT→ RαKO chimera, but not RαKO→RαKO chimera (Fig. 2 B, top). Importantly, comparable numbers of NK cells were readily observed in the spleens and peripheral blood of both WT/RαKO→RαKO and RβKO/RαKO→RαKO chimera (Fig. 2 B, top). The presence of IL-15Rα–competent cells, regardless of their ability to express IL-2/15Rβ, is therefore sufficient to support the development and survival of peripheral NK cells. This finding indicates that hematopoietic cells do not need to transduce IL-2/15Rβ–dependent signals to support NK cells in trans.
We then investigated the cell-autonomous role of IL-2/15Rβ expression in supporting NK cells by examining the genotype of surviving NK cells in WT/RαKO→RαKO and RβKO/RαKO→RαKO chimera via congenic markers. Consistent with the data above, the percentages of NK cells derived from IL-15Rα−/− and IL-15Rα+/− bone marrow stem cells were similar to the percentages of non–IL-15Rα–dependent lymphocytes (B and CD4+ T cells) in WT/RαKO→RαKO chimera (Fig. 2 B, bottom left). By contrast, the percentage of total NK cells that were derived from Ly5.1+ IL-2Rβ−/− cells (8.4%) was dramatically reduced when compared with the percentage of other Ly5.1+ lymphocytes (41%) reconstituted in RβKO/RαKO→ RαKO chimera (Fig. 2 B, bottom right). These data indicate that IL-2/15Rβ expression is required on NK cells for their development and maintenance.
Finally, as both WT/RαKO→RαKO and RβKO/RαKO→RαKO chimera generally contained reduced percentages of NK cells compared with WT→RαKO chimera, we hypothesized that the percentage of peripheral NK cells might be a function of the relative percentage of IL-15Rα–competent hematopoietic cells. To investigate this possibility, we reconstituted IL-15Rα−/− mice with either 1:1 or 1:4 mixtures of IL-15Rα−/− and either WT or IL-2/15Rβ−/− bone marrow stem cells. Examination of the percentage of NK cells in these chimera revealed that the numbers of NK cells decreased as the proportion of IL-15Rα–competent (either WT or IL-2/15Rβ−/−) bone marrow stem cells decreased (Fig. 2 C). These findings suggest that the relative frequency of IL-15Rα–competent hematopoietic cells regulates the size of the peripheral NK cell pool.
IL-2/15Rβ−/− Hematopoietic Cells Support Memory CD8+ T Cells.
Memory phenotype CD8+ T cells are dependent upon both IL-15 and IL-15Rα for their development and peripheral survival (16, 17, 22). To investigate whether IL-2/15Rβ−/− cells could support memory phenotype CD8+ T cells in a non-cell–autonomous fashion, we examined the reconstitution of this population in RβKO/RαKO→RαKO chimera. CD44hi IL-2/15Rβhi CD8+ T cells were readily observed in both WT→RαKO and RβKO/RαKO→RαKO chimera, but not in RαKO→RαKO chimera (Fig. 3 A). Notably, there was no obvious population of IL-2/15Rβ−/− CD44hi CD8+ T cells present in RβKO/RαKO→RαKO chimera, suggesting that IL-2/15Rβ, but not IL-15Rα, expression by CD44hi CD8+ memory phenotype cells is critical for their peripheral maintenance (Fig. 3 A and not depicted).
Figure 3.
IL-15Rα, but not IL-2/15Rβ, expression is required by hematopoietic cells to support CD8+ memory T cell homeostasis. IL-15Rα−/− mice were lethally irradiated and reconstituted with bone marrow from either IL-15Rα+/− (Wt) or IL-15Rα−/− (RαKO) mice, or a mixture of IL-2/15Rβ−/− and IL-15Rα−/− (RβKO/RαKO) bone marrow. (A) Flow cytometric analyses of endogenous CD44hi IL-2/15Rβhi (memory phenotype) CD8+ T cells in spleens of chimeric mice. The percentages of total CD8+ T cells that are memory phenotype CD8+ T cells in the indicated chimeric mice were assessed 8 wk after reconstitution. The percentage of CD8+ T cells in each quadrant is shown. Plots are gated on CD8+ T cells and are representative of at least three mice per condition. (B) Graphic representation of peripheral blood H2Kb-OVA+ CD8+ T cells after immunization of chimeric mice. 8 wk after reconstitution, mice received OT-1+ CD8+ T cells and were immunized with OVA and poly I:C. The percentage of total lymphocytes that were H2Kb-OVA+ CD8+ T cells was quantitated by flow cytometric analyses of serial peripheral blood samples after immunization. Data represent mean ± SEM of at least two mice per group.
CD44hi IL-2/15Rβhi CD8+ T cells include antigen-experienced memory cells as well as cells that may have been activated via alternate mechanisms (e.g., homeostatic expansion). To directly assess the ability of IL-2/15Rβ−/− hematopoietic cells to support antigen-experienced memory CD8+ T cells, we adoptively transferred naive OT-1+ RAG-1−/− CD8+ T cells into WT→RαKO, RαKO→RαKO, WT/RαKO→ RαKO, or RβKO/RαKO→RαKO chimera 8 wk after reconstitution. These chimera were then immunized with OVA and poly I:C, and the frequency of OT-1+ CD8+ T cells was serially examined as described above. Similar primary responses of OT-1+ CD8+ T cells were observed in all types of chimera during the first 20–30 d after immunization. However, after ∼50 d, progressive loss of these cells was noted in RαKO→RαKO chimera, but not in the other types of chimera, all of which contained IL-15Rα–competent hematopoietic cells (Fig. 3 B). Thus, in parallel to our findings with NK cells, IL-2/15Rβ−/− hematopoietic cells are capable of supporting memory CD8+ T cells in a non-cell–autonomous fashion, and this finding suggests that hematopoietic cells use IL-15Rα to support memory CD8+ T cells exclusively by a trans presentation mechanism (Fig. 2 A).
Coordinate Expression of IL-15 and IL-15Rα by Hematopoietic Cells Is Required for Supporting NK Cells In Vivo.
The findings described above support a model whereby RAG-1–independent hematopoietic cells use IL-15Rα, but not IL-2/15Rβ, to present IL-15 in trans to IL-2/15Rβ–, but not IL-15Rα–, dependent receptors on NK and memory CD8+ T cells. However, it remained unclear why NK and memory CD8+ T cells are able to respond to soluble or plate-bound IL-15 in vitro, but apparently fail to receive IL-15 signals in mice that are IL-15Rα deficient but IL-15 competent. One possible explanation would be that IL-15Rα is not only required on accessory cells for trans presenting IL-15, but is also required for making IL-15 bioavailable for trans presentation. In this scenario, IL-15 would not be freely available in the serum of mice to be bound by IL-15Rα–presenting cells, but might need to be produced by the same cells that produce IL-15Rα. To test this hypothesis, we investigated whether coordinate expression of IL-15 and IL-15Rα was required to support NK and memory CD8+ T cells in vivo (Fig. 4). Lethally irradiated IL-15Rα−/− mice were reconstituted with WT (WT→RαKO), IL-15Rα−/− (RαKO→RαKO), IL-15−/− (15KO→RαKO), a mixture of WT and IL-15Rα−/− (WT/RαKO→RαKO), or a mixture of IL-15−/− and IL-15Rα−/− bone marrow stem cells (15KO/RαKO→RαKO). Roughly half of the hematopoietic cells in 15KO/RαKO→RαKO chimera should express IL-15Rα, but not IL-15, whereas the other half of hematopoietic cells and all residual stromal cells should express IL-15, but not IL-15Rα. Thus, if IL-15 can be secreted from IL-15Rα−/− cells to bind to IL-15Rα expressed on IL-15−/− cells, then 15KO/RαKO→RαKO chimera should support IL-15–dependent lymphocytes as well as WT/RαKO→RαKO chimera. Alternatively, if IL-15Rα is required for IL-15 elaboration, then 15KO/RαKO→RαKO chimera would not be able to support NK and CD8+ memory T cells. Analyses of NK cells in these various chimera 8 wk after reconstitution revealed that WT→RαKO and WT/RαKO→RαKO chimera contained significant numbers of peripheral NK cells, whereas 15KO→RαKO, RαKO→RαKO, and 15KO/RαKO→ RαKO chimera failed to reconstitute these cells (Fig. 5 A). Importantly, IL-15Rα was readily observed on the surface of a variety of IL-15−/− cell types, including IL-15−/− dendritic cells (Fig. 5 B). Taken together, these findings suggest that IL-15 and IL-15Rα must be coordinately expressed by hematopoietic cells to support the development of NK cells via trans presentation.
Figure 4.
Two models for the relationship of IL-15–producing and –presenting cells. In model 1, IL-15 is secreted by a distinct IL-15–producing cell, and subsequently bound and presented by an IL-15Rα–competent cell. In model 2, coordinate expression of IL-15 and IL-15Rα is required for efficient presentation of IL-15 to responding cells.
Figure 5.
Coordinate expression of IL-15 and IL-15Rα is required for NK cell development and maintenance. IL-15Rα−/− mice were lethally irradiated and reconstituted with bone marrow cells from either IL-15Rα+/− (Wt), IL-15Rα−/− (RαKO), or IL-15−/− (15KO), mice, or with 1:1 mixtures of either IL-15Rα+/− and IL-15Rα−/− bone marrow cells (Wt/RαKO), or IL-15Rα−/− and IL-15−/− bone marrow cells (15KO/RαKO). (A) Flow cytometric analyses of endogenous NK cells in bone marrow (BM) and spleens (SPL) of chimeric mice 8 wk after reconstitution with the indicated bone marrow genotypes. Note that endogenous NK cells are not supported in chimera generated from a mixture of IL-15−/− and IL-15Rα2/2 bone marrows. Data are representative of at least four mice per group. (B) Flow cytometric analysis of IL-15Rα expression on LPS-stimulated, bone marrow–derived dendritic cells of the indicated genotypes. Bone marrow–derived dendritic cells were stimulated with LPS for 24 h in vitro and stained for IL-15Rα expression. (C) Graphic representation of survival of adoptively transferred NK cells after transfer into the indicated mixed chimera. The percentage of total lymphocytes that were adoptively transferred NK cells (i.e., CFSE+ NK1.1+ CD3− cells) is indicated at each time point. Note that adoptively transferred NK cells are not supported in chimera generated from a mixture of IL-15−/− and IL-15Rα2/2 bone marrows. Data represent mean ± SEM of at least two mice per group.
The failure to reconstitute peripheral NK cells in 15KO/RαKO→RαKO chimera may reflect a failure of development or peripheral maintenance of NK cells, or both. To directly investigate whether 15KO/RαKO→RαKO chimera are capable of supporting the survival of mature peripheral NK cells, congenic splenic NK cells were adoptively transferred into 15KO/RαKO→RαKO as well as control chimera. Serial peripheral blood analyses of these chimera revealed that transferred NK cells persisted for >2 d in WT→RαKO and WT/RαKO→RαKO chimera, but not in RαKO→RαKO, 15KO→RαKO, or most notably, 15KO/RαKO→RαKO chimera (Fig. 5 C). Therefore, coordinate expression of IL-15 and IL-15Rα is required to support peripheral NK cell survival.
Coordinate Expression of IL-15 and IL-15Rα by Hematopoietic Cells Is Required for Supporting Memory CD8+ T Cells In Vivo.
Next, we investigated whether coordinate expression of IL-15Rα and IL-15 is required for the development and maintenance of memory phenotype CD8+ T cells. Analyses of tissues from various chimera revealed that IL-2/15Rβhi CD8+ T cells were readily observed in WT→RαKO chimera, but not in RαKO→RαKO, 15KO→RαKO, or 15KO/RαKO→RαKO chimera (Fig. 6 A). Therefore, coordinate expression of both IL-15Rα and IL-15 is necessary for maintenance of memory phenotype CD8+ T cells.
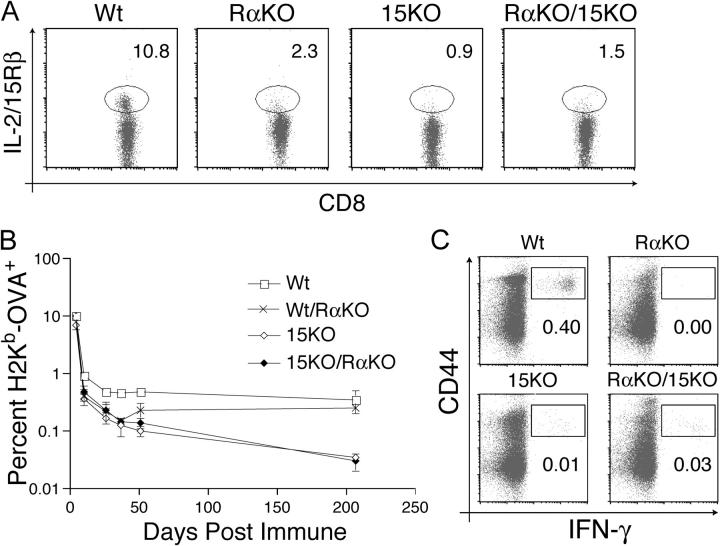
Figure 6.
Homeostasis of memory phenotype and memory CD8+ T cells requires coordinate expression of IL-15 and IL-15Rα. IL-15Rα−/− mice were lethally irradiated and reconstituted with bone marrow from either IL-15Rα+/− (Wt), IL-15Rα−/− (RαKO), or IL-15−/− (15KO) mice, or with a 1:1 mixture of IL-15Rα−/− and IL-15−/− bone marrow cells (RαKO/15KO). (A) Flow cytometric analysis of IL-2/15Rβhi memory phenotype CD8+ T cells 8 wk after reconstitution. The percentage of total CD8+ T cells that are IL-2/15Rβhi memory phenotype CD8+ T cells is indicated. Data are representative of at least five mice per condition. (B) Graphic representation of the percentages of memory H2Kb-OVA+ CD8+ T cells after immunization of the indicated chimeric mice. 8 wk after reconstitution, mice received OT-1+ CD8+ T cells and were then immunized with OVA and poly I:C. The percentage of total lymphocytes that are H2Kb-OVA+ CD8+ T cells after immunization was quantitated by serial peripheral blood analyses. Data represent mean ± SEM of at least three mice per group. (C) Flow cytometric analysis of IFN-γ production by memory CD8+ T cells in chimeric mice. IL-15 and IL-15Rα must be expressed by the same cell for optimal memory CD8+ T cell function. 8 wk after reconstitution, chimeric mice received OT-1+ CD8+ T cells and were then immunized with OVA and poly I:C. 90 d after immunization, splenocytes were stimulated with SIINFEKL, and IFN-γ production was assessed by intracellular staining. The percentage of total lymphocytes that are CD8+ CD44hi IFN-γ+ is indicated. Plots are gated on CD8+ cells and are representative of at least three mice.
Finally, we examined whether coordinate expression of IL-15 and IL-15Rα is required for the generation and maintenance of antigen-experienced memory CD8+ T cells. We adoptively transferred OT-1+ CD8+ T cells into WT→RαKO, WT/RαKO→RαKO, RαKO→RαKO, 15KO→RαKO, and 15KO/RαKO→RαKO chimera, immunized these mice with poly I:C and OVA 2 d later, and examined the kinetics of transgenic T cell responses. Although the primary expansions of these cells were similar 4 d after immunization in the various chimera, memory OT-1+ CD8+ T cells were subsequently maintained in WT→RαKO and WT/RαKO→RαKO chimera, but not in RαKO→RαKO, 15KO→RαKO, and 15KO/RαKO→RαKO chimera (Fig. 6 B and not depicted). This selective loss of memory OT-1+ CD8+ T cells in 15KO→RαKO and 15KO/RαKO→RαKO chimera was particularly evident when the numbers of functional memory OT-1+ CD8+ T cells were assessed by analyzing IFN-γ production in response to the cognate peptide SIINFEKL. Although WT→RαKO chimera had significant numbers of IFN-γ+ CD44hi CD8+ T cells 90 d after immunization, RαKO→RαKO, 15KO→RαKO, and 15KO/RαKO→ RαKO chimera possessed negligible numbers of SIINFEKL-responsive cells (Fig. 6 C). Thus, like NK cells, functional memory CD8+ T cells require coordinate expression of IL-15Rα and IL-15 for their maintenance.
Discussion
RAG-1–independent, IL-15Rα–competent Cells Define Homeostatic Space for NK Cell Survival and Memory CD8+ T Cell Homeostasis.
IL-15 regulates the homeostasis of NK and memory CD8+ T cells, and the high affinity IL-15R, IL-15Rα, is critical for mediating IL-15's functions in vivo. These observations suggest that the bioavailability of IL-15 and IL-15Rα defines a homeostatic space that regulates the numbers of these lymphocytes that an organism possesses at any one time. However, the cellular and molecular bases of these homeostatic interactions are poorly understood. In this work, we have examined the cellular mechanisms by which IL-15Rα supports lymphoid homeostasis in vivo. Our experiments indicate that RAG-1–independent hematopoietic cells comprise the predominant cell types that provide IL-15Rα–dependent homeostatic support. In this regard, myeloid cells such as macrophages and dendritic cells express low levels of both IL-15 and IL-15Rα constitutively, and express higher levels in response to proinflammatory stimuli. We have also found that the proportion of IL-15Rα–competent hematopoietic cells in WT/RαKO→RαKO mixed chimera correlates directly with the number of NK cells maintained in these mice. Thus, the number of IL-15Rα–competent accessory cells might be a limiting resource for NK and CD8+ T cells in resting animals. We have separately examined both the survival of peripheral NK cells and the generation and maintenance of memory CD8+ T cells in various mixed chimera, and found similar IL-15Rα requirements for these distinct populations. As the peripheral homeostasis of NK cells is largely maintained by cell survival, while memory CD8+ T cell homeostasis is supported by both survival and proliferation, these results suggest that IL-15Rα regulates multiple cellular processes in a cell type– and context-dependent fashion. Taken together, these experiments help define the nature of “homeostatic space” available to IL-15–responsive lymphocytes.
Trans Presentation as the Dominant Physiological Mechanism by Which IL-15Rα Supports NK and CD8+ Memory T Cells In Vivo.
Previous studies indicated that IL-15Rα supports NK cell and CD8+ memory T cell homeostasis in a non-cell–autonomous fashion in vivo (4, 5, 18). The non-cell–autonomous mechanism(s) by which IL-15Rα–competent hematopoietic cells support NK and CD8+ T cells could occur via two nonexclusive mechanisms. First, IL-15Rα–competent accessory cells could transduce signals through their heterotrimeric IL-15Rs and synthesize secondary proteins that support NK and CD8+ T cells. Alternatively, IL-15Rα–competent accessory cells could use IL-15Rα to directly present IL-15 in trans to NK and CD8+ T cells. Previous studies have shown that IL-2/15Rβ expression is required for IL-15–induced proliferative responses (7, 12). Moreover, IL-2/15Rβ−/−, but not IL-2Rα−/−, mice lack NK cells, similar to both IL-15−/− and IL-15Rα−/− mice. Taken together, these data suggest that IL-2/15Rβ is critical for IL-15 responses. Hence, our finding that IL-2/15Rβ−/− hematopoietic cells perform as well as WT hematopoietic cells in supporting NK and CD8+ T cells indicates that accessory cells mediate this function without transducing IL-15 signals themselves, and without producing secondary proteins that in turn support lymphocytes. Therefore, these accessory cells use IL-15Rα exclusively to directly present IL-15 in trans to NK cells and CD8+ memory T cells. This finding is consistent with recent findings that IL-2/15Rβ−/− hematopoietic cells can support intraepithelial lymphocyte homeostasis (19). Thus, trans presentation is likely to be the exclusive physiological mechanism by which IL-15Rα supports NK and CD8+ T cells in vivo.
Our studies also shed light on the cell-autonomous requirements for IL-15R signaling in lymphoid homeostasis. Analyses of congenic NK cells recovered from RβKO/RαKO→RαKO mixed chimera indicate that IL-2/15Rβ expression by IL-15–responsive NK and memory CD8+ T cells is required for their homeostasis. This finding confirms previous suggestions that high expression levels of IL-2/15Rβ on these cells correlates with their sensitivity to IL-15–dependent signals in vivo (23, 24). Meanwhile, analyses of congenic NK cells recovered from our WT/RαKO→RαKO mixed chimera indicate that IL-15Rα on IL-15–dependent NK and memory CD8+ T cells is entirely dispensable for their homeostasis in vivo. These in vivo data are consistent with the fact that IL-15Rα+/− and IL-15Rα−/− memory CD8+ T cells respond similarly to a given dose of IL-15 in vitro, regardless of whether it is provided as a plate-bound IL-15/IL-15Rα–γc complex or as a soluble cytokine (unpublished data). Hence, despite the fact that IL-15Rα can augment signaling responses to soluble IL-15 in transfected cells, it is unlikely that IL-15Rα on NK and memory CD8+ T cells facilitates binding of IL-15 to IL-2/15Rβ and γc receptor chains on these cells in vivo. Similarly, it is unlikely that IL-15Rα on accessory cells transfers IL-15 to IL-15Rα on responding NK and CD8+ memory T cells. Taken together, these studies indicate that IL-2/15Rβ receptors are critical, whereas IL-15Rα receptors are entirely dispensable on NK and memory CD8+ T cells for their homeostasis.
Trans presentation is a novel mechanism by which cytokine signals are transduced. Although a previous report suggested that IL-2Rα could present IL-2 in trans (25), IL-2Rα alone binds IL-2 with low affinity (K d ∼10−8 M) and in vivo studies with IL-2Rα−/− T cells indicated that IL-2Rα plays a cell-autonomous role in supporting T cells (26–28). Thus, trans presentation is unlikely to be the physiological mechanism by which IL-2Rα supports T cells. Signaling through the IL-6 receptors, IL-6Rα and gp130, more closely resembles IL-15R signaling. Soluble IL-6Rα is produced by both proteolytic cleavage of IL-6Rα and alternative splicing. Soluble IL-6Rα binds IL-6 in solution and IL-6–sIL-6Rα complexes then bind to gp130 receptors on cell surfaces to initiate signal transduction events (29, 30). Nevertheless, there might be fundamental differences between IL-6Rα– and IL-15Rα–mediated signaling. Specifically, in contrast to IL-6Rα, it is unclear if IL-15 and IL-15Rα can form soluble complexes that can signal to IL-2/15Rβ–γc receptors on responding cells (11, 31). Therefore, the ability of IL-15Rα on the surface of accessory hematopoietic cells to present IL-15 in trans to IL-2/15Rβ and γc low affinity dimeric receptors on NK and memory CD8+ T cells in vivo may represent a novel mechanism of cytokine signaling that may involve cell to cell contact.
Coordinate Expression of IL-15 and IL-15Rα by Trans Presenting Accessory Cells.
Our studies with 15KO/RαKO→RαKO mixed chimera indicate that IL-15 and IL-15Rα must be expressed by the same accessory cells to support both NK and memory CD8+ T cells in vivo. As IL-15−/− cells express normal levels of cell surface IL-15Rα (Fig. 5 B), and as IL-15Rα−/− cells express normal levels of IL-15 mRNA (5, 18), the inability of 15KO/RαKO→RαKO chimera to support NK and memory CD8+ T cells suggests that IL-15Rα−/− cells may not elaborate IL-15 protein. This surprising finding would explain why IL-15Rα–competent lymphocytes respond to heterologous IL-15 in vitro, but fail to respond to IL-15 elaborated from IL-15–competent cells in vivo. Thus, IL-15Rα might be essential for either the translation of IL-15 mRNA or the trafficking of IL-15 protein to the cell surface. With regards to the latter possibility, it is known that the signal sequences of IL-15 mediate protein secretion poorly (32). As IL-15Rα associates with IL-15 with high affinity, one intriguing possibility is that IL-15Rα may bind to IL-15 intracellularly and facilitate trafficking of IL-15/IL-15Rα to the surface of accessory cells. Intracellular association of IL-15 and IL-15Rα has recently been described in several contexts, including endosomal recycling of internalized IL-15Rα–IL-15 complexes that follow binding of extracellular IL-15 to surface IL-15Rα (20, 33, 34). By contrast, our current findings suggest that the critical interactions between IL-15 and IL-15Rα occur within IL-15–producing cells, before IL-15's emergence on the plasma membrane. In addition, as soluble IL-15 has been difficult to document in mice, it is possible that IL-15Rα recognizes and binds to IL-15 exclusively within cells that synthesize both proteins. Therefore, these experiments indicate novel cell biological requirements for the regulation of IL-15, and also provide a compelling explanation for why trans presentation is the physiological mechanism by which IL-15 supports lymphoid homeostasis.
In summary, we have examined the mechanism by which IL-15Rα supports NK cell survival and CD8+ memory T cell proliferation in vivo. Our findings indicate that myeloid accessory cells do not use IL-15Rs to transduce signals leading to the elaboration of secondary proteins that support lymphoid homeostasis. Instead, these cells use IL-15Rα to present IL-15 in trans to IL-2/15Rβ–bearing receptors on the surface of NK and CD8+ memory T cells. These accessory cells must coordinately synthesize IL-15 and IL-15Rα to present IL-15 in trans. Therefore, the critical events regulating homeostatic niches for NK and CD8+ memory T cells in vivo can be focused upon the production and trans presentation of IL-15 by IL-15Rα–expressing myeloid cells.
Acknowledgments
We thank D. Boone for critically reading the manuscript.
This work was supported by National Institutes of Health (NIH) RO1 grants AI45860 and AI59827 (to A. Ma), NIH T32DK60414 (to R. Koka), and the Sandler Family Foundation.
The authors have no conflicting financial interests.
P. Burkett and R. Koka contributed equally to this work.
Abbreviation used in this paper: γc, γ chain.
References
- 1.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 2.Becker, T.C., E.J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldrath, A.W., P.V. Sivakumar, M. Glaccum, M.K. Kennedy, M.J. Bevan, C. Benoist, D. Mathis, and E.A. Butz. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkett, P.R., R. Koka, M. Chien, S. Chai, F. Chan, A. Ma, and D.L. Boone. 2003. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA. 100:4724–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koka, R., P.R. Burkett, M. Chien, S. Chai, F. Chan, J.P. Lodolce, D.L. Boone, and A. Ma. 2003. Interleukin (IL)-15Rα–deficient natural killer cells survive in normal but not IL-15Rα–deficient mice. J. Exp. Med. 197:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, M.A., J.E. Bush, T.A. Fehniger, J.B. VanDeusen, R.E. Waite, Y. Liu, H.L. Aguila, and M.A. Caligiuri. 2002. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 100:3633–3638. [DOI] [PubMed] [Google Scholar]
- 7.Giri, J.G., S. Kumaki, M. Ahdieh, D.J. Friend, A. Loomis, K. Shanebeck, R. DuBose, D. Cosman, L.S. Park, and D.M. Anderson. 1995. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 14:3654–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulanova, E., V. Budagian, T. Pohl, H. Krause, H. Durkop, R. Paus, and S. Bulfone-Paus. 2001. The IL-15R alpha chain signals through association with Syk in human B cells. J. Immunol. 167:6292–6302. [DOI] [PubMed] [Google Scholar]
- 9.Bulanova, E., V. Budagian, Z. Orinska, H. Krause, R. Paus, and S. Bulfone-Paus. 2003. Mast cells express novel functional IL-15 receptor alpha isoforms. J. Immunol. 170:5045–5055. [DOI] [PubMed] [Google Scholar]
- 10.Bulfone-Paus, S.S., E. Bulanova, T. Pohl, V. Budagian, H. Durkop, R. Ruckert, U. Kunzendorf, R. Paus, and H. Krause. 1999. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 13:1575–1585. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, D.M., S. Kumaki, M. Ahdieh, J. Bertles, M. Tometsko, A. Loomis, J. Giri, N.G. Copeland, D.J. Gilbert, N.A. Jenkins, et al. 1995. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J. Biol. Chem. 270:29862–29869. [DOI] [PubMed] [Google Scholar]
- 12.Bamford, R.N., A.J. Grant, J.D. Burton, C. Peters, G. Kurys, C.K. Goldman, J. Brennan, E. Roessler, and T.A. Waldmann. 1994. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 91:4940–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri, J.G., M. Ahdieh, J. Eisenman, K. Shanebeck, K. Grabstein, S. Kumaki, A. Namen, L.S. Park, D. Cosman, and D. Anderson. 1994. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 13:2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiSanto, J.P., W. Muller, D. Guy-Grand, A. Fischer, and K. Rajewsky. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 92:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki, H., T.M. Kundig, C. Furlonger, A. Wakeham, E. Timms, T. Matsuyama, R. Schmits, J.J. Simard, P.S. Ohashi, H. Griesser, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 268:1472–1476. [DOI] [PubMed] [Google Scholar]
- 16.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodolce, J.P., P.R. Burkett, D.L. Boone, M. Chien, and A. Ma. 2001. T cell–independent interleukin 15Rα signals are required for bystander proliferation. J. Exp. Med. 194:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schluns, K.S., E.C. Nowak, A. Cabrera-Hernandez, L. Puddington, L. Lefrancois, and H.L. Aguila. 2004. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc. Natl. Acad. Sci. USA. 101:5616–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois, S., J. Mariner, T.A. Waldmann, and Y. Tagaya. 2002. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 17:537–547. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura, T., R. Koka, A. Ma, and V. Kumar. 2003. Differential roles for IL-15R alpha-chain in NK cell development and Ly-49 induction. J. Immunol. 171:5085–5090. [DOI] [PubMed] [Google Scholar]
- 22.Judge, A.D., X. Zhang, H. Fujii, C.D. Surh, and J. Sprent. 2002. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns, K.S., K.D. Klonowski, and L. Lefrancois. 2003. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 103:988–994. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 25.Eicher, D.M., and T.A. Waldmann. 1998. IL-2R alpha on one cell can present IL-2 to IL-2R beta/gamma(c) on another cell to augment IL-2 signaling. J. Immunol. 161:5430–5437. [PubMed] [Google Scholar]
- 26.D'Souza, W.N., K.S. Schluns, D. Masopust, and L. Lefrancois. 2002. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 168:5566–5572. [DOI] [PubMed] [Google Scholar]
- 27.Leung, D.T., S. Morefield, and D.M. Willerford. 2000. Regulation of lymphoid homeostasis by IL-2 receptor signals in vivo. J. Immunol. 164:3527–3534. [DOI] [PubMed] [Google Scholar]
- 28.Willerford, D.M., J. Chen, J.A. Ferry, L. Davidson, A. Ma, and F.W. Alt. 1995. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 3:521–530. [DOI] [PubMed] [Google Scholar]
- 29.Kallen, K.J. 2002. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim. Biophys. Acta. 1592:323–343. [DOI] [PubMed] [Google Scholar]
- 30.Rose-John, S. 2003. Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochim. Pol. 50:603–611. [PubMed] [Google Scholar]
- 31.Dubois, S., F. Magrangeas, P. Lehours, S. Raher, J. Bernard, O. Boisteau, S. Leroy, S. Minvielle, A. Godard, and Y. Jacques. 1999. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J. Biol. Chem. 274:26978–26984. [DOI] [PubMed] [Google Scholar]
- 32.Kurys, G., Y. Tagaya, R. Bamford, J.A. Hanover, and T.A. Waldmann. 2000. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J. Biol. Chem. 275:30653–30659. [DOI] [PubMed] [Google Scholar]
- 33.Pereno, R., J. Giron-Michel, A. Gaggero, E. Cazes, R. Meazza, M. Monetti, E. Monaco, Z. Mishal, C. Jasmin, F. Indiveri, et al. 2000. IL-15/IL-15Ralpha intracellular trafficking in human melanoma cells and signal transduction through the IL-15Ralpha. Oncogene. 19:5153–5162. [DOI] [PubMed] [Google Scholar]
- 34.Ruckert, R., K. Brandt, E. Bulanova, F. Mirghomizadeh, R. Paus, and S. Bulfone-Paus. 2003. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur. J. Immunol. 33:3493–3503. [DOI] [PubMed] [Google Scholar]