Abstract
The signaling events leading to the activation of integrins and firm arrest of rolling neutrophils in inflamed venules have yet to be elucidated. In vitro assays suggest that both E-selectin and chemokines can trigger arrest of rolling neutrophils, but E-selectin−/− mice have normal levels of adherent neutrophils in inflamed venules. To test whether chemokine-induced neutrophil arrest in vivo can be unmasked by blocking E-selectin, we investigated neutrophil adhesion in inflamed cremaster muscle venules in tumor necrosis factor (TNF)-α–treated CXCR2−/− or wild-type (WT) mice injected with E-selectin blocking monoclonal antibody (mAb) 9A9. To block chemokine receptor signaling, we investigated E-selectin−/− or WT mice treated with pertussis toxin (PTx) intravenously. Neutrophil adhesion was unchanged in CXCR2−/−, E-selectin−/−, PTx-treated WT, or mAb 9A9–treated WT mice. However, TNF-α–induced neutrophil adhesion was almost completely abrogated in E-selectin−/− mice treated with PTx and significantly reduced in CXCR2−/− mice treated with the E-selectin blocking mAb. In thioglycollate-induced peritonitis, PTx treatment blocked neutrophil recruitment into the peritoneum of E-selectin−/− mice, but had only a partial effect in WT animals. These data show that E-selectin– and chemokine-mediated arrest mechanisms are overlapping in this model and identify CXCR2 as an important neutrophil arrest chemokine in vivo.
Keywords: E-selectin, chemokine, neutrophils, G protein–coupled receptors, pertussis toxin
Introduction
Neutrophil accumulation into sites of inflammation proceeds as a sequence of overlapping steps required for a cell to exit the vascular pool and enter the tissue. This process occurs as a result of molecular changes on the surface of the endothelium in response to inflammatory stimuli (1). Neutrophils may process numerous activating signals during rolling, and the transition from slow rolling to arrest occurs as a result of β2 integrin activation. This activation allows high avidity interaction with their ligands on the endothelium (2, 3), which can be triggered by immobilized chemokines. Such arrest chemokines are presented on the endothelial surface and are sufficient to induce neutrophil arrest from rolling. IL-8 arrests rolling human neutrophils in a parallel plate flow chamber in vitro (4). So far, no neutrophil arrest chemokine has been demonstrated in vivo, although exogenous application of many chemokines and other chemoattractants is known to induce neutrophil arrest.
Selectin binding can lead to increased β2 integrin–dependent adhesion (5). This integrin activation pathway appears to be relevant for neutrophils rolling on E-selectin in vitro, but conflicting data was also reported (5–7). In vivo, the number of adherent neutrophils per unit surface area was reduced in E-selectin−/− mice (8). Although P-selectin glycoprotein ligand (PSGL)-1 can bind E-selectin, E-selectin–dependent rolling and adhesion is only mildly impaired in PSGL-1−/− mice (9, 10). Human, but not mouse, L-selectin binds E-selectin (11), and the physiologically relevant E-selectin ligand has not been identified (12).
This work was designed to test the hypothesis that chemokine- and E-selectin–mediated activation cooperate to arrest rolling neutrophils in vivo. To block chemokine-mediated signaling, pertussis toxin (PTx) was injected i.v. into WT or E-selectin−/− mice in which inflammation was induced by intrascrotal TNF-α. To identify a relevant chemokine receptor, CXCR2−/− mice were investigated with and without treatment with an E-selectin blocking mAb. To test the relevance of these results, thioglycollate was used to induce peritonitis in WT or E-selectin−/− mice with and without PTx treatment.
Materials and Methods
Reagents.
WT C57Bl/6, WT BALB/c, and CXCR2−/− mice on BALB/c background were obtained from The Jackson Laboratory. E-selectin−/− mice on a C57Bl/6 background were derived from founders provided by A. Beaudet (Baylor College of Medicine, Houston, TX; reference 13), PSGL-1−/− mice on a C57Bl/6 background were provided by R. McEver (Oklahoma Medical Research Foundation, Oklahoma City, OK; reference 10), L-selectin−/− mice on a C57Bl/6 background were from T. Tedder (Duke University Medical Center, Durham, NC), and ST3Gal-IV−/− mice on a C57Bl/6 background were from J. Marth (University of California, San Diego, La Jolla, CA; reference 12). All animal experiments were approved by the Animal Care and Use Committee of the University of Virginia. PTx from Bordetella pertussis (lyophilized powder; Sigma-Aldrich) was dissolved in physiological saline. Murine recombinant TNF-α and keratinocyte-derived chemokine (KC) were obtained from R&D Systems and PeproTech, respectively.
Intravital Microscopy.
3 h before cremaster muscle exteriorization, mice received an intrascrotal injection of 500 ng TNF-α in 0.25 ml of saline. Some animals also received tail vein injections of 4 μg PTx suspended in 0.5 ml of saline, 5 min before TNF-α injection. Mice were anesthetized with an i.p. injection of 125 mg/kg ketamine (Sanofi Winthrop Pharmaceuticals), 12.5 mg/kg xylazine (Phoenix Scientific), and 0.025 mg/kg atropine sulfate (Fujisawa), and placed on a 38°C heating pad. The trachea was intubated using polyethylene 90 tubing (ID: 0.86 mm, OD: 1.27 mm; Becton Dickinson). The left carotid artery was cannulated using PE10 tubing (ID: 0.28 mm, OD: 0.61 mm). The cremaster was exteriorized, pinned to the stage, and superfused with thermocontrolled bicarbonate-buffered saline (131.9 mM NaCl, 18 mM NaHCO3, 4.7 mM KCl, 2.0 mM CaCl2 • 2H20, and 1.2 mM MgCl2) equilibrated with 5% CO2 in N2.
Microscopic observations were made on an intravital microscope (Axioskop; Carl Zeiss MicroImaging, Inc.) with a saline immersion objective (SW 40/0.75) visualized under bright field illumination. Recordings were made through a CCD camera (model VE-1000CD; Dage-MTI) on a Panasonic S-VHS recorder. In TNF-α–inflamed cremaster muscles, randomly selected venules with diameters between 19 and 49 μm were recorded for 2 min.
Separate experiments without TNF-α were designed to test the efficacy of PTx treatment. Venules were recorded for 2 min before and after injection of 600 ng KC (in 0.05 ml of physiological saline) intraarterially (i.a.). This dose matches the IL-8 dose previously used on a per kilogram basis to arrest rolling neutrophils in rabbit mesentery (14). Rolling flux and numbers of adherent cells per 200-μm length of venule were measured 1 min before and after KC administration. Vessels in this group had diameters between 25 and 30 μm.
Vessel centerline blood velocity was measured using a dual photodiode and digital cross-correlation program (Circusoft Instrumentation). Mean blood velocity, V b, was approximated by multiplying the centerline blood velocity by 0.625 (15). The interfacial shear rate, γi, is the slope of the velocity profile at the interface of the endothelial surface layer and the vessel lumen, and it was estimated as γi = 4.9*8*[V b /d], where d is the diameter of the vessel and 4.9 is a mean empirical correction factor (16). Systemic leukocyte counts were measured from 25 μl of carotid blood samples in a Hemavet 850 (CDC Technologies).
Peritonitis Model.
Peritoneal recruitment of leukocytes was induced using thioglycollate according to previously published methods (17). Some mice received tail vein injections of 4 μg PTx 2 h before thioglycollate injection. Mice were injected i.p. with 1 ml of sterile water containing 4% thioglycollate medium (Sigma-Aldrich). After 4 h, mice were killed and the peritoneum was rinsed with 5 ml PBS (containing 5 mM EDTA and 10 U/ml heparin) and massaged. The peritoneum was then opened, and the total neutrophils and mononuclear cells/milliliter of draining fluid was determined using Kimura-stained samples.
Data Analysis.
A MicroMotion DC30 video compression card (Pinnacle Systems) was used to digitize video recordings from a JVC HR-53600U VHS recorder into a Macintosh computer (Adobe Premiere software). Digitized video clips were analyzed with the public domain NIH Image program with custom-written macros. Adherent cells were defined as leukocytes that did not move for at least 30 s and normalized by surface area for comparison between groups. Rolling leukocyte flux was measured by counting the number of cells that rolled past a line perpendicular to the vessel axis and expressed as leukocytes per minute. Data are presented as mean ± SEM. Individual comparisons between groups were calculated using a one-tailed t test with P < 0.05.
Results and Discussion
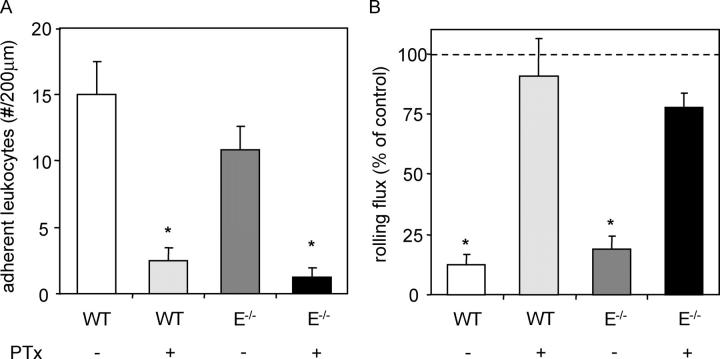
To confirm that systemically applied PTx was sufficient to block Gαi signaling events leading to firm arrest, WT mice were pretreated with 4 μg PTx i.v. 3 h before exteriorization of the cremaster muscle. In this trauma model, neutrophils roll, but rarely adhere, in cremaster venules due to P-selectin expression on the endothelial surface. 600 ng of recombinant murine chemokine KC, which binds CXCR2, was injected i.a., and rapidly triggered firm adhesion of rolling leukocytes in WT control venules (15 ± 2.5 adherent cells per 200-μm length of venule above baseline), but not in venules of mice treated with systemic PTx (2.5 ± 1.0 adherent cells; Fig. 1 A). In addition, KC injection resulted in a reduction in leukocyte rolling flux from 70.6 ± 15.5 to 5.3 ± 1.3 cells/min in untreated mice, but did not significantly reduce rolling flux in PTx-treated animals (Fig. 1 B). Both effects occurred within 15 s of KC injection and were fully recapitulated in E-selectin−/− mice.
Figure 1.
Adherent leukocytes per 200-μm length of vessel (increase above background; A) and percent reduction in leukocyte rolling flux (B) due to injection of 600 ng KC into the carotid artery cannula. Data was acquired 1 min before and after injection into WT (7 venules in 3 mice; white bars), WT plus 4 μg PTx 3 h before cremaster exteriorization (10 venules in 4 mice; light gray bars), E-selectin−/− (6 venules in 2 mice; dark gray bars), and E-selectin−/− plus PTx (5 venules in 2 mice; black bars). *, P < 0.05 versus WT control (A) or rolling flux before KC injection (B).
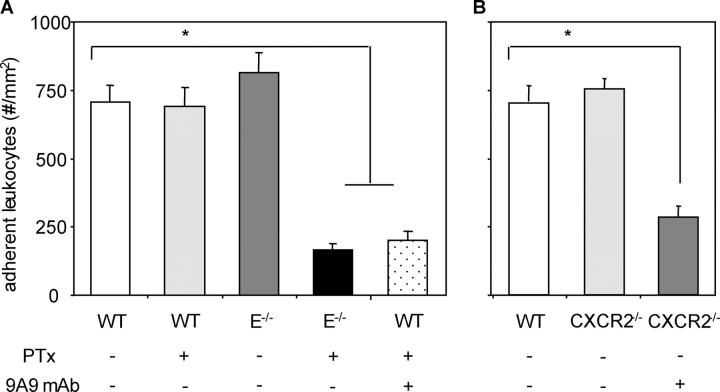
3-h intrascrotal TNF-α treatment in mice results in dense neutrophil rolling and adhesion mediated through the selectins and CD18 integrins (18). In this model, >90% of all intravascular, as well as transmigrated extravascular, leukocytes are neutrophils in both WT and E-selectin−/− mouse cremaster venules after 3-h intrascrotal TNF-α stimulation, a stimulus time course identical to the one used here (19). WT mice treated with 4 μg PTx via tail vein injection 5 min before intrascrotal injection of 500 ng TNF-α showed no reduction in leukocyte adhesion (689 ± 72 firmly adherent cells per mm2) versus WT mice that did not receive PTx treatment (706 ± 61 cells/mm2; Fig. 2 A). However, PTx treatment of E-selectin−/− (164 ± 24 cells/mm2) or WT mice that also received 60 μg of the α-E-selectin mAb 9A9 at the time of PTx injection, caused a significant reduction in neutrophil adhesion (202 ± 33 cells/mm2) relative to E-selectin−/− controls (815 ± 73 cells/mm2; Fig. 2 A). Shear rates and diameters were similar between different groups, excluding a hemodynamic contribution to reduced leukocyte adhesion in PTx-treated E-selectin−/− mice or WT mice that received both PTx and mAb 9A9 (Table I). The interfacial shear rates presented in Table I are significantly higher than historical values in the literature for wall shear rate because of the slope of the velocity profile of blood in small venules and the presence of the endothelial surface layer (16).
Figure 2.
Numbers of adherent cells per mm2 in murine cremaster muscle venules. Cremaster muscle exteriorized 3 h after intrascrotal injection of 500 ng TNF-α in WT (11 venules in 3 mice), WT plus 4 μg PTx 5 min before TNF-α injection (12 venules in 3 mice), E-selectin−/− (13 venules in 3 mice), E-selectin−/− plus PTx (17 venules in 3 mice), and WT plus PTx plus 60 μg α–E-selectin mAb 9A9 5 min before TNF-α injection (10 venules in 2 mice) using mice on a C57Bl/6 background (A), and WT (11 venules in 2 mice), CXCR2−/− (13 venules in 3 mice), and CXCR2−/− plus mAb 9A9 (11 venules in 2 mice) using mice on a BALB/c background (B). *, P < 0.05 versus all other groups.
Table I.
Mean ± SEM of Diameter, Interfacial Shear Rate γi and Newtonian Wall Shear Rate of γw of Venules in TNF-α–treated WT or E-Selectin−/− Mice with (+) or without (−) PTx Treatment
| PTx | 9A9 | dia | γi | Newtonian γw |
|
|---|---|---|---|---|---|
| μm | s−1 | s−1 | |||
| C57B1/6 | |||||
| WT | − | − | 36.2 ± 3 | 1,433 ± 218 | 292 ± 44 |
| + | − | 35.3 ± 3 | 1,052 ± 122 | 215 ± 25 | |
| + | + | 30.5 ± 4 | 1,070 ± 160 | 218 ± 33 | |
| E-selectin−/− | − | − | 33.8 ± 2 | 1,265 ± 179 | 258 ± 37 |
| + | − | 29.1 ± 2 | 1,419 ± 216 | 290 ± 44 | |
| BALB/c | |||||
| WT | − | − | 33.5 ± 2 | 1,362 ± 144 | 278 ± 29 |
| CXCR2−/− | − | − | 31.5 ± 2 | 1,278 ± 48 | 261 ± 20 |
| − | + | 34.8 ± 2 | 1,106 ± 112 | 226 ± 23 |
To determine which chemokine receptor might be responsible for neutrophil arrest in E-selectin−/− mice, CXCR2−/− mice were treated with the function blocking α-E-selectin mAb 9A9. CXCR2−/− mice retain normal levels of neutrophil arrest after 3 h of TNF-α stimulation (755 ± 76 adherent cells/mm2) versus control BALB/c WT mice (684 ± 56 adherent cells/mm2), whereas mAb 9A9 treatment dramatically reduced adhesion almost to baseline levels (283 ± 42 adherent cells/mm2; Fig. 2 B). Taken together, CXCR2 binding chemokines and E-selectin are responsible for firm adhesion of neutrophils in inflamed venules. These data are consistent with findings by Simon et al. (5) who showed that neutrophil interaction with L cells transfected with E-selectin caused neutrophil activation through a p38 MAP kinase–dependent pathway.
The E-selectin ligands responsible for neutrophil activation are unknown. Although PSGL-1 tethers neutrophils to E-selectin in vivo, other unidentified ligands mediate slow rolling. This was demonstrated in TNF-α–treated cremaster venules of PSGL-1−/− mice, which showed a significant reduction in the number, but not velocity, of neutrophils rolling on E-selectin (10). A study in inflamed cremaster muscle postcapillary venules of ST3Gal-IV−/− mice demonstrated that E-selectin–mediated rolling velocities are significantly increased, whereas the number of rolling leukocytes was not affected, suggesting that the unidentified E-selectin ligand(s) is modified by ST3Gal-IV (12). In this work, PTx treatment had no effect on neutrophil arrest in PSGL-1−/−, L-selectin−/−, or ST3Gal-IV−/− mice (not depicted). Thus, E-selectin–dependent neutrophil arrest occurs through unidentified or overlapping ligands.
To determine if E-selectin ligation during rolling is sufficient to directly lead to neutrophil arrest, a recently described autoperfused flow chamber was used on which recombinant murine E-selectin and intercellular adhesion molecule (ICAM)-1 were coadsorbed (20). The autoperfused flow chamber is advantageous because it does not require leukocyte subset isolation leading to neutrophil cell activation. At an E-selectin coating concentration sufficient to result in robust neutrophil tethering and rolling (∼12 rolling cells per 520 by 470 μm field of view), neutrophil rolling at mean wall shear stresses of 1.4 ± 0.2 dyn/cm2 did not lead to firm arrest (not depicted). Infusion of soluble recombinant CXCR2 binding chemokine KC or coadsorption of KC with both E-selectin and ICAM-1 resulted in robust firm adhesion to the substrate (not depicted). This adhesion was dependent on ICAM-1, as coadsorption of KC on E-selectin alone did not lead to neutrophil firm arrest (not depicted). These data suggest that E-selectin binding to its ligand(s) on neutrophils is not sufficient to induce arrest.
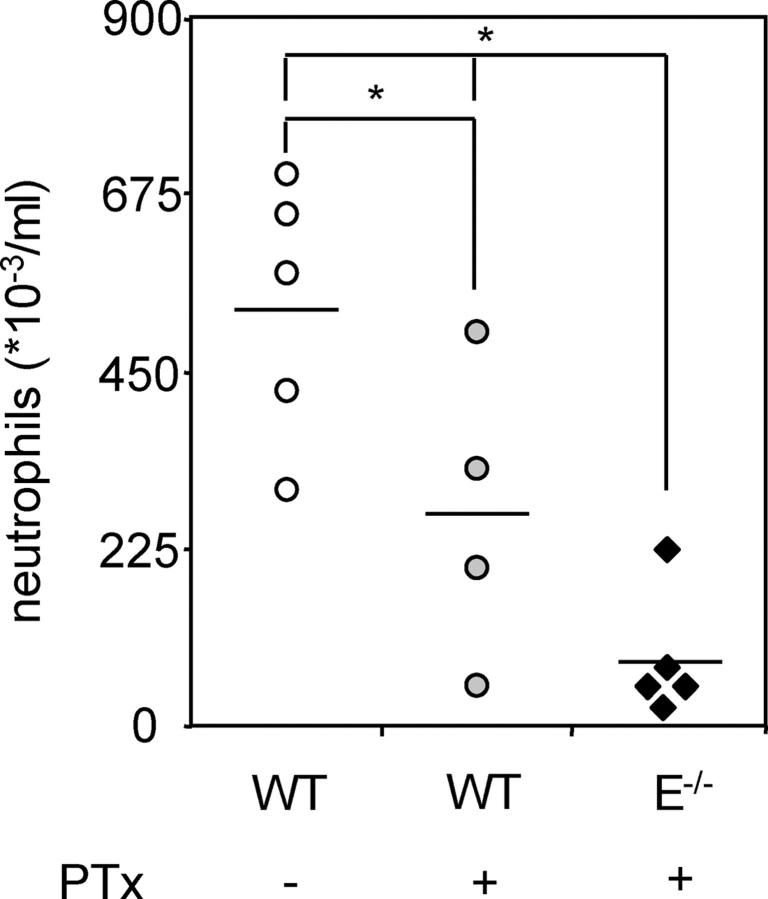
The inability of neutrophils to firmly adhere in cremaster venules of PTx-treated E-selectin−/− mice suggests that blockade of both E-selectin– and chemokine-mediated adhesion, but not either one alone, may prevent neutrophil influx into inflamed tissues. To test this hypothesis, neutrophil influx into thioglycollate-induced peritonitis was investigated in WT and E-selectin−/− mice with or without PTx treatment. PTx treatment reduced, but did not eliminate, neutrophil recruitment to the peritoneum 4 h after thioglycollate injection into WT mice (269 ± 95 in PTx-treated vs. 530 ± 74*103 cells/ml in control WT mice). As reported previously (21), neutrophil influx was not reduced in E-selectin−/− mice (not depicted), but neutrophil influx was absent in PTx-treated E-selectin−/− animals (85 ± 36*103 cells/ml; Fig. 3) and significantly lower than recruitment in all other groups. Although PTx treatment probably results in inhibition of both neutrophil arrest and migration, these data show that neutrophil arrest inhibition through blockade of E-selectin and G protein–coupled receptor–dependent signals is physiologically relevant. Thus, antiinflammatory strategies targeting neutrophils must now account for both modes of neutrophil arrest in parallel because blockade of only E-selectin or G protein–coupled receptor results in no or only partial inhibition of neutrophil recruitment in a model of peritonitis.
Figure 3.
Peritoneal neutrophil influx 4 h after 1 ml injection of 4% thioglycollate into WT (five mice; open circles), WT plus 4 μg PTx 3 h before thioglycollate injection (four mice; light gray circles), E-selectin−/− (four mice; dark gray bars), and E-selectin−/− plus PTx (five mice; closed diamonds). Data expressed as numbers of neutrophils (×103) per ml peritoneal lavage fluid counted using Kimura-stained samples. *, P < 0.05.
The dependence of neutrophil arrest in vivo on CXCR2 or binding to E-selectin may also be responsible for the qualitative difference between lymphocyte arrest, which is instantaneous (22), and neutrophil arrest, which can require several minutes of rolling (2). The overlapping function of E-selectin and CXCR2 is reminiscent of the observation that blocking both E-selectin and P-selectin is necessary to block neutrophil recruitment into the inflamed peritoneum (21). Blocking E-selectin and P-selectin blocks tethering and rolling, whereas blocking E-selectin and CXCR2 prevents neutrophil arrest, as demonstrated here.
In conclusion, these data indicate that E-selectin and CXCR2 chemokines serve a redundant role in the arrest of rolling neutrophils. Normal levels of adhesion in WT PTx-treated or E-selectin mAb-treated animals, untreated E-selectin−/− animals, or CXCR2−/− mice show that E-selectin or CXCR2 chemokines individually are sufficient to trigger the transition from slow rolling to arrest. This robust mechanism is responsible for intact neutrophil recruitment even without chemokines, and explains the previous inability to identify arrest chemokines for neutrophils.
Acknowledgments
We thank Michele Kirkpatrick for animal husbandry. We gratefully acknowledge Jessica L. Dunne for assistance with intravital microscopy in CXCR2−/− mice and Michael Steele for assistance with data analysis. We thank Dr. Barry Wolitzky for the mAb 9A9 and Drs. Thomas Tedder, Arthur Beaudet, Jamey Marth, and Rodger McEver for mice.
This work was supported by National Institutes of Health (NIH) grant HL54136 to K. Ley. M.L. Smith is supported by NIH training grant T32 GM 08715-01A1. T.S. Olson is supported by NIH training grant GM07267-23.
The authors have no conflicting financial interests.
M.L. Smith and T.S. Olson contributed equally to this work.
References
- 1.Springer, T.A. 1995. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57:827–872. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel, E.J., J.L. Dunne, and K. Ley. 2000. Leukocyte arrest during cytokine-dependent inflammation in vivo. J. Immunol. 164:3301–3308. [DOI] [PubMed] [Google Scholar]
- 3.Constantin, G., M. Majeed, C. Giagulli, L. Piccio, J.Y. Kim, E.C. Butcher, and C. Laudanna. 2000. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 13:759–769. [DOI] [PubMed] [Google Scholar]
- 4.Rainger, G.E., A.C. Fisher, and G.B. Nash. 1997. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am. J. Physiol. 272:H114–H122. [DOI] [PubMed] [Google Scholar]
- 5.Simon, S.I., Y. Hu, D. Vestweber, and C.W. Smith. 2000. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 164:4348–4358. [DOI] [PubMed] [Google Scholar]
- 6.Kuijpers, T.W., B.C. Hakkert, M. Hoogerwerf, J.F.M. Leeuwenberg, and D. Roos. 1991. Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells: ELAM-1 mediated CD18 activation. J. Immunol. 147:1369–1376. [PubMed] [Google Scholar]
- 7.Repo, H., Y.P. Rochon, B.R. Schwartz, S.R. Sharar, R.K. Winn, and J.M. Harlan. 1997. Binding of human peripheral blood polymorphonuclear leukocytes to E-selectin (CD62E) does not promote their activation. J. Immunol. 159:943–951. [PubMed] [Google Scholar]
- 8.Milstone, D.S., D. Fukumura, R.C. Padgett, P.E. O'Donnell, V.M. Davis, O.J. Benavidez, W.L. Monsky, R.J. Melder, R.K. Jain, and M.A. Gimbrone. 1998. Mice lacking E-selectin show normal numbers of rolling leukocytes but reduced leukocyte stable arrest on cytokine-activated microvascular endothelium. Microcirculation. 5:153–171. [PubMed] [Google Scholar]
- 9.Yang, J., T. Hirata, K. Croce, G. Merrill-Skoloff, B. Tchernychev, E. Williams, R. Flaumenhaft, B.C. Furie, and B. Furie. 1999. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin–mediated but not E-selectin–mediated neutrophil rolling and migration. J. Exp. Med. 190:1769–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia, L., M. Sperandio, T. Yago, M. McDaniel, R.D. Cummings, S. Pearson-White, K. Ley, and R.P. McEver. 2002. P-selectin glycoprotein ligand-1 deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Invest. 109:939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zollner, O., M.C. Lenter, J.E. Blanks, E. Borges, M. Steegmaier, H.G. Zerwes, and D. Vestweber. 1997. L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J. Cell Biol. 136:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellies, L.G., M. Sperandio, G.H. Underhill, J. Yousif, M. Smith, J.J. Priatel, G.S. Kansas, K. Ley, and J.D. Marth. 2002. Sialyltransferase specificity in selectin ligand formation. Blood. 100:3618–3625. [DOI] [PubMed] [Google Scholar]
- 13.Bullard, D.C., E.J. Kunkel, H. Kubo, M.J. Hicks, I. Lorenzo, N.A. Doyle, C.M. Doerschuk, K. Ley, and A.L. Beaudet. 1996. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J. Exp. Med. 183:2329–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley, K., J.B. Baker, M.I. Cybulsky, M.A. Gimbrone, and F.W. Luscinskas. 1993. Intravenous interleukin-8 inhibits granulocyte emigration from rabbit mesenteric venules without altering L-selectin expression or leukocyte rolling. J. Immunol. 151:6347–6357. [PubMed] [Google Scholar]
- 15.Lipowsky, H.H., and B.W. Zweifach. 1978. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc. Res. 15:93–101. [DOI] [PubMed] [Google Scholar]
- 16.Smith, M.L., D.S. Long, E.R. Damiano, and K. Ley. 2003. Near-wall μ-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys. J. 85:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forlow, S.B., and K. Ley. 2001. Selectin-independent rolling and adhesion in mice deficient in E-, P- and L-selectin and ICAM-1. Am. J. Physiol. 280:H634–H641. [DOI] [PubMed] [Google Scholar]
- 18.Jung, U., K.E. Norman, K. Scharffetter-Kochanek, A.L. Beaudet, and K. Ley. 1998. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 102:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forlow, S.B., E.J. White, S.C. Barlow, S.H. Feldman, H. Lu, G.J. Bagby, A.L. Beaudet, D.C. Bullard, and K. Ley. 2000. Severe inflammatory defect and reduced viability in CD18 and E-selectin double mutant mice. J. Clin. Invest. 106:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, M.L., M. Sperandio, E.V. Galkina, and K. Ley. 2004. Autoperfused mouse flow chamber reveals synergistic neutrophil accumulation through P-selectin and E-selectin. J. Leukoc. Biol. In press. [DOI] [PubMed] [Google Scholar]
- 21.Labow, M.A., C.R. Norton, J.M. Rumberger, K.M. Lombard-Gillooly, D.J. Shuster, J. Hubbard, R. Bertko, P.A. Knaack, R.W. Terry, M.L. Harbison, et al. 1994. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1:709–720. [DOI] [PubMed] [Google Scholar]
- 22.Campbell, J.J., J. Hedrick, A. Zlotnik, M.A. Siani, D.A. Thompson, and E.C. Butcher. 1998. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 279:381–384. [DOI] [PubMed] [Google Scholar]