Abstract
The molecular basis of how Borrelia burgdorferi (Bb), the Lyme disease spirochete, maintains itself in nature via a complex life cycle in ticks and mammals is poorly understood. Outer surface (lipo)protein A (OspA) of Bb has been the most intensively studied of all borrelial molecular constituents, and hence, much has been speculated about the potential role(s) of OspA in the life cycle of Bb. However, the precise function of OspA (along with that of its close relative and operonic partner, outer surface [lipo]protein B [OspB]) heretofore has not been directly determined, due primarily to the inability to generate an OspA/B-deficient mutant from a virulent strain of Bb. In this study, we created an OspA/B-deficient mutant of an infectious human isolate of Bb (strain 297) and found that OspA/B function was not required for either Bb infection of mice or accompanying tissue pathology. However, OspA/B function was essential for Bb colonization of and survival within tick midguts, events crucial for sustaining Bb in its natural enzootic life cycle.
Keywords: Lyme disease, OspA, lipoprotein, arthropod vector, Borrelia burgdorferi
Introduction
Lyme disease is the most prevalent arthropod-born infection in the United States (1). The agent of Lyme disease, Borrelia burgdorferi (Bb), maintains itself in nature via a complex life cycle involving ticks and mammals (2). Since the discovery of the etiological agent, outer surface (lipo)protein A (OspA) has been a central focus of Lyme disease research (3, 4). OspA (a) has comprised the first Lyme disease vaccine (5), (b) represents a paradigm of differential lipoprotein expression (6–8), (c) has been implicated in the late sequelae (chronic arthritis) of Lyme disease (9), and (d) has been crystallized (10). OspA is abundantly expressed by Bb within the midguts of unfed ticks but is down-regulated in Bb as ticks take a blood meal and as borreliae are transmitted to the mammalian host (6–8). These observations have led to speculation that OspA may be involved in some aspect(s) of the tick phase of Bb's life cycle (11, 12). Subsequent studies have postulated that OspA may promote tick colonization, in that purified recombinant OspA bound to extracts of tick midgut cells (13) and adherence of Bb to tick midgut epithelium was markedly reduced in the presence of OspA antibodies (14). Despite these intensive efforts, direct evidence to demonstrate a role for OspA either in the tick-born or mammalian phase of Bb's life cycle heretofore has been lacking (11).
A major obstacle to defining the function of OspA has been that virulent strains of Bb are refractory to genetic manipulation (15, 16), thereby hindering the generation of OspA-deficient mutants of the spirochete. Producing such OspA-deficient variants is essential for establishing a causal relationship between OspA and any given phenotype. Recent successes in genetically manipulating virulent Bb and the development of new shuttle vectors (17–22) prompted renewed efforts to generate an OspA-deficient mutant. In an effort to satisfy “molecular” Koch's postulates (23), in the current study an OspA/B-deficient mutant of Bb 297 and genetically complemented strains were generated and examined for phenotypic traits pertaining to mouse infectivity, pathogenicity, and their colonization of and transmission by Ixodes scapularis ticks.
Materials and Methods
Bacterial Strains and I. scapularis.
An infectious clone of Bb, BbAH130, was recovered after plating Bb strain 297 (297) on Barbour-Stoenner-Kelly (BSK) agar medium. The clone was then needle inoculated (intradermally) into mice and recovered from cultures of ear punch biopsies. BbAH130 possesses all 21 of the parental plasmids (determined by PCR) found in strain 297 (19) and is infectious for mice. Bb strain 48081, lacking the lp54 plasmid (24), was provided by John F. Anderson (Connecticut Agricultural Experimental Station, New Haven, CT). Borreliae were cultivated in vitro in BSK-H liquid medium at 37°C (Sigma-Aldrich). Escherichia coli strain TOP 10 (Invitrogen) was used as a cloning host. Adult female I. scapularis ticks were collected in Connecticut as a source of eggs. The egg masses were laid in the laboratory. Hatched larvae were allowed to feed on pathogen-free C3H/HeN mice to produce sterile nymphs. The absence of bacteria in nymphs was verified by performing dark field microscopy on midgut contents from several ticks within each reared colony. All tick rearing was performed in an incubator at 26°C with 85% relative humidity and using a controlled light–dark photoperiod.
Construction of Suicide Plasmids.
All recombinant DNA experiments and the use of antibiotic resistance markers in strain 297 were reviewed and approved by the University of Texas Southwestern Medical Center Biological and Chemical Safety Advisory Committee. A 4-kb DNA fragment containing ospAB (genes BBA15 and BBA16) with flanking sequence was obtained by PCR amplification from 297 genomic DNA. PCR was performed by using the Expand High Fidelity PCR System (Roche Diagnostics). The primer design was based on published gene sequences for Bb strain B31 (25) (primers 1 and 2; Table S1, available at http://www.jem.org/cgi/content/full/jem.20031960/DC1). The PCR fragment was then cloned into the pCR-XL-TOPO cloning vector (Invitrogen). The resulting plasmid was designated as pXT-OspAB. Then, an aadA cassette (which confers streptomycin [Strep] resistance to Bb [26]) was inserted at the AccI site within the ospA gene, resulting in plasmid pXT-OspAB-Strep (Fig. 1 A). To generate the ospAB mutant, 20 μg of pXT-OspA-Strep plasmid DNA was electroporated into BbAH130. The transformation mixture was then diluted into 100 ml of BSK-H medium. After overnight recovery, Strep was added to the transformation mixture at a final concentration of 50 μg/ml, and the mixtures were aliquotted into multiple 96-well tissue culture plates (200 μl/well). 2–3 wk after plating, the wells that contained positive cultures were identified by color change of the medium; the presence of viable spirochetes subsequently was verified by dark field microscopy. In general, <10% of microtiter plate wells contained positive cultures, suggesting that each well culture was clonal in origin. PCR amplification with various primer pairs (Table S1) was performed on whole cell lysates of these transformants to verify that the streptomycin-resistance (Strepr) transformants contained the appropriate aadA insertion (Fig. 1). Although highly unlikely, the possibility that the suicide plasmid inserted into more than one site would not have influenced our studies because the mutant remained infectious and complementation was successful in restoring OspA/B function (see Results and Discussion). Also, plasmid contents of the OspA/B− mutants were examined by PCR as described previously (19, 27, 28).
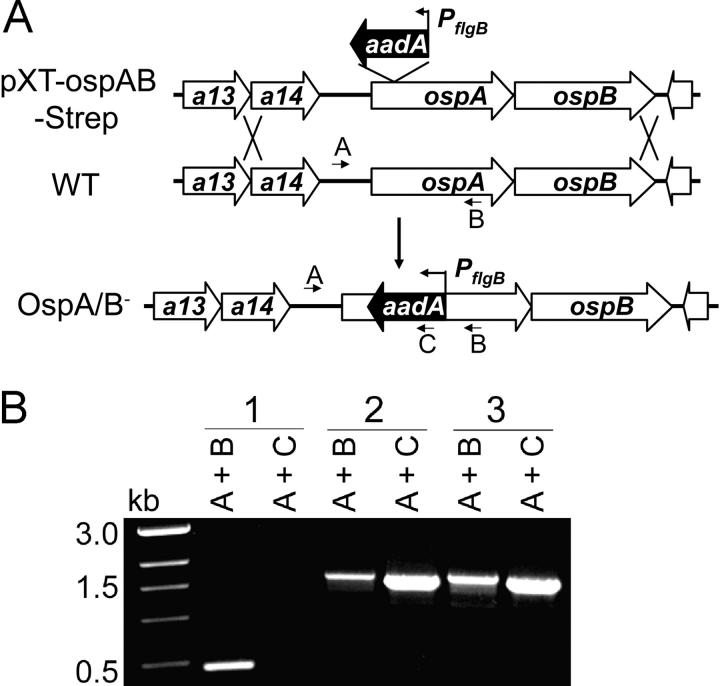
Figure 1.
Construction of the OspA/B− mutant of Bb. (A) Strategy for insertional (aadA) inactivation of the ospAB operon. The top line diagram denotes the suicide plasmid (pXT-OspAB-Strep) used for transformation into Bb and subsequent homologous recombination (double crossover) with the wild-type (WT) genome in Bb. Only the relevant portions of the plasmid and genome are shown. Arrows denote positions of oligonucleotide primers used for PCR. (B) PCR analysis of Bb variants. Letter combinations denote primer pairs used for PCR. 1, wild-type Bb; 2, the OspA/B− mutant; 3, spirochetes recovered from mice infected with the OspA/B− mutant.
Complementation of the OspA/B− Mutant.
To complement the OspA/B− mutant with a wild-type copy of ospAB, a 2-kb DNA fragment containing the wild-type ospAB genes first was PCR amplified from Bb strain 297 using primers 3 and 4 (Table S1). This fragment subsequently was cloned into pCR-XL-TOPO (Invitrogen), resulting in plasmid pXT-ospAB-2kb. pXT-ospAB-2kb was then digested with SacI and XbaI, and the 2-kb fragment containing the ospAB genes was inserted into the corresponding sites of pBSV2 (22) (provided by Philip E. Stewart and Patricia Rosa, National Institutes of Health Rocky Mountain Laboratories, Hamilton, MT). The resulting plasmid was designated pOspA/B (see Fig. 6 A). To complement the OspA/B− mutant with only wild-type ospA, pOspA/B was digested with AspE1 and BstAp1 (cleaving within the ospB gene). The linear plasmid with the ospB deletion was then blunt ended and ligated closed, culminating in plasmid pOspA. For complementation, 20 μg of pOspA/B or pOspA DNA was electroporated into the OspA/B− mutant, and ∼20 kanamycin-resistant/Strepr clones were obtained. To confirm that these clones contained the pOspA/B or pOspA plasmid, whole cell lysates of kanamycin-resistant/Strepr cells were used to transform TOP10 E. coli. Plasmid was then rescued from these E. coli transformants, and restriction digestions were performed to verify recovery of the respective plasmid.
Figure 6.
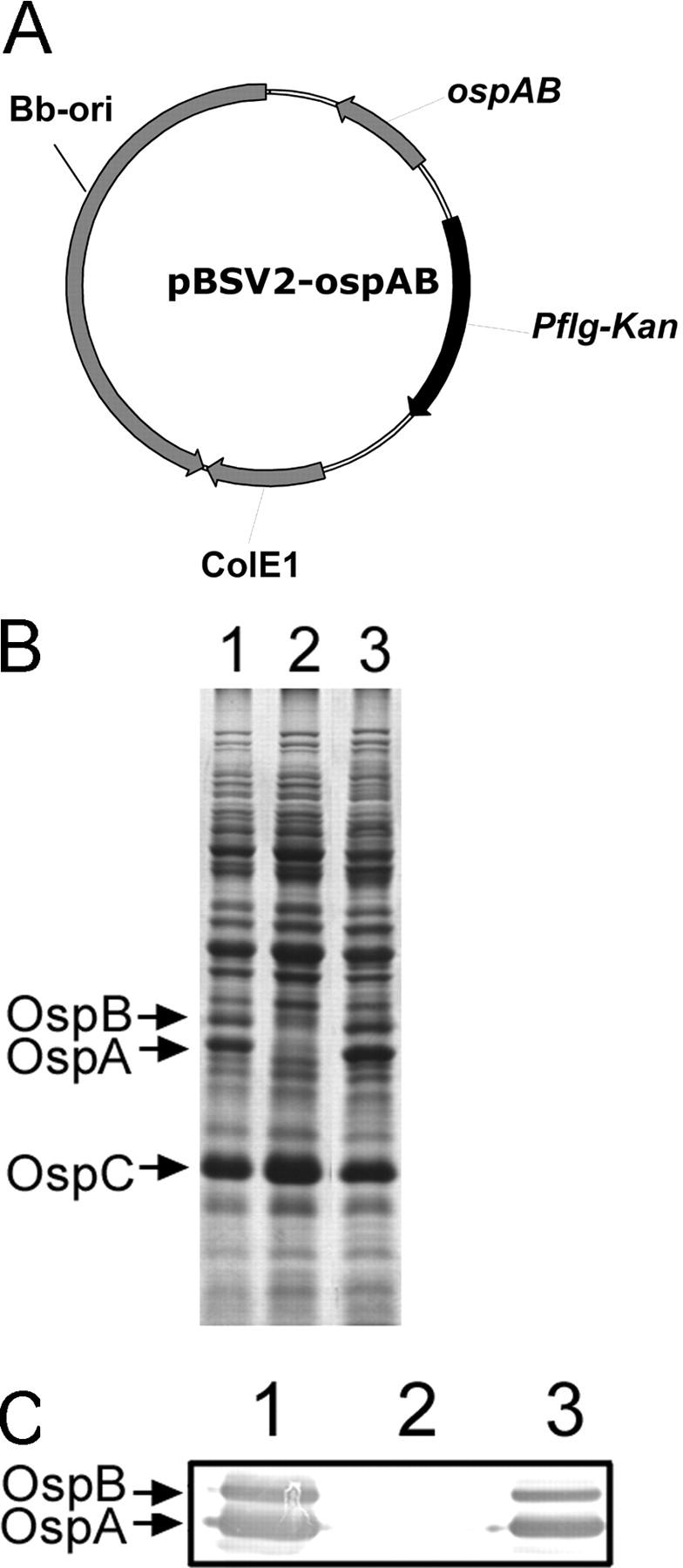
Strategy for transcomplementation of the OspA/B− mutant. (A) Diagram of the shuttle vector pOspA/B used for complementation. (B) SDS-PAGE (Coomassie blue stain) and (C) immunoblot of whole cell lysates of Bb strains. For immunoblotting, antibodies directed against OspA and OspB were pooled. Lanes 1, wild-type Bb; lanes 2, the OspA/B− mutant; lanes 3, the OspA/B− mutant complemented with pOspA/B.
Bb Infection of Mice via Needle Inoculation.
Groups of five 3-wk-old C3H/HeN mice (Jackson Laboratories) were needle inoculated (intradermally) with either 102, 103, or 104 wild-type 297 or OspA/B− mutants. 3 wk later, tibiotarsal and knee joints were evaluated for swelling (29). Ear punch biopsies were taken and cultured in BSK-H medium at 37°C. Skin, hearts, and joint tissues also were harvested for DNA extraction. Joint tissues were additionally subjected to histopathology analysis and were scored in blinded fashion; ranking was performed on the basis of relative degrees of leukocyte and fibrin exudation and synovial proliferation (29).
Transmission of Bb from Infected Mice to Sterile Ticks.
To study the transmission of various Bb strains from mice to ticks, naive nymphal forms of I. scapularis were allowed to feed on Bb-infected (needle inoculated) mice. Actively feeding nymphs were forcibly detached from the skin of mice at 12-h intervals after the onset of attachment. Other nymphs were allowed to feed to repletion and detach naturally from the mice; these nymphs were collected at 24-h intervals for up to 96 h postfeeding. Midguts from each group of nymphs were microscopically dissected in the presence of PBS. The midgut luminal contents (including the blood meal) and the midgut tissues that were washed with PBS to ensure the removal of luminal contents were isolated and subjected to immunofluorescence confocal microscopy (13). Spirochetes in the samples were then enumerated microscopically as described previously (13, 14).
Microinjection of Bb into Ticks.
A microinjection delivery system was developed and used to introduce spirochetes into the gut of I. scapularis nymphs. For microinjection, each Bb variant was cultivated under normal conditions in BSK-H medium in the presence or absence of selective antibiotics. Bacteria were harvested by centrifugation and concentrated in fresh BSK-H medium to a density of 109 spirochetes per ml. 10 μl of the cell suspension was then loaded into a 1-mm diameter glass capillary needle (World Precision Instruments Inc.) using a microloader (Eppendorf AG). Unfed nymphs were attached with the ventral side facing up onto a glass slide using double-sided tape. With the aid of a stereo-dissecting binocular microscope, tips of the capillary needles harboring the spirochete suspension were carefully inserted into the rectal aperture through forced opening of the rectal valves; the suspension was then injected into the tick using a femtojet microinjector system (Eppendorf AG). The parameters for injection were a pressure of 1,000 hPa, injection time of 1 s, and a compensation pressure of 8 hPa. After microinjection, ticks were reared at room temperature in a humid chamber for 24–72 h. Groups of ticks were killed and assessed for spirochete content by dark field microscopy, culture, and RT-PCR analysis. Groups of ticks with spirochete infection efficiencies of nearly 100% were then used for further colonization evaluations.
PCR, RT-PCR, and Real-Time PCR.
Total RNA from wild-type BbAH130 or mutants was isolated using the NucleoSpin RNA II purification kit (BD Biosciences) according to instructions provided by the manufacturer. RT-PCR reactions for amplification of ospA and flaB transcripts were performed using ∼50 ng of total RNA, pertinent oligonucleotide primers, and the Titan One Tube RT-PCR System (Roche Diagnostics).
For RT-PCR analysis of Bb RNA in ticks, total RNA was isolated from I. scapularis using the NucleoSpin II RNA isolation kit (CLONTECH Laboratories Inc.). 2 μg of total RNA was converted to cDNA using ProSTAR first strand RT-PCR kit (Stratagene) with random hexamers. The cDNA was then used for PCR amplification of flaB using oligonucleotide primers 7 and 8. In selected tubes, RT-PCR was conducted without the addition of reverse transcriptase to ensure the absence of DNA contamination. An aliquot of the RT-PCR product was analyzed on a 1.5% agarose gel. As an internal control, primers 5 and 6 (Table S1) were used to amplify tick β-actin mRNA (GenBank/EMBL/DDBJ accession no. AF426178).
For quantitative analysis of Bb loads in mouse tissues, real-time PCR was performed to detect Bb flaB. To accomplish this, total DNA was isolated from the skin, hearts, or joints of infected mice using the DNAeasy kit (QIAGEN). 70-bp flaB amplicons were quantitated using oligonucleotide primers 9 and 10 and a labeled probe (primer 11). The DNA samples were then subjected to serial 10-fold dilutions added to a 50-μl reaction mixture containing 0.2 μM of primers and probe. PlatinumTaq (Invitrogen) in the presence of 2 mM MgSO4 was used to perform the thermal cycling as follows: 95°C for 4 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. Standard curves for flaB were prepared using 10-fold serial dilutions of known quantities (10 ng to 0.1 fg) of Bb DNA or the plasmid pCR 2.1–β-actin. Equal amounts of mouse DNA were then used for measuring levels of Bb flaB.
Antibodies, Antisera, and Protein Analyses.
Monoclonal antibodies (14D2–27 and 8H3–33) directed against OspA and FlaB, respectively, were reported previously (30). Rat polyclonal antisera directed against OspB was described previously (31). SDS-PAGE and immunoblotting were performed as reported (30).
Online Supplemental Material.
Table S1 (available at http://www.jem.org/cgi/content/full/jem.20031960/DC1) shows oligonucleotide primers used in this study.
Results and Discussion
Generation of the OspA/B-deficient Mutant.
ospA and its downstream operonic partner ospB are encoded on the 54-kb linear plasmid (lp54) (25). OspA and OspB are 53% identical and 63% similar, suggesting that these two lipoproteins arose from a gene duplication event. OspB also has the same temporal and topological (membrane) expression pattern as OspA (32), and thus OspA and OspB likely perform similar, if not identical, physiological function(s). Therefore, to ensure that a particular borrelial phenotype could be attributed to a complete loss of function, our strategy for creating an ospA-deficient mutant of Bb included inactivating the entire ospAB operon. The generation of such ospAB mutants appeared feasible given that “escape” mutants lacking the ospAB genes have been isolated from high passage, noninfectious strains of Bb after treatment with OspA or OspB bactericidal antibodies (33, 34).
To create an ospAB mutant from the infectious 297 strain of Bb, we first constructed a suicide vector containing the ospAB operon disrupted by insertion of the Strepr marker, aadA, into ospAB (26) (Fig. 1 A). Because it has been noted that noninfectious “escape” mutants of Bb lacking OspA/B expression (isolated after antibody selection in vitro) aggregate and have reduced plating efficiency on semisolid medium (35), we modified the established protocol for Bb transformation (36) by first diluting the transformation mixture in BSK-H liquid medium and then aliquoting it into 96-well tissue culture plates. Strepr cultures arising from individual clones were readily obtained in these wells (indicated by color change of the medium). Subsequent plating revealed that, in comparison with wild-type 297, these clones were severely impaired for growth on semisolid pBSK agar medium, a condition that likely contributed to past failures to isolate OspA-deficient mutants of infectious strains of Bb. Of note, the method of plating the transformation mixture into 96-well plates has increased our efficiency in obtaining many other types of Bb mutants. Further analyses of the Strepr clones indicated that they contained the appropriate aadA marker insertion within ospAB (Fig. 1 B) and did not produce either OspA or OspB (Fig. 2, A and B, lanes 2). Because infectious Bb tends to lose its endogenous plasmids upon in vitro cultivation and genetic manipulation, we screened each clone by PCR for plasmid content. Most clones had plasmid profiles identical to parental (wild-type) 297, although some lacked cp9 (nonessential). One clone containing the full complement of plasmids was chosen for further study.
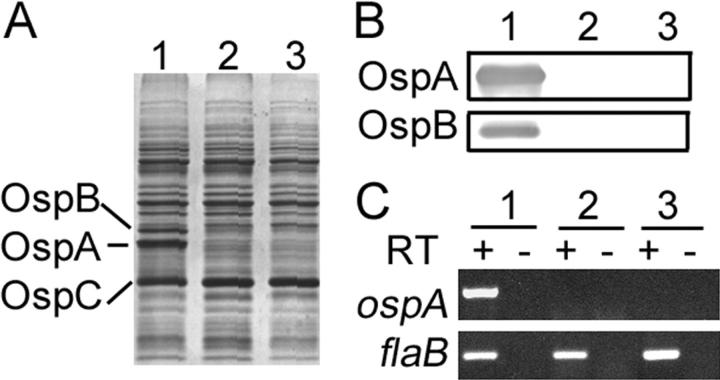
Figure 2.
Characterization of the OspA/B− mutant of Bb. (A) SDS-PAGE, (B) immunoblot, and (C) RT-PCR analyses of wild-type 297 (lanes 1), OspA/B− mutant (lanes 2), and the OspA/B− mutant recovered from mice needle inoculated with the OspA/B− mutant (lanes 3). + and −, with or without reverse transcriptase (RT), respectively. flaB transcription was assessed as an internal control.
Influence of OspA/B Deficiency on Bb Infectivity for Mice.
To examine a potential role for OspA/B in the infectious phenotype of Bb, groups of five C3H/HeN mice were inoculated intradermally with varying numbers (105, 104, 103, or 102) of either the OspA/B-deficient mutant (OspA/B−) or wild-type 297. 2 wk after injection, ear punch biopsies were harvested and cultured in BSK-H medium (absent antibiotic selection). Both wild-type 297 and OspA/B− spirochetes were readily observed in all cultures of ear punch biopsies, indicating that wild-type 297 and the mutant had similar infectious phenotypes. Further analyses indicated that the OspA/B− mutant maintained its mutant genotype and phenotype after recovery from mice (Fig. 2 A–C, lanes 3). To further assess tissue dissemination by the OspA/B− mutant, we next used quantitative PCR for flaB as a surrogate indicator of spirochete loads in mouse skin, joints, and hearts. Mice infected with the OspA/B− mutant had similar (skin and heart) or even higher (joint) levels of spirochetes when compared with mice infected by wild-type 297 (Fig. 3 A). Further analysis of joint swelling and histopathology (29) revealed that the OspA/B− mutant elicited arthritis more pronounced than that induced by wild-type 297 (Fig. 3 B), perhaps as a consequence of the higher numbers of spirochetes detected in joints (Fig. 3 A) (37). Nonetheless, the combined data indicated that OspA/B function is not essential for Bb infection, replication, dissemination, or pathogenesis in the murine model of Lyme borreliosis.
Figure 3.
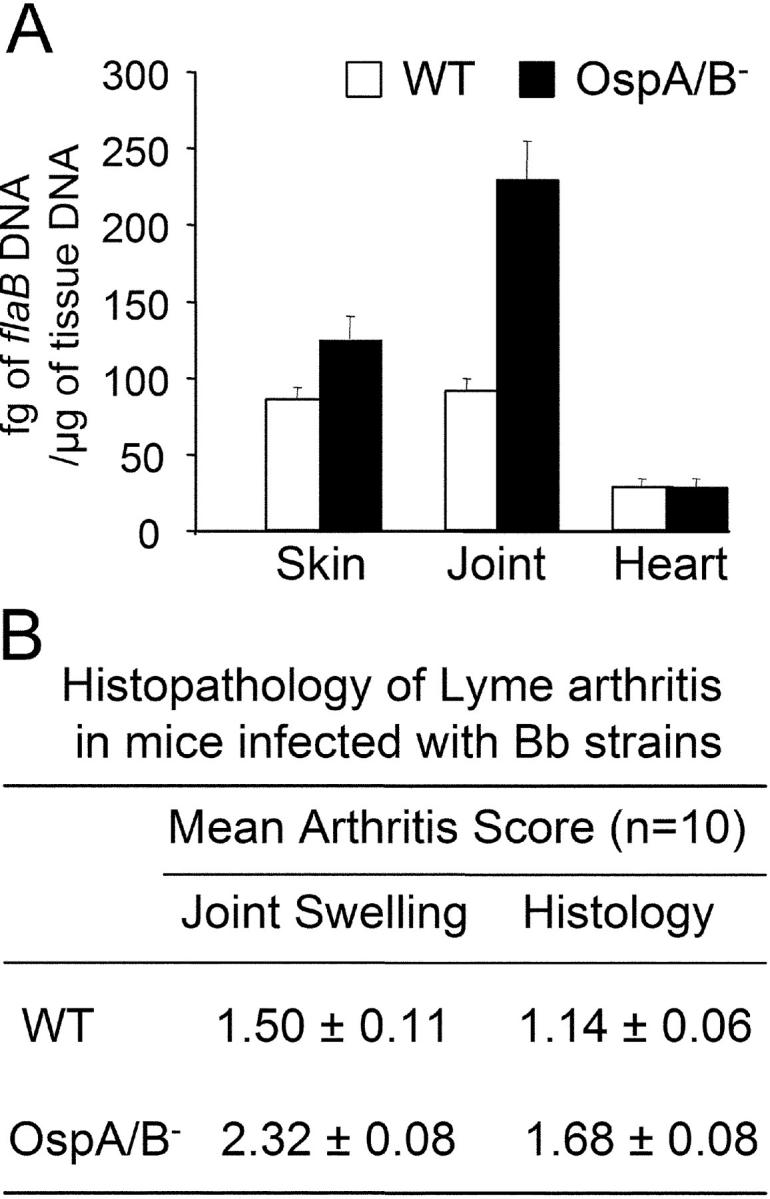
Characterization of the OspA/B− mutant in the murine model of Lyme borreliosis. (A) Quantitative PCR assessment of spirochete loads in mouse tissues 3 wk postinfection; units on the y-axis are fg of flaB DNA per μg of mouse tissue DNA. (B) Quantitative assessment of arthritis in infected mice. Arthritis was evaluated on a scale of 0–3 (absent, mild, moderate, or severe) for joint swelling and exudation of fibrin and leukocytes into joint spaces, synovial thickening due to proliferation, and hypertrophy of synovial cells. Both tibiotarsal and knee joints were examined in each animal.
It is well established that Bb down-regulates the expression of OspA and OspB during its transmission into mammalian hosts (7, 8). This observation has given rise to the hypothesis that OspA/B function may not be required for the early event(s) of mammalian infection. Our data provide the first direct evidence that OspA/B is not essential for either early stage mammalian infection or pathogenicity by Bb. However, our results do not exclude a role for OspA in the pathogenesis of late stage Lyme disease. In this regard, it has been shown that patients with late stage Lyme disease have detectable levels of serum OspA antibodies (38), suggesting that OspA may be expressed by Bb late in the course of infection. Further studies have revealed that an immunodominant T cell epitope of OspA is homologous to a sequence within human leukocyte function–associated antigen-1, implying that autoimmunity induced by OspA may, in part, drive the clinical sequelae of chronic (treatment-resistant) Lyme arthritis (9). Because there is no murine model of chronic Lyme arthritis, the possible contribution of OspA to the development of chronic Lyme arthritis could not be addressed in our studies but warrants further investigation.
Influence of OspA/B Deficiency on Bb's Ability to Colonize Ticks.
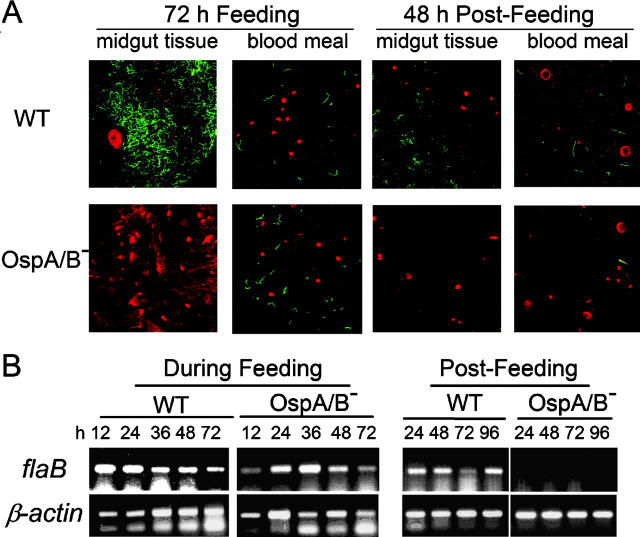
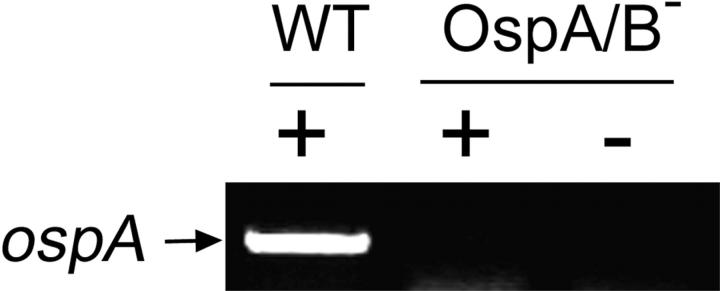
The availability of the OspA/B-deficient mutant of infectious Bb made it possible to assess directly the influence of the OspA/B deficiency on the natural transmission of Bb from infected mice to I. scapularis ticks. To accomplish this, sterile nymphs were allowed to feed on mice 3 wk after infection (needle inoculation) with either wild-type 297 or the OspA/B− mutant (same group of mice used in Fig. 3). Nymphs were forcibly removed from mice every 12 h during the feeding phase (generally lasting 72–96 h). After the feeding phase, naturally detached ticks were collected at 24-h intervals for an additional 96-h postfeeding. Tick midgut luminal contents (including the blood meal) as well as midgut tissues washed free of luminal contents were isolated and subjected to immunofluorescence confocal microscopy. In ticks that fed on wild-type spirochete-infected mice, bacteria were first detected in tick midgut tissue samples at 48 h of tick feeding. At 72 h of tick feeding, striking numbers of wild-type spirochetes were associated with tick midgut tissues (Fig. 4 A). In contrast, spirochetes were not observed in midgut tissue samples of ticks that fed on OspA/B−-infected mice (Fig. 4 A), suggesting that the mutant was defective in tick midgut colonization. This defect could not be attributed to a deficiency in transmission from the mouse to the tick, inasmuch as the OspA/B− mutant was readily detected in the blood meal at levels comparable to those of wild-type 297 (Fig. 4 A); this finding was supported by RT-PCR (for flaB transcripts) of total RNA from parallel groups of ticks (Fig. 4 B). Furthermore, RT-PCR confirmed that ospA transcripts were not detectable from ticks fed on OspA/B−-infected mice (Fig. 5). In the postfeeding phase, wild-type spirochetes, but not the OspA/B− mutant, again were found associated with tick midgut tissues (Fig. 4 A). However, in contrast to blood meal results obtained from the feeding ticks, OspA/B− spirochetes were not detected in the blood meals of postfeeding ticks (Fig. 4 A). Additional RT-PCR analyses corroborated this finding (Fig. 4 B). Thus, the combined findings of Fig. 4, A and B, suggest that although the OspA/B− mutant was transmissible from mouse tissues to tick midguts, it failed to colonize tick midgut tissue. Furthermore, a particularly surprising finding was the inability of the OspA/B− mutant to survive (even at 48 h postfeeding) within tick midguts, a key niche for Bb in nature.
Figure 4.
Detection of wild-type or OspA/B− spirochetes in ticks by (A) confocal microscopy or (B) RT-PCR. I. scapularis nymphs feeding on mice infected previously with either wild-type 297 (WT) or the OspA/B− mutant were removed from mice every 12 h; after this feeding phase, naturally detached ticks were collected at 24-h intervals. Tick midgut luminal contents (including the blood meal) and midgut tissues washed free of luminal contents were then isolated. Red denotes tick tissues or blood cells; green indicates spirochetes. Amplicons of flaB transcripts were used to signify the presence of spirochetes; tick β-actin was used as an internal control for RT-PCR.
Figure 5.
RT-PCR analysis of ospA from Bb in ticks after 72 h of tick feeding. RNAs were exacted from ticks forcibly removed from mice infected with either wild-type Bb (WT) or the OspA/B− mutant. + and −, the presence or absence, respectively, of reverse transcriptase in the RT-PCR reactions.
That the OspA/B− mutant was defective in tick midgut tissue colonization could have been due to suboptimal levels of mutant spirochetes transmitted to the tick gut or a general inability of the mutant to survive for any significant period of time in ticks. To address these possibilities, as opposed to using the more conventional capillary feeding (39), we chose a direct microinjection approach that could be more easily controlled. With this new method, it is possible to deliver an equal number of mutant or wild-type spirochetes with 100% efficiency into sterile nymph midguts by direct microinjection into tick rectums. A relatively large number (104) of spirochetes per tick also can be introduced, such that colonization could be visualized at a time point (24 h) earlier than was possible via natural tick feeding (72 h) and that presumably preceded loss of spirochete viability. Microinjected nymphs then were allowed to feed on naive mice. At 24 h of tick feeding, spirochetes in the blood meals of ticks harboring either wild-type 297 or the OspA/B− mutant were abundant and comparable, whereas wild-type spirochetes but not the OspA/B− mutant were found associated with tick midgut tissues (see Fig. 7). In addition, ticks containing wild-type 297 were able to transmit their spirochetes to uninfected mice but ticks harboring the OspA/B− mutant were not. We conclude from these data that the OspA/B− mutant cannot colonize and survive within tick midgut tissues and therefore cannot complete the transmission cycle.
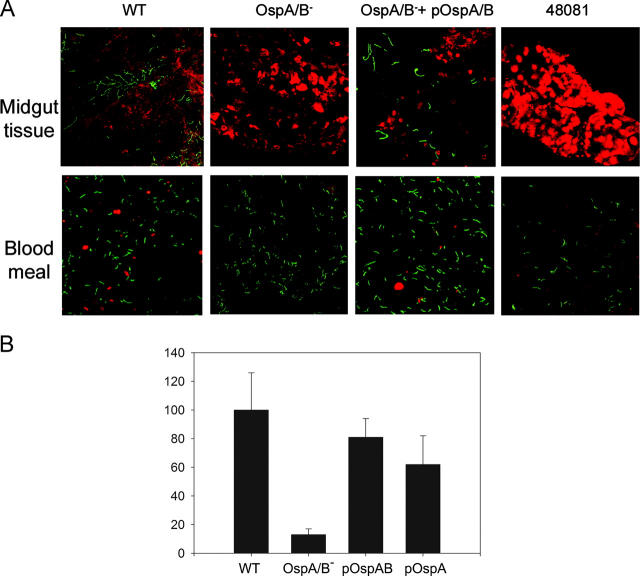
Figure 7.
Detection of spirochetes in I. scapularis nymphs microinjected with either wild-type 297 (WT), the OspA/B− mutant, the OspA/B− mutant complemented with ospAB or ospA, or strain 48081 (lacking lp54 encoding the ospAB operon). (A) Confocal immunofluorescence microscopy. Microinjected ticks were allowed to feed on naive mice; samples collected at 24 h after the onset of feeding were prepared as described in Fig. 4 legend. Red denotes tick tissues or blood cells; green indicates spirochetes. (B) Quantitative assessment (relative Bb number per microscope field) of data shown in A. pOspA/B, the OspA/B− mutant complemented with the ospAB genes. pOspA, the OspA/B− mutant complemented with ospA. Binding of wild-type (WT) Bb was considered as the 100% value.
To confirm in the context of “molecular” Koch's postulates (23) that the loss of ospAB expression accounted for the inability of the OspA/B− mutant to colonize tick midgut tissues, we constructed a strain of the mutant complemented in trans with a wild-type copy of the ospAB operon on a shuttle plasmid (Fig. 6 A). Expression of OspA and OspB was readily restored in this complemented strain (OspA/B− + pOspA/B) (Fig. 6, B and C). Again taking advantage of the tick microinjection approach, we observed that in contrast to the OspA/B− mutant, the transcomplemented strain readily colonized tick midgut tissues (Fig. 7, A and B). To examine whether the ospA gene alone (i.e., in the absence of ospB) could complement the colonization defect, the mutant also was transformed with a shuttle plasmid harboring a wild-type copy of ospA. As shown in Fig. 7 B, ospA function also was able to restore the midgut colonization phenotype to the OspA/B− mutant.
Of additional potential relevance to our study, naturally occurring Borrelia variants, ostensibly Bb that lack lp54 encoding the ospAB operon, occasionally have been isolated from unfed and feeding I. scapularis or I. dentatus ticks (24). The existence in nature of such OspA/B− variants of Borrelia seemingly contradicts our findings that OspA/B function is vital for Bb colonization and survival in ticks in the wild. To assess the behavior of such an OspA/B− variant in ticks, strain 48081 (lacking lp54) (24) also was microinjected into nymphs. Like our OspA/B− mutant, strain 48081 was readily detected within the blood meal but was unable to colonize tick midgut tissue (Fig. 7). The paradox that strain 48081 was isolated from wild ticks (24) but was unable to colonize ticks in our experiments may be explained by the fact that such isolates were obtained after growth in semisolid medium, a method prone to inducing plasmid loss in Bb. Moreover, such isolates are unable to infect laboratory mice (24), calling into question their relevance overall as natural agents of Lyme borreliosis.
Summary and Implications.
The complex enzootic life cycle of Bb can be envisioned to occur in distinct substages. Upon first feeding of sterile tick larvae on Bb-infected (white-footed) mice, spirochetes colonize the tick midgut. A resident Bb population persists there as the larval forms molt into nymphs. Infected nymphs then take a second blood meal in the Spring of the following year, whereupon Bb replicates, emigrates from the midgut to the salivary glands, and then is deposited into the intended (mice, deer) or unintended (human) host (2). Our data demonstrate an essential role for OspA/B in tick colonization by Bb. Furthermore, results from genetic complementation studies (Fig. 7 B) suggest that adherence and colonization of Bb to tick midgut tissue is mediated largely, if not solely, by OspA. Our additional surprising finding that the OspA/B− mutant was unable to survive in tick midguts suggests that adherence to tick midgut cells also is crucial for some as yet unknown aspect(s) of spirochete survival. Nonetheless, the blockage in attachment of Bb to tick midgut tissue and subsequent loss of spirochete viability effectively obstructs all subsequent downstream transmission events, thereby disrupting the entire enzootic transmission cycle of Bb.
Acknowledgments
We thank Philip E. Stewart and Patricia Rosa for providing pBSV2, D. Scott Samuels for providing the aadA marker, John Anderson for providing strain 48081, Debby Beck for technical assistance, and Eric J. Hansen and Kevin McIver for critical reviews of the manuscript.
This work was supported by National Institutes of Health grants AI-45538 (to M.V. Norgard), AI-32947 and AI-49200 (to E. Fikrig), and R03-AR-47640 (U. Pal), and by a grant (Advanced Technology Program) (to M.V. Norgard) from the Texas Higher Education Coordinating Board.
The online version of this article includes supplemental material.
X.F. Yang and U. Pal contributed equally to this work.
Abbreviations used in this paper: Bb, Borrelia burgdorferi; OspA, outer surface (lipo)protein A; OspB, outer surface (lipo)protein B; Strep, streptomycin; Strepr, streptomycin resistance.
References
- 1.Steere, A.C. 2001. Lyme disease. N. Engl. J. Med. 345:115–125. [DOI] [PubMed] [Google Scholar]
- 2.Hayes, E.B., and J. Piesman. 2003. How can we prevent Lyme disease? N. Engl. J. Med. 348:2424–2430. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorfer, W., A.G. Barbour, S.F. Hayes, J.L. Benach, E. Grunwaldt, and J.P. Davis. 1982. Lyme disease, a tick-borne spirochetosis? Science. 216:1317–1319. [DOI] [PubMed] [Google Scholar]
- 4.Philipp, M.T. 1998. Studies on OspA: a source of new paradigms in Lyme disease research. Trends Microbiol. 6:44–47. [DOI] [PubMed] [Google Scholar]
- 5.Steere, A.C., V.K. Sikand, F. Meurice, D.L. Parenti, E. Fikrig, R.T. Schoen, J. Nowakowski, C.H. Schmid, S. Laukamp, C. Buscarino, D.S. Krause, and The Lyme Disease Vaccine Study Group. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 6.Schwan, T.G., J. Piesman, W.T. Golde, M.C. Dolan, and P.A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA. 92:2909–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery, R.R., S.E. Malawista, K.J.M. Feen, and L.K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva, A.M., S.R. Telford, L.R. Brunet, S.W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross, D.M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z.A. Nagy, J.A. Field, A.C. Steere, and B.T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 281:703–706. [DOI] [PubMed] [Google Scholar]
- 10.Li, H., J.J. Dunn, B.J. Luft, and C.L. Lawson. 1997. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc. Natl. Acad. Sci. USA. 94:3584–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwan, T.G. 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem. Soc. Trans. 31:108–112. [DOI] [PubMed] [Google Scholar]
- 12.Schwan, T.G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal, U., A.M. de Silva, R.R. Montgomery, D. Fish, J. Anguita, J.F. Anderson, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Invest. 106:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal, U., R.R. Montgomery, D. Lusitani, P. Voet, V. Weynants, S.E. Malawista, Y. Lobet, and E. Fikrig. 2001. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J. Immunol. 166:7398–7403. [DOI] [PubMed] [Google Scholar]
- 15.Cabello, F.C., M.L. Sartakova, and E.Y. Dobrikova. 2001. Genetic manipulation of spirochetes—light at the end of the tunnel. Trends Microbiol. 9:245–248. [DOI] [PubMed] [Google Scholar]
- 16.Tilly, K., A.F. Elias, J.L. Bono, P. Stewart, and P. Rosa. 2000. DNA exchange and insertional inactivation in spirochetes. J. Mol. Microbiol. Biotechnol. 2:433–442. [PubMed] [Google Scholar]
- 17.Sartakova, M., E. Dobrikova, and F.C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA. 97:4850–4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubner, A., X. Yang, D.M. Nolen, T.G. Popova, F.C. Cabello, and M.V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA. 98:12724–12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggers, C.H., M.J. Caimano, M.L. Clawson, W.G. Miller, D.S. Samuels, and J.D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281–295. [DOI] [PubMed] [Google Scholar]
- 20.Hubner, A., A.T. Revel, D.M. Nolen, K.E. Hagman, and M.V. Norgard. 2003. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect. Immun. 71:2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purser, J.E., M.B. Lawrenz, M.J. Caimano, J.K. Howell, J.D. Radolf, and S.J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753–764. [DOI] [PubMed] [Google Scholar]
- 22.Stewart, P.E., R. Thalken, J.L. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714–721. [DOI] [PubMed] [Google Scholar]
- 23.Falkow, S. 1988. Molecular Koch's postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10:S274–S276. [DOI] [PubMed] [Google Scholar]
- 24.Anderson, J.F., R.A. Flavell, L.A. Magnarelli, S.W. Barthold, F.S. Kantor, R. Wallich, D.H. Persing, D. Mathiesen, and E. Fikrig. 1996. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J. Clin. Microbiol. 34:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser, C.M., S. Casjens, W.M. Huang, G.G. Sutton, R. Clayton, R. Lathigra, O. White, K.A. Ketchum, R. Dodson, E.K. Hickey, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 390:580–586. [DOI] [PubMed] [Google Scholar]
- 26.Frank, K.L., S.F. Bundle, M.E. Kresge, C.H. Eggers, and D.S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purser, J.E., and S.J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA. 97:13865–13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labandeira-Rey, M., and J.T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthold, S.W., D.S. Beck, G.M. Hansen, A.A. Terwilliger, and K.D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133–138. [DOI] [PubMed] [Google Scholar]
- 30.Yang, X., T.G. Popova, K.E. Hagman, S.K. Wikel, G.B. Schoeler, M.J. Caimano, J.D. Radolf, and M.V. Norgard. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radolf, J.D., K.W. Bourell, D.R. Akins, J.S. Brusca, and M.V. Norgard. 1994. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J. Bacteriol. 176:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox, D.L., D.R. Akins, K.W. Bourell, P. Lahdenne, M.V. Norgard, and J.D. Radolf. 1996. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc. Natl. Acad. Sci. USA. 93:7973–7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman, J.L., R.C. Rogers, and J.L. Benach. 1992. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect. Immun. 60:3098–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadziene, A., P.A. Rosa, P.A. Thompson, D.M. Hogan, and A.G. Barbour. 1992. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J. Exp. Med. 176:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadziene, A., D.D. Thomas, and A.G. Barbour. 1995. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect. Immun. 63:1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuels, D.S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods in Molecular Biology. J.A. Nickoloff, editor. Humana Press, Totowa, NJ. 253–259. [DOI] [PMC free article] [PubMed]
- 37.Ma, Y., K.P. Seiler, E.J. Eichwald, J.H. Weis, C. Teuscher, and J.J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalish, R.A., J.M. Leong, and A.C. Steere. 1995. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect. Immun. 63:2228–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broadwater, A.H., D.E. Sonenshine, W.L. Hynes, S. Ceraul, and A.M. de Silva. 2002. Glass capillary tube feeding: a method for infecting nymphal Ixodes scapularis (Acari: Ixodidae) with the Lyme disease spirochete Borrelia burgdorferi. J. Med. Entomol. 39:285–292. [DOI] [PubMed] [Google Scholar]