Abstract
The immune response to infection must be controlled to ensure it is optimal for defense while avoiding the consequences of excessive inflammation, which include fatal septic shock. Mice deficient in FcγRIIb, an inhibitory immunoglobulin G Fc receptor, have enhanced immune responses. Therefore, we examined whether FcγRIIb controls the response to Streptococcus pneumoniae. Macrophages from FcγRIIb-deficient mice showed increased antibody-dependent phagocytosis of pneumococci in vitro, and consistent with this infected FcγRIIb-deficient mice demonstrated increased bacterial clearance and survival. In contrast, previously immunized FcγRIIb-deficient mice challenged with large inocula showed reduced survival. This correlated with increased production of the sepsis-associated cytokines tumor necrosis factor α and interleukin 6. We propose that FcγRIIb controls the balance between efficient pathogen clearance and the cytokine-mediated consequences of sepsis, with potential therapeutic implications.
Keywords: FcγRIIb, Streptococcus pneumoniae, septic shock, tumor necrosis factor, interleukin 6
Introduction
The outcome of the immune response to infection is determined, in part, by the degree of inflammation it generates. Inflammatory cytokines, produced by cells including macrophages and neutrophils, are important in generating an effective primary immune response and in clearing infection (1, 2). However, in severe infection, the production of proinflammatory cytokines, such as TNF-α, IL-1, and IL-6, causes septic shock, which has a mortality of >50% even when appropriate antibiotic therapy is administered (3). The immune system must, therefore, closely regulate the inflammatory response to infection to optimize beneficial effects and minimize harmful ones. Inhibitory receptors have been shown to control aspects of immune reactivity and the development of autoimmunity (4, 5), but their role in controlling inflammation and the outcome of infection has not been extensively studied. FcγRIIb (CD32) is a candidate to control this vital balance. It is an IgG Fc receptor expressed on immune cells that inhibits activation by the B cell receptor and activatory FcRs (4). FcγRIIb-deficient mice have increased antibody responses, cytokine production, macrophage activation (6, 7), and immune-mediated pathology including spontaneous SLE (4). Streptococcus pneumoniae is an encapsulated Gram-positive organism that is a major cause of human disease, particularly pneumonia, peritonitis, and meningitis (8). It is also a pathogen of mice and has been extensively studied in this context. Defense against S. pneumoniae is dependent on antibody (9) and FcR-mediated clearance (10), making it an appropriate organism with which to study the control of responses to infection by FcγRIIb. Therefore, we examined whether FcγRIIb controlled the balance between defense and septic shock in the response to S. pneumoniae. FcγRIIb-deficient mice showed increased phagocytosis of pneumococci by macrophages in vitro and increased bacterial clearance and survival in vivo. However, previously immunized FcγRIIb-deficient mice challenged with large inocula showed reduced survival. This correlated with increased production of the sepsis-associated cytokines TNF-α and IL-6. Thus, FcγRIIb controls the balance between efficient pathogen clearance and the cytokine-mediated consequences of sepsis.
Materials and Methods
Mice.
FcγRII-deficient mice on BALB/c and C57BL/6 backgrounds (backcrossed for at least eight generations) were provided by J. Ravetch and S. Bolland (Rockefeller University, New York, NY). All other mice were obtained from Charles River Laboratories.
Antibodies.
M1/70 (anti–MAC-1) antibody and avidin–Texas red conjugate were purchased from BD Biosciences. Horseradish peroxidase–conjugated goat anti–mouse IgM, IgG, and IgG3 were obtained from Southern Biotechnology Associates, Inc.
Microscopy.
Peritoneal macrophages were incubated for 2 h in serum-free RPMI on 1% Alcian blue–coated coverslips. Immunofluorescence confocal microscopy (TCS 4D; Leica) was performed after staining with M1/70 and 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes) to identify macrophages and nuclei, respectively.
Bacteria.
S. pneumoniae type 2 strain D39 (provided by J.S. Brown, Imperial College School of Medicine, London, UK) or type 14 (provided by D. Goldblatt, University College Hospital, London, UK) were cultured overnight on blood agar plates (5% CO2, 95% air, 37°C), inoculated into Todd-Hewitt broth (Oxoid Ltd.) supplemented with 0.5% yeast extract (Oxoid Ltd.), cultured for 4–5 h, and then washed and resuspended at 109 CFU/ml (estimated by OD660 = 1). Aliquots were stored at −70°C and made up in sterile PBS for use. Their concentration was verified by serial dilution and culture on blood agar plates.
S. pneumoniae Peritonitis.
Groups of 7–13 male or female, age-matched control and FcγRII-deficient mice (8–16 wk of age) were inoculated i.p. with 200 μl PBS containing S. pneumoniae. Mice were observed at least every 4 h for the first 72 h, every 8 h until 96 h, and daily thereafter (11). During observation, mice were scored by a blinded observer for the presence or absence of physical signs of progressive sepsis (12). Mice that became moribund were considered to have reached the end point of the experiment and were killed. Tail bleeds were performed at 7 and/or 24 h after infection and blood was cultured for bacterial growth for 24 h and assayed for cytokines. Survival data was analyzed using Kaplan-Meier graphs and log-rank tests. All animal experiments were performed in accordance with Home Office regulations.
Antibody Responses.
Control and FcγRIIb-deficient mice were immunized with 1 μg Pneumovax II (Aventis Pasteur MSD) s.c. diluted in 200 μl sterile PBS. Serum was collected at 14 and 21 d.
ELISA Assays.
Anti-phosphorylcholine (PC) and anti-pneumococcal polysaccharide antibodies were measured by ELISA as described in Supplemental Materials and Methods, available at http://www.jem.org/cgi/content/full/jem.20032197/DC1.
Phagocytosis Assay.
S. pneumoniae type 14 was cultured to log phase in Todd-Hewitt broth with 0.5% yeast extract (Oxoid Ltd.), heat inactivated at 60°C for 1 h, and labeled with FITC (Sigma-Aldrich; reference 13). FITC-labeled S. pneumoniae were incubated in PBS or dilutions of heat-inactivated serum at 37°C for 1 h before washing. Immune serum used for opsonization was taken from five pneumovax-immunized mice 21 d after challenge. Peritoneal macrophages or RAW-297 cells were adhered to plastic and aliquots of serum-opsonized and nonopsonized FITC-labeled pneumococci were added at 37°C for 30 min (along with 4°C control) plates. Adhered macrophages were then washed, harvested, and analyzed by flow cytometry (FACSCalibur™; Becton Dickinson). Peritoneal macrophages were identified by scatter characteristics and MAC-1 staining. The percentage of FITC+ macrophages and the geometric mean fluorescence of FITC+ macrophages were used as a measure of phagocytosis. Duplicate or triplicate wells were processed for each serum sample and results were compared using the Student's t test.
Cytokine Quantification.
TNF-α and IL-6 levels in serum and macrophage culture supernatant were measured using Cytometric Bead Array (BD Biosciences) according to the manufacturer's instructions (see Supplemental Materials and Methods).
Online Supplemental Material.
Supplemental Materials and Methods describes ELISA assays and cytokine quantification. Fig. S1 shows natural anti-PC antibody titres in FcγRIIb−/− and control mice and Fig. S2 shows a phagocytosis assay. Fig. S3 illustrates signs of sickness in infected FcγRIIb−/− and control mice. Supplemental Materials and Methods and Figs. S1–S3 are available at http://www.jem.org/cgi/content/full/jem.20032197/DC1.
Results and Discussion
Normal Natural Anti-PC Antibody Titres, but Increased Antibody Responses to Vaccination, in FcγRIIb-deficient Mice.
Natural antibody against bacterial cell wall PC is critical for defense against pneumococcal infection in naive mice (9). We found similar titres of anti-PC IgM and IgG in FcγRIIb-deficient mice and controls (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20032197/DC1), and no significant differences in the B1 or marginal zone B cell populations (unpublished data), thought to be the major sources of such antibody (14). Vaccination raises protective antibodies against capsular polysaccharide antigens (8). When immunized with the T-independent anti-pneumococcal vaccine Pneumovax II, FcγRIIb-deficient mice produced increased titres of such antibodies (Fig. 1 A), as seen by others using model T–independent antigens (6) and T-dependent pneumococcal vaccines (15).
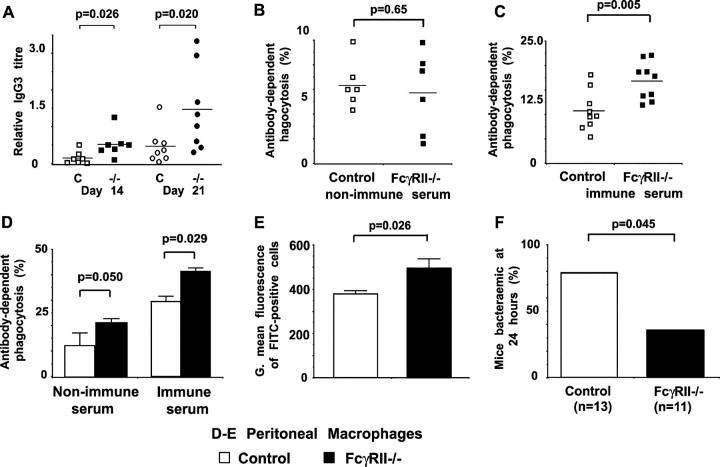
Figure 1.
Anti-pneumococcal antibody production and phagocytosis in FcγRIIb-deficient mice. (A) Anti-pneumococcal polysaccharide IgG3 titres in control BALB/c (□) and FcγRIIb−/− (▪) mice 14 and 21 d after immunization with 1 μg Pneumovax II. Each point represents data from an individual mouse expressed relative to a positive control. The horizontal bar is the mean. (B–E) The effect of FcγRIIb on the phagocytosis of S. pneumoniae in vitro. The RAW-297 macrophage cell line (B and C) or peritoneal macrophages (D and E) were incubated with FITC-labeled S. pneumoniae opsonized with heat-inactivated serum, followed by flow cytometric analysis. Antibody-dependent phagocytosis is expressed as percent FITC+ cells relative to nonopsonized sample (see Fig. S2). (B) Serum from unimmunized control (□) and FcγRIIb−/− (▪) mice provides equivalent opsonization, whereas (C) serum from immunized FcγRIIb−/− mice enhances uptake. (D) FcγRIIb−/− peritoneal macrophages show increased phagocytosis of opsonized S. pneumoniae. Phagocytosis was assessed as above but using peritoneal macrophages from control BALB/c (□) and FcγRIIb−/− (▪) mice, and serum from control mice only. (E) Macrophages from FcγRIIb−/− mice phagocytose a larger number of bacteria per macrophage than control mice, estimated by the geometric mean fluorescence of FITC+ cells (see Fig. S2). (B–E) Values represent mean of triplicates, the experiments shown are representative of two, and p-values were obtained using an unpaired Student's t test. (F) 24 h after inoculation with S. pneumoniae, tail bleeds were performed on C57BL/6 control (n = 11) and FcγRIIb−/− (n = 13) mice and blood cultured for bacterial growth. Fewer FcγRIIb−/− mice were bacteremic (results from two experiments combined; Chi-square test).
Increased Serum-dependent Uptake of Pneumococci by FcγRIIb-deficient Macrophages.
We investigated the role of FcγRIIb in phagocytosis of pneumococci using an in vitro system in which FITC-conjugated pneumococci are fed to macrophages and analyzed by flow cytometry (Fig. S2, A–C, available at http://www.jem.org/cgi/content/full/jem.20032197/DC1). Macrophages were chosen because they are the major phagocytes in the uninflamed peritoneal cavity and we planned to use peritonitis as our model of pneumococcal infection. Opsonization of pneumococci with serum from nonimmunized FcγRIIb-deficient or control mice increased uptake equally (Fig. 1 B), consistent with their having similar titres of “natural” anti-PC antibody. Serum from FcγRIIb-deficient mice immunized with Pneumovax was a more efficient opsonogen (Fig. 1 C), consistent with the increased anti-PC antibodies they produce. Findings using the RAW mouse macrophage cell line were confirmed in BALB/c peritoneal macrophages (Fig. S2, D and E). Comparison of phagocytosis by control and FcγRIIb-deficient macrophages, keeping the opsonizing serum constant, showed that more macrophages from FcγRIIb-deficient mice consumed a larger number of opsonized pneumococci per cell more quickly than controls (Fig. 1, D and E, and Fig. S2 F). Thus, FcγRIIb dampens macrophage uptake of pneumococci both by an effect on the macrophage itself and by reducing opsonizing antibody titres after immunization (but not via natural antibody).
FcγRIIb-deficient Mice Are Resistant to Pneumococcal Peritonitis.
Increased antibody-dependent phagocytosis of pneumococci in vitro suggested that FcγRIIb-deficient mice might clear S. pneumoniae more efficiently after intraperitoneal infection. Mice of both the BALB/C and C57BL/6 backgrounds were used, the latter being more susceptible to pneumococcal infection (16). Less than 200 CFU did not cause death (unpublished data), but when 105 (C57Bl/6) or 107 (BALB/C) CFU were used a survival advantage for FcγRIIb-deficient mice was seen compared with controls. At higher doses all mice died (Fig. 2). Consistent with this, more efficient bacterial clearance was seen in FcγRIIb-deficient mice (Fig. 1 F). Supporting these findings is the recent observation that FcγRIIb deficiency protects against death from staphylococcal infection in unimmunized mice (17).
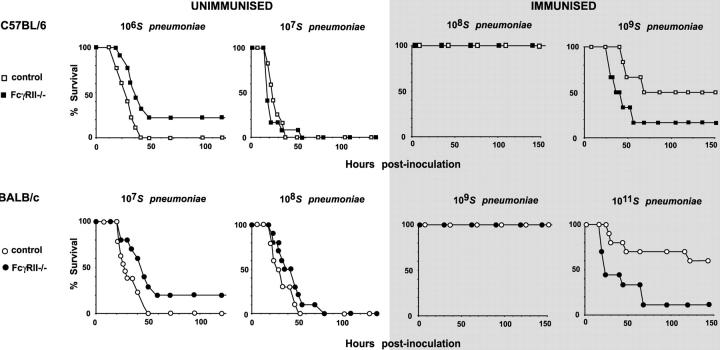
Figure 2.
Survival after S. pneumoniae infection. Unimmunized FcγRIIb−/− or control mice were inoculated with S. pneumoniae type 2 i.p. Both C57BL/6 (106 CFU) and BALB/c (107 CFU) FcγRIIb−/− mice have increased survival (P = 0.027 and P = 0.026, respectively). When unimmunized mice were challenged with higher doses of S. pneumoniae (107 in C57BL/6, 108 in BALB/c), both FcγRII−/− and control mice succumbed to infection. FcγRIIb−/− and control mice were immunized with 1 μg pneumococcal polysaccharide (Pneumovax II) s.c. and 21–28 d later mice were challenged with an intermediate dose of S. pneumoniae type 2 or a high dose of S. pneumoniae type 2 (gray shading). At intermediate doses, both strains were protected by immunization. However, at high doses of S. pneumoniae, both C57BL/6 and BALB/c FcγRIIb−/− mice showed increased mortality (P = 0.017 and P = 0.012, respectively). Each experiment shown is representative of at least two, and p-values were obtained with a log-rank test.
Immunized FcγRIIb-deficient Mice Infected with Higher Doses of Bacteria Have an Increased Mortality.
When immunized mice were infected with intermediate numbers of bacteria (107–109), both strains were equally protected and all survived (Fig. 2 C; reference 15). As immunized FcγRIIb-deficient mice showed both increased macrophage phagocytosis in vitro and higher titres of antipolysaccharide antibody we predicted that immunized mice might, if challenged with sufficient bacteria to overcome protection, again demonstrate enhanced survival. Against expectations, challenge of immunized mice with higher doses of bacteria resulted in increased death of FcγRIIb-deficient mice (Fig. 2 D, also with the low virulence serotype 14; unpublished data).
Increased Proinflammatory Cytokine Production and Signs of Sepsis in FcγRIIb-deficient Mice.
Most death from Gram-positive organisms is due not purely to the infection itself, but from the hypotension and end organ failure characteristic of septic shock. This is a clinical manifestation of the uncontrolled release of proinflammatory cytokines such as TNF-α and IL-6, which has a mortality of >50% (3, 18). Infusion of TNF-α alone can cause fatal septic shock at serum levels similar to those seen in animals with bacterial sepsis (18). As proinflammatory cytokines are released in response to FcR cross-linking and FcγRIIb can control such release (7), it seemed likely that the high degree of FcR cross-linking on macrophages that would occur when large doses of bacteria met high levels of anti-pneumococcal antibody could result in uncontrolled cytokine production and septic shock. Consistent with this, commonly accepted signs of illness (e.g., piloerection) occurred more quickly in infected immunized FcγRIIb-deficient mice, but not in infected naive mice (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20032197/DC1). This was independent of bacterial division, as similar results were obtained when mice were challenged with heat-killed bacteria (Fig S3). Thus, the increased mortality of immunized FcγRIIb-deficient mice could be due to the inflammatory response to sepsis itself.
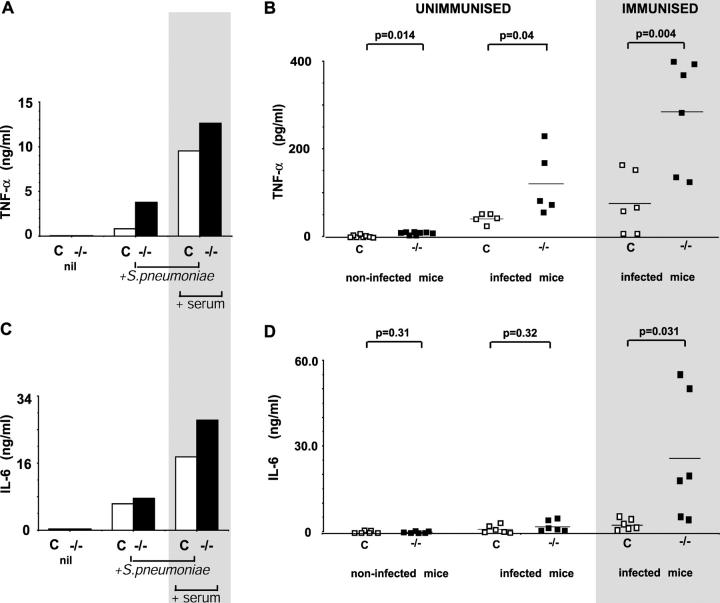
To test this we measured TNF-α and IL-6 production by FcγRIIb-deficient mice; TNF-α because it has been directly implicated in causing death (18) and IL-6 as its serum levels correlate best with mortality due to septic shock (19). Peritoneal macrophages from FcγRIIb-deficient mice produced similar levels of TNF-α to control when cultured alone, but enhanced production when unopsonized or opsonized pneumococci were added (Fig. 3 A). In vivo, in the absence of infection, levels of TNF-α were low but significantly higher in FcγRIIb-deficient mice, suggesting that FcγRIIb controls “basal” TNF-α production. Unimmunized FcγRIIb-deficient mice produced increased titres of TNF-α in response to infection. However, when previously immunized mice were challenged with high doses of pneumococcus, increases in TNF-α were particularly prominent (Fig. 3 B), reaching serum levels shown to be associated with fatal septic shock (20, 21). In vitro IL-6 production by macrophages showed a similar pattern to TNF-α (Fig. 3 C). In vivo, IL-6 levels were low in uninfected and infected unimmunized mice. No significant difference was seen between FcγRIIb-deficient and control mice. In contrast, IL-6 levels in infected, immunized FcγRIIb-deficient mice were markedly elevated to levels ∼10 times those seen in control mice (Fig. 3 D). Thus, both the cytokine pattern and clinical picture seen in infected, immunized mice are consistent with death being due to septic shock.
Figure 3.
Proinflammatory cytokine production in response to S. pneumoniae in control and FcγRIIb−/− mice. (A) Peritoneal macrophages from C57BL/6 and FcγRIIb−/− mice were cultured for 12 h alone, with unopsonized, heat-killed S. pneumoniae, or with heat-killed S. pneumoniae opsonized with heat-inactivated immune serum. TNF-α levels, measured using a cytometric bead assay, and were higher in FcγRIIb−/− culture supernatant in all conditions, but particularly when opsonized bacteria were used. The experiment shown is representative of two. (B) Serum TNF-α levels were higher in FcγRIIb−/− mice whether unimmunized (left), 7 h after inoculation with S. pneumoniae (middle), and in particular, in mice immunized and subsequently inoculated with S. pneumoniae (right, gray shading). (C) IL-6 levels in peritoneal macrophage culture supernatant. (D) Serum IL-6 levels in control (□) and FcγRIIb−/− (▪) mice, uninfected or 7 h after inoculation with S. pneumoniae either with (gray shading) or without prior immunization. Values are from individual mice, experiments shown are representative of two, and p-values were obtained using an unpaired Student's t test.
Unimmunized FcγRIIb-deficient mice demonstrated a clear survival advantage when challenged with pneumococcus, probably due primarily to increased FcR-dependent bacterial uptake by phagocytes freed of FcγRIIb-mediated suppression. The modest increase in proinflammatory cytokine production observed in unimmunized FcγRIIb-deficient mice, both before and after infection, may also be important, as these cytokines have been shown to be important in defense against pneumococcus (2). The other effects of FcγRIIb seem less likely to be involved. Natural anti-PC antibody levels were similar, and neither increased antigen presentation (22) nor antibody production (Fig. 1 A; reference) would have had time to have an effect.
There was a striking contrast when previously immunized mice were challenged with pneumococcus. In this situation FcγRIIb deficiency resulted in rapid and increased mortality. This was likely to be due to septic shock associated with excessive release of proinflammatory cytokines, serum levels of which reached those shown to cause death in models of sepsis in mice (20) and other species (21). Treatment of septic shock with anticytokine therapy has been disappointing, particularly in Gram-positive infection. Our attempts to use treatment with anti–TNF-α to reduce mortality in immunized infected mice met with similar failure (unpublished data), consistent with the conflicting effects seen in other Gram-positive models (23). A number of explanations for this have been put forward (24). Two seem particularly relevant and also underline the potential for manipulation of inhibitory receptors to provide novel therapeutic approaches in septic shock. First, treatment with anti–TNF-α increases mortality in naive mice infected with pneumococcus (1), and experiments in TNF and TNF receptor–deficient mice confirm that normal production of TNF-α is necessary for survival from pneumococcal infection (2). TNF-α blockade does not normalize TNF-α levels but abolishes them, and would therefore be expected to reduce the risk of septic shock but at the same time cause death from the infection itself. Second, although serum TNF-α levels seen in our in vivo experiments are similar to those causing death in other studies (0.1–10 ng/ml; references 20 and 21), it is clear that TNF-α does not act alone. Neutralization (or, rather, normalization) of a number of components of the “cytokine storm” seen in septic shock may well be required to improve prognosis. Inhibitory receptors can control a number of cytokines at once, making them attractive targets for novel therapeutic strategies addressing this important clinical condition. Manipulation of the expression or function of FcγRIIb or other inhibitory receptors may therefore normalize proinflammatory cytokines in a “global” fashion, offering a route to effective therapy in sepsis and other inflammatory conditions.
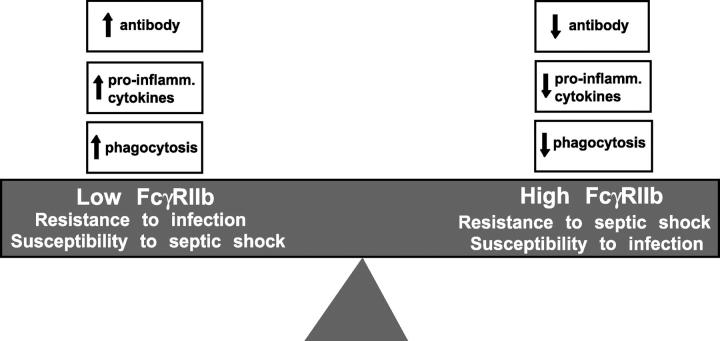
FcγRIIb has opposing effects on infection in different circumstances; damping down the immune response to pneumococci in naive mice, but preventing death from the inflammatory consequences of sepsis in immunized ones. The physiological role of FcγRIIb thus appears to be to help control the “inflammatory threshold,” balancing the inflammatory response to infection to optimize survival (Fig. 4). This role would explain the need for complex regulation of FcγRIIb expression and function on different cell types (25, 26). It could also result in evolutionary pressures underlying the distribution of SLE-associated FcγRIIb polymorphisms in humans (27, 28) and mice (29, 30). It remains to be seen if FcγRIIb controls other infections in the same way, or if other inhibitory receptors operate in an analogous fashion, although both seem likely. Understanding the exact role of inhibitory receptors in setting the inflammatory threshold in different infections, and understanding the mechanism by which these receptors are themselves controlled, is likely to be of significant therapeutic importance in treating both primary infection and septic shock.
Figure 4.
The physiological role of FcγRIIb appears to be to control cytokine release, antibody production, and phagocytosis to balance the inflammatory response to infection to optimize survival in different circumstances. Factors that determine the level of expression of FcγRIIb include the cytokine milieu and naturally occurring FcγRIIb promoter polymorphisms (references 29 and 30).
Acknowledgments
We would like to thank David Goldblatt, Jerry Brown, Nick Pritchard, Tony Cutler, and Liz Walker for technical advice and help, and Patrick Sissons and Alex Betz for critical comments on the manuscript. Dr. Jeff Ravetch and Silvia Bolland kindly provided the FcγRII−/− mice.
M.R. Clatworthy is funded by a Wellcome Trust Clinical Training Fellowship (065770) and The Sackler Fund. K.G.C. Smith is supported by a Wellcome Research Leave Award for Clinical Academics (grant 067543AIA) and the Medical Research Council (grant 9805187).
The online version of this article contains supplemental material.
References
- 1.Takashima, K., K. Tateda, T. Matsumoto, Y. Iizawa, M. Nakao, and K. Yamaguchi. 1997. Role of TNF alpha in pathogenesis of pneumococcal pneumonia in mice. Infect. Immun. 65:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellmer, A., J. Gerber, J. Ragheb, G. Zysk, T. Kunst, A. Smirnov, W. Bruck, and R. Nau. 2001. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 69:6881–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138–150. [DOI] [PubMed] [Google Scholar]
- 4.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 5.Pritchard, N.R., and K.G.C. Smith. 2003. B cell inhibitory receptors and autoimmunity. Immunology. 108:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai, T., M. Ono, M. Hikida, H. Ohmori, and J.V. Ravetch. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 379:346–349. [DOI] [PubMed] [Google Scholar]
- 7.Clynes, R., J.S. Maizes, R. Guinamard, M. Ono, T. Takai, and J.V. Ravetch. 1999. Modulation of immune complex–induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 189:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuorimaa, T., and H. Kayhty. 2002. Current state of pneumococcal vaccines. Scand. J. Immunol. 56:111–129. [DOI] [PubMed] [Google Scholar]
- 9.Briles, D.E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mold, C., B. Rodic-Polic, and T.W. Du Clos. 2002. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J. Immunol. 168:6375–6381. [DOI] [PubMed] [Google Scholar]
- 11.Brown, J.S., A.D. Ogunniyi, M.C. Woodrow, D.W. Holden, and J.C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.1994. Pain and distress in laboratory rodents and lagomorphs. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Pain and Distress accepted by the FELASA Board of Management November 1992. Lab. Anim. 28:97–112. [DOI] [PubMed]
- 13.Martinez, J.E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G.M. Carlone. 1999. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, F., A.M. Oliver, and J.F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 14:617–629. [DOI] [PubMed] [Google Scholar]
- 15.Saeland, E., J.H. Leusen, G. Vidarsson, W. Kuis, E.A. Sanders, I. Jonsdottir, and J.G. van de Winkel. 2003. Role of leukocyte immunoglobuin G receptors in vaccine-induced immunity to Streptococcus pneumoniae. J. Infect. Dis. 187:1686–1693. [DOI] [PubMed] [Google Scholar]
- 16.Gingles, N.A., J.E. Alexander, A. Kadioglu, P.W. Andrew, A. Kerr, T.J. Mitchell, E. Hopes, P. Denny, S. Brown, H.B. Jones, et al. 2001. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect. Immun. 69:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gjertsson, I., S. Kleinau, and A. Tarkowski. 2002. The impact of Fcgamma receptors on Staphylococcus aureus infection. Microb. Pathog. 33:145–152. [PubMed] [Google Scholar]
- 18.Tracey, K.J., B. Beutler, S.F. Lowry, J. Merryweather, S. Wolpe, I.W. Milsark, R.J. Hariri, T.J. Fahey III, A. Zentella, J.D. Albert, et al. 1986. Shock and tissue injury induced by recombinant human cachectin. Science. 234:470–474. [DOI] [PubMed] [Google Scholar]
- 19.Casey, L.C., R.A. Balk, and R.C. Bone. 1993. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann. Intern. Med. 119:771–778. [DOI] [PubMed] [Google Scholar]
- 20.Teti, G., G. Mancuso, F. Tomasello, and M.S. Chiofalo. 1992. Production of TNF-alpha and IL-6 in mice infected with group B streptococci. Circ. Shock. 38:138–144. [PubMed] [Google Scholar]
- 21.Suitters, A.J., R. Foulkes, S.M. Opal, J.E. Palardy, J.S. Emtage, M. Rolfe, S. Stephens, A. Morgan, A.R. Holt, L.C. Chaplin, et al. 1994. Differential effect of isotype on efficacy of anti–TNF-α chimeric antibodies in experimental septic shock. J. Exp. Med. 179:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minskoff, S.A., K. Matter, and I. Mellman. 1998. Fc gamma RII-B1 regulates the presentation of B cell receptor-bound antigens. J. Immunol. 161:2079–2083. [PubMed] [Google Scholar]
- 23.Wayte, J., A.T. Silva, T. Krausz, and J. Cohen. 1993. Observations on the role of TNF-alpha in a murine model of shock due to Streptococcus pyogenes. Crit. Care Med. 21:1207–1212. [DOI] [PubMed] [Google Scholar]
- 24.Abraham, E. 1999. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 25:556–566. [DOI] [PubMed] [Google Scholar]
- 25.Pricop, L., P. Redecha, J.L. Teillaud, J. Frey, W.H. Fridman, C. Sautes-Fridman, and J.E. Salmon. 2001. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 166:531–537. [DOI] [PubMed] [Google Scholar]
- 26.Rudge, E.U., A.J. Cutler, N.R. Pritchard, and K.G.C. Smith. 2002. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and FcγRII-mediated B cell suppression. J. Exp. Med. 195:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyogoku, C., H.M. Dijstelbloem, N. Tsuchiya, Y. Hatta, H. Kato, A. Yamaguchi, T. Fukazawa, M.D. Jansen, H. Hashimoto, J.G. van de Winkel, et al. 2002. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 46:1242–1254. [DOI] [PubMed] [Google Scholar]
- 28.Siriboonrit, U., N. Tsuchiya, M. Sirikong, C. Kyogoku, S. Bejrachandra, P. Suthipinittharm, K. Luangtrakool, D. Srinak, R. Thongpradit, K. Fujiwara, et al. 2003. Association of Fcgamma receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 61:374–383. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard, N.R., A.J. Cutler, S. Uribe, S.J. Chadban, B.J. Morley, and K.G.C. Smith. 2000. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr. Biol. 10:227–230. [DOI] [PubMed] [Google Scholar]
- 30.Xiu, Y., K. Nakamura, M. Abe, N. Li, X.S. Wen, Y. Jiang, D. Zhang, H. Tsurui, S. Matsuoka, Y. Hamano, et al. 2002. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J. Immunol. 169:4340–4346. [DOI] [PubMed] [Google Scholar]