Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of autoantibodies to certain cellular macromolecules, such as the small nuclear ribonucleoprotein particles (snRNPs), which had been considered to be passive targets of the autoimmune response. SLE is also characterized by the increased expression of type I interferon (IFN), which appears to be associated with the development and severity of disease. Here, we show that specific, highly conserved RNA sequences within snRNPs can stimulate Toll-like receptors (TLRs) 7 and 8 as well as activate innate immune cells, such as plasmacytoid dendritic cells (pDCs), which respond by secreting high levels of type I IFN. SLE patient sera containing autoantibodies to snRNPs form immune complexes that are taken up through the Fc receptor γRII and efficiently stimulate pDCs to secrete type I IFNs. These results demonstrate that a prototype autoantigen, the snRNP, can directly stimulate innate immunity and suggest that autoantibodies against snRNP may initiate SLE by stimulating TLR7/8.
Autoimmunity in systemic lupus erythematosus (SLE) can attack virtually any organ in the body. Nevertheless, the failure in immune tolerance that leads to disease in SLE is not generalized, but rather is highly specific: the earliest targets of the autoimmune response are certain nucleic acid–containing macromolecules, such as chromatin and the U1 as well as other small nuclear ribonucleoprotein particles (snRNPs). Intriguingly, these autoantigens are clustered together at the membranes of cells undergoing apoptosis (for review see reference 1). Apoptosis is generally considered to be a noninflammatory type of cell death, and many studies have reported tolerogenic effects of apoptotic cells. On the other hand, injection of large numbers of apoptotic cells into normal mice reportedly induces autoantibody production (2). An association between apoptosis and autoimmunity has been described in human SLE patients who have reduced clearance of apoptotic cells and increased circulating levels of nucleosomes (3–5). Moreover, mice or humans with deficiencies in DNase or defects in the clearance of apoptotic cells develop autoimmunity, providing increasing evidence for the hypothesis that exposure to elevated levels of nucleosomes or snRNPs can overcome self-tolerance (6–12).
This association of nucleosomal antigens with autoimmunity has been hypothesized to result from apoptosis-induced cleavage or other alterations in the antigen structures, making them immunogenic. However, this hypothesis does not explain the observed abrogation of immune tolerance in mice injected with apoptotic cells, which implies the existence of some simultaneous immune activation, perhaps triggered by the apoptotic debris itself. In considering possible mechanisms through which apoptotic material could conceivably induce autoimmunity, it is intriguing that phagosomes containing apoptotic cell particles are adjacent to Toll-like receptor (TLR)7 (13), which is known to be activated by single-stranded RNA molecules (14–16). Because the snRNPs within apoptotic blebs contain single-stranded RNA, they are potential TLR7 ligands.
In this study, we tested whether the U1 snRNP, a prototype target of the autoimmune response, could stimulate immunity directly. We demonstrate that the U1 snRNP can stimulate TLR7, leading to IFN-α secretion from plasmacytoid DCs (pDCs), and TLR8, leading to TNF-α secretion from monocytes. These stimulatory effects require the RNA component of the particle and can be reproduced by synthetic oligoribonucleotides (ORNs) as short as nine bases containing certain conserved U-rich autoantigen or autoantibody binding sites from the U1 or other snRNAs. Stimulation requires transfection or the formation of immune complexes comprised of anti-RNP antibodies from SLE patients and snRNP, which pDCs appear to take up through the FcγRII. Inhibitory oligodeoxynucleotides (ODNs) and “antimalarial” small molecules are shown to block these immunostimulatory effects with potential therapeutic implications.
Results
Immune stimulation by purified U1 snRNP through TLR7
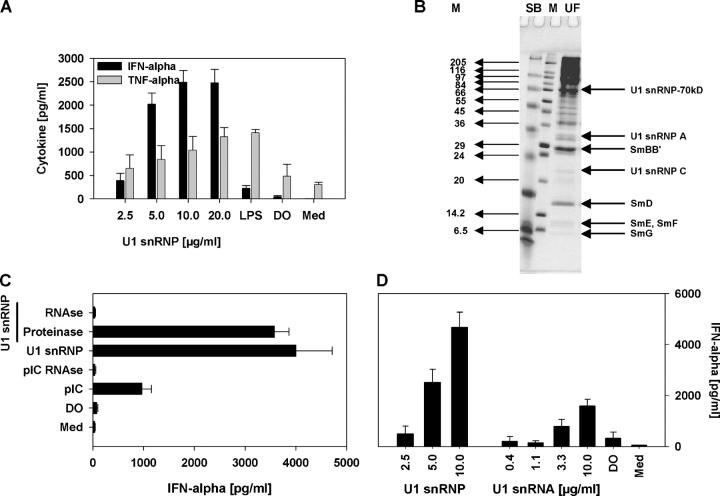
Apoptotic cells or apoptotic material in the presence of some SLE sera has been reported to induce PBMC secretion of IFN-α, which was attributed to the formation of stimulatory immune complexes because stimulation reportedly required the addition of SLE serum (17). In preliminary studies, we found that apoptotic cell debris induced IFN-α secretion from normal human PBMCs, even in the absence of SLE serum (not depicted). To test the possibility that U1 snRNP within the apoptotic material may be responsible for this stimulatory effect, human PBMCs were incubated with U1 snRNP, revealing a dose-dependent increase in IFN-α and TNF-α secretion (Fig. 1 A). Mammalian U1 snRNP consists of a complex of 10 different proteins and the 165-nucleotide U1 RNA molecule (18). The stimulatory activity of U1 snRNP only required the U1 RNA because treatment with RNase, but not proteinase, led to complete loss of IFN-α secretion (Fig. 1 C). Highly purified U1 snRNA stimulated dose-dependent production of IFN-α (Fig. 1 D). However, the level of IFN-α secretion induced by the U1 snRNA was lower than that from the snRNP, suggesting the possibility that the protein component of the snRNP may in some way enhance the immune effect of the RNA. In contrast, total cellular RNA isolated from the human T cell line Jurkat induced little or no IFN-α secretion, even at RNA concentrations as high as 100 μg/ml (not depicted).
Figure 1.
U1 snRNP induces type I IFN production. (A) Human PBMCs were stimulated with U1 snRNP (concentration given for total RNA plus protein) complexed to DOTAP (DO), 100 ng/ml LPS, or DOTAP alone, and cytokines were measured. Med, medium control. (B) SDS page of U1 snRNP, 4% stacking, 13.5% separation gel under reducing conditions, and Coomassie blue staining. Lane UF, RNP/Sm antigen, 8.8 μg protein; lane M, SigmaMarker, wide molecular weight range. (C) PBMCs were cultured with 20 μg/ml snRNP, 10 μg/ml poly rI:rC (pIC), snRNP, or poly rI:rC pretreated with RNase A or snRNP pretreated with proteinase K complexed to DOTAP. (D) PBMCs were stimulated with U1 snRNP or U1 snRNA complexed to DOTAP or DOTAP alone. The stimulatory properties of U1 snRNP or snRNA required the presence of an uptake enhancer (not depicted). All experiments show mean ± SEM of one representative out of two or more experiments, each with at least three to six donors.
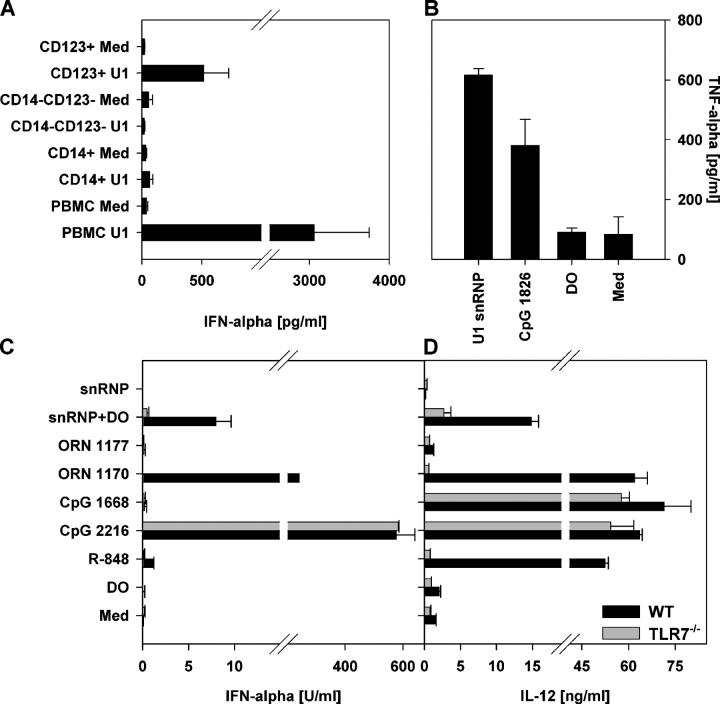
The high level of snRNP-induced IFN-α secretion suggested the potential involvement of pDCs, which are the primary IFN-α–secreting cells in response to viral infection (19). Double-stranded RNAs activate TLR3 (20), and single-stranded RNA molecules have been reported to stimulate murine TLR7 and human TLR8 (14–16). Of these, only TLR7 is expressed within pDCs. Enrichment of pDCs or monocytes from human PBMCs demonstrated snRNP-mediated stimulation of IFN-α production from the pDC-enriched (CD123+ CD14−), but not the monocyte-enriched (CD123− CD14+) or double-negative (CD123− CD14−), fraction (Fig. 2 A). A lower production of IFN-α induced by the U1 snRNP in the pure pDCs may reflect some beneficial effect of cell–cell interactions, which will need to be elucidated in further studies. Experiments with HEK293 cells stably transfected with human TLR8 and an NF-κB luciferase reporter construct demonstrated only modest TLR8-mediated signaling induced by the U1 snRNPs (U1 snRNP at 100 μg/ml: fold induction luciferase activity: 2.02 ± 0.69; R-848 at 25 μM: 11.21 ± 0.84), perhaps due to a relatively low sensitivity of TLR8 as described previously (21). Surprisingly, we were unable to demonstrate direct stimulation of TLR7 by snRNPs in stably transfected HEK293 cells (not depicted), despite finding strong stimulation using the ligand R-848 (21). As an alternative approach to testing for possible TLR7 activation, we used the murine macrophage-like cell line RAW246, which expresses TLR7 but no functional TLR8. U1 snRNP stimulated TNF-α production in these cells (Fig. 2 B). Although these experiments on human pDCs and murine RAW246 cells suggested that the U1 snRNP can stimulate TLR7, they did not indicate whether TLR7 was required for the innate immune response to snRNP. To more directly address this question, we compared the ability of snRNP to induce IFN-α and IL-12 secretion in DCs from Fms-like tyrosine kinase 3 ligand (Flt3L)-induced bone marrow cultures of wild-type mice or mice genetically deficient in TLR7 (Fig. 2, C and D). These experiments revealed that TLR7 is almost completely required for the snRNP-induced IFN-α response and is important but not absolutely required for the snRNP-induced IL-12 response. As expected, TLR7 was absolutely required for responses to the TLR7 ligand R-848 but was not required for responses to the TLR9 ligands CpG 2216 and CpG 1668 (Fig. 2, C and D). Thus, the snRNPs directly stimulate TLR7 within murine immune cells, but additional stimulation of other pathways may contribute to the low but consistent IL-12 response in TLR7−/− cells.
Figure 2.
TLR7 is required for the immune response to U1 snRNP. (A) Human PBMCs, CD14-enriched PBMCs (CD14+), CD14- and CD123-depleted PBMCs (CD14− CD123−), or CD123-enriched PBMCs (CD123+) were stimulated with 20 μg/ml U1 snRNP complexed to DOTAP (DO), and cytokines were measured. Med, medium control. Mean ± SEM of three donors. (B) Murine RAW246 macrophages were stimulated for 16 h with 20 μg/ml U1 snRNP complexed to DOTAP, 1.0 μM CpG ODN 1826, or DOTAP alone. (C–D) Murine DCs derived from Flt3L-induced bone marrow cultures from C57/B6 wild-type or TLR7−/− mice were cultured with 20 μg/ml U1 snRNP or ORN 1170 or ORN 1177 alone or complexed to DOTAP, 2 μg/ml R-848, or 1 μM CpG ODN 1668 or ODN 2216. Percentage of pDCs was 44% for wild type and 45% for TLR7−/− bone marrow (measured as CD45RA and CD11chigh cells).
Sequence-specific immune activation
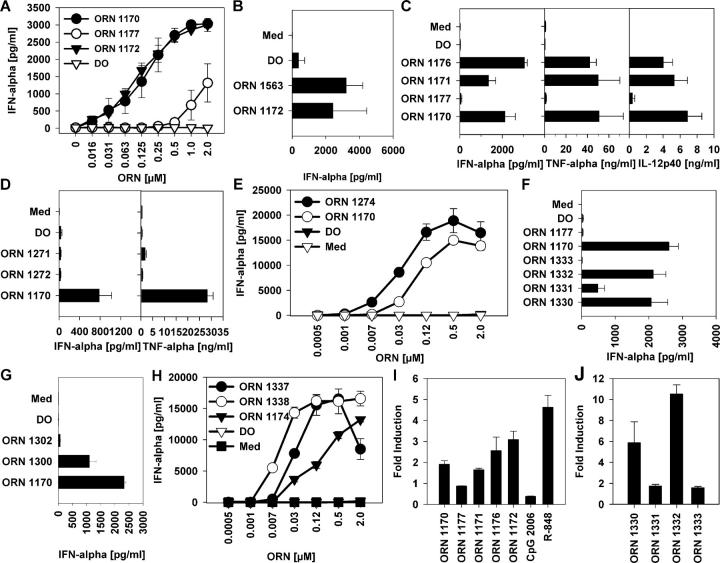
The Sm proteins are common to all U snRNPs, whereas the U1-70K, U1-A, and U1-C proteins are specific to U1. The U1 snRNA forms four hairpin loops (loop I to IV), the stems of which are connected by a four-way junction. Stem/loop IV is linked to this junction by a short single-stranded sequence to which the Sm proteins bind. U1-70K and U1-A bind directly to U1 RNA stem/loop I and II, respectively (18). To determine whether any of these U1 RNA sequences are particularly responsible for the U1 snRNA–induced immune stimulation, we synthesized ORNs comprising the binding sites for the U1-70K (1176; Table I), U1-A (1171), or Sm (1170) proteins (22) and/or containing autoantibody binding sites (1170 and 1171; references 23 and 24). U1 snRNA–derived ORNs (1170, 1171, and 1176) stimulated the secretion of IFN-α, TNF-α, and IL-12p40 in a dose-dependent fashion, comparable to a previously reported immune stimulatory sequence derived from the U5 region of HIV-1 RNA (Fig. 3, A and C; reference 15).
Table I.
Oligonucleotides (ONs)
| ON | Sequence 5′–3′ | Length | Position/description | S |
|---|---|---|---|---|
| 1170 | GGACUGCGUUCGCGCUUUCC | 20 | Stem/Loop IV | + |
| 1171 | GGCUUAUCCAUUGCACUCCGGA | 22 | Stem/Loop II | + |
| 1172 | GACUAGCUUGCUGUUU | 16 | 3′ Stem hY5 RNA | + |
| 1174 | UUUGUGGUAGUGGGGGACUG | 20 | Single-stranded region 5′ loop IV U1 RNA | + |
| 1176 | ACGAAGGUGGUUUUCCCAG | 19 | 3′ Stem I | + |
| 1177 | AAACAACAAACACACAAACC | 20 | U, G →A 1170 | − |
| 1271 | ACCUGGCAGGGGAGA | 15 | 5′ Stem I | − |
| 1272 | CCCAGGGCGAGGC | 13 | 5′ Stem II | − |
| 1274 | GGACUGCGUUGUGGCUUUCC | 20 | ORN 1170 with G/U-rich region | + |
| 1300 | GAUACUUACCUG | 12 | 5′ end U1 snRNA | + |
| 1302 | GAUACU | 6 | 5′ end U1 snRNA upon apoptotic cleavage | − |
| 1330 | AAUUUUUGA | 9 | Sm Site U4 snRNA | + |
| 1331 | AACCCCCGA | 9 | Sm Site U4 snRNA U to C | − |
| 1332 | AAUUUGUGG | 9 | Sm Site U1 snRNA | + |
| 1333 | AACCCGCGG | 9 | Sm Site U1 snRNA U to C | − |
| 1337 | GUAGUGUUUGUGGGGGACUG | 20 | ORN 1174 5′ UUUGUG shift center | + |
| 1338 | GUAGUGGGGGACUGUUUGUG | 20 | ORN 1174 5′ UUUGUG shift 3′ end | + |
| 1563 | GACUAGCCUUU | 11 | 3′ Stem hY3 RNA | + |
| 1668 (DNA) | TCCATGACGTTCCTGATGCT | 20 | Murine CpG B-Class | + |
| 1826 (DNA) | TCCATGACGTTCCTGACGTT | 20 | Murine CpG B-Class | + |
| 2006 (DNA) | TCGTCGTTTTGTCGTTTTGTCGTT | 24 | CpG B-Class | + |
| 2088 (DNA) | TCCTGGCGGGGAAGT | 15 | S-Class | − |
| 2216 (DNA) | GGG-G-G-A-C-G-A-T-C-G-T-CGGGGG | 19 | CpG A-Class (− indicates phosphodiester linkages) | + |
| 2395 (DNA) | TCGTCGTTTTCGGCGCGCGCCG | 22 | CpG C-Class | + |
All ONs have phosphorothioate backbones for nuclease resistance, except as indicated for ON 2216, and all are RNA with the exception of the last six, which are DNA as indicated. S + or − indicates stimulatory capacity of the ONs.
Figure 3.
U1 snRNA ORNs stimulate cytokine production and TLR8 signaling. PBMCs were stimulated with DOTAP alone (DO) or complexed to ORN at the indicated concentrations (A, E, and H) at 0.25 μM (C, D and F) or 0.5 μM (B and D), and cytokines were measured. Med, medium control. Panels show one representative of up to three independent experiments (each with three donors; mean ± SEM) or one representative of three donors (E and H; mean ± SD). (I) hTLR8-HEK293 cells were incubated with 3 μM ORN complexed to DOTAP, 10 μM R-848, or 10 μM CpG ODN for 16 h. Stimulation indices represent -fold NF-κB activation compared with transfected, nonstimulated cells. (J) hTLR8-HEK293 cells were incubated with 10 μM ORN complexed to DOTAP for 16 h.
Autoantibodies frequently target Ro RNPs, which consist of at least one of two polypeptides (Ro and La) associated with one of four small cytoplasmic RNAs (hY1, hY3, hY4, and hY5; references 25 and 26). ORNs 1172 and 1563 derived from the hY5 and hY3 RNA, both containing the binding site for the La autoantigen, stimulated IFN-α production from human PBMCs (Fig. 3, A and B). Interestingly, the La binding site of all human hY RNAs consists of U-rich regions preceded by at least one C or G (Table S1, available at http://www.jem.org/cgi/content/full/jem.20051696/DC1). Similar sequences can be found within the 3′ regions of human small RNAs synthesized by the RNA polymerase III (Table S1), suggesting that such endogenous RNAs may also exert immune stimulatory effects. Moreover, some U1 snRNA–derived ORNs failed to induce substantial cytokine production (ORNs 1271 and 1272; Fig. 3 D). On examination of the U1 snRNA ORN sequences, we noted that the stimulatory ones (ORNs 1170, 1171, 1174, and 1176) contained G/U-rich domains, but the nonstimulatory ones (ORNs 1271 and 1272) had only G-rich regions (Table I). To test the hypothesis that immune stimulation required these G/U-rich domains, we synthesized ORN 1177, in which all U and G nucleotides of ORN 1170 were replaced by A. ORN 1177 stimulated minimal IFN-α or other cytokine production (Fig. 3, A and C, and not depicted). In contrast, introducing a G/U-rich sequence derived from the previously described HIV-1 RNA sequence (15) into ORN 1170 led to an enhancement of IFN-α secretion (Fig. 3 E). These results confirm a requirement for U1-derived G/U-rich sequences for the stimulatory effects.
The Sm protein binding sites (as well as the hY RNA La binding sites) differ from the previously described G/U-rich TLR7-activating sequences. The U1 Sm binding site consists of an adenosine at the 5′ side of an oligo(U) tract containing just one guanosine (Table S1; references 27 and 28). Nevertheless, ORN with only this highly conserved 9-mer U-rich sequence from the U4 or U1 Sm binding sites (ORNs 1330 and 1332, respectively) stimulated IFN-α (Fig. 3 F) and TNF-α (not depicted) production from PBMCs. The U specificity of these effects was confirmed by the inactivity of control ORN with the same sequence but a U to C exchange (ORNs 1331 and 1333). The Sm protein binding site on the various snRNAs is highly conserved throughout different species (Table S1), suggesting a general potential for immune stimulatory effects of snRNPs across these species. These results expand the range of ORNs known to activate TLR7 and TLR8 to U-rich sequences containing as little as one guanosine.
The U1 snRNP is only slightly cleaved during apoptosis, and cleavage of the U1 snRNA is very specific. Only the first five to six 5′ nucleotides protruding from the U1 snRNP complex are removed. ORN 1300 consisting of just the 12 5′ snRNA nucleotides stimulated IFN-α production from human PBMCs (Fig. 3 G and Table I). ORN 1302 containing only six 5′ snRNA nucleotides was minimally stimulatory compared with ORNs with nine or more nucleotides, demonstrating length dependence of the RNA-mediated effects. Another U1 snRNA–derived ORN derived from the 5′ single-stranded region of loop IV (ORN 1174) contains a 5′ G/U-rich sequence. To determine the influence of position of the G/U-rich sequence on immune stimulation, the 5′ sequence (5′-UUUGUG-3′) was shifted stepwise to the 3′ end, demonstrating a position-dependent effect of ORN-mediated cytokine production favoring the 3′ end (Fig. 3 H).
Stimulation of TLRs, pDCs, and monocytes by ORNs
Previous studies have demonstrated that in addition to the well-established stimulation of TLR3 by double-stranded RNA, a variety of single-stranded RNAs can activate mouse immune cells through TLR7 and human immune cells through TLR8, but the question of whether the effects are sequence-specific or not has been controversial (14, 15, 29, 30). To investigate the involvement of TLRs in the snRNA-derived ORN-mediated cytokine production, HEK293 cells stably transfected with human TLR8 and an NF-κB luciferase reporter construct were incubated with various ORNs, the TLR9 ligand CpG ODN 2006, or the TLR7/8 ligand R-848 (21). All of the U-rich ORNs activated TLR8, but the mutated control ORNs 1177, 1331, and 1333 did not (Fig. 3, I and K), demonstrating sequence dependency of TLR8-mediated NF-κB activation. In contrast to the U1 snRNP, all ORN immune stimulatory effects were abolished in DCs generated from TLR7−/− mice (Fig. 2, C and D), confirming that their activity on murine immune cells depended entirely on this innate immune receptor. Additional studies will be needed to understand the mechanism of the difference between ORN- and snRNP-mediated immune effects in wild-type versus TLR7−/− cells. None of these ORNs induced detectable TLR7-dependent signaling in transfected HEK293 cells, despite appropriate positive controls (not depicted), which suggests either that the HEK293 cells may lack some cofactor specifically required for ORN-induced activation of TLR7, or that the NF-κB luciferase reporter used for these assays might be insufficient to detect the ORN effects. Additional studies will be required to resolve this question.
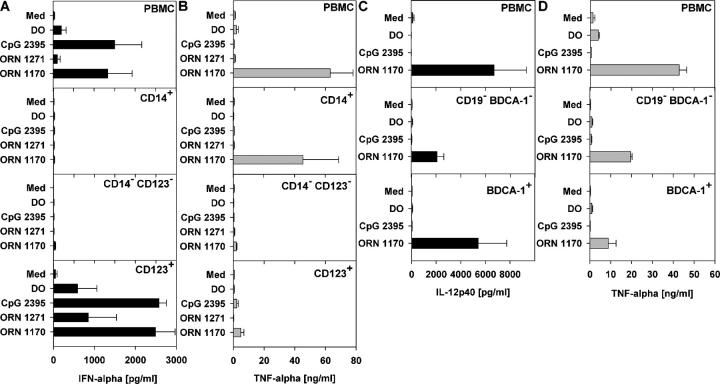
To determine whether the ORNs would stimulate RNA-responsive TLRs in primary immune cells, monocytes (TLR8+), myeloid DCs (mDCs) (TLR8+), or pDCs (TLR7+) were isolated from human PBMCs and cultured with ORN 1170 or ORN 1271, or the TLR9 agonist CpG ODN 2395 (31). PBMCs and CD123+ pDCs were highly stimulated by ORN 1170 or CpG ODN 2395 to secrete IFN-α, whereas CD14+ monocytes or CD123-/CD14-depleted PBMCs were not, and the control ORN 1271 was inactive (Fig. 4 A). This indicated the direct ability of the U-rich ORN to activate the pDCs through TLR7. Conversely, ORN 1170 induced high levels of TNF-α production from PBMCs, mDCs, and CD14+ monocytes as well as high IL-12 production from mDCs, indicating the direct ability of the U-rich ORN to activate monocytes and mDCs through TLR8 (Fig. 4, B–D).
Figure 4.
U1 snRNA ORNs induce cytokine secretion from purified human monocytes, pDCs, or mDCs. Human PBMCs, CD14-enriched PBMCs (CD14+), CD14- and CD123-depleted PBMCs (CD14− CD123−), CD123- enriched PBMCs (CD123+), CD19- and CD1c-depleted PBMCs (CD14− BDCA-1−), or CD1c-enriched PBMCs (BDCA-1+) were stimulated with 0.5 μM ORN 1170 and/or 1271 complexed to DOTAP (DO), DO alone, or 0.5 μM CpG ODN and tested for (A) IFN-α, (B and D) TNF-α, and (C) IL-12p40 secretion. Mean ± SEM of two (A) or three (B–D) donors. Med, medium control.
Inhibition by chloroquine and suppressive ODNs
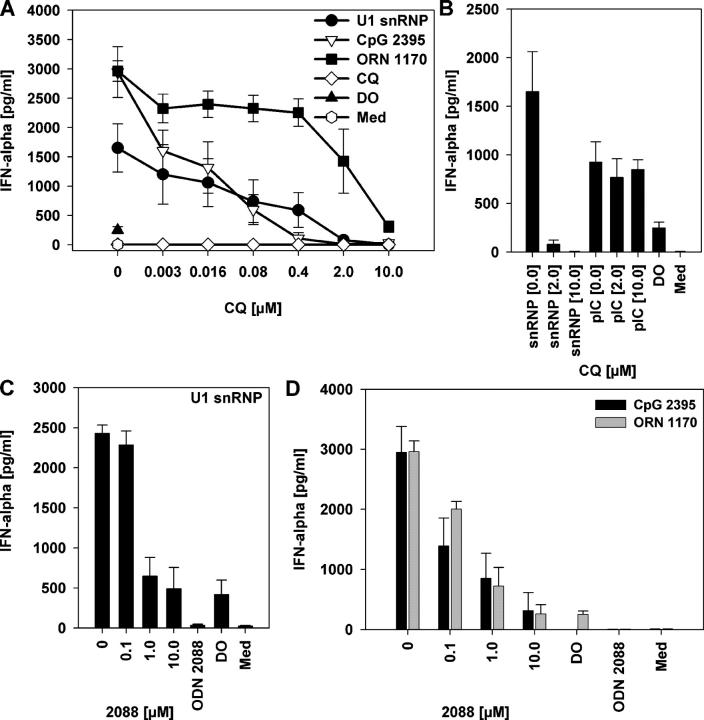
Chloroquine and related compounds inhibit CpG DNA–driven signaling through TLR9 (32). Chloroquine inhibited IFN-α secretion from PBMCs stimulated by U1 snRNP, ORN 1170, or CpG ODN 2395 in a dose-dependent manner (Fig. 5 A). Compared with the U1 snRNP, IFN-α secretion induced by the ORN required higher chloroquine concentrations for inhibition, perhaps because of the nuclease-resistant phosphorothioate backbone of the ORN. However, chloroquine failed to inhibit poly rI:rC–mediated IFN-α production, even at high concentrations (Fig. 5 B). Because poly rI:rC is a TLR3 ligand, this result points to a difference in the mechanism of TLR3-mediated immune stimulation compared with the chloroquine-sensitive effects mediated through TLR7–9 and suggests that the chloroquine-sensitive effects of the snRNP and ORN are not mediated through TLR3. The specific inhibitor of the endosomal and lysosomal V-type ATPase, bafilomycin A1, had a similar inhibitory effect on IFN-α secretion by snRNP or ORN to that of chloroquine (see Fig. 7 B and not depicted). Certain suppressive G-rich ODNs (“S-Class”) inhibit immune activation elicited by CpG DNA and can be used to treat lupus in animal models (33). The S-Class ODN 2088 suppressed snRNP-, ORN 1170–, and CpG ODN 2395–mediated IFN-α production (Fig. 5, C and D), demonstrating that its inhibitory effects are not limited to TLR9, but extend to TLR7.
Figure 5.
Chloroquine and S-Class ODN 2088 block the immune stimulatory effects of U1 snRNP and U1-derived ORNs. (A and B) Human PBMCs were stimulated with medium (Med), 10 μg/ml U1 snRNP complexed to DOTAP (DO), 0.5 μM CpG ODN 2395, 0.125 μM ORN 1170 complexed to DOTAP, or 30 μg/ml poly rI:rC (pIC) alone or in the presence of the indicated concentrations of chloroquine (CQ) or (C and D) S-Class ODN 2088, or with 10 μg/ml of ODN 2088 alone. ODN 2088 also inhibited ORN-induced TNF-α secretion (not depicted). Mean ± SEM of one representative out of two to three independent experiments (n = 3 donors).
Figure 7.
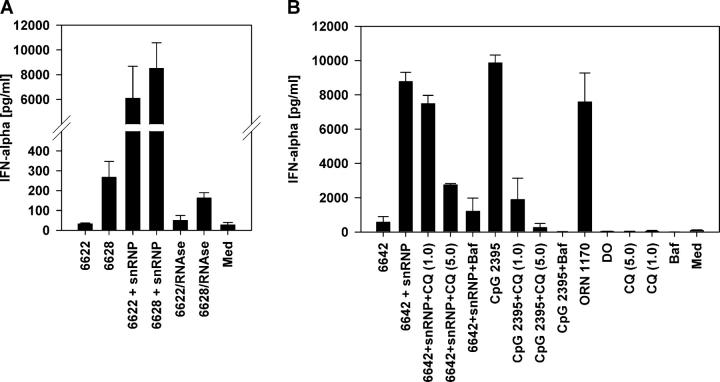
U1 snRNP and anti-RNP+ SLE patient sera stimulate RNA-dependent IFN-α production inhibited by chloroquine and bafilomycin. (A) SLE patient sera 6622 and 6628 with 10 μg/ml U1 snRNP pretreated with RNase A were used to stimulate human PBMCs as described in Fig. 6. Mean ± SEM for three donors. (B) Normal human PBMCs were stimulated with SLE serum 6642 alone or with 10 μg/ml U1 snRNP or 0.5 μM CpG ODN 2395 in the presence or absence of 1.0 or 5.0 μM chloroquine (CQ) or 500 nM bafilomycin (Baf). Controls included chloroquine or bafilomycin alone or 0.5 μM ORN 1170. Mean ± SEM for two donors.
CD32-dependent activation of pDCs by lupus sera and snRNPs
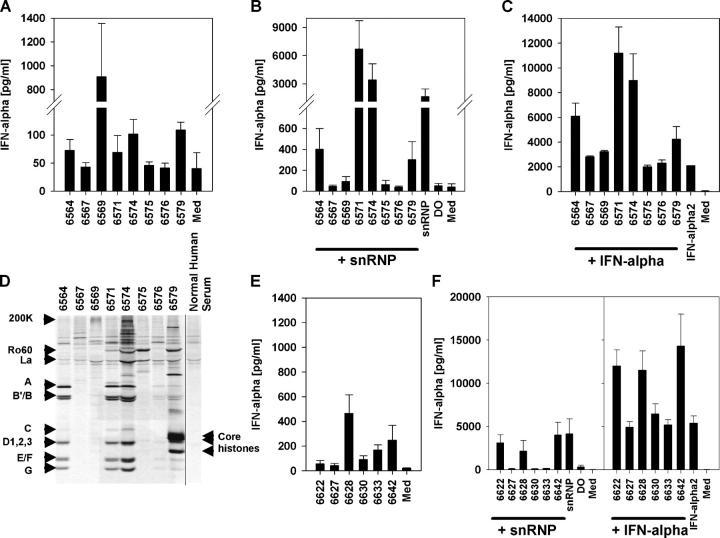
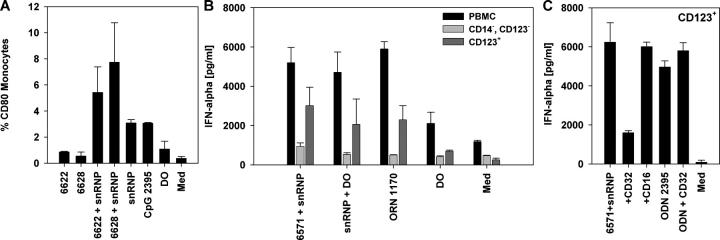
Immune stimulation by the purified snRNPs and synthetic ORNs required their delivery with cationic lipids. We hypothesized that in SLE patients, delivery of the immune stimulatory U-rich RNA sequences could be facilitated if the sequences were within immune complexes. To test this hypothesis, we mixed purified snRNP with 14 separate SLE patient sera containing various autoantibody specificities (Table II and Fig. 6 D). Some of the SLE patient sera alone induced IFN-α secretion from normal human PBMCs (Fig. 6, A and E). However, all of the sera that contained at least moderate levels of immunoprecipitating anti-RNP antibody induced strong IFN-α production when supplemented with purified U1 snRNP (Fig. 6, B and F). Preincubation of PBMCs with IFN-α led to increased IFN-α secretion by these anti-RNP sera that was more than additive above the level of IFN-α2 added to the cultures (Fig. 6, C and F). Stimulation of IFN-α production in the presence of snRNPs was decreased to levels of roughly those observed in the absence of snRNP upon RNase treatment (Fig. 7 A). Chloroquine or bafilomycin severely diminished IFN-α production (Fig. 7 B). U1 snRNP alone as well as sera supplemented with snRNP stimulated monocyte CD80 expression, indicating the maturation of these cells (Fig. 8 A). Positive SLE patient sera combined with snRNPs stimulated IFN-α production from PBMCs or purified pDCs to a similar level as that induced by snRNPs or ORN 1170 transfected with DOTAP (Fig. 8 B). The IgG receptor CD32 (FcγRII) binds avidly to IgG present in immune complexes and is expressed on human pDCs where it mediates IFN-α production by SLE patient serum combined with apoptotic cells or DNA immune complexes (34, 35). By adding blocking antibodies against CD16 (FcγRIII) or CD32, we found that only anti-CD32 antibody blocked the IFN-α secretion from purified pDCs (Fig. 8 C). In contrast, IFN-α secretion stimulated by CpG ODN 2395 was not affected by the anti-CD32 antibody. Therefore, CD32 appears to be responsible for delivering RNA immune complexes to one or more pDC intracellular compartments containing TLR7.
Table II.
Autoantigen-precipitating activity of SLE patient sera
| Patient | RNP | Sm | Ro60 | La | Core histones | RNAPII | Su | DNA-PK | OJ |
|---|---|---|---|---|---|---|---|---|---|
| 6564 | X | ||||||||
| 6567 | |||||||||
| 6569 | |||||||||
| 6571 | X | X | |||||||
| 6574 | X | X | X | X | |||||
| 6575 | X | ||||||||
| 6576 | X | ||||||||
| 6579 | X | X | X | ||||||
| 6622 | X | X | X | X | |||||
| 6627 | X | ||||||||
| 6628 | X | X | |||||||
| 6630 | X | X | |||||||
| 6633 | X | ||||||||
| 6642 | X | X |
Immunoprecipitations were performed as described in Materials and methods, and as shown in Fig. 6 D. X indicates a positive result as defined for the following specificities: RNP, immunoprecipitation of A, B′/B, C, D, E, F, and G, but not U5 200K protein; Sm, immunoprecipitation of A, B′/B, C, D, E, F, and G plus U5 200K protein; Ro60, immunoprecipitation of 60 kD protein; La, immunoprecipitation of 45 kD protein; Core histones, immunoprecipitation of H2A/H2B plus unidentified band; RNAPII, immunoprecipitation of RNA polymerase II, two largest subunits; DNA-PK, immunoprecipitation of DNA-PKcs, Ku70, and Ku80 proteins; OJ, immunoprecipitation of multiprotein complex.
Figure 6.
SLE patient sera containing anti-RNP antibodies combine with purified U1 snRNP to induce IFN-α production. (A and B) Normal human PBMCs were stimulated in 87.5% culture medium with 12.5% of eight (A–C) or six (E and F) SLE patient sera without (A and E) or with (B and F) 10 μg/ml U1 snRNP, or were pretreated with 500 IU/ml IFN-α2b (C and F) and assayed for IFN-α. Controls included medium (Med) and DOTAP (DO) alone or with 10 μg/ml U1 snRNP. Mean ± SEM for three donors. (D) Eight coded SLE patient sera (for all sera see Table II) were tested for the presence and level of various autoantibodies, including RNP components by radioimmunoprecipitation. Assessment of anti-RNP levels within these eight sera revealed two strongly positive sera (6571 and 6574), two moderate positive (6564 and 6579), and one very weak positive (6576), with the others negative.
Figure 8.
Anti-RNP+ SLE patient sera and U1 snRNP stimulate monocyte maturation and CD32-dependent IFN-α production from pDCs. (A) PBMCs were stimulated with SLE patient sera 6622 and 6628 with 10 μg/ml U1 snRNP, snRNP complexed to DOTAP (DO), or with 0.5 μM CpG ODN 2395 as described in Fig. 6. CD80 expression on CD14+ cells was measured by flow cytometry. Mean ± SEM for two donors. (B) Whole PBMCs, CD14- and CD123-depleted PBMCs (CD14− CD123−), or purified CD123+ pDCs were cultured as shown. Mean ± SEM for three donors. (C) Purified CD123+ pDCs were incubated with SLE patient sera 6571 (or 6574; not depicted) and 10 μg/ml U1 snRNP, or 0.5 μM of positive control CpG ODN 2395 ± 5 μg/ml antibody to CD16 or CD32. Mean ± SD for one out of two experiments.
Discussion
There has been much interest in understanding why the autoimmune response in SLE is targeted selectively at certain nucleic acid–containing macromolecules that are concentrated within apoptotic cellular blebs. A popular hypothesis has been that these autoantigens become immunogenic during apoptosis, which then may drive the autoimmune response. The present results demonstrate that the prototype autoantigen U1 snRNP, which is highly represented within apoptotic cells, is not simply a passive target of the autoimmune response, but rather is directly immune stimulatory when delivered into innate immune cells expressing either TLR7 or TLR8. Delivery of U1 snRNP can occur either via transfection or more physiologically by the uptake of snRNP-containing immune complexes formed by SLE patient sera containing anti-RNP antibody through FcγRIIa (CD32) present on pDCs. The stimulatory effects require the RNA component of the particle and appear to be mediated predominately through TLR7. A modest response is seen in TLR7−/− mice lacking functional TLR8 (15), suggesting an additional involvement of other immune stimulatory pathways. Immune activation could be reproduced by purified U1 snRNA or by synthetic ORNs containing certain conserved U-rich autoantigen or autoantibody binding sites from the U1 snRNAs or hY RNAs. Some of these U-rich regions are conserved across various small RNAs and different species, indicating that they may stimulate TLR7-mediated immune responses, at least when present in or reaching TLR7-containing compartments. Stimulation by synthetic ORNs was mediated by G/U- or U-rich sequences containing as little as one guanosine and was dependent on the length and position of the U-rich sequence.
Type I IFN promotes T cell responses (36) and induces autoimmunity in up to 20% of humans treated with recombinant IFN-α (for review see reference 37). Increased expression of type I IFN occurs in the pDCs of lupus patients, is associated with disease severity, and may contribute to disease development (38–41), thereby linking pDC activation to SLE pathogenesis. Activation of the IFN-α pathway in SLE patients is significantly associated with the presence of certain autoantibody specificities, especially anti-RNP and other RNA-associated factors, but not with antiphospholipid antibodies (40). Human pDCs express TLR7, but not the other RNA-activated TLRs, TLR3 or TLR8 (42, 43), that localize preferentially near phagosomes containing apoptotic particles (13). Therefore, inappropriate or excessive activation of TLR7 by U-rich RNA sequences within snRNP could trigger the observed activation of the IFN-α pathway in SLE patients. All these TLRs are in endoplasmic reticulum or endosomal/lysosomal compartments (44), which explains the requirement we found for transfection of the purified snRNPs or snRNA-derived ORNs. Our results suggest a possible mechanism for the observed linkage between defects in apoptotic cell clearance and SLE. We propose that under normal physiologic conditions, the small amounts of apoptotic cellular debris might be cleared too rapidly to activate the TLR pathways. However, in susceptible individuals with defective clearance, or under such abnormal conditions as during an immune response to a virus or other agent, the apoptotic debris in the form of immune complexes might be taken up by pDCs at high enough levels so that the U-rich sequences within the enclosed snRNP activate TLR7, thereby initiating type I IFN production and autoimmunity. In light of our results, the recently observed association of EBV infection with SLE could be explained by the homology and antigenic cross reactivity between the EBV nuclear antigen 1 and the snRNP and other autoantigens that are associated with apoptotic blebs (45). Molecular mimicry between EBV nuclear antigen 1 and snRNP or other lupus autoantigens would lead to the production of autoantibodies reactive with apoptotic debris. Once an individual made autoantibodies that cross reacted with any component of the apoptotic cells or snRNP, these would form immune complexes that enhance the uptake of the nucleosome and snRNP nucleic acids through FcγRII on the pDCs. The autoimmune process would then become self-perpetuating, further enhanced by autocrine type I IFN production by pDCs and exacerbated by any defect in the clearance of apoptotic debris or immune complexes. DNA-containing immune complexes from SLE sera stimulate pDCs to secrete IFN-α through TLR9 (35, 41, 46). These results demonstrate that immune complexes upon intracellular delivery via CD32 can activate pDCs not only through TLR9 as reported previously (35, 47–49), but also through TLR7, demonstrating a strong link between the pDCs and autoimmunity to both DNA- and RNA-containing autoantigens. Because the snRNAs also have dsRNA regions, we cannot completely exclude the possibility that they may also stimulate TLR3 or other pathogen-associated molecular patterns, causing additional immune stimulatory effects on cells other than pDCs (20, 50). But the complete inhibition of the effects by chloroquine, which fails to block activation by the TLR3 ligand poly rI:rC, suggests that any such TLR3-mediated effect would be quite small.
Apoptosis may exert additional effects on nucleosomal material that contribute to the development and direction of the subsequent autoimmune response. The U1 snRNP in contrast to other RNPs is only slightly cleaved during apoptosis, which could enable the U1 snRNA to better activate the DCs taking up these particles, resulting in the presentation of the associated 70K protein and Sm proteins on a mature DC that might drive a T cell response to the antigens through epitope spreading. Thus, the RNA component of the snRNPs would act as a built-in adjuvant to drive autoimmune responses against the associated proteins, explaining their strong and unique association with systemic autoimmunity.
All of the immune stimulatory effects of the U1 snRNP or snRNA ORNs are completely blocked by chloroquine at relatively low concentrations that occur in patients taking this class of drug for the treatment of SLE. These results suggest that the mechanism of action of chloroquine in the treatment of systemic autoimmunity, which had been unknown, could be through inhibition of one or more of TLR7/8/9. Our data support the concept that chloroquine-related compounds and suppressive ODNs, which were previously reported to block DNA-mediated TLR9 stimulation, may prevent apoptotic debris from stimulating and sustaining autoimmunity. Thus, we have identified a new potential therapeutic mechanism of action for these agents, making improved TLR7/8/9 antagonists interesting candidates for therapeutic development.
Materials and Methods
ODNs and reagents.
ODNs (Coley Pharmaceutical GmbH) and ORNs (BioSpring GmbH) were suspended in sterile, endotoxin-free Tris-EDTA (Sigma-Aldrich) or DNase- and RNase-free dH2O (Life Technologies), respectively. Sequences are listed in Table I. LPS from Escherichia coli and chloroquine were purchased from Sigma-Aldrich. Recombinant IFN-α2b (IntronA) was obtained from SP Europe, and poly rI:rC was from GE Healthcare. R-848 was commercially synthesized by GLSynthesis. U1 snRNP was purchased from Sweden Diagnostic (gel of the preparation used is shown in Fig. 1 B). U1 snRNA was extracted from U1 snRNPs affinity purified from K562 cells as described previously (51). Whole human RNA was extracted from Jurkat cells (American Type Culture Collection LGC Promochem) with the RNeasy Kit (QIAGEN).
Isolation of cells and cell culture.
Peripheral blood buffy coat preparations from healthy male and female human donors were obtained from the Institute for Hemostaseology and Transfusion Medicine of the University Hospital of Düsseldorf as described previously (31). pDCs and monocytes were isolated with the BDCA-4 pDC or CD14 monocyte isolation kit (Miltenyi Biotec). mDCs were isolated with the BDCA-1 (CD1c) mDC isolation kit after depleting CD19+ B cells (Miltenyi Biotec). Purity was confirmed by staining with mAb to CD11c (Diaclone), CD14, HLA-DR, and CD123 (all from BD Biosciences) and was typically >80%. Purity of mDC fractions was confirmed by staining with mAb to CD14, CD19, and streptavidin. Antibody to CD80, CD16, and CD32 (PE labeled) was from BD Biosciences, and unlabeled blocking antibody to CD32 was from CellSystems Biotechnologie Vertrieb GmbH. For some of the assays, PBMCs were precultured at 37°C for 4 h with recombinant IFN-α2b before adding stimulatory substances. SLE patient serum was obtained at the Rheumatology Clinic, University of Florida, under a protocol approved by the institutional review board. Murine DCs were derived from Flt3L-induced bone marrow cultures from C57/B6 wild-type or TLR7−/− mice (protocol approved by the Munich Institute for Medical Microbiology Animal Use Committee) as described previously (15).
Reporter gene assay.
HEK293 cells bearing a luciferase gene under the control of a 6×NF-κB promoter construct and additionally constitutively expressing the gene for human TLR8 (“hTLR8-HEK293”; reference 21) were incubated for 16 h at 37°C in a humidified incubator with reagents as indicated. Cells were lysed and the amount of luciferase was determined with BriteLite on a luminometer (both from PerkinElmer).
Cytokine detection.
Human or murine cells were added to 96-well round-bottomed plates with or without the addition of TLR ligands or reagents as indicated. Enhanced stability and intracellular delivery of U1 snRNP, RNA, or ORN was achieved by DOTAP (Roche) at concentrations ranging from 5 to 50 μg/ml (snRNP typically with 25 μg/ml DOTAP). In some experiments, snRNP or poly rI:rC was pretreated with RNase A or proteinase K (both from QIAGEN). Culture supernatants were collected at 24 h or at the indicated time points. Amounts of cytokines in the culture supernatants were assessed using commercially available ELISA kits (human TNF-α from Diaclone; human and mouse IL-12p40 and mouse TNF-α from BD Biosciences) or an in-house ELISA developed using commercially available antibodies (for detection of human or murine IFN-α; PBL Biomedical Laboratories).
Immunoprecipitation.
K562 cells were cultured overnight with [35S]methionine/cysteine and the proteins were extracted, mixed with 5 μl of the patient serum, and incubated with protein A sepharose beads followed by washing of the beads, protein gel electrophoresis, and autoradiography.
Online supplemental material.
Conserved U-rich motifs within U snRNA and hY RNA are shown in Table S1, which is available at http://www.jem.org/cgi/content/full/jem.20051696/DC1.
Acknowledgments
We thank Dr. Bernhard Noll and Tanja Sniatala for performing quality control for identity and purity on the synthetic ODNs and ORNs, and Andrea Kritzler and Carmen Montino for excellent technical assistance. We also thank Drs. Ann Marshak-Rothstein, Christian Schetter, Grayson Lipford, and Ian R. Rifkin for reviewing the manuscript and for helpful discussions. We are grateful to Heike Berthold of Sweden Diagnostic (Germany) GmbH, an affiliate of PharmaDiagnostics AB (Freiburg, Germany), for providing SDS Page of purified U1 snRNP.
S. Bauer is supported by DFG Priorityprogram SP1110 “Innate Immunity” and SFB 576. J. Vollmer, M. Jurk, A. Forsbach, S. Bauer, and A.M. Krieg are inventors on one or more patents relating to the TLR technology.
All of the authors except K.M. Kelly, S. Akira, S. Bauer, S. Hamm, and W.H. Reeves are employees of Coley Pharmaceutical Group and may have a financial interest in the study's outcome.
Abbreviations used: Flt3L, Fms-like tyrosine kinase 3 ligand; mDC, myeloid DC; ODN, oligodeoxynucleotide; ON, oligonucleotide; ORN, oligoribonucleotide; pDC, plasmacytoid DC; SLE, systemic lupus erythematosus; snRNP, small nuclear ribonucleoprotein particle; TLR, Toll-like receptor.
References
- 1.Hall, J.C., L. Casciola-Rosen, and A. Rosen. 2004. Altered structure of autoantigens during apoptosis. Rheum. Dis. Clin. North Am. 30:455–471. [DOI] [PubMed] [Google Scholar]
- 2.Mevorach, D., J.L. Zhou, X. Song, and K.B. Elkon. 1998. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amoura, Z., J.C. Piette, H. Chabre, P. Cacoub, T. Papo, B. Wechsler, J.F. Bach, and S. Koutouzov. 1997. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. 40:2217–2225. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann, M., R.E. Voll, O.M. Zoller, M. Hagenhofer, B.B. Ponner, and J.R. Kalden. 1998. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41:1241–1250. [DOI] [PubMed] [Google Scholar]
- 5.Rumore, P.M., and C.R. Steinman. 1990. Endogenous circulating DNA in systemic lupus erythematosus. Occurrence as multimeric complexes bound to histone. J. Clin. Invest. 86:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickerstaff, M.C., M. Botto, W.L. Hutchinson, J. Herbert, G.A. Tennent, A. Bybee, D.A. Mitchell, H.T. Cook, P.J. Butler, M.J. Walport, and M.B. Pepys. 1999. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 5:694–697. [DOI] [PubMed] [Google Scholar]
- 7.Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, P.L., R. Caricchio, V. Abraham, T.D. Camenisch, J.C. Jennette, R.A. Roubey, H.S. Earp, G. Matsushima, and E.A. Reap. 2002. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 196:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napirei, M., H. Karsunky, B. Zevnik, H. Stephan, H.G. Mannherz, and T. Moroy. 2000. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25:177–181. [DOI] [PubMed] [Google Scholar]
- 10.Qian, Y., H. Wang, and S.H. Clarke. 2004. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J. Immunol. 172:625–635. [DOI] [PubMed] [Google Scholar]
- 11.Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 411:207–211. [DOI] [PubMed] [Google Scholar]
- 12.Yasutomo, K., T. Horiuchi, S. Kagami, H. Tsukamoto, C. Hashimura, M. Urushihara, and Y. Kuroda. 2001. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat. Genet. 28:313–314. [DOI] [PubMed] [Google Scholar]
- 13.Nishiya, T., E. Kajita, S. Miwa, and A.L. DeFranco. 2005. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 280:37107–37117. [DOI] [PubMed] [Google Scholar]
- 14.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 15.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 16.Lund, J.M., L. Alexopoulou, A. Sato, M. Karow, N.C. Adams, N.W. Gale, A. Iwasaki, and R.A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 101:5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovgren, T., M.L. Eloranta, U. Bave, G.V. Alm, and L. Ronnblom. 2004. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50:1861–1872. [DOI] [PubMed] [Google Scholar]
- 18.Achsel, T., H. Stark, and R. Luhrmann. 2001. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc. Natl. Acad. Sci. USA. 98:3685–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 21.Jurk, M., F. Heil, J. Vollmer, C. Schetter, A.M. Krieg, H. Wagner, G. Lipford, and S. Bauer. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 22.Stark, H., P. Dube, R. Luhrmann, and B. Kastner. 2001. Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature. 409:539–542. [DOI] [PubMed] [Google Scholar]
- 23.Deutscher, S.L., and J.D. Keene. 1988. A sequence-specific conformational epitope on U1 RNA is recognized by a unique autoantibody. Proc. Natl. Acad. Sci. USA. 85:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoet, R.M., B. Kastner, R. Luhrmann, and W.J. van Venrooij. 1993. Purification and characterization of human autoantibodies directed to specific regions on U1RNA; recognition of native U1RNP complexes. Nucleic Acids Res. 21:5130–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granger, D., A. Tremblay, C. Boulanger, B. Chabot, H.A. Menard, and G. Boire. 1996. Autoantigenic epitopes on hY5 Ro RNA are distinct from regions bound by the 60-kDa Ro and La proteins. J. Immunol. 157:2193–2200. [PubMed] [Google Scholar]
- 26.Wolin, S.L., and J.A. Steitz. 1984. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc. Natl. Acad. Sci. USA. 81:1996–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinrichs, V., W. Hackl, and R. Luhrmann. 1992. Direct binding of small nuclear ribonucleoprotein G to the Sm site of small nuclear RNA. Ultraviolet light cross-linking of protein G to the AAU stretch within the Sm site (AAUUUGUGG) of U1 small nuclear ribonucleoprotein reconstituted in vitro. J. Mol. Biol. 227:15–28. [DOI] [PubMed] [Google Scholar]
- 28.Raker, V.A., K. Hartmuth, B. Kastner, and R. Luhrmann. 1999. Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol. Cell. Biol. 19:6554–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koski, G.K., K. Kariko, S. Xu, D. Weissman, P.A. Cohen, and B.J. Czerniecki. 2004. Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J. Immunol. 172:3989–3993. [DOI] [PubMed] [Google Scholar]
- 30.Scheel, B., S. Braedel, J. Probst, J.P. Carralot, H. Wagner, H. Schild, G. Jung, H.G. Rammensee, and S. Pascolo. 2004. Immunostimulating capacities of stabilized RNA molecules. Eur. J. Immunol. 34:537–547. [DOI] [PubMed] [Google Scholar]
- 31.Vollmer, J., R. Weeratna, P. Payette, M. Jurk, C. Schetter, M. Laucht, T. Wader, S. Tluk, M. Liu, H.L. Davis, and A.M. Krieg. 2004. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 34:251–262. [DOI] [PubMed] [Google Scholar]
- 32.Rutz, M., J. Metzger, T. Gellert, P. Luppa, G.B. Lipford, H. Wagner, and S. Bauer. 2004. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 34:2541–2550. [DOI] [PubMed] [Google Scholar]
- 33.Dong, L., S. Ito, K.J. Ishii, and D.M. Klinman. 2005. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB × NZW mice. Arthritis Rheum. 52:651–658. [DOI] [PubMed] [Google Scholar]
- 34.Bave, U., M. Magnusson, M.L. Eloranta, A. Perers, G.V. Alm, and L. Ronnblom. 2003. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J. Immunol. 171:3296–3302. [DOI] [PubMed] [Google Scholar]
- 35.Means, T.K., E. Latz, F. Hayashi, M.R. Murali, D.T. Golenbock, and A.D. Luster. 2005. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 115:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tough, D.F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 272:1947–1950. [DOI] [PubMed] [Google Scholar]
- 37.Ioannou, Y., and D.A. Isenberg. 2000. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 43:1431–1442. [DOI] [PubMed] [Google Scholar]
- 38.Baechler, E.C., F.M. Batliwalla, G. Karypis, P.M. Gaffney, W.A. Ortmann, K.J. Espe, K.B. Shark, W.J. Grande, K.M. Hughes, V. Kapur, P.K. Gregersen, and T.W. Behrens. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 100:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanco, P., A.K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 294:1540–1543. [DOI] [PubMed] [Google Scholar]
- 40.Kirou, K.A., C. Lee, S. George, K. Louca, M.G. Peterson, and M.K. Crow. 2005. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52:1491–1503. [DOI] [PubMed] [Google Scholar]
- 41.Ronnblom, L., and G.V. Alm. 2001. A pivotal role for the natural interferon α–producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 194:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 43.Kadowaki, N., S. Ho, S. Antonenko, M. R. de Waal, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latz, E., A. Schoenemeyer, A. Visintin, K.A. Fitzgerald, B.G. Monks, C.F. Knetter, E. Lien, N.J. Nilsen, T. Espevik, and D.T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190–198. [DOI] [PubMed] [Google Scholar]
- 45.McClain, M.T., L.D. Heinlen, G.J. Dennis, J. Roebuck, J.B. Harley, and J.A. James. 2005. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 11:85–89. [DOI] [PubMed] [Google Scholar]
- 46.Vallin, H., A. Perers, G.V. Alm, and L. Ronnblom. 1999. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-α inducer in systemic lupus erythematosus. J. Immunol. 163:6306–6313. [PubMed] [Google Scholar]
- 47.Boule, M.W., C. Broughton, F. Mackay, S. Akira, A. Marshak-Rothstein, and I.R. Rifkin. 2004. Toll-like receptor 9–dependent and –independent dendritic cell activation by chromatin–immunoglobulin G complexes. J. Exp. Med. 199:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]
- 49.Viglianti, G.A., C.M. Lau, T.M. Hanley, B.A. Miko, M.J. Shlomchik, and A. Marshak-Rothstein. 2003. Activation of autoreactive B cells by CpG dsDNA. Immunity. 19:837–847. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman, R.W., T. Gazitt, M.F. Foecking, R.A. Ortmann, M. Misfeldt, R. Jorgenson, S.L. Young, and E.L. Greidinger. 2004. U1 RNA induces innate immunity signaling. Arthritis Rheum. 50:2891–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reeves, W., M. Satoh, and D. McCauliffe. 2002. Autoantibody testing by non-FITC methods. In Manual of Clinical Laboratory Immunology. N. Rose, editor. American Society of Microbiology Press, Washington, DC. 933–950.