Abstract
The role of natural CD4+CD25+ regulatory T (T reg) cells in the control of allergic asthma remains poorly understood. We explore the impact of T reg cell depletion on the allergic response in mice susceptible (A/J) or comparatively resistant (C3H) to the development of allergen-induced airway hyperresponsiveness (AHR). In C3H mice, anti-CD25–mediated T reg cell depletion before house dust mite treatment increased several features of the allergic diathesis (AHR, eosinophilia, and IgE), which was concomitant with elevated T helper type 2 (Th2) cytokine production. In similarly T reg cell–depleted A/J mice, we observed a moderate increase in airway eosinophilia but no effects on AHR, IgE levels, or Th2 cytokine synthesis. As our experiments suggested that T reg cell depletion in C3H mice before sensitization was sufficient to enhance the allergic phenotype, we characterized dendritic cells (DCs) in T reg cell–depleted C3H mice. T reg cell–depleted mice had increased numbers of pulmonary myeloid DCs with elevated expression of major histocompatibility complex class II, CD80, and CD86. Moreover, DCs from T reg cell–depleted mice demonstrated an increased capacity to stimulate T cell proliferation and Th2 cytokine production, which was concomitant with reduced IL-12 expression. These data suggest that resistance to allergen-driven AHR is mediated in part by CD4+CD25+ T reg cell suppression of DC activation and that the absence of this regulatory pathway contributes to susceptibility.
Allergic asthma is a chronic inflammatory disease of the lung whose incidence and morbidity continues to rise in developed nations. Asthma is thought to occur as a result of inappropriate type 2–dominated immune responses to otherwise innocuous environmental allergens. In particular, type 2 cytokines (i.e., IL-4, IL-5, and IL-13) have been shown to regulate the pathological features of disease, including airway hyperresponsiveness (AHR), eosinophilic pulmonary inflammation, airway wall remodeling, and IgE synthesis (1). Despite an increasingly sophisticated understanding of the role of these cytokines in disease pathogenesis, the mechanism underlying the development of skewed T helper type 2 (Th2) immune responses in certain individuals remains unknown. An improved understanding of the mechanisms of regulation of T cell activity in allergic disease may lead to the development of effective therapeutic strategies for the treatment of this debilitating disease.
Regulatory T (T reg) cells are T cells functionally distinguished by their capacity to limit T cell proliferation and effector function (2, 3). Although there is extensive evidence that T reg cells play a major role in controlling autoimmune responses (4), studies exploring the role of T reg cells in allergic disease have primarily focused on inducible T reg cells (referred to as Tr1 or Th3 cells). In humans, the reduction of allergic symptoms after successful allergen immunotherapy was associated with the appearance of IL-10–producing T reg cells (5, 6). In animal studies, a population of CD4+ T cells induced by OVA (alum) immunization has been shown to inhibit the development of IgE responses (7). Inhalational tolerance is also mediated by ICOS/ICOS-L–driven expansion of IL-10–producing T reg cells (8). Additionally, exposure to inhaled allergen before systemic sensitization induces decreased IgE synthesis and airway eosinophilia by promoting the development of CD4+ T reg cells expressing surface-bound TGF-β (9). Interestingly, exposure to heat-killed Mycobacterium vaccae gives rise to a population of IL-10– and TGF-β–producing T reg cells that limit synthesis of IgE, type 2 cytokine production, and AHR after subsequent allergen exposure (10). In a similar manner, systemic allergen sensitization in the presence of killed Listeria monocytogenes markedly reduces airway inflammation and AHR by facilitating the development of a unique population of T reg cells that produce IL-10 and IFN-γ and express ICOS, T-bet, and Foxp3 (11). Collectively these studies provide compelling evidence that induction of T reg cell populations (Tr1, Th3, or Th1-like Tr cells) can facilitate the suppression of allergic inflammation and AHR through increased IL-10 and/or TGF-β production.
Naturally occurring regulatory cells are a distinct, thymus-derived population of regulatory CD4+ T cells that constitutively express the IL-2 receptor α chain, CD25. Expression of Foxp3, a forkhead transcription factor induced early in thymic T cell development after TCR–MHC interactions confers regulatory activity to the CD4+ CD25+ T cell population (12). In contrast to the consistently reported inhibitory effect of inducible T reg cells on allergic responses, the few studies exploring the importance of naturally occurring T reg cells in control of allergic disease have yielded conflicting data. Although the development of AHR is consistently unaffected by altering the number of CD4+CD25+ T reg cells present (13, 14), the development of eosinophilic airway inflammation is reported to be both increased (13) and, paradoxically, decreased (15) after depletion of naturally occurring CD4+ CD25+ T reg cells. Interestingly, the role of naturally occurring T reg cells has only been evaluated in strains of mice that are genetically predisposed to the development of experimental asthma, which may confound determination of the role they normally play in controlling the development of allergic asthma in genetically resistant hosts.
In this paper, we determined the extent to which naturally occurring T reg cells limit the development of experimentally induced asthma in mice genetically predisposed (A/J) or comparatively resistant (C3H/HeJ) to the development of AHR. We find that in vivo depletion of natural CD4+ CD25+ T reg cells in resistant animals before initial antigen contact increases AHR, airway eosinophilia, IgE synthesis, and is concomitant with type 2 cytokine production. Despite effective in vivo depletion of T reg cells and an equivalent ability of T reg cells from susceptible mice to suppress T effector function in vitro, T reg cell depletion has no effect on allergen-driven AHR in the A/J mice. Enhancement of the allergic phenotype in T reg cell–depleted C3H mice is associated with an enhanced ability of pulmonary DCs to stimulate T cell proliferation and Th2 cytokine production in vitro and an up-regulation of MHC class II and co-stimulatory molecule expression on the myeloid DC (mDC) population. Thus, we conclude that naturally occurring CD4+ CD25+ T reg cells exert their suppressive effects in vivo not through direct effects on T cells, but rather by limiting DC activation.
Results
T reg cell depletion renders normally resistant C3H mice susceptible to the development of AHR
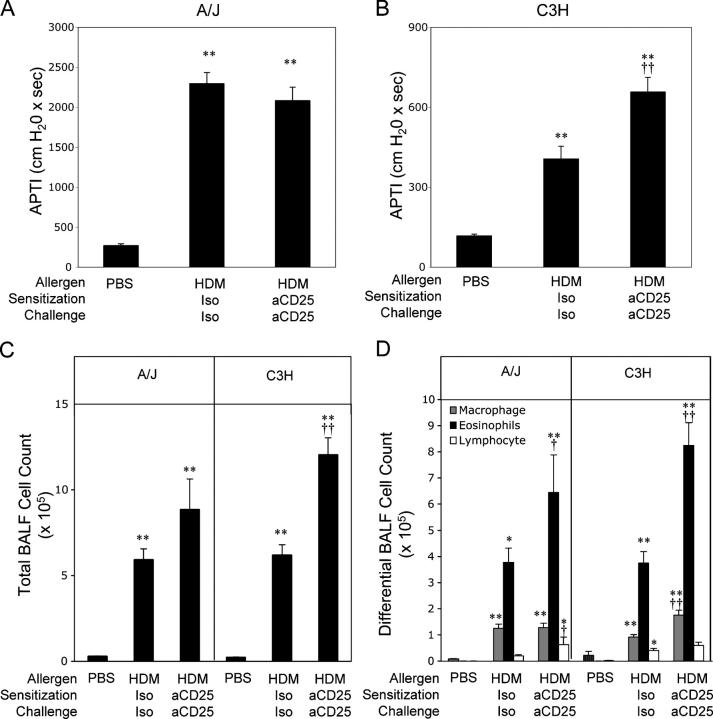
To directly assess the importance of T reg cells in the control of experimentally induced asthma, we examined AHR in T reg–depleted allergen-sensitized and -challenged mice susceptible (A/J) or resistant (C3H) to the development of allergen-induced AHR (16). To deplete T reg cells throughout the duration of the experiment, mice were treated with the T reg cell–depleting anti-CD25 mAb PC61 (17, 18) or an isotype control before sensitization (day –2) and during the effector phase (day 12) of the immune response. As shown in Fig. 1 A, intratracheal (i.t.) house dust mite (HDM) instillation in isotype-treated A/J mice resulted in a greater than eightfold increase in AHR compared with PBS-treated animals. Anti-CD25 treatment did not significantly alter AHR levels in A/J mice (Fig. 1 A). In contrast, although HDM exposure increased AHR approximately threefold in C3H mice, anti-CD25 treatment resulted in an additional 75% increase in AHR above that seen in C3H mice treated with control mAb (Fig. 1 B). These results suggest that natural T reg cells normally exert regulatory control that limits the development of allergen-induced AHR in resistant but not susceptible mice.
Figure 1.
Increased bronchial hyperresponsiveness and airway inflammation in anti-CD25–treated C3H mice. A/J and C3H animals were sensitized and challenged with PBS or HDM as described in Materials and methods. HDM-treated animals were treated with isotype control mAb (HDM Iso) or anti-CD25 (HDM aCD25) throughout the protocol. AHR of A/J (A) and C3H (B) mice was measured. Total (C) and differential (D) BALF cell counts. Data represent means + SEM (n = 20 mice/group). * or **, P < 0.05 or P < 0.001, respectively, compared with PBS-sensitized/challenged animals; † or ††, P < 0.05 or P < 0.001, respectively, compared with HDM isotype–treated animals.
To examine the effects of T reg cell depletion on allergen-induced pulmonary inflammation, bronchoalveolar lavage fluid (BALF) was collected and cell counts were performed. In both A/J and C3H mice exposed to i.t. HDM, a dramatic increase in BALF cellularity was seen (Fig. 1 C), of which eosinophils constituted 65 and 55% in A/J and C3H animals, respectively (Fig. 1 D). Depletion of T reg cells resulted in a significant increase in the number of eosinophils in the BALF of both A/J and C3H anti-CD25–treated mice compared with those receiving isotype control mAb (70 and 120% increases in A/J and C3H mice, respectively; Fig. 1 D). Significant increases in BALF macrophage and lymphocyte numbers were also seen in anti-CD25–treated C3H and A/J mice, respectively (Fig. 1 D). It is particularly interesting to note that anti-CD25 treatment of both strains of mice resulted in enhanced eosinophilia (albeit not as dramatically in the A/J mice), yet only C3H mice had a concomitant increase in AHR. This suggests that there is a disassociation between control of eosinophilic inflammation and AHR by natural CD4+CD25+ T reg cells.
Depletion of T reg cells enhances IgE synthesis and Th2 cytokine production in resistant mice
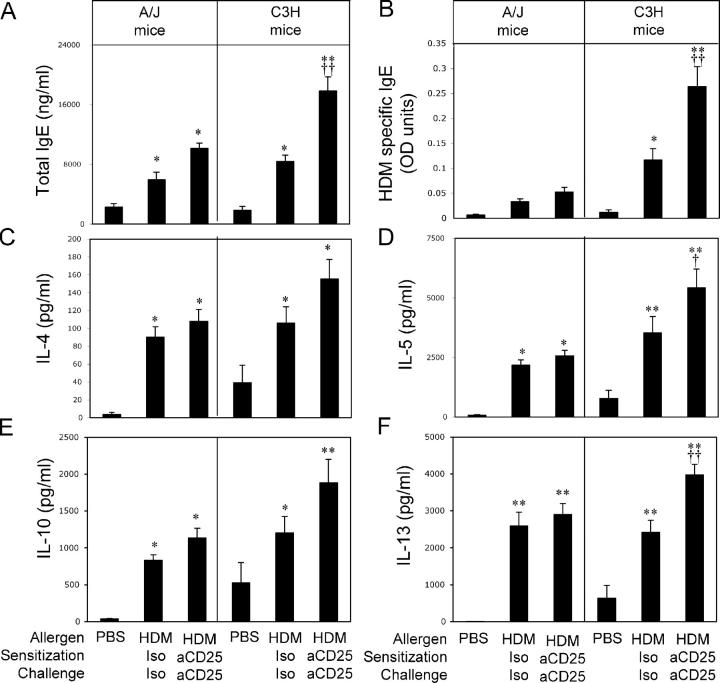
To measure the impact of T reg cell depletion on in vivo production of IgE, animals were bled immediately before death for measurement of total and HDM-specific IgE levels. Both A/J and C3H mice exposed to HDM produced elevated levels of total (Fig. 2 A) and HDM-specific IgE in comparison with their respective controls (Fig. 2 B). Although levels of total and HDM-specific IgE were similar in anti-CD25– and control mAb–treated A/J mice, there was a dramatic increase in IgE production in T reg cell–depleted C3H mice compared with C3H mice treated with control antibodies (Fig. 2, A and B).
Figure 2.
T reg cell depletion increases intensity of immune responses in C3H but not A/J animals. A/J and C3H animals were sensitized and challenged with PBS or HDM as described in Materials and methods. HDM-treated animals were treated with isotype control mAb (HDM Iso) or anti-CD25 (HDM aCD25) throughout the protocol. Serum levels of total IgE (A) and HDM-specific IgE (B) were measured by ELISA at death, 72 h after final HDM challenge. Production of IL-4 (C), IL-5 (D), IL-10 (E), and IL-13 (F), measured by ELISA, of lung cells cultured ex vivo for 72 h in the presence of 30 μg/ml HDM extract. Data represent means + SEM (n = 20 mice/group). * or **, P < 0.05 or P < 0.001, respectively, compared with PBS-sensitized/challenged animals; † or ††, P < 0.05 or P < 0.001, respectively, compared with HDM isotype–treated animals.
As the pathophysiological manifestations of allergic asthma are typically accompanied by robust Th2 immune responses, we next examined the effect of T reg cell depletion on the intensity of the HDM-specific type 2 immune response. To measure the intensity of the allergic response in anti-CD25–treated A/J and C3H mice, we examined HDM-stimulated cytokine production in whole lung cell cultures. Lung cells from HDM-sensitized and -challenged A/J and C3H mice both produced readily detectable quantities of the type 2 cytokines IL-4, IL-5, IL-10, and IL-13 upon in vitro HDM restimulation (Fig. 2, C–F). In cultures from A/J mice, HDM-stimulated type 2 cytokine production from anti-CD25–treated cells was indistinguishable from that seen in cultures from control mice (Fig. 2, C–F). In contrast, production of IL-5 and IL-13 was significantly increased in lung cultures from anti-CD25–treated C3H mice (Fig. 2, D and F). Although there was also clearly elevated IL-4 and IL-10 production in cultures from anti-CD25–treated C3H mice, these values did not reach statistical significance (Fig. 2, C and E). IFN-γ production was undetectable in HDM-stimulated lung cell cultures from both strains of mice under all conditions demonstrating a strong type 2 bias of the immune response (unpublished data). Thus, although natural CD4+CD25+ T reg cells have the capacity to limit airway eosinophilia in both strains of animals, other parameters of the asthmatic response such as IgE synthesis, type 2 cytokine production, and AHR are only under the regulatory control of T reg cells in genetically resistant animals. These results suggest that resistance to the development of asthma may result from a population of CD4+CD25+ T reg cells with a more potent regulatory capacity or that “target” cells from resistant individuals are uniquely sensitive to the suppressive influence of T reg cells.
Failure of anti-CD25 treatment of A/J mice to enhance immunity is not caused by incomplete T reg cell depletion
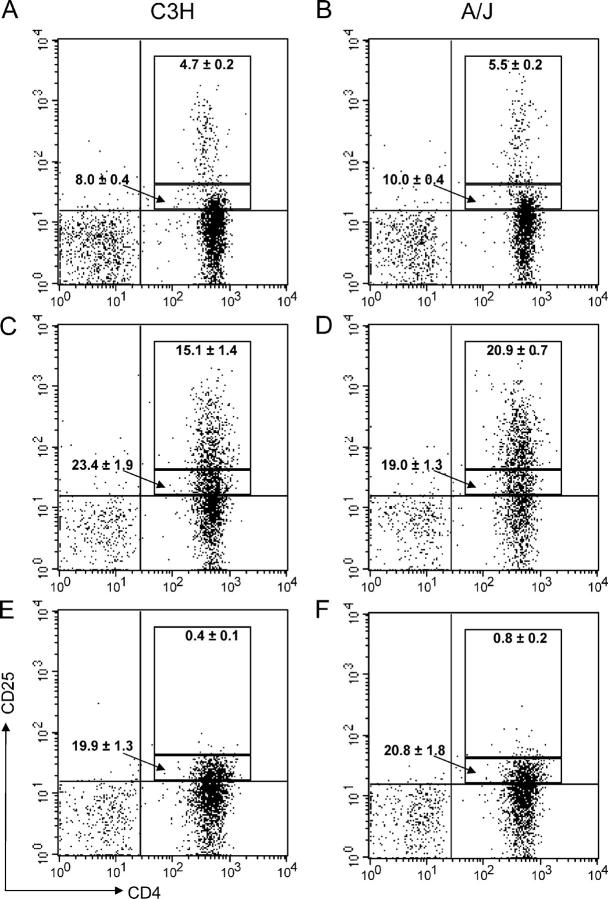
To determine whether the differences in the effect of T reg cell depletion on the allergic response in the two strains is caused by differences in the numbers of T reg cells in the lungs, we examined the numbers of T reg cells in the lungs of susceptible A/J and resistant C3H mice. Flow cytometry experiments confirmed that the lungs of naive C3H and A/J mice contained similar numbers of CD4+CD25bright cells (T reg cells) and CD4+CD25dim cells (effector T cells), demonstrating that there is not a baseline deficiency of T reg cells in A/J animals (Fig. 3, A and B). Sorted CD4+CD25bright T cells had ∼25-fold higher expression levels of Foxp3 compared with CD4+CD25− cells, confirming an enrichment in T reg cells (unpublished data). After sensitization and challenge with HDM, there is a similar four- to fivefold increase in the percentage of T reg cells in the lungs of both strains, again suggesting that A/J and C3H mice do not differ in their ability to recruit CD4+CD25+ cells to the site of inflammation (Fig. 3, C and D). Finally, when the levels of T reg cells were determined in anti-CD25–treated animals, the CD4+CD25bright population was virtually eliminated in both strains of mice, demonstrating that the anti-CD25 mAb was equally effective in each (Fig. 3, E and F). The anti-CD25 clone 7D4 was also used to stain cells with similar results (unpublished data). Furthermore, there was no significant difference in Foxp3 expression in cells from A/J and C3H mice (P = 0.55), again suggesting that there is no difference in T reg cell numbers in the two strains (unpublished data). These data clearly demonstrate that the inability of anti-CD25 treatment to enhance type 2 immunity in A/J mice is not a result of incomplete T reg cell depletion or a lack of T reg cells in the lungs of this strain.
Figure 3.
Anti-CD25 treatment efficiently depletes T reg cells in A/J mice. Lung cells from C3H (A, C, and E) and A/J (B, D, and F) animals treated with PBS (A and B), HDM (C and D), or HDM + anti-CD25 (E and F) were stained with specific antibodies to CD4 and CD25 for quantification of CD4+CD25bright T reg cells and CD4+CD25dim effector T cells. Values indicate means ± SEM of frequency of CD4+CD25dim and CD4+CD25bright populations from groups of 16 mice.
Comparison of CD4+CD25+ cell-induced suppression of effector T cell proliferation in A/J and C3H mice
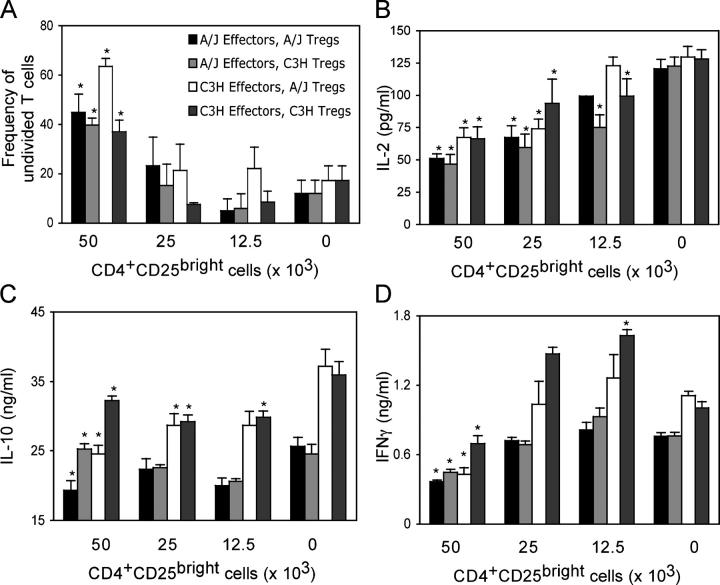
The demonstration that T reg cell depletion does not enhance the Th2 immune response in susceptible A/J mice despite similar numbers of CD4+CD25+ cells suggests that A/J mice may possess an intrinsic defect in T reg cell suppressive capacity. To directly examine the regulatory capacity of T reg cells from the lungs of A/J and C3H mice, we co-cultured sorted T reg cells (CD4+CD25bright, >95% purity) from C3H or A/J mice with carboxyfluorescein succinimidyl ester (CFSE)–labeled effector T cells (CD4+CD25−, 99% purity) taken from the same strain (i.e., A/J T reg cells with A/J effectors) or the opposite strain (i.e., A/J T reg cells with C3H effectors). Both CD4+CD25− and CD4+CD25bright T cell populations were purified from HDM-sensitized and -challenged animals. As MHC mismatch between A/J and C3H mice prevented the use of APCs in these cultures, cells were stimulated with anti-CD3/CD28–coated latex beads. When purified CD4+CD25bright cells were included in the cultures, there was a significant decrease in the proliferation (Fig. 4 A) of effector T cells, demonstrating that the CD4+CD25bright population possesses suppressive activity and that it was equally suppressive in A/J and C3H animals (compare closed with light gray bars). Additionally, examination of effector T cell cytokine production in co-culture supernatants revealed that CD4+CD25bright T reg cells isolated from the lungs of both A/J and C3H animals have the capacity to inhibit the synthesis of the effector T cell cytokines IL-2 and IFN-γ (Fig. 4, B and D) and IL-4 (unpublished data). CD4+CD25bright T reg cells from the lungs do not limit effector T cell activation by up-regulating expression of IL-10, as levels of this cytokine were also reduced in the presence of T reg cells (Fig. 4 C). It is also interesting to note that CD4+CD25− T cell populations from the two strains were equally sensitive to suppression of proliferation and cytokine synthesis by T reg cells from A/J mice (Fig. 4, A and D, compare filled with open bars), demonstrating that T reg cells from A/J mice are not only capable of suppression but also that effector T cell targets are sensitive to their suppressive influence. Similar regulatory capacity and effector T cell sensitivity in the two strains argues that CD4+ CD25+ T reg cells limit the severity of AHR in C3H but not A/J mice through influences on a non–T cell population.
Figure 4.
Natural CD4+CD25+ T reg cells are equally functional in A/J and C3H animals. CD4+CD25− and CD4+CD25bright cells were purified from the lungs of HDM-sensitized and -challenged A/J or C3H mice and stained with CFSE. 50,000 CFSE-labeled CD4+CD25− T cells were co-cultured with the indicated numbers of CD4+CD25bright and stimulated with anti-CD3/-CD28–coated latex beads as described in Materials and methods. Co-cultures consisted of CD4+CD25−/CD4+CD25bright population from A/J/A/J, A/J/C3H, C3H/A/J, or C3H/C3H mice as indicated. TCR-β+ T cells were detected via flow cytometry, and intensity of CFSE staining was used to identify T cells that remained undivided after 5 d of culture (A). Levels of IL-2 (B), IL-10 (C), or IFN-γ (D) were assessed in tissue culture supernatant by ELISA. Data represent means ± SEM. *, P < 0.05 between cultures stimulated in the presence and absence of CD4+CD25bright.
T reg cell depletion during the initiation of immune responses is sufficient to enhance allergen-induced AHR in resistant C3H mice
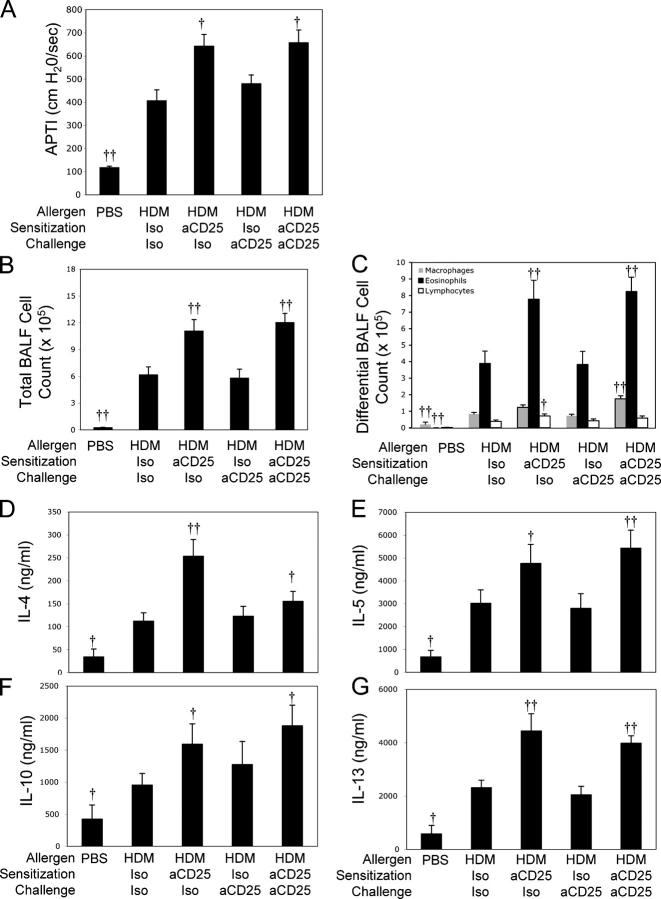
We next determined at what stage of the immune response the presence of T reg cells was required for limiting the development of AHR in C3H mice. To this end, we administered anti-CD25 only during induction of the immune response (day –2), during the development of the recall immune response (day 12), or throughout the allergen treatment protocol (day –2 and day 12) and examined the resulting allergic responses. As previously demonstrated, treatment of C3H mice with anti-CD25 throughout the course of the experiment results in enhanced AHR (Figs. 1 D and 5 A). Treatment of mice with anti-CD25 before the initiation of the immune response was sufficient to increase AHR to levels similar to that seen in mice receiving anti-CD25 treatment throughout (Fig. 5 A). Similar results were found for BAL eosinophilia: a single dose of anti-CD25 before sensitization enhanced eosinophil counts to a level indistinguishable from those seen in mice receiving anti-CD25 throughout (Fig. 5 C). In contrast, treatment of mice with only anti-CD25 during the effector phase of the immune response did not significantly enhance AHR or BALF cellularity compared with mice receiving the isotype control mAbs (Fig. 5, A–C).
Figure 5.
T reg cell depletion during initiation of immune response is sufficient to exacerbate AHR. C3H mice were treated with PBS or HDM as in Materials and methods. HDM-treated mice were treated with isotype/isotype (Iso Iso), aCD25/isotype (anti-CD25 Iso), isotype/anti-CD25 (Iso aCD25), or anti-CD25/anti-CD25 (aCD25 aCD25) during the initiation and effector phases, respectively. AHR (A) to i.v. acetylcholine was assessed before death, 72 h after final HDM challenge. BALF was harvested at time of death and total BAL cells (B) and differential cell counts (C) were performed. Lung cells from the indicated groups were cultured in the presence of 30 μg/ml HDM, and tissue culture supernatants were harvested after 72 h to determine production of IL-4 (D), IL-5 (E), IL-10 (F), and IL-13 (G). Data represent means + SEM (n = 12 mice/group). † or ††, P < 0.05 or P < 0.001, respectively, compared with HDM Iso/Iso–treated animals.
To examine the intensity of the type 2 immune response, production of Th2 cytokines was examined in HDM-stimulated whole lung cell cultures from mice that received treatment with anti-CD25 before the initiation of the immune response, during the effector phase, or throughout the experiment. HDM-stimulated type 2 cytokine production in mice depleted of T reg cells during the initiation of the immune response was indistinguishable or, in the case of IL-4, slightly elevated compared with cultures from mice given anti-CD25 throughout the experiment (Fig. 5, D–G). In contrast, cultures of HDM-stimulated lung cells from mice treated with anti-CD25 during the effector phase of the immune response did not consistently produce more cytokines compared with those treated with isotype control antibodies (Fig. 5, D–G). Although administration of anti-CD25 mAb during the effector phase of the immune response consistently failed to enhance the intensity of airway responses or cytokine production, it is important to point out that treatment with anti-CD25 also failed to diminish any features of the allergic phenotype as expected if anti-CD25 treatment was depleting CD25+ effector T cells. This confirms that the CD25bright population depleted after anti-CD25 administration consists primarily of natural Foxp3+ T reg cells, as reported elsewhere (12). Collectively, these data demonstrate that in our model of HDM-stimulated experimental asthma, the principal endogenous controls that limit the development of allergen-induced AHR in naturally resistant hosts occur very early in the immune response and not at the effector T cell level.
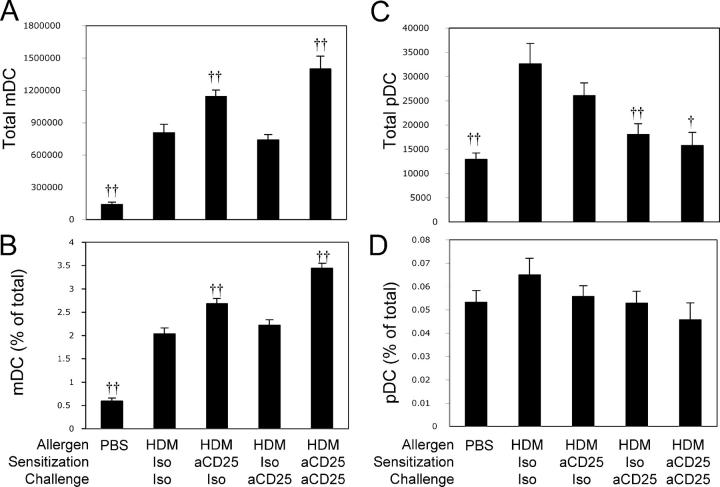
Depletion of T reg cells increases the frequency of immunogenic pulmonary DCs
The data in Fig. 5 demonstrate that T reg cell–mediated suppression after initiation of the immune response is not required to control AHR in C3H mice. Because DCs are known for their ability to initiate an immune response and pulmonary DCs have potent antigen-presenting capacity (19), we assessed the impact of anti-CD25 treatment on the numbers and phenotype of DCs found in the lung after allergen challenge. We were particularly interested in plasmacytoid DCs (pDCs [CD11c+, CD11b−, Gr1+, and B220+]) and mDCs (CD11c+, CD11b+, Gr1−, and B220−), as these subsets had been ascribed immunosuppressive and immunostimulatory roles, respectively, in experimental asthma models (20). Compared with PBS-treated C3H mice, HDM-challenged C3H mice treated with control mAbs displayed a fivefold increase in the number of both pulmonary mDCs and pDCs after HDM sensitization/challenge (Fig. 6). Treatment with anti-CD25 before the initiation of the immune response or throughout the entire treatment protocol further increased the number (Fig. 6 A) and frequency (Fig. 6 B) of mDCs found in the lungs of HDM-exposed C3H mice. In contrast, when T reg cell depletion was delayed until the effector phase, the anti-CD25–induced increase in mDC number and frequency was completely abrogated (Fig. 6, A and B). Interestingly, treatment with anti-CD25, particularly during the effector phase, resulted in a decrease in the number and frequency of pDCs found in the lung to levels similar to those seen in naive mice (Fig. 6, C and D). In contrast, anti-CD25 treatment of A/J mice did not significantly increase the number of mDCs (556,776 ± 69,067 vs. 571,492 ± 74,718 for Iso/Iso- and anti-CD25/anti-CD25–treated A/J mice, respectively) or significantly decrease the number of pDCs (26,268 ± 2,082 vs. 29,556 ± 2,992 for Iso/Iso- and anti-CD25/anti-CD25–treated A/J mice, respectively) in the lungs of HDM-sensitized and -challenged mice. Thus, although natural T reg cells limit the number of immunogenic mDCs in the lungs of resistant hosts, pulmonary DCs from normally susceptible animals appear refractory to similar T reg cell–mediated suppression.
Figure 6.
T reg cell depletion before HDM sensitization increases the frequency of pulmonary mDCs. Mice were treated as in Fig. 5. Total number (A) and frequency (as a function of total lung cells) of mDCs (CD11c+, CD11b+, Gr1−; B) and total number (C) and frequency (as a function of total lung cells; D) of pDCs (CD11c+, CD11b−, Gr1+) were determined by flow cytometry. Data represent means + SEM (n = 12 mice/group). † or ††, P < 0.05 or P < 0.001, respectively, compared with HDM Iso/Iso–treated animals.
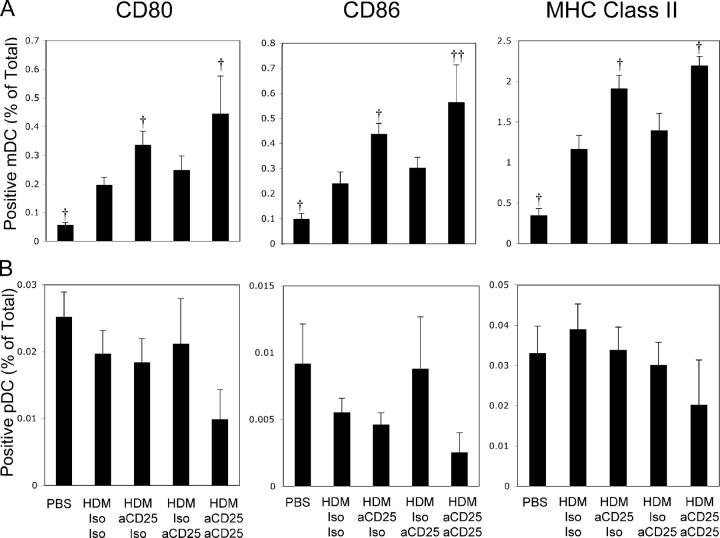
Pulmonary DCs from anti-CD25–treated animals have elevated expression of co-stimulatory molecules
As DC expression of ICOS-L or PD-L1/PD-L2 facilitates down-regulation of T cell activity by signaling through T cell–expressed ICOS or PD-1 (8, 21), we hypothesized that reduced expression of these coinhibitory molecules may promote the enhanced asthmatic phenotype observed after T reg cell depletion in C3H mice. However, treatment with anti-CD25 did not significantly decrease HDM-induced ICOS-L (mDC, P = 0.89; pDC, P = 0.97), PD-L1 (mDC, P = 0.36; pDC, P = 0.69), or PD-L2 (mDC, P = 0.21; pDC, P = 0.96) expression on mDC or pDC populations (unpublished data). Although the frequency of ICOS+ T cells did decrease as a result of anti-CD25 treatment, the remaining T cells expressing ICOS had a greater level of expression on a per-cell basis (unpublished data). The failure of anti-CD25 treatment to consistently alter expression of ICOS/ICOS-L or PD-L1/PD-L2/PD-1 family members led us to conclude that these pathways were not of critical importance in our model system.
We next examined the T cell stimulatory capacity of pulmonary DCs taken from anti-CD25– or isotype-treated allergen-primed C3H mice. To this end, expression of MHC class II, CD80, and CD86 were examined on pulmonary mDC and pDC subsets by flow cytometry in mice treated with anti-CD25 before the initiation of the immune response, during the effector phase, or both. As shown in Fig. 7 A, compared with PBS-treated control C3H mice, the frequency of CD80-, CD86-, and MHC class II–expressing mDCs (as a function of total lung cells) was significantly increased in mice treated with HDM and control mAb. Interestingly, concomitant with observed increases in AHR, expression of all three co-stimulatory molecules examined was significantly increased only in mice that received anti-CD25 before the initiation of the immune response (Fig. 7 A). In contrast, mice treated with only anti-CD25 during the effector phase of the immune response demonstrated no appreciable change in the frequency of CD80+, CD86+, or MHC class II+ mDCs (Fig. 7 A). The frequency of pulmonary pDC expression of MHC class II, CD80, and CD86 was not significantly affected by HDM treatment, and though there was a trend toward decreased pDC expression of CD80, CD86, and MHC class II in mice receiving anti-CD25 treatment throughout the protocol, this did not reach statistical significance (Fig. 7 B). It is important to stress that even though the increases in the frequency of CD80/86/MHC class II–expressing mDCs appears minor in the context of total lung cells, a 0.1% increase in frequency (as seen in CD80+ mDCs in anti-CD25/Iso–treated mice compared with Iso/Iso-treated mice) represents as many as 60,000 CD80+ mDCs. It is also important to note that expression of CD25 was not detectable on any subset of DCs (unpublished data), and, thus, alterations of pDC or mDC numbers are not caused by direct effects of the mAb. These data demonstrate that changes in DC phenotype and stimulatory capacity are strongly correlated with enhanced airway and cytokine responses seen after T reg cell depletion in naturally resistant mice.
Figure 7.
Depletion of CD4+CD25+ cells during the initiation of the immune responses increases the frequency of MHC class II, CD80, and CD86 expression mDCs. Mice were treated as in Fig. 5. Frequency (as a function of total lung cells) of CD80-, CD86-, and MHC class II–expressing mDCs (A) and pDCs (B) were determined by flow cytometry. Data represent means + SEM (n = 12 mice/group). † or ††, P < 0.05 or P < 0.001, respectively, compared with HDM Iso/Iso–treated animals.
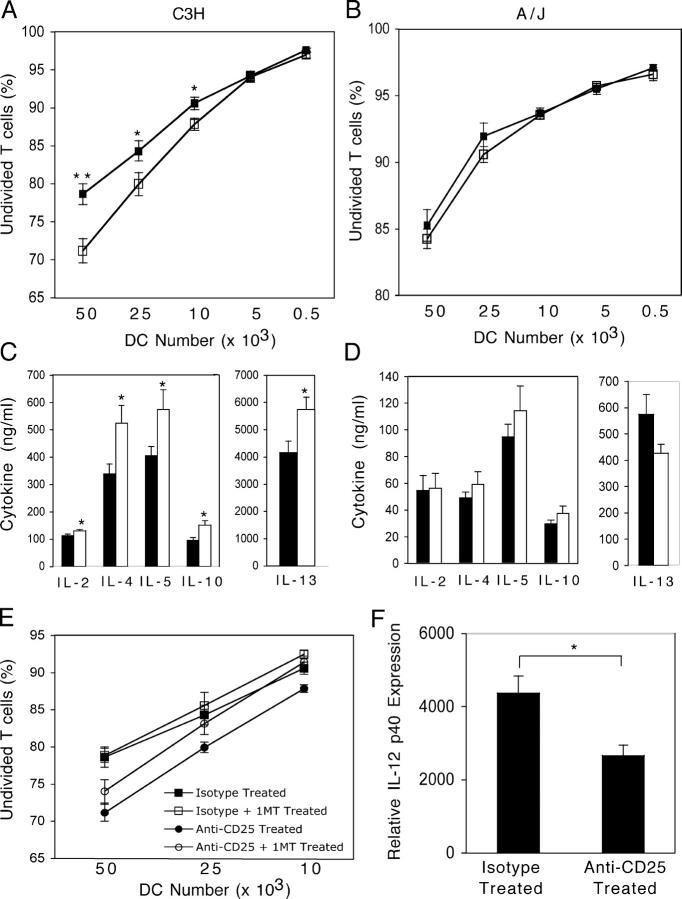
Pulmonary DCs from anti-CD25–treated C3H mice have increased T cell stimulatory capacity
The fact that T reg cell depletion increases the numbers of mDCs and their expression of antigen-presenting and co-stimulatory molecules suggests that perhaps T reg cells regulate the immunostimulatory capacity of DCs. To directly test this hypothesis, we purified CD11c+ DCs from the lungs of isotype control– or anti-CD25–treated HDM-sensitized mice 72 h after the final HDM challenge. Purified DCs were co-cultured with CFSE-labeled lung T cells (from mice treated with HDM alone, 72 h after final challenge) at various DC/T cell ratios, and the resultant T cell proliferation and cytokine production was quantified. As shown in Fig. 8 A, the number of undivided T cells in C3H cultures stimulated with DCs from anti-CD25–treated animals was significantly lower than that seen in cultures stimulated with DCs from isotype-treated animals. In contrast, anti-CD25 treatment of A/J mice does not result in a population of DCs that demonstrate increased T cell stimulatory capacity (Fig. 8 B). When T cell cytokine production was examined in DC/T cell co-cultures from anti-CD25–treated C3H mice (1:1 ratio), there was a substantial increase in the production of IL-4 (55%), IL-5 (42%), IL-10 (58%), and IL-13 (38%) and a small but significant increase in IL-2 (15%) synthesis (Fig. 8 C). Cytokine production in cultures stimulated with DCs from anti-CD25–treated A/J mice was identical to that seen in cultures stimulated with DCs from isotype-treated A/J mice (Fig. 8 D). IFN-γ was not produced in detectable quantities in any culture (unpublished data).
Figure 8.
T reg cell depletion enhances the T cell stimulatory capacity of pulmonary DCs from C3H but not A/J mice. CD11c+ DCs with low autofluorescence were sorted from lungs of isotype-treated (closed squares and bars) or anti-CD25–treated (open circles and bars) HDM-sensitized/challenged mice. DCs were co-cultured at the indicated ratios with CFSE-labeled CD4+ T cells purified from the lungs of HDM-sensitized/challenged mice in the presence of 30 μg/ml HDM. The frequency of undivided T cells was determined after 3 d of co-culture via flow cytometry in cultures from C3H (A) and A/J (B) mice. Cytokine levels in HDM-stimulated DC/T cell co-cultures (1:1) from C3H (C) and A/J (D) mice. (E) The effect of IDO inhibition in DC/T cell co-cultures was assessed by inclusion of 1-methyl-dl-tryptophan in co-cultures from isotype- and anti-CD25–treated C3H mice. (F) IL-12 p40 expression was examined in DCs purified from C3H mice as described above. Data represent means + SEM (n = 8 mice/group). * or **, P < 0.05 or P < 0.001, respectively, compared with cultures containing DCs from HDM Iso/Iso–treated animals.
To further explore potential mechanisms through which T reg cell–DC interactions may limit the development of AHR, we focused on indoleamine 2,3-dioxygenase (IDO) (22), an enzyme implicated in negative regulation of T cell activation (23). By adding the IDO inhibitor 1-methyl-dl-tryptophan to the DC/T cell co-cultures described above, we found that inhibition of IDO activity did not significantly alter the capacity of pulmonary DCs from isotype- or anti-CD25–treated animals to stimulate T cell proliferation. These data suggest that activity of this enzyme in pulmonary DCs is not significantly altered by T reg cell depletion. In contrast, the expression of IL-12 p40, a DC-derived cytokine involved in limiting the development of AHR in resistant C3H mice (24), was significantly lower (∼50%) in DCs isolated from anti-CD25–treated C3H mice as compared with levels seen in DCs from isotype-treated animals (Fig. 8 F). Similar inhibition of IL-12 p40 expression was not seen in anti-CD25–treated A/J mice (unpublished data). Thus, depletion of T reg cells in resistant animals does not diminish the activity of regulatory enzymes (like IDO), but rather renders pulmonary DCs less capable of limiting the development of a Th2-dominated immune response by limiting their capacity to produce IL-12. These data suggest that there are regulatory influences exerted by natural T reg cells on DCs that are unique to those hosts resistant to the development of AHR.
Discussion
Although the role of naturally occurring T reg cells in control of autoimmune disease is becoming increasingly apparent, their potential involvement in limiting the development of allergic asthma remains poorly understood. In this paper, we examined the role that natural T reg cells play in limiting the development of AHR by comparing the impact of CD4+CD25+ T reg cell depletion on airway responses in A/J (susceptible to development of AHR after allergen exposure) with C3H mice (resistant to development of AHR after allergen exposure). We demonstrate that depletion of CD4+CD25+ T cells significantly enhances AHR, airway inflammation, Th2 cytokine production, and in vivo IgE synthesis in C3H but not A/J animals. Interestingly, the results of our study suggests that naturally occurring CD4+ CD25+ T reg cells normally limit the development of AHR in resistant hosts by limiting expression of CD80, CD86, and MHC class II while promoting IL-12 production in pulmonary DCs, thereby preventing T cell proliferation and Th2 cytokine production. Furthermore, these data suggest that natural CD4+CD25+ T reg cells function through mechanisms distinct from antigen-inducible T reg cells and that failure of T reg cells to limit DC activation in normally susceptible animals contributes to the development of allergen-induced AHR.
We demonstrate that depletion of CD4+CD25+ T cells using an mAb to CD25 results in a significant enhancement of airway eosinophilia (Fig. 1 B), in vivo IgE synthesis (Fig. 2, A and B), and Th2 cytokine production (Fig. 2, C–F) in naturally resistant C3H mice. Consistent with increased intensity of type 2 immunity and increased airway eosinophilia, we also observed significant increases in AHR after depletion of CD4+CD25+ T reg cells. In contrast, despite dramatic reductions in the number of CD4+CD25+ T reg cells found in the lung (Fig. 3 F), similar treatment of susceptible A/J animals resulted in only a modest increase in airway eosinophilia (Fig. 1 D) without concomitant enhancement of AHR, type 2 cytokine production, or IgE serum levels (Fig. 2, A–F). Our findings are consistent with studies conducted in other asthma-prone murine strains, such as BALB/c, in which T reg cells were also shown to limit eosinophilia but not type 2 cytokine production or AHR (13, 14). Collectively, these results suggest that naturally occurring T reg cells have different levels of activity in hosts that are susceptible and resistant to the development of asthma. Consistent with this concept, recent experiments conducted in human subjects show that, compared with healthy controls, atopic subjects possessed reduced T reg cell numbers or defects in T reg cell function, demonstrating that allergy is accompanied by defects in the regulatory capacity of the host (25, 26).
One caveat to our study, as well as others using anti-CD25 to identify natural T reg cell populations, is that CD25 and other markers commonly used to identify T reg cells (ICOS, GITR, and CD103) are also expressed on recently activated effector T cells, making it impossible to rule out the possibility that effects of the mAb relate to depletion of activated T cells. However, the anti-CD25 mAb (PC61) used in our experiments has been used in other studies to transiently deplete T reg cells in vivo (17, 18, 27). Furthermore, the increased intensity of the immune response observed in C3H mice and, to a lesser extent, in A/J animals after administration of anti-CD25 is more consistent with the removal of a negative regulatory population than depletion of an effector T cell population. Interestingly, the anti-CD25 mAb used selectively depleted those cells expressing the highest levels of CD25 (Fig. 3, E and F), a population recently demonstrated to comprise primarily Foxp3+ cells (12, 28). As Foxp3 expression remains the most specific T reg cell marker identified to date and indeed confers regulatory capacity (12, 28), it seems likely that the anti-CD25 administration in this model depletes T reg and not effector T cells.
It is generally thought that T reg cell–mediated suppression of immune responses occurs at the level of the effector T cell either through regulation of IL-2 production by target T cell populations and/or expression of IL-10, CTLA-4 and/or TGF-β (29–32). Our data suggest, however, that natural T reg cells do not limit the development of AHR in resistant animals through interactions with effector T cells. This is supported by the following observations: (a) despite the dramatically different outcomes of T reg cell ablation in vivo in A/J and C3H mice, these mice do not markedly differ in either the suppressive capacity of their respective T reg cells or the sensitivity of their effector T cells to T reg cell–mediated suppression (Fig. 4); (b) in C3H mice, T reg cell depletion during the effector phase of the immune response fails to increase AHR, whereas depletion before the initiation of the immune response results in substantial increases in AHR (Fig. 5 A); and (c) pulmonary DCs purified from anti-CD25–treated C3H mice stimulate a greater level of T cell proliferation and cytokine production than those taken from mice with an intact T reg cell compartment (Fig. 8, A and C). Furthermore, we find increased IL-10 production after T reg cell depletion (Fig. 2 C) and no correlation between the observed changes in ICOS/ICOS-L (unpublished data) and the changes in AHR after T reg cell depletion (Fig. 5 A). This suggests that the effects of T reg cells in our model are not mediated through IL-10– or ICOS/ICOS-L–dependent pathways, as has been previously reported for T reg cells induced by exposure to mucosally administered allergen or exposure to bacterial products (8, 10, 11). These differences may reflect distinct roles of natural and inducible T reg cells (i.e., Tr1 or Tr3) in regulating immune responses in vivo.
In examining the impact of T reg cell depletion on pulmonary DC populations, several important observations were made. First, we demonstrate that T reg cell depletion before initiation of the immune response, but not during the effector phase, results in an increase in the number of pulmonary mDCs (Fig. 6, A and B). These changes in the frequency of mDCs correlate well with observed changes in AHR after anti-CD25 administration (Fig. 5 A). A recent study by de Heer et al. demonstrated that in murine models of allergic asthma, mDCs were particularly immunogenic in murine, whereas pDCs promoted tolerance to allergen exposure (20).
Thus, the observed shift toward increased frequency of mDCs in the lungs of anti-CD25–treated C3H mice represents the development of a highly immunogenic population of pulmonary DCs. Indeed, when the T cell stimulatory capacity of pulmonary DCs was examined, it was determined that T reg cell depletion enhanced the ability of DCs from C3H but not A/J animals to stimulate T cell proliferation and cytokine production (Fig. 8). Furthermore, enhanced expression of classical co-stimulatory molecules as a result of anti-CD25 treatment was confirmed by the observation of significantly enhanced expression of MHC class II, CD80, and CD86 in C3H mice depleted of natural T reg cells (Fig. 7 A). Similar negative regulation of MHC class II and CD80/CD86 expression by T cells has been observed when anergic T cells or T cells taken from cardiac allograft–tolerant mice were co-cultured with Ag-pulsed DCs (33, 34). Moreover, natural CD4+CD25+ T reg cells isolated from naive mice directly regulate DC expression of CD80 (by down-regulating CD80 mRNA levels) and CD86 (through unknown mechanisms) in a contact-dependent mechanism (35).
Although we cannot yet identify the precise mechanism by which T reg cells negatively regulate the phenotype and activation state of DCs, our results indicate that this regulation does not occur at the level of IDO activity (Fig. 8 E) in our model, as suggested elsewhere (22). Interestingly, our data do support a role for T reg cells in promoting the production of IL-12, as DC populations from T reg cell–depleted mice demonstrated reduced IL-12 p40 mRNA levels (Fig. 8F). Reduced IL-12 availability in “susceptible” C3H mice after T reg cell depletion is wholly consistent with our previous report demonstrating that IL-12 neutralization is sufficient to trigger robust airway responses in C3H mice (24). Although we are unaware of any report of direct T reg cell regulation of DC IL-12 synthesis, T reg cells do enhance DC IFN-γ production (22), and IFN-γ enhances IL-12 p40 expression (36), suggesting a potential mechanism by which T reg cells may promote IL-12 production. Although the observation of reduced IL-12 production is sufficient to enhance airway responses in resistant mice, we are presently unable to ascertain whether reducing IL-12 synthesis represents the specific mechanism by which T reg cells influence DC activation or whether depressed IL-12 p40 expression is a manifestation of other regulatory influences. Moreover, the lack of effect of T reg cell depletion on DC activation and p40 production in the susceptible strain may represent an inability of endogenous CD4+CD25+ T reg cells to limit DC activation or refractoriness of the DCs to the inhibitory effects of T reg cells. Further studies are required to elucidate the exact mechanisms underlying these genetic differences.
In summary, the findings reported here demonstrate that naturally occurring T reg cells play a major role in conferring natural resistance to the development of allergen-induced AHR. Moreover, our findings highlight a novel mechanism by which T reg cells exert their regulatory control on the allergic response, that of controlling DC activation and antigen presentation rather than through direct effects on effector T cells. Collectively, these results provide support for the hypothesis that development of allergic asthma may be a result of an inefficient regulatory network, one of the central tenets of the hygiene hypothesis (37, 38). Accordingly, these findings suggest that modulation of this pathway may provide an effective means of controlling aberrant immune responses to harmless airborne antigens.
MaterialS and Methods
Mice
5–6-wk-old male A/J and C3H/HeJ mice were purchased from the Jackson Laboratory and housed in a specific pathogen-free facility at Cincinnati Children's Hospital Medical Center. Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee approved all animal studies.
Characterization of allergen-induced asthma phenotype
A/J and C3H mice were given 100 μg HDM extract (Greer Laboratories) or 40 μl PBS i.t. on days 0, 14, and 21. To deplete T reg cells in vivo, mice were treated with 1 mg anti-CD25 (mAb PC61) or a rat IgG1 isotype control (mAb GL113; a gift of F. Finkelman, Cincinnati Children's Hospital, Cincinnati, OH) administered i.p. on day –2 and/or day 12. To evaluate airway responses, mice were anaesthetized, intubated, and respirated at a rate of 120 breaths per minute with a constant tidal volume (0.2 ml) and paralyzed with 25 mg/kg decamethonium bromide 72 h after final allergen challenge. After a stable baseline was achieved, 50 mg/kg acetylcholine was injected into the inferior vena cava, and dynamic airway pressure (cm H2O × sec) was followed for 5 min. Immediately after AHR measurements, blood was collected. Serum samples were tested for total and HDM-specific IgE using antibodies from BD Biosciences. To collect BALF, lungs were lavaged three times with a 1.0-ml aliquot of cold Hanks' balanced salt solution (Invitrogen). Recovered lavage fluid (70–80%) was centrifuged at 300 g for 8 min, and the cell pellet was resuspended in 1.0 ml of 10% FBS in PBS. Total cells were counted with a hemocytometer (Hauser Scientific). Slides were prepared by cytocentrifugation (Cytospin 3; Shandon Instruments) and stained with Diff-Quik (Dade Behring). BAL cell differential counts were determined using morphologic criteria under a light microscope (Optical Elements Corporation) with the evaluation of ≥500 cells/slide.
Lung cell isolation
After collection of BAL, lungs were perfused with 5 ml of ice-cold PBS and removed. Lungs were minced and placed in 6 ml RPMI 1640 containing 0.5 mg/ml Liberase CI (Roche Diagnostics) and 0.5 mg/ml DNase I (Sigma-Aldrich) and incubated at 37°C for 45 min. To make a single cell suspension, the remaining tissue was forced through a 70-μm cell strainer. Cells were pelleted and red blood cells were lysed using 1 ml of ACK lysis buffer (Biosource International). The remaining cells were washed with RPMI 1640 containing 10% FBS, and viable cells were counted via trypan blue exclusion and used in subsequent experiments. Cells were cultured at 8.3 × 105 cells/ml in a total volume of 300 μl. Cells were stimulated with culture medium alone or HDM at 30 μg/ml. Tissue culture supernatant was harvested at 72 h and frozen at –70°C until cytokine concentrations were assayed.
Determination of cytokine concentration
Cytokine levels in lung cell culture supernatants were measured by ELISA using matched antibody pairs purchased from BD Biosciences (IL-4 and IL-5) or R&D Systems (IL-10 and IL-13). Cytokine concentrations in tissue culture supernatants from DC/T cell co-cultures were measured using a Bio-Plex suspension array system (Bio-Rad Laboratories). Multiplex assay kits were purchased from Biosource International and used according to the manufacturer's instructions.
Flow cytometry
Staining reactions were performed at 4°C after incubation of cells with anti-CD16/32 mAb (clone 2.4G2) for 30 min. mDCs (CD11c+, CD11b+, and Gr1−) and pDCs (CD11c+, CD11b−, and Gr1+) were quantified using antibodies to CD11c (HL3), CD11b (M1/70), and Gr1 (RB6-8C5). Co-stimulatory molecule expression was examined by using antibodies to CD80 (16-10A1), CD86 (GL1), MHC class II I-Ek (14-4-4s), ICOS-L (HK5.3), B7-H1 (MIH5), and B7-DC (TY25). T cell subsets were examined using mAbs to CD4 (RM4-5), CD25 (PC61 or 7D4), and ICOS (7E.17G9), and proliferation was followed using CFSE (Invitrogen). Dead cells were excluded using the viability dye 7-amino-actinomycin D (7-AAD). All antibodies were purchased from BD Biosciences or eBioscience. All samples were analyzed for six colors using a FACSVantage SE flow cytometer equipped with 488-nm argon and 633-nm HeNe lasers and digital DiVa software. Spectral overlap was compensated using the DiVa software (BD Biosciences). Analysis was done using FlowJo software (Tree Star, Inc.).
T reg cell inhibition assay
The capacity of T reg cells from A/J and C3H mice to inhibit proliferation of T effectors from A/J and/or C3H mice was determined using an in vitro assay. Lungs of HDM-sensitized and -challenged A/J and C3H mice were removed, and T reg and effector T cell populations were sorted using a FACSVantage SE. T reg cells were defined as CD4+CD25bright, and effector T cells were defined as CD4+CD25−. Purified cells were >95 and 99% pure, respectively. 105 CD4+CD25− effectors were stained with CFSE and cultured in 96-well U-bottom plates with CD4+ CD25bright T reg cells at the ratios indicated in the figures. To avoid using APCs in these cultures (because of A/J–C3H MHC mismatch), cultures were stimulated with nothing or 5-μm latex beads (105 beads/well; Interfacial Dynamics Corporation) coated with anti-CD3 (145-2C11) and anti-CD28 (37.51). Cells were harvested after 3 d, and the proliferation index was determined based on CFSE dilution using FlowJo.
DC/T cell co-culture
The ability of purified pulmonary DCs to stimulate effector T cell proliferation and cytokine production was determined using an in vitro assay. CD11c+ 7-AAD− cells were purified from HDM-sensitized mice treated with isotype- or anti-CD25–treated A/J and C3H mice 72 h after final HDM exposure using a FACSVantage SE. To remove contaminating alveolar macrophages, which also express CD11c, cells with low autofluorescence in FL1 were then purified as previously described (39). The resulting population was >95% CD11c+, and 85% had low autofluorescence. CD4+ T cells were purified from the lungs of HDM-sensitized A/J or C3H animals (>99% purity). 5 × 104 T cells labeled with CFSE were cultured with varying numbers of purified DCs in the presence of 30 μg/ml HDM. Cells were harvested after 3 d of culture, and CFSE dilution was used to determine the degree of proliferation of TCR-β+CD4+ T cells using FlowJo. Day 3 tissue culture supernatants were frozen at –80°C for determination of T cell cytokine production.
Quantitative real-time RT-PCR
To measure IL-12 p40 and IDO message expression in flow cytometry–purified pulmonary DCs isolated from isotype- or anti-CD25–treated, HDM-sensitized and -challenged A/J and C3H mice, we used quantitative RT-PCR assays as described previously (40). PCR primer pairs were designed using Beacon Designer software (Premier Biosoft International) and were as follows: IL-12 p40 sense, CAGAAGCTAACCATCTCCTGG, and IL-12 p40 anti-sense, TCAGGCGTGTCACAGGTGAG.
Statistical analysis
For comparison between multiple groups, one-way analysis of variance was used to determine differences between groups with post hoc comparisons using Fisher's method. For comparison between two groups, Student's t test was performed. In both cases, significance was assumed at P < 0.05.
Acknowledgments
We would like to thank D. Marmer for FACS sorting and helpful discussions about flow cytometric analysis of DC populations.
This work was supported by National Heart, Lung, and Blood Institute grants HL67736 and HL076383 to M. Wills-Karp.
The authors have no conflicting financial interests.
Abbreviations used: AHR, airway hyperresponsiveness; BALF, bronchoalveolar lavage fluid; CFSE, carboxyfluorescein succinimidyl ester; HDM, house dust mite; IDO, indoleamine 2,3- dioxygenase; i.t., intratracheal; mDC, myeloid DC; pDC, plasmacytoid DC.
References
- 1.Wills-Karp, M. 2004. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 202:175–190. [DOI] [PubMed] [Google Scholar]
- 2.Piccirillo, C.A., and E.M. Shevach. 2004. Naturally-occurring CD4+ CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin. Immunol. 16:81–88. [DOI] [PubMed] [Google Scholar]
- 3.Coombes, J.L., N.J. Robinson, K.J. Maloy, H.H. Uhlig, and F. Powrie. 2005. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 204:184–194. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi, S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6:345–352. [DOI] [PubMed] [Google Scholar]
- 5.Francis, J.N., S.J. Till, and S.R. Durham. 2003. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 111:1255–1261. [DOI] [PubMed] [Google Scholar]
- 6.Jutel, M., M. Akdis, F. Budak, C. Aebischer-Casaulta, M. Wrzyszcz, K. Blaser, and C.A. Akdis. 2003. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 33:1205–1214. [DOI] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille, M.A., S. Muriglan, M.J. Sunshine, Y. Lei, N. Kutchukhidze, G.C. Furtado, A.K. Wensky, D. Olivares-Villagomez, and J.J. Lafaille. 2001. Hyper immunoglobulin E response in mice with monoclonal populations of B and T lymphocytes. J. Exp. Med. 194:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbari, O., G.J. Freeman, E.H. Meyer, E.A. Greenfield, T.T. Chang, A.H. Sharpe, G. Berry, R.H. DeKruyff, and D.T. Umetsu. 2002. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 9.Ostroukhova, M., and A. Ray. 2005. CD25+ T cells and regulation of allergen-induced responses. Curr. Allergy Asthma Rep. 5:35–41. [DOI] [PubMed] [Google Scholar]
- 10.Zuany-Amorim, C., E. Sawicka, C. Manlius, A. Le Moine, L.R. Brunet, D.M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625–629. [DOI] [PubMed] [Google Scholar]
- 11.Stock, P., O. Akbari, G. Berry, G.J. Freeman, R.H. Dekruyff, and D.T. Umetsu. 2004. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat. Immunol. 5:1149–1156. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 13.Jaffar, Z., T. Sivakuru, and K. Roberts. 2004. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J. Immunol. 172:3842–3849. [DOI] [PubMed] [Google Scholar]
- 14.Hadeiba, H., and R.M. Locksley. 2003. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J. Immunol. 170:5502–5510. [DOI] [PubMed] [Google Scholar]
- 15.Suto, A., H. Nakajima, S.I. Kagami, K. Suzuki, Y. Saito, and I. Iwamoto. 2001. Role of CD4(+) CD25(+) regulatory T cells in T helper 2 cell-mediated allergic inflammation in the airways. Am. J. Respir. Crit. Care Med. 164:680–687. [DOI] [PubMed] [Google Scholar]
- 16.Ewart, S.L., D. Kuperman, E. Schadt, C. Tankersley, A. Grupe, D.M. Shubitowski, G. Peltz, and M. Wills-Karp. 2000. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am. J. Respir. Cell Mol. Biol. 23:537–545. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu, J., S. Yamazaki, and S. Sakaguchi. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218. [PubMed] [Google Scholar]
- 18.McHugh, R.S., and E.M. Shevach. 2002. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 168:5979–5983. [DOI] [PubMed] [Google Scholar]
- 19.Julia, V., E.M. Hessel, L. Malherbe, N. Glaichenhaus, A. O'Garra, and R.L. Coffman. 2002. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 16:271–283. [DOI] [PubMed] [Google Scholar]
- 20.de Heer, H.J., H. Hammad, T. Soullie, D. Hijdra, N. Vos, M.A. Willart, H.C. Hoogsteden, and B.N. Lambrecht. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwald, R.J., G.J. Freeman, and A.H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548. [DOI] [PubMed] [Google Scholar]
- 22.Fallarino, F., U. Grohmann, K.W. Hwang, C. Orabona, C. Vacca, R. Bianchi, M.L. Belladonna, M.C. Fioretti, M.L. Alegre, and P. Puccetti. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4:1206–1212. [DOI] [PubMed] [Google Scholar]
- 23.Mellor, A.L., and D.H. Munn. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4:762–774. [DOI] [PubMed] [Google Scholar]
- 24.Keane-Myers, A., M. Wysocka, G. Trinchieri, and M. Wills-Karp. 1998. Resistance to antigen-induced airway hyperresponsiveness requires endogenous production of IL-12. J. Immunol. 161:919–926. [PubMed] [Google Scholar]
- 25.Akdis, M., J. Verhagen, A. Taylor, F. Karamloo, C. Karagiannidis, R. Crameri, S. Thunberg, G. Deniz, R. Valenta, H. Fiebig, et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling, E.M., T. Smith, X.D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V.A. Carr, and D.S. Robinson. 2004. Relation of CD4+ CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363:608–615. [DOI] [PubMed] [Google Scholar]
- 27.Haeryfar, S.M., R.J. DiPaolo, D.C. Tscharke, J.R. Bennink, and J.W. Yewdell. 2005. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 174:3344–3351. [DOI] [PubMed] [Google Scholar]
- 28.Wan, Y.Y., and R.A. Flavell. 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 102:5126–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura, K., A. Kitani, I. Fuss, A. Pedersen, N. Harada, H. Nawata, and W. Strober. 2004. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 172:834–842. [DOI] [PubMed] [Google Scholar]
- 31.Sundstedt, A., E.J. O'Neill, K.S. Nicolson, and D.C. Wraith. 2003. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 170:1240–1248. [DOI] [PubMed] [Google Scholar]
- 32.Thornton, A.M., E.E. Donovan, C.A. Piccirillo, and E.M. Shevach. 2004. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J. Immunol. 172:6519–6523. [DOI] [PubMed] [Google Scholar]
- 33.Min, W.P., D. Zhou, T.E. Ichim, G.H. Strejan, X. Xia, J. Yang, X. Huang, B. Garcia, D. White, P. Dutartre, et al. 2003. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J. Immunol. 170:1304–1312. [DOI] [PubMed] [Google Scholar]
- 34.Frasca, L., C. Scotta, G. Lombardi, and E. Piccolella. 2002. Human anergic CD4+ T cells can act as suppressor cells by affecting autologous dendritic cell conditioning and survival. J. Immunol. 168:1060–1068. [DOI] [PubMed] [Google Scholar]
- 35.Cederbom, L., H. Hall, and F. Ivars. 2000. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 30:1538–1543. [DOI] [PubMed] [Google Scholar]
- 36.Ma, X., J.M. Chow, G. Gri, G. Carra, F. Gerosa, S.F. Wolf, R. Dzialo, and G. Trinchieri. 1996. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 183:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romagnani, S. 2004. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology. 112:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wills-Karp, M., J. Santeliz, and C.L. Karp. 2001. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat. Rev. Immunol. 1:69–75. [DOI] [PubMed] [Google Scholar]
- 39.Vermaelen, K., and R. Pauwels. 2004. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 61:170–177. [DOI] [PubMed] [Google Scholar]
- 40.Finkelman, F.D., M. Yang, C. Perkins, K. Schleifer, A. Sproles, J. Santeliz, J.A. Bernstein, M.E. Rothenberg, S.C. Morris, and M. Wills-Karp. 2005. Suppressive effect of IL-4 on IL-13-induced genes in mouse lung. J. Immunol. 174:4630–4638. [DOI] [PubMed] [Google Scholar]