Abstract
Long-lived plasma cells, residing primarily in the bone marrow, continuously secrete antibody and provide an important component of humoral memory. However, when such cells secrete autoantibodies or become transformed, they can be pathogenic. We have shown recently that the transcriptional repressor B lymphocyte–induced maturation protein 1 (Blimp-1) is required for the formation of plasma cells. To determine what role Blimp-1 might play in maintenance of plasma cells, we generated mice in which the gene encoding Blimp-1 could be deleted in an inducible manner. Deletion of Blimp-1 either in vitro or in vivo leads to loss of previously formed B220LOCD138HI plasma cells. Using BrdU incorporation, we confirmed that Blimp-1 is required for the maintenance of nondividing, long-lived plasma cells in the bone marrow. Blimp-1 is also required for long-term maintenance of antigen-specific immunoglobulin in serum. Thus Blimp-1 is required not only for the formation but also for the maintenance of long-lived plasma cells. This finding provides the possibility of new drug design strategies for autoimmunity and multiple myeloma focused on blocking Blimp-1 expression or activity.
Upon initial encounter with pathogens, B cells can differentiate into two types of cells that provide humoral memory as follows: (a) memory cells that differentiate into Ig-secreting plasma cells upon secondary antigenic challenge and (b) plasma cells that survive in the bone marrow, continuously secreting Ig (1). Long-lived plasma cells in the marrow are germinal center–experienced cells (2) that survive for months to years (3) in the absence of antigen (4) or cell division (5). These cells reside in a limited number of niches, primarily in the bone marrow, that provide them with survival signals (6). The antibodies that these long-lived plasma cells secrete provide protection for future encounters with the pathogens that led to their formation. Although long-lived plasma cells are critical for humoral memory, they can also be pathogenic when they express autoantibodies in diseases such as lupus erythematosus (7) or become transformed in multiple myeloma. Treatments designed to target B lineage cells, such as radiation, prednisone, cyclophosphamide, and anti-CD20 antibodies (8), do not eliminate nondividing long-lived plasma cells. Thus, in lupus and multiple myeloma, these treatments often do not lead to resolution of disease.
Despite the physiological and pathological significance of long-lived plasma cells, little is known about their maintenance. There is increased understanding, however, of how plasma cell formation is regulated (6). B lymphocyte–induced maturation protein-1 (Blimp-1) is a transcriptional repressor that is found both necessary (9) and sufficient (10) for plasma cell differentiation. Blimp-1 is called a master regulator of plasma cell differentiation because it directly represses transcription factors that, in turn, regulate several important gene programs (11). Blimp-1 represses c-myc and other genes involved in cell cycle progression and cell division (11, 12). Blimp-1 represses Bcl-6 (11), a key germinal center factor, and blocks other germinal center activities. Finally, Blimp-1 represses Pax-5 (13), which is required for B cell identity, germinal center function, and repression of XBP-1 (14); J chain; and Ig heavy and light chain transcription. By relieving Pax-5–dependent repression of these genes, Blimp-1 drives plasmacytic differentiation and Ig secretion (9, 15). Thus, Blimp-1 both induces plasmacytic differentiation and inhibits the alternate mature B cell fate.
Blimp-1 requires association with Groucho and histone deacetylases (16, 17) and the G9a histone methyltransferase (18) for its repressive activity. Nucleosomes near functional Blimp-1 binding sites have deacetylated H3 lysines in a plasmacytoma expressing endogenous Blimp-1 (13) and methylated H3 lysine 9 in cells ectopically expressing Blimp-1 (18). Although histone acetylation/deacetylation is known to be dynamic, histone methylation appears to be more stable. Blimp-1–dependent chromatin modifications might be stable because terminally differentiated plasma cells do not divide. However, Blimp-1 is expressed in bone marrow plasma cells and multiple myeloma cells, suggesting its continued presence could be required (11).
Here, using a mouse where the gene encoding Blimp-1 can be inducibly deleted, we show that Blimp-1 is required not only for the formation of plasma cells, but also for their maintenance as long-lived Ig-secreting cells in the bone marrow. This sheds light on the biology of these important cells and challenges the idea that plasma cells have a stable gene expression program. Additionally, the discovery that Blimp-1 is required to maintain long-lived plasma cells suggests that interfering with Blimp-1 may provide a new rationale for designing drugs to treat autoimmune diseases or multiple myeloma.
RESULTS AND DISCUSSION
Blimp-1 is required for plasma cell maintenance in vitro
To determine if Blimp-1 is required for maintenance of plasma cells after they form, we created mice in which deletion of prdm1, encoding Blimp-1, could be regulated by treatment with tamoxifen. Prdm1 Flox/Flox mice (9) were crossed with mice in which cDNA encoding Cre recombinase fused to a mutated estrogen receptor ligand binding domain (ERCre) was inserted into the ROSA 26 gene.
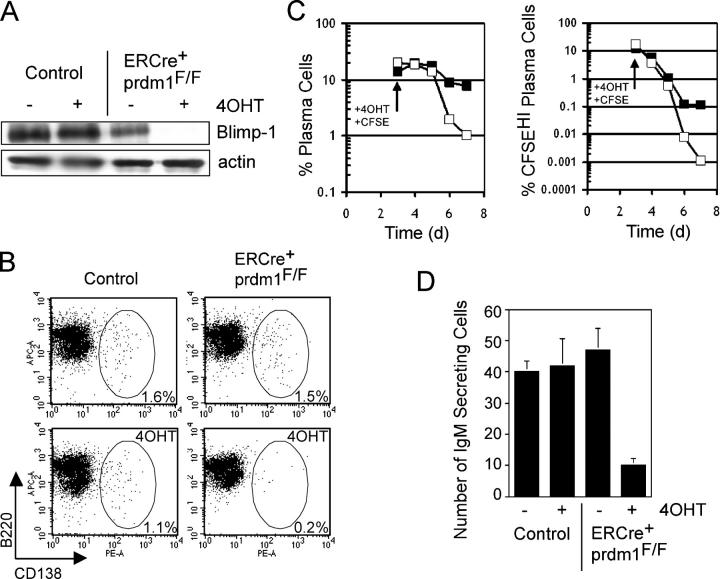
Short-lived plasma cells can be generated in vitro by treating splenocytes with the polyclonal activator, LPS. We harvested splenocytes from ERCre + prdm1 F/F and control mice and cultured them in LPS for 3 d. After 3 d, 98% of cells were of the B cell lineage (B220+) and 12–20% were CD138HI plasma cells in both cultures (unpublished data). We treated the cells with either 4-hydroxytamoxifen (4OHT) or vehicle for an additional 4 d. At the end of the treatment, Blimp-1 was detected by immunoblotting in control cultures, but not in ERCre + prdm1 F/F cultures treated with 4OHT (Fig. 1 A), indicating that deletion in vitro was efficient and Blimp-1 was no longer present. After 7 d, control cultures treated with 4OHT had a 30% reduction in the frequency of CD138HI plasma cells compared with vehicle treated cultures, whereas ERCre + prdm1 F/F cultures treated with 4OHT showed 90% reduction in plasma cell frequency compared with vehicle-treated cultures (Fig. 1 B). A similar difference was observed in multiple experiments. In one experiment, B cells in LPS culture were labeled with a division-tracking dye at the time 4OHT was added, to distinguish newly forming plasma cells (B220LOCD138HICSFELO/−) from nondividing, previously formed plasma cells (B220LO CD138HICSFEHI) (Fig. 1 C). After 3–4 d of 4OHT treatment, both total and CSFEHI plasma cells disappeared more rapidly and to a greater overall extent in cultures of ERCre + prdm1 F/F B cells than in controls. Consistent with this, ELISPOT analyses showed a substantial number of IgM secreting cells in control cultures at the end of the culture period, but in the ERCre + prdm1 F/F cultures treated with 4OHT, the number of IgM-secreting cells was greatly reduced (Fig. 1 D). Thus, Blimp-1 is required to maintain previously formed plasma cells in vitro.
Figure 1.
Loss of Blimp-1 in vitro. Splenocytes were treated with LPS for 3 d in vitro followed by 4 d of 4-hydroxytamoxifen (4OHT) or sham treatment. (A) Western blot for Blimp-1 and actin protein in ERCre + prdm1 F/F and control cultures treated with 4OHT and sham. (B) CD138 vs. B220 staining of splenocyte cultures. (left) Control; (right) ERCre + prdm1 F/F cultures; (top) sham; (bottom) 4OHT treated. The frequency of gated CD138HI plasma cells is indicated. Depicted results are representative of five experiments. (C) Kinetics of plasma cell disappearance after 4OHT treatment. (left) The percent of total B220LOCD138HI plasma cells in control (solid square) and ERCre + prdm1 F/F (open square) cultures for 4 d after 4OHT treatment. (right) The percent of CFSEHI plasma cells in these cultures. (D) IgM-secreting cells as assessed by ELISPOT analysis for ERCre + prdm1 F/F and control cultures treated with 4OHT and sham. The y axis represents number of IgM-secreting cells per 2,000 cells. C and D are representative of two experiments.
Blimp-1 is required for plasma cell maintenance in vivo
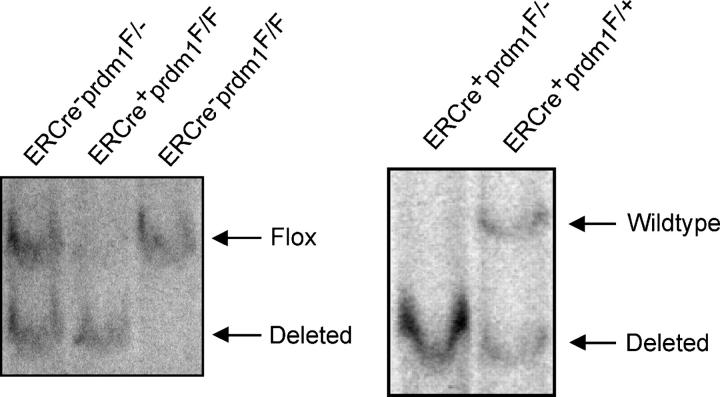
We expanded these studies to long-lived plasma cells in the bone marrow of mice. 3–4 wk after intraperitoneal injection of mice with tamoxifen, deletion of prdm1 in bone marrow cells was assessed by Southern blotting. In ERCre + prdm1 F/F mice, deletion of the floxed allele was very efficient (Fig. 2). Because Blimp-1 mRNA and protein have half lives of <2 and 4 h, respectively (unpublished data), Blimp-1 protein disappears soon after gene deletion. The presence of B220LOCD138HI plasma cells was assessed by flow cytometry. ERCre + prdm1 F/F mice had a more than fourfold reduction in the frequency of plasma cells in the bone marrow compared with control mice (not depicted), suggesting that Blimp-1 is required to maintain these cells. Both ERCre + prdm1F/ + and ERCre−prdm1F/F mice were used as controls in this and other experiments and no differences were observed between them.
Figure 2.
Loss of Blimp-1 in vivo. Southern blots of DNA from total bone marrow harvested 3.5 wk after tamoxifen treatment. Bands for deleted, wild type, and floxed prdm1 are indicated.
Blimp-1 is required for the maintenance of long-lived, nondividing plasma cells
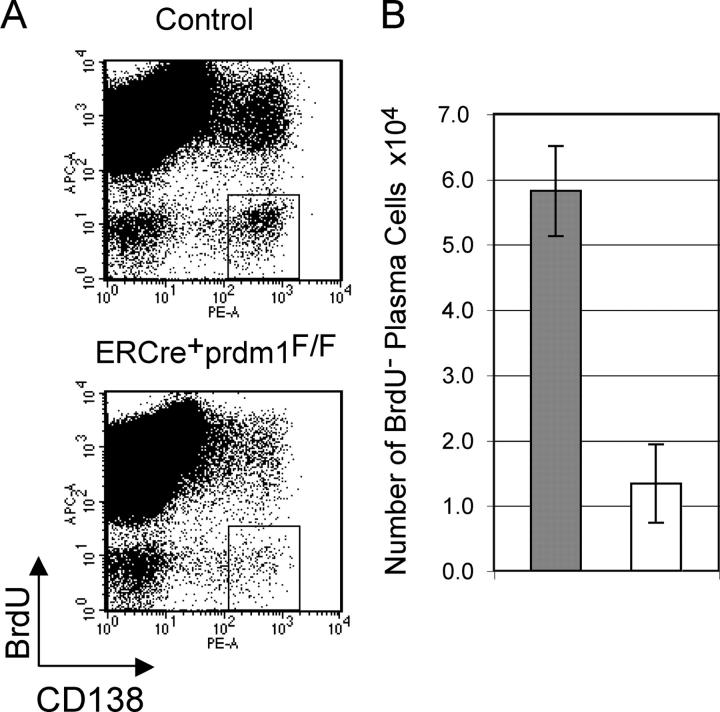
Because Blimp-1 is known to be required for the differentiation of B cells into plasma cells and because our initial studies did not fully distinguish loss of previously formed plasma cells from inability to form new plasma cells, we studied this further. Mice were fed BrdU in drinking water from the time of tamoxifen treatment until bone marrow harvest. B cells (19) and plasma cell precursors (20) proliferate during plasmacytic differentiation so that plasma cells formed after tamoxifen treatment would be BrdU+, whereas previously formed, long-lived plasma cells would be BrdU−. The ratio of BrdU+/BrdU− plasma cells shows the distribution of these two populations. In bone marrow 3–4 wk after tamoxifen treatment (Fig. 3 A), we observed the anticipated decrease B220LOCD138HIBrdU+ plasma cells, confirming our previous report that plasma cell formation requires Blimp-1 (11). In addition, we observed a fourfold decrease in BrdU− plasma cells (Fig. 3, A and B). This decrease shows that the continued presence of long-lived, nondividing B220LOCD138HI plasma cells in the bone marrow requires Blimp-1.
Figure 3.
Loss of long-lived bone marrow plasma cells after deletion of prdm1. (A) Plots of CD138 vs. BrdU for bone marrow from ERCre + prdm1 F/F and control mice that received tamoxifen 3–4 wk previously and were fed BrdU from the time of tamoxifen treatment until harvest. Boxes represent CD138HI plasma cells that did not incorporate BrdU. (B) Total BrdU− B220LOCD138HI plasma cells after tamoxifen treatment (averages and SEM for three ERCre+prdm1F/F and three control mice). Gray bars represent control and white bars represent ERCre + prdm1 F/F.
Blimp-1 is required for long-term maintenance of serum Ig
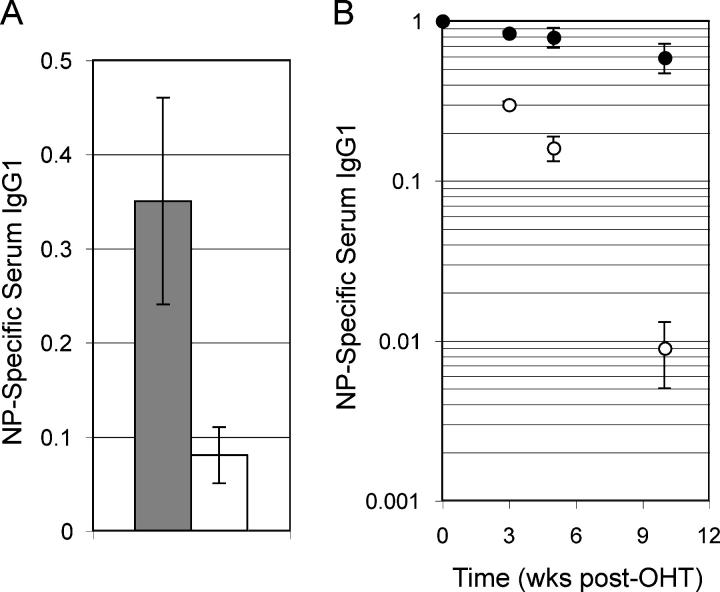
We wished to determine if Ig secretion was affected when prdm1 was deleted in long-lived plasma cells, as suggested by the decrease in B220LOCD138HI cells. ERCre + prdm1 F/F and control mice were immunized with NP-KLH. 6 wk after immunization, when both the early and the post–germinal center plasma cell responses had subsided, serum was harvested before mice were treated with tamoxifen. 5 wk after tamoxifen treatment (11 wk postimmunization), serum was again harvested. When final NP-specific IgG1 levels were compared with the initial levels before tamoxifen treatment, control mice had 35% remaining, but ERCre + prdm1 F/F mice had only 8% remaining, a fourfold lower amount (Fig. 4 A). Thus, we conclude that Blimp-1 is required for continued Ig secretion by long-lived plasma cells. Decreased serum Ig is also consistent with cell loss rather than egress of Ig-secreting cells from the bone marrow to other peripheral sites.
Figure 4.
Loss of antigen-specific Ig after deletion of prdm1. (A) 6 wk after immunization with NP-KLH, ERCre + prdm1 F/F and control mice were treated with tamoxifen. The levels of NP-specific serum IgG1 at 5 wk after tamoxifen treatment divided by the levels immediately before tamoxifen treatment for three ERCre + prdm1 F/F and control mice were averaged (with SEM) and graphed. Gray bars represent control and white bars represent ERCre + prdm1 F/F. (B) Bone marrow from ERCre + prdm1 F/F or control NP-KLH immunized mice was transferred to naive hosts. After engraftment, tamoxifen was administered. Serum was harvested on the day of the first tamoxifen dose and at later dates. Graph depicts the fraction of posttamoxifen NP-specific serum IgG1 at 3, 5, and 10 wk divided by pretamoxifen NP-specific serum IgG1 (averages with SEM for three ERCre + prdm1 F/F and control donors). Closed circles represent control and open circles represent ERCre + prdm1 F/F.
When ERCre + prdm1 F/F mice are treated with tamoxifen, prdm1 is deleted in all cells due to the ubiquitous expression of ERCre. Because Blimp-1 is expressed in epithelial cells (unpublished data), we reasoned that the requirement for Blimp-1 could reside in bone marrow stromal cells. To test this possibility, we designed an adoptive transfer experiment in which bone marrow cells from immunized mice were transferred to naive mice in which normal bone marrow stroma was present. ERCre + prdm1 F/F and control mice were immunized and later boosted with NP-KLH. 7–9 wk after secondary immunization, bone marrow was transferred to sublethally irradiated naive hosts. After allowing levels of NP-specific serum Ig to stabilize in the recipients, tamoxifen was administered and serum was harvested 3, 5, and 10 wk later to assess levels of NP-specific IgG1 by ELISA. Although NP-specific IgG1 decreased over time in the mice with control bone marrow, NP-specific IgG1 decreased to a substantially greater degree in mice with ERCre + prdm1 F/F bone marrow (Fig. 4 B). 10 wk posttamoxifen, NP-specific IgG1 had fallen 66-fold more in mice that received ERCre + prdm1 F/F bone marrow compared with those receiving control marrow (control 59% of initial Ig level vs. ERCre + prdm1 F/F 0.9% of initial Ig level). Thus, the requirement for Blimp-1 does not reside in the stromal cells but appears to be intrinsic to the transferred plasma cells. This conclusion is also consistent with the B cell intrinsic requirement for Blimp-1 to maintain plasma cells in vitro (Fig. 1).
Blimp-1 and plasma cell maintenance
Although much is known about genes involved in the differentiation of B cells to plasma cells (6), there is less information about genes required for maintenance of plasma cells in the bone marrow. XBP-1, a transcriptional activator required for plasma cell formation (21), is required continuously for survival of myeloma cells because treatment with a proteasome inhibitor, which reduces levels of activated XBP-1, causes apoptosis due to ER stress (22). XBP-1 is probably required for maintenance of long-lived plasma cells, but this has not been tested directly. Blimp-1 is required for XBP-1 induction during plasmacytic differentiation (9, 15) and lack of XBP-1 may be an important component in the requirement for Blimp-1 shown in this study. Aiolos, an Ikaros family member, is required for long-lived plasma cells to appear in the bone marrow, but it is unclear if it affects formation, migration, and/or maintenance of these cells (23).
Mice lacking BCMA, a receptor for the TNF superfamily factors BAFF and APRIL, have reduced bone marrow plasma cells, suggesting that BAFF and/or APRIL are necessary for survival of these cells (24). However, BAFF enhances survival of human plasmablasts, but not plasma cells (25). Interestingly, APRIL binds not only to BCMA and TACI receptors, but also to the proteoglycan CD138, which is highly expressed on plasma cells (26). Thus, APRIL may be an important survival factor for bone marrow plasma cells. IL-6 supports the survival of isolated bone marrow plasma cells and is a survival factor for myeloma cells; however, IL-6−/− mice have no abnormalities of plasma cell maintenance in vivo (27), suggesting other survival factors can compensate for its absence. In other settings, STAT3 (28) (which is activated by IL-6), NF-κB (unpublished data), and AP-1 (29) (when activated by toll-like receptors and possibly TNF family receptors) induce prdm1 transcription. Thus, an important function of survival signals from the bone marrow stroma to plasma cells may be activation of transcription factors required for transcription of prdm1.
Our data have interesting implications for the molecular mechanisms by which Blimp-1 represses target gene transcription. As discussed before, Blimp-1's repressive function involves both histone deacetylation and histone methylation, the latter being thought to be a relatively stable chromatin modification. The results reported here suggest that the covalent chromatin modifications associated with Blimp-1–dependent repression are not stable, even in the absence of cell division, and show that the continuous presence of Blimp-1 is required to keep its target genes repressed. Such a mechanism would fit with a model where survival signals drive continued Blimp-1 expression in plasma cells that inhabit specific survival niches. Eventual loss of Blimp-1 in plasma cells that are ejected from their bone marrow niches by competitors or never reach such niches could be the physiological basis for plasma cell loss.
Our data do not distinguish whether long-lived plasma cells die or dedifferentiate upon the loss of Blimp-1. The requirement for XBP-1 in the survival of myeloma cells and the role of Blimp-1 in inducing XBP-1 suggest that plasma cells lacking Blimp-1 might die due to ER stress secondary to absence of XBP-1. However, a recent study has shown that the plasma cell phenotype of a myeloma line can be reversed by forced coexpression of the germinal center proteins MTA3 and Bcl-6 (30). In these experiments, dedifferentiation included reduction of Blimp-1 and XBP-1 mRNAs. Because Blimp-1 represses Bcl-6 (11), deletion of prdm1 could derepress Bcl-6, causing dedifferentiation with loss of Ig secretion and CD138 expression, which are the two plasma cell phenotypes we monitored.
Long-lived plasma cells can be pathogenic in autoimmune diseases such as systemic lupus erythematosus (7), where autoantibodies contribute to pathology, and in multiple myeloma, where CD138+ tumor cells in the bone marrow apparently represent the transformed counterparts of long-lived plasma cells. However, as discussed before, long-lived plasma cells are resistant to the treatments often used in these diseases. Thus, regardless of the ultimate fate of plasma cells lacking Blimp-1, our data show that targeting Blimp-1 or its regulators provides a novel approach in drug design strategies to eliminate long-lived plasma cells or their transformed counterparts in bone marrow.
Materials and Methods
Mice.
Prdm1 F/F and prdm1 F/− mice were crossed with ERCre mice to generate controls (ERCre + prdm1 F/+ , ERCre − prdm1 F/F, or ERCre − prdm1 F/−) and experimental (ERCre + prdm1 F/F or ERCre + prdm1 F/−) mice. No differences were observed in control or experimental mice with varying genotypes; in the text, experimental mice are labeled ERCre + prdm1 F/F for simplicity. The ERCre mice were a gift from T. Ludwig (Columbia University, New York, NY). All procedures with mice were approved by the Institutional Animal Care and Use Committee of Columbia University.
In vitro cultures.
Splenocytes were cultured with LPS as previously described (11) and were treated with 100 nM 4-hydroxytamoxifen in ethanol or sham treated (ethanol or no treatment) on day 3 of culture. Splenocytes treated with CFSE were prewashed with PBS and incubated with 25 nM of CFDA SE dye (GE Healthcare) following the manufacturer's instructions. Aliquots of cells were harvested daily for 4 d and stained for FACS (see Flow cytometry section), plated for ELISPOT analysis, or lysed for Western blot analysis. The methods for the latter two experiments were described previously (11).
In vivo tamoxifen treatment.
Mice were injected intraperitoneally on three consecutive days with 0.5 ml of 10 mg/ml tamoxifen in sunflower seed oil.
Immunization.
Mice were immunized i.p. and later boosted i.p. in appropriate experiments, with 400 μg NP-KLH in Ribi adjuvant (Corixa).
BrdU feeding.
Mice were fed drinking water with 1.0 mg/ml BrdU starting at the time of tamoxifen injection until bone marrow harvest 3–4 wk later. Water was changed every other day and protected from light.
Bone marrow transfer.
Recipient C57B/6 or B6/SJL congenic mice were irradiated at 680 rad and rested for 1 d. BM was harvested from control and ERCre + prdm1 F/F mice 7–9 wk after NP-KLH boost and 6.5–7.9 × 106 total BM cells were transferred by tail vein injection to recipients. Mice were fed water with Baytril for the duration of the experiment.
Southern blot.
DNA was extracted from BM harvested from control and ERCre + prdm1 F/F mice and Southern blotting was performed as described previously (9). Wild type, floxed, and deleted prdm1 were detected as 15-, 13.5-, and 10-kb bands, respectively.
Flow cytometry.
BM was harvested from control and ERCre + prdm1 F/F mice 3–4 wk after tamoxifen treatment, red blood cells were lysed, and cells were stained with α-CD138-PE (BD Biosciences) and α-B220-allophycocyanin or α-B220-FITC (eBioscience). The buffer for all antibody incubations and washes was PBS with 1% BSA, 2% FCS, 0.03% NaN3, and 2 mM EDTA. For BrdU experiments, after B220 and CD138 staining, cells were washed, resuspended in ice-cold 0.15 M NaCl, and permeabilized with ice-cold 100% ethanol. After washing, cells were fixed with 1% paraformaldehyde, washed, treated with DNase I (Sigma-Aldrich), and washed before incubation with α-BrdU-allophycocyanin (GE Healthcare) for flow cytometry.
ELISA.
Serum was harvested from mice at various times, processed and, diluted to an appropriate range for ELISA analysis to detect NP-specific IgG1 as described previously (9).
Acknowledgments
We thank Drs. S. Anderson and M. Shlomchik for advice on the BrdU studies, and the Calame lab for helpful discussions.
This work was supported by National Institutes of Health grant nos. RO1AI50659 and RO1AI43576 (to K. Calame).
The authors have no conflicting interests.
K-I. Lin's present address is The Genomics Research Center, Academia Sinica, Taipei 115, Taiwan.
References
- 1.Dorner, T., and A. Radbruch. 2005. Selecting B cells and plasma cells to memory. J. Exp. Med. 201:497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson, L.D., B.G. Durell, L.A. Vogel, B.P. O'Connor, M. Cascalho, T. Yasui, H. Kikutani, and R.J. Noelle. 2002. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J. Clin. Invest. 109:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slifka, M.K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manz, R.A., M. Lohning, G. Cassese, A. Thiel, and A. Radbruch. 1998. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10:1703–1711. [DOI] [PubMed] [Google Scholar]
- 5.Manz, R.A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature. 388:133–134. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro-Shelef, M., and K. Calame. 2005. Regulation of plasma cell development. Nat. Rev. Immunol. 5:230–242. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer, B.F., K. Moser, A.E. Hauser, A. Peddinghaus, C. Voigt, D. Eilat, A. Radbruch, F. Hiepe, and R.A. Manz. 2004. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman, G.J., and S. Weisman. 2003. Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum. 48:1484–1492. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro-Shelef, M., K.-I. Lin, L. McHeyzer-Williams, J. Liao, M. McHeyzer-Williams, and K. Calame. 2003. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory cells. Immunity. 19:607–620. [DOI] [PubMed] [Google Scholar]
- 10.Turner, C.A., Jr., D.H. Mack, and M.M. Davis. 1994. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 77:297–306. [DOI] [PubMed] [Google Scholar]
- 11.Shaffer, A.L., K.I. Lin, T.C. Kuo, X. Yu, E.M. Hurt, A. Rosenwald, J.M. Giltnane, L. Yang, H. Zhao, K. Calame, and L.M. Staudt. 2002. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 17:51–62. [DOI] [PubMed] [Google Scholar]
- 12.Lin, Y., K. Wong, and K. Calame. 1997. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science. 276:596–599. [DOI] [PubMed] [Google Scholar]
- 13.Lin, K.I., C. Angelin-Duclos, T.C. Kuo, and K. Calame. 2002. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol. Cell. Biol. 22:4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reimold, A.M., P.D. Ponath, Y.S. Li, R.R. Hardy, C.S. David, J.L. Strominger, and L.H. Glimcher. 1996. Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. J. Exp. Med. 183:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer, A.L., M. Shapiro-Shelef, N.N. Iwakoshi, A.H. Lee, S.B. Qian, H. Zhao, X. Yu, L. Yang, B.K. Tan, A. Rosenwald, et al. 2004. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 21:81–93. [DOI] [PubMed] [Google Scholar]
- 16.Yu, J., C. Angelin-Duclos, J. Greenwood, J. Liao, and K. Calame. 2000. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol. Cell. Biol. 20:2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren, B., K.J. Chee, T.H. Kim, and T. Maniatis. 1999. PRDI-BF1/Blimp-1 repression is mediated by corepressors of the Groucho family of proteins. Genes Dev. 13:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyory, I., J. Wu, G. Fejer, E. Seto, and K.L. Wright. 2004. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5:299–308. [DOI] [PubMed] [Google Scholar]
- 19.Jelinek, D.F., and P.E. Lipsky. 1983. The role of B cell proliferation in the generation of immunoglobulin-secreting cells in man. J. Immunol. 130:2597–2604. [PubMed] [Google Scholar]
- 20.O'Connor, B.P., M. Cascalho, and R.J. Noelle. 2002. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 195:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reimold, A.M., N.N. Iwakoshi, J. Manis, P. Vallabhajosyula, E. Szomolanyi-Tsuda, E.M. Gravallese, D. Friend, M.J. Grusby, F. Alt, and L.H. Glimcher. 2001. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 412:300–307. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A.H., N.N. Iwakoshi, K.C. Anderson, and L.H. Glimcher. 2003. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. USA. 100:9946–9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortes, M., and K. Georgopoulos. 2004. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J. Exp. Med. 199:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor, B.P., V.S. Raman, L.D. Erickson, W.J. Cook, L.K. Weaver, C. Ahonen, L.L. Lin, G.T. Mantchev, R.J. Bram, and R.J. Noelle. 2004. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avery, D.T., S.L. Kalled, J.I. Ellyard, C. Ambrose, S.A. Bixler, M. Thien, R. Brink, F. Mackay, P.D. Hodgkin, and S.G. Tangye. 2003. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingold, K., A. Zumsteg, A. Tardivel, B. Huard, Q.G. Steiner, T.G. Cachero, F. Qiang, L. Gorelik, S.L. Kalled, H. Acha-Orbea, et al. 2005. Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 201:1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassese, G., S. Arce, A.E. Hauser, K. Lehnert, B. Moewes, M. Mostarac, G. Muehlinghaus, M. Szyska, A. Radbruch, and R.A. Manz. 2003. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171:1684–1690. [DOI] [PubMed] [Google Scholar]
- 28.Reljic, R., S.D. Wagner, L.J. Peakman, and D.T. Fearon. 2000. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J. Exp. Med. 192:1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasanwala, F.H., S. Kusam, L.M. Toney, and A.L. Dent. 2002. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J. Immunol. 169:1922–1929. [DOI] [PubMed] [Google Scholar]
- 30.Fujita, N., D.L. Jaye, C. Geigerman, A. Akyildiz, M.R. Mooney, J.M. Boss, and P.A. Wade. 2004. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 119:75–86. [DOI] [PubMed] [Google Scholar]