Abstract
Recent studies suggest that human regulatory T (T reg) cells protect against the development of allergic and asthmatic disease and that their function is impaired during active disease. Two new studies contribute to our understanding of the role that T reg cells play in the control of allergic airway disease in mice. However, these studies also highlight several outstanding questions in the field.
Human disease versus animal models
Human allergic airway disease is a complex condition characterized by increased amounts of systemic IgE, elevated allergen-specific T helper (Th)2 cells and their products, airway hyperreactivity (AHR), and structural changes in the lung. Animal models of both acute and chronic allergic airway responses have been described, although none of these models captures all aspects of human disease (1). Nonetheless, animal models have provided invaluable insights into the molecular mechanisms of allergic airway disease, including the role of Th2 cytokines in disease pathology and, more recently, the role of regulatory pathways in the control of disease. These models thus provide an important preclinical tool for dissecting the biological mechanisms involved in the sensitization to allergens and the effector mechanisms associated with disease, as well as for the investigation of novel therapies for human disease.
Regulatory T cells
T reg cells control peripheral immune responses and are likely to play a central role in determining the incidence and severity of several immune pathologies, including autoimmune, infectious, allergic, and asthmatic diseases (2). One eventual hope for the treatment of allergic airway disease, as for other immune-mediated pathologies, is to design therapies that boost endogenous allergen-specific T reg cell function or that induce de novo T reg cell activity. Long-term, allergen-specific relief from disease symptoms provided by T reg cells would likely be preferable to the nonspecific, noncurative regimens that are currently prescribed.
There are two major categories of T reg cells described to date. The first is the naturally occurring, thymically derived CD4+CD25+ T reg cells that express high levels of the transcription factor Foxp3, which is essential for their development and function (3–6). The other category is the antigen-specific T reg cells, which can be induced in vitro and in vivo under particular conditions of antigenic stimulation. These antigen-specific T reg cells secrete antiinflammatory cytokines such as interleukin (IL)-10 and/or TGFβ, and regulate immune responses and inflammatory pathologies (2, 7). Induced T reg cells that secrete IL-10 are often referred to as IL-10–T reg cells, or Tr1 cells; those that secrete TGFβ have been referred to as Th3 cells. However, it is likely that both categories of T reg cells require one or both of these cytokines to evoke their suppressive functions during different stages or types of inflammatory responses. Many questions remain to be answered regarding the distinct roles of these T reg cell subsets during immune responses. Still unknown, for example, is whether these cells exhibit complementary functions, regulate each other's activity, or use common inhibitory mechanisms.
T reg cells in health and disease
Recent studies have suggested that naturally occurring T reg cells and antigen-induced IL-10–producing T reg cells have a physiological role in protecting against human allergic disease. A rare mutation in the gene encoding FOXP3 results in a disease called IPEX (immunedysregulation, polyendocrinopathy and enteropathy, X-linked) syndrome. Individuals with IPEX syndrome suffer from a range of autoimmune endocrine pathologies as well as allergic symptoms including severe eczema, increased serum IgE levels, eosinophilia, and food allergies (6, 8). Peripheral blood mononuclear cells (PBMCs) from atopic or allergic patients generally proliferate more extensively and produce more Th2 cytokines in response to allergen stimulation than PBMCs from nonatopic control subjects. When CD25+ T cells were depleted from PBMC cultures derived from the control subjects in these studies, these responses were significantly enhanced (9, 10). Collectively, these studies suggest that active T reg cell–mediated suppression of allergen-specific immune responses occurs in healthy individuals.
Additional evidence for a physiological role for IL-10–producing T reg cells in suppressing allergic inflammation comes from several recent studies. The suppressive cytokine IL-10 inhibits the activation of many cell types and effector functions that are associated with allergic disease (for review see reference 2). A recent study by Akdis et al. showed that the frequency of allergen-specific, IL-10–secreting T cells was significantly increased in nonatopic individuals compared with allergic patients, whereas the reverse was true for T cells producing the Th2 cytokine IL-4 (11). Several other studies similarly suggested that IL-10 levels were inversely correlated with the severity of human disease (12–14). Finally, therapies shown to be beneficial for the treatment of allergy and asthma, such as allergen immunotherapy or glucocorticoid therapy, have been shown to increase IL-10 synthesis by T cells (15–17). Whether the IL-10 is produced by T reg cells, activated effector T cells, and/or non–T cells is likely to vary in different experimental and clinical systems, and the predominant cellular source may even vary at different times during the course of an immune response. Nevertheless, these studies suggest a central role for IL-10 in disease control.
T reg cells and control of allergic airway disease in mice
Two studies in this issue clarify the role of CD4+CD25+ T reg cells in the regulation of allergic responses and show several similarities to observations made in humans. The study by Lewkowich et al. (p. 1549) investigates the impact of depleting CD4+CD25+ T reg cells on sensitization to allergens in susceptible and resistant strains of immunocompetent mice (18). The study by Kearley et al. (p. 1539) examines the effect of adoptively transferring CD4+CD25+ T reg cells into allergen-sensitized mice before antigen challenge (19).
As both articles point out, these are not the first reports to show that naturally occurring CD4+CD25+ T reg cells can control allergic airway disease. However, none of the earlier studies used wild-type, immunocompetent animals nor did they use as direct an experimental approach; overall the results of past studies have been conflicting. For example, in one of the earliest studies to deplete CD4+CD25+ T cells in allergic airway disease, the removal of these cells paradoxically ameliorated inflammation and AHR. These data are difficult to interpret but could potentially reflect the depletion of CD4+CD25+ effector T cells in addition to the T reg cells (20), as effector T cells up-regulate CD25 upon activation (5). Using a more indirect approach, another recent study showed that transfer of ovalbumin-specific CD4+ T cells that were depleted of CD25+ cells induced more airway inflammation upon transfer than did the nondepleted population. However, in the same study CD4+CD25+ T reg cells that were expanded in vitro before transfer exhibited no regulatory activity in vivo (21). This result either calls into question the ability of these cells to ameliorate disease or suggests that in vitro expansion in some way altered their suppressive capacity. In a model of chronic airway inflammation, CD4+ CD25+ T cells infiltrated the lung and inhibited airway inflammation but had no effect on AHR (22). Finally, using a transgenic model of hyper-IgE responses, Curotto et al. showed that both CD4+ CD25+ and CD4+CD25− T cells inhibited the development of IgE responses and Th2 differentiation (23). Although the likely consensus from these past studies is that CD4+CD25+ T reg cells can prevent allergic airway inflammation, both the mechanism of inhibition and effects on other aspects of disease were unresolved.
In their new study, Lewkowich et al. examined the capacity of CD4+ CD25+ T reg cells to act before allergic sensitization to control the development of allergic airway disease. They used an anti-CD25 antibody to deplete CD25+ cells in vivo and showed that the treatment greatly diminished the number of lung-infiltrating CD4+ T cells that express high levels of the CD25 antigen, but did not change the number of T cells expressing intermediate levels of CD25. Depletion of CD25+ cells before delivery of a house dust mite allergen led to significantly increased Th2 cytokine responses, IgE levels, eosinophilia, and AHR in allergy-resistant mice (C3H strain), but had comparatively little effect in the allergy-sensitive mice (A/J strain). The authors observed no difference in the ex vivo regulatory activity of CD25+ T reg cells from the sensitive or resistant mouse strains nor in the susceptibility of effector T cells to regulation. These data led them to attribute the observed differences between the sensitive and resistant mice to a non–T cell population.
The non–T cell population in question appeared to be a subset of dendritic cells (DCs). CD25+ cell depletion resulted in a greater frequency of immunogenic pulmonary myeloid DCs in the lungs of the resistant mice, and these DCs expressed elevated levels of MHC class II, and the costimulatory molecules CD80 and CD86. When cultured with T cells, the DCs triggered enhanced production of Th2 cytokines (such as IL-4, IL-5, and IL-13) from the T cells. The authors explained this phenomenon by showing that depletion of CD25+ cells resulted in reduced production of the Th1-inducing cytokine IL-12p40 by the myeloid DCs. Interestingly, the frequency of another type of pulmonary DC—plasmacytoid DCs—although present at a considerably lower frequency than myeloid DCs, appeared to be reduced by CD25+ cell depletion.
Lewkowich et al. went on to demonstrate that although depletion of CD4+CD25+ T reg cells before sensitization resulted in significant increases in AHR, depletion of pulmonary CD25+ T reg cells during the effector phase of the immune response did not enhance AHR. One potential caveat in this study is that although the number of lung CD4+ T cells expressing intermediate levels of the CD25 antigen was unaltered by the CD25 antibody treatment, it is still possible that subtle alterations in the effector CD4+ T cell or CD4−CD25+ cell populations occurred. A unique and interesting aspect of the study by Lewkowich et al. is the use of an animal that is relatively resistant to allergic sensitization, which arguably parallels the human situation more closely than many other models.
The IL-10 question
Using a complementary approach—adoptive transfer of CD4+CD25+ T reg cells—Kearley et al. explored the capacity of purified antigen-specific CD4+CD25+ T reg cells to inhibit disease when administered before challenge of immunocompetent mice with an antigen (ovalbumin) to which the mice had previously been sensitized. In this study, transfer of T reg cells inhibited AHR, eosinophil recruitment, and Th2 cytokine production. The T reg cell–mediated inhibition was dependent on the production of IL-10, as blocking the IL-10 receptor in vivo reversed the inhibition. However, CD4+CD25+ T reg cells derived from IL-10–deficient mice were still able to suppress allergic inflammation, implying that the IL-10 need not be produced by the T reg cells themselves. Indeed, the authors found that IL-10 was produced by host T cells in the lung.
The study by Kearley et al. highlights a major unresolved issue in the field of CD4+CD25+ T reg cell biology, namely how these cells act in vivo. Although naturally occurring T reg cells are proposed to act via cell contact–dependent mechanisms in vitro, they have been proposed to work variously through inhibitory cytokines or non–cytokine-dependent mechanisms in vivo, depending on the model being studied (5). Additional complexity arises from the fact that Th2 cells are reportedly less sensitive than Th1 cells to regulation by CD4+CD25+ T reg cells (24).
Kearley et al. suggest that IL-10 production by host T cells is required for suppression; the study by Lewkowich did not formally investigate whether IL-10 was required for suppression in their system. Surprisingly, Kearley et al. did not observe increased IL-10 production by lung macrophages or DC populations, as might perhaps have been predicted by an earlier independent study (25). However, this disparity might simply reflect the different systems used and the different time points at which the response was analyzed. In the earlier study, the investigators suggested that induction of T cell tolerance after respiratory exposure to high doses of allergen inhibited the development of AHR, and showed that pulmonary DCs synthesized IL-10 and promoted the induction of IL-10–producing T reg cells (Fig. 1). The reasons why the initial source of IL-10 differed in the two reports will be of considerable interest to determine in future studies.
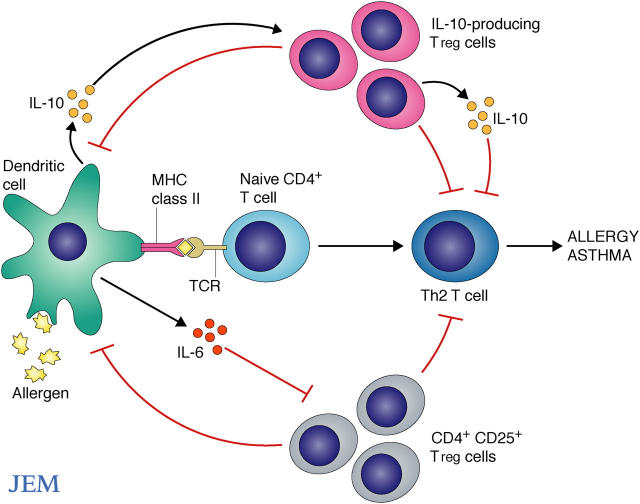
Figure. 1.
Control of allergic airway disease by regulatory T cells. Allergic airway disease is caused by inappropriate Th2-driven immune responses to “harmless” antigens, or allergens, in the environment. CD4+CD25+ and IL-10–producing T reg cells can regulate allergic sensitization in vivo through inhibitory effects on Th2 cells or on dendritic cells (DCs) in the lung. T reg cells can also induce the production of the immunosuppressive cytokine IL-10 by host lung T cells (not shown). In turn, DCs can produce immunomodulatory cytokines such as IL-6, which has been shown to inhibit CD4+CD25++ T reg cell function, and IL-10, which has been shown to induce IL-10–producing T reg cells.
Notably, parallels exist between the Kearley et al. study and an in vitro study in which human CD4+CD25+ cells were shown to induce an IL-10–dependent regulatory phenotype in CD4+ CD25− T cells (26). Indeed, most studies suggest that CD4+CD25+ T reg cells are not major producers of IL-10. However, it may be possible to boost their capacity for IL-10 production, albeit to a lesser extent than in CD4+ T cells not enriched for CD25+ cells (2). It will be of interest for future studies in both mice and humans to resolve which T cell–derived or non–T cell–derived sources of IL-10 are critical for mediating disease protection or resolution.
Control of T reg cell function in disease
Studies using PBMCs from humans demonstrate that allergen-specific Th2 cells from allergic patients who exhibit strong in vitro responses to allergen, and patients with active hay fever, are less sensitive to inhibition by CD4+CD25+ T reg cells. (10, 27). If loss of inhibitory function is associated with more active disease, one prediction is that the loss of T reg cell activity will be most apparent at the disease site. Although I am unaware of studies that directly address this question in human allergy or asthma, studies of autoimmune pathologies have analyzed tissue-derived CD4+CD25+ T reg cell function. One report showed that CD25+ T reg cells isolated from the joint synovium of rheumatoid arthritis patients were functional when removed from their inflammatory environment. In that study, environmental signals in the inflamed joint, in this case IL-7 and IL-15, exerted a negative effect on T reg cell function that likely prevented them from suppressing inflammation in vivo (28).
Pasare and Medzhitov previously demonstrated that IL-6, produced by activated DCs, impaired the regulatory activity of CD4+CD25+ T reg cells (29) (Fig. 1). However, other studies suggest that IL-6 may also play a role in controlling regulatory functions in allergic disease. Indeed, this cytokine is increased in patients with allergic asthma (30). Furthermore, cross-linking of CD40 on human respiratory tract DCs enhances their IL-6 production (31). A study by Doganci et al. published earlier this year suggested a role for naturally occurring T reg cells and IL-6 in controlling allergic airway disease in mice (32). This study confirmed earlier work supporting a role for IL-6 in promotion of Th2 responses (33), and showed that blocking IL-6 leads to local expansion of Foxp3+ CD4+CD25+ T reg cells in the lung that have an increased suppressive capacity. Thus, IL-6 and IL-10 emerge as two cytokines with the potential to contribute to CD4+CD25+ T reg cell activity in disease (Fig. 1).
The future
Although mouse models are imperfect, they are needed to further dissect T reg cell function in allergic airway disease and to facilitate comparisons with emerging data from human studies. Evidence exists for a role for CD4+ CD25+ T reg cells in limiting allergic sensitization, airway inflammation, and even AHR if delivered before allergen challenge. However, critical questions remain that will be most readily addressed in murine models. These include whether CD4+CD25+ T reg and IL-10–producing T reg cells can act in a therapeutic context once disease is already established (for example, to reverse structural remodelling of the airways), which critical mechanisms are involved, and which local signals regulate the regulator cells. Clearly, further studies will be required to fully elucidate the relative importance of and the relationship between CD25+ T reg cells and IL-10–producing T reg cells in controlling Th2-mediated allergic inflammation.
Acknowledgments
I gratefully acknowledge support from Asthma UK and Euro-Thymaide (European Union).
References
- 1.Boyce, J.A., and K.F. Austen. 2005. No audible wheezing: nuggets and conundrums from mouse asthma models. J. Exp. Med. 201:1869–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawrylowicz, C.M., and A. O'Garra. 2005. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5:271–283. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 4.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 5.Shevach, E.M. 2004. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 6.Ramsdell, F. 2003. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 19:165–168. [DOI] [PubMed] [Google Scholar]
- 7.Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. [DOI] [PubMed] [Google Scholar]
- 8.Chatila, T.A., F. Blaeser, N. Ho, H.M. Lederman, C. Voulgaropoulos, C. Helms, and A.M. Bowcock. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taams, L.S., M. Vukmanovic-Stejic, J. Smith, P.J. Dunne, J.M. Fletcher, F.J. Plunkett, S.B. Ebeling, G. Lombardi, M.H. Rustin, J.W. Bijlsma, et al. 2002. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 32:1621–1630. [DOI] [PubMed] [Google Scholar]
- 10.Ling, E.M., T. Smith, X.D. Nguyen, C. Pridgeon, M. Dallman, J. Arbery, V.A. Carr, and D.S. Robinson. 2004. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 363:608–615. [DOI] [PubMed] [Google Scholar]
- 11.Akdis, M., J. Verhagen, A. Taylor, F. Karamloo, C. Karagiannidis, R. Crameri, S. Thunberg, G. Deniz, R. Valenta, H. Fiebig, et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borish, L., A. Aarons, J. Rumbyrt, P. Cvietusa, J. Negri, and S. Wenzel. 1996. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97:1288–1296. [DOI] [PubMed] [Google Scholar]
- 13.Lim, S., E. Crawley, P. Woo, and P.J. Barnes. 1998. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 352:113. [DOI] [PubMed] [Google Scholar]
- 14.Heaton, T., J. Rowe, S. Turner, R.C. Aalberse, N. de Klerk, D. Suriyaarachchi, M. Serralha, B.J. Holt, E. Hollams, S. Yerkovich, et al. 2005. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 365:142–149. [DOI] [PubMed] [Google Scholar]
- 15.Akdis, C.A., T. Blesken, M. Akdis, B. Wuthrich, and K. Blaser. 1998. Role of interleukin 10 in specific immunotherapy. J. Clin. Invest. 102:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawrylowicz, C., D. Richards, T.K. Loke, C. Corrigan, and T. Lee. 2002. A defect in corticosteroid-induced IL-10 production in T lymphocytes from corticosteroid-resistant asthmatic patients. J. Allergy Clin. Immunol. 109:369–370. [DOI] [PubMed] [Google Scholar]
- 17.Xystrakis, E., S. Kusumakar, S. Boswell, E. Peek, P. Lavender, Z. Urry, D.F. Richards, A. Adikibi, C. Pridgeon, M. Dallman, T.-K. Loke, D.S. Robinson, F. Barrat, A. O'Garra, T.H. Lee, C. Corrigan, and C.M. Hawrylowicz. 2006. Reversing the defective induction of IL-10 secreting T regulatory cells in glucocorticoid resistant asthma patients. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewkowich, I.P., N.S. Herman, K.W. Schleifer, M.P. Dance, B.L. Chen, A.A. Sproles, J.S. Shah, Y. Belkaid, and M. Wills-Karp. 2005. CD4+CD25+ T cells protect against experimentally-induced asthma by altering the dendritic cell phenotype and activation state. J. Exp. Med. 202:1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearley, J., J.E. Barker, D.S. Robinson, and C.M. Lloyd. 2005. Resolution of allergen-induced airway inflammation and hyperreactivity by transfer of allergen-specific CD4+ CD25+ regulatory T cells in vivo: role of IL-10. J. Exp. Med. 202:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suto, A., H. Nakajima, S.I. Kagami, K. Suzuki, Y. Saito, and I. Iwamoto. 2001. Role of CD4(+) CD25(+) regulatory T cells in T helper 2 cell-mediated allergic inflammation in the airways. Am. J. Respir. Crit. Care Med. 164:680–687. [DOI] [PubMed] [Google Scholar]
- 21.Jaffar, Z., T. Sivakuru, and K. Roberts. 2004. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J. Immunol. 172:3842–3849. [DOI] [PubMed] [Google Scholar]
- 22.Hadeiba, H., and R.M. Locksley. 2003. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J. Immunol. 170:5502–5510. [DOI] [PubMed] [Google Scholar]
- 23.Curotto de Lafaille, M.A., S. Muriglan, M.J. Sunshine, Y. Lei, N. Kutchukhidze, G.C. Furtado, A.K. Wensky, D. Olivares-Villagomez, and J.J. Lafaille. 2001. Hyper immunoglobulin E response in mice with monoclonal populations of B and T lymphocytes. J. Exp. Med. 194:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosmi, L., F. Liotta, R. Angeli, B. Mazzinghi, V. Santarlasci, R. Manetti, L. Lasagni, V. Vanini, P. Romagnani, E. Maggi, et al. 2004. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 103:3117–3121. [DOI] [PubMed] [Google Scholar]
- 25.Akbari, O., R.H. DeKruyff, and D.T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725–731. [DOI] [PubMed] [Google Scholar]
- 26.Dieckmann, D., C.H. Bruett, H. Ploettner, M.B. Lutz, and G. Schuler. 2002. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J. Exp. Med. 196:247–253 (corrected). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellinghausen, I., B. Klostermann, J. Knop, and J. Saloga. 2003. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J. Allergy Clin. Immunol. 111:862–868. [DOI] [PubMed] [Google Scholar]
- 28.Ruprecht, C.R., M. Gattorno, F. Ferlito, A. Gregorio, A. Martini, A. Lanzavecchia, and F. Sallusto. 2005. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 201:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 30.Yokoyama, A., N. Kohno, S. Fujino, H. Hamada, Y. Inoue, S. Fujioka, S. Ishida, and K. Hiwada. 1995. Circulating interleukin-6 levels in patients with bronchial asthma. Am. J. Respir. Crit. Care Med. 151:1354–1358. [DOI] [PubMed] [Google Scholar]
- 31.Faith, A., J. McDonald, E. Peek, D. Richards, J. Caulfield, E. Chevretton, D. Roberts, T. Lee, C. Corrigan, and C. Hawrylowicz. 2005. Functional plasticity of human respiratory tract dendritic cells: GM-CSF enhances TH2 development. J. Allergy Clin. Immunol. 116:1136–1143. [DOI] [PubMed] [Google Scholar]
- 32.Doganci, A., T. Eigenbrod, N. Krug, G.T. De Sanctis, M. Hausding, V.J. Erpenbeck, B. Haddad el, H.A. Lehr, E. Schmitt, T. Bopp, et al. 2005. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 115:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rincon, M., J. Anguita, T. Nakamura, E. Fikrig, and R.A. Flavell. 1997. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]