Abstract
Like the immune response itself, our efforts to understand the “rules” for self–nonself discrimination are constantly evolving. The discovery of pattern recognition receptors—the Toll-like receptor (TLR) family in particular—shifted the emphasis of self–nonself recognition from lymphocytes functioning in the adaptive immune system to antigen-presenting cells (APCs) functioning in the innate immune system. Two new articles, one in a recent issue (1) and one in this issue (see Vollmer et al. [2] on p. 1575), demonstrate that antigen–antibody complexes containing RNAs activate B lymphocytes and dendritic cells (DCs) through interaction with TLR7 and/or TLR8. From these and other papers, one begins to see how specific types of autoantigens—by virtue of their capacity to act as TLR ligands—favor autoantibody production. This is known as the Toll hypothesis.
Recent discoveries set the stage for the experiments described by Lau et al. (1) and Vollmer et al. (2). A major observation was that mammalian DNA (likely in the form of chromatin) activated B cells or DCs when endocytosed through the B cell receptor (BCR) or FcγR, respectively (3, 4). This debunked the concept that TLRs dependably distinguish self from foreign and thus alert the host to “danger.” In addition to mammalian DNA, certain single stranded viral RNAs and mammalian RNAs, especially those rich in uridine (U) or uridine and guanine (UG), were found to be biological agonists for TLR7/8 (5, 6), which are expressed in human B cells (TLR7), plasmacytoid DCs (TLR7), and myeloid DCs (TLR7/8) (7). Finally, Ronnblom and colleagues (8) made the observation that serum from patients with lupus stimulated type I interferon (IFN) production when incubated with apoptotic cells, and that this response was attenuated by RNase.
The article by Lau et al. (1) extends the “two receptor paradigm” previously described for endogenous DNA–Ig immune complexes to complexes containing endogenous RNA. The authors observed that IFN-primed B cells obtained from mice transgenic for rheumatoid factor proliferated after exposure to Sm–ribonucleoprotein (RNP) immune complexes. This activity was both RNase sensitive and TLR7 dependent. Furthermore, they observed that autoantibody production was significantly ameliorated in the absence of the TLR adaptor protein MyD88 in lupus-prone mice.
Vollmer et al. (2) also demonstrated the ability of mammalian RNP particles to stimulate innate responses. Using a series of synthetic oligoribonucleotides, they showed that highly conserved U-rich RNAs activated plasmacytoid DCs via TLR7 and monocytes and myeloid DCs via TLR8. Vollmer et al. also showed that sera from lupus patients with anti-RNP antibodies stimulated IFN production from PBMCs or plasmacytoid DCs via CD32 (FcγRIII).
Here, we discuss autoantibody formation in systemic lupus erythematosus (SLE), where the Toll hypothesis is particularly appealing. SLE is an autoimmune disease characterized in part by the presence of serum autoantibodies that recognize nucleoprotein antigens, such as Sm and RNP. These endogenous core proteins are tightly bound to U-rich small nucleotide (sn)RNAs (including U1, 2, 4, 5, and 6). The fact that these snRNAs are U rich makes them appropriate targets to test for immune stimulation through TLR7/8. Although the studies by Lau et al. and Vollmer et al. come to similar conclusions with regard to self RNA-induced immune activation, they raise questions that are central to our understanding of autoantibody generation and autoimmunity in general. Some of these questions are discussed below.
Do RNPs both initiate and perpetuate autoimmunity?
Perhaps the most fundamental question to arise from these and other studies is whether TLR activation is critical for both the initiation and perpetuation of autoantibody production. Mammalian DNA or RNA must enter discrete endocytic compartments within the cell in order to interact with intracellular TLRs (such as TLR3, 7, 8, and 9). The only way that this has been achieved experimentally without the use of transfection is through engagement of IgG–nucleoprotein complexes with the antigen receptor on B cells or Fcγ receptors on DCs (8). Furthermore, the most robust responses were observed when the target cells were first “primed” by exposure to IFN-α or CD40 ligand (CD40L) (1, 2), a phenomenon explained in part by the IFN-α– or CD40L-induced up-regulation of TLR7. From these in vitro studies, one can infer that during immune activation and after the production of antinucleoprotein autoantibodies—in other words, when tolerance has already been broken—the pathways described in these articles are likely to perpetuate immune activation and autoimmunity in vivo.
Could the same pathways be invoked in the initiation of autoimmunity? Viglianti et al. (9) showed that chromatin ingestion can occur through direct binding of chromatin to the BCR on B cells derived from transgenic mice expressing a DNA-specific BCR, and that this chromatin ingestion triggers B cell proliferation. If B cell proliferation equates with maturation and autoantibody production (as yet unproven assumptions, as discussed below), then the uptake of nucleoproteins directly through the BCR may initiate autoantibody production. However, as low-affinity antibodies specific for self-DNA or RNA are thought to be abundant in the peripheral circulation (10), the avidity of interaction between the BCR and nucleoprotein, as well as the downstream signaling responses triggered by this interaction, and/or other environmental influences may be critical in determining whether pathogenic autoantibodies are produced. Perhaps exposure to IFN-α or other inflammatory cytokines, produced in response to inflammatory proteins released by dying cells (11), are necessary coconspirators that are required to prime the immune system toward exaggerated responses. It is also possible that this process is sustained by defective clearance of apoptotic cells (12) (Fig. 1 A). In addition, failure to establish self-tolerance during early B cell development allows the escape of autoreactive B cells, as recently shown in a small number of untreated SLE patients (13). If these B cells have sufficient avidity for self-nucleoproteins with repetitive epitopes that cross-link the BCR, the cells could become activated, endocytose the nucleoprotein, and be stimulated through their TLRs (Fig. 1 B and Fig. 2).
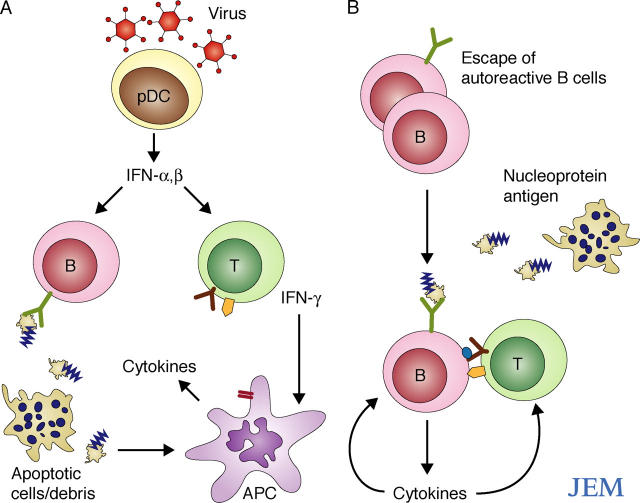
Figure 1.
Possible mechanisms by which nucleoprotein antigens initiate autoantibody production. (A) A virus infection is sensed by TLRs in plasmacytoid dendritic cells (pDCs) resulting in the production of large concentrations of type I interferon (IFN-α/β). IFNs prime the adaptive immune system to respond to other signals that may include nucleoprotein antigens released from dead and dying cells. Virus persistence and/or defective clearance of apoptotic cells might drive chronic, self-perpetuating autoimmunity through uptake of nucleoprotein antigen–antibody complexes (Fig. 2). (B) Defective B cell tolerance leads to capture of nucleoprotein antigens by autoreactive B cells, thereby triggering B cell activation, TLR stimulation, and antigen presentation to T cells. Apoptotic cells are abundant in germinal centers, and nucleoprotein antigens may be released at other sites due to abnormal cell death or defective cell clearance.
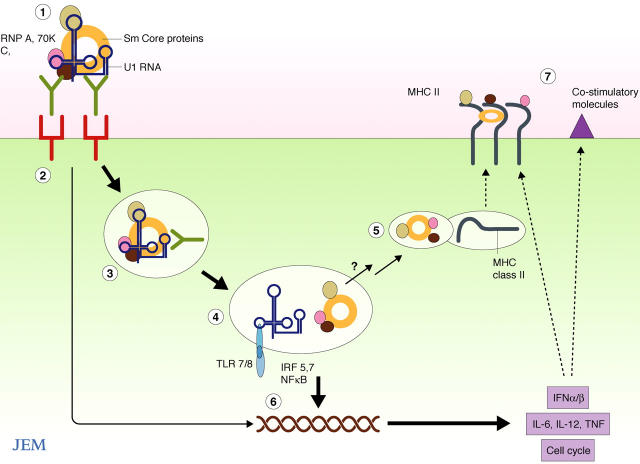
Figure 2.
Possible mechanisms by which engagement of the U1 snRNP antigen activates APCs. (1) The U1 snRNP binds to the BCR on a B cell or FcγR on a DC. (2) Cross-linking of the receptor results in an activation signal. (3) The receptor is endocytosed, and the endosome matures into a late endosome/lysosome containing TLR7 and 8. (4) The U-rich RNA, initially protected by the SmRNP proteins and/or the antibody, now engages the TLR triggering activation of IRF and NF-κB signal transduction pathways. (5) The protein component dissociates and is degraded by lysosomal enzymes with membrane fusion to vesicles containing MHC class II. (6) Activated transcription factors induce expression of proinflammatory cytokines such as type I IFNs, IL-12, IL-6, and TNF which (7) up-regulate MHC class II and costimulatory molecule expression. Cell activation may also lead to cell proliferation.
Does TLR binding by nucleoproteins explain autoantibody specificity?
Throughout the decades after the discovery of autoantibodies in patients with lupus and related diseases, interest in these antibodies has waxed and waned. Although often dismissed as epiphenomena, the remarkable disease specificity of these autoantibodies, as well as the association between certain autoantibodies and disease manifestations (10), suggest that an understanding of how these antibodies are generated might provide insight into the mechanisms of disease. As autoantibodies in SLE patients recognize components of chromatin (such as dsDNA, histones, and nucleosomes) and U-rich ribonucleoproteins (such as RNP and Sm)—all particles that are now known to activate TLRs (10)—it can be proposed that selection of these particles for immune attack is based on their intrinsic ability to activate the TLRs—the Toll hypothesis. This hypothesis would explain why most autoantigens in SLE and related diseases are nucleoproteins—the nucleic acid component could serve as the adjuvant that stimulates cytokine production and the up-regulation of costimulatory molecules, thus facilitating the presentation of the associated peptides to T cells (Fig. 2).
Sm/RNP is a particularly appropriate model antigen, as the highly conserved domains of Sm that make up the hexameric or heptameric rings of the protein (Fig. 2) bind to the oligo-U consensus sequence RAU5GR (where R is any purine) on U snRNAs (for review see reference 14). If the Toll hypothesis is correct, other common RNP antigens that are targeted in SLE, such as Ro (SSA) and La (SSB), should also be TLR agonists. Vollmer et al. (2) report that two small Ro RNAs areTLR7/8 agonists. But what about La? La associates with the 3′ termini of a variety of newly synthesized small RNAs (including the RNA polymerase III transcripts, Ro Y RNAs, U6, pre-tRNAs, pre-5S, and 7SL RNA, as well as small viral RNAs) and, at least in yeast, with polymerase II transcripts (15). A unifying explanation for the binding of RNPs to these nascent transcripts is recognition of a short 3′ oligo U tract, UUU-OH.
It remains to be experimentally tested whether Ro, La, and other ribonucleoprotein antigens that are targeted in SLE are preferential activators of TLR7/8. But the large number of possible U-rich regions in RNAs of the snRNP family (which includes at least 12 members), of small nucleolar RNAs (which number in the hundreds), and of mRNAs that contain 3′ U-rich regions, suggest that the property of being U, UG, or G rich is itself unlikely to be a sufficient explanation for autoantibody selection through TLR activation. Could there be stimulatory sequences within these RNAs similar to those described for DNA and, more recently, for small interfering RNAs (16)? Could one or more of the more than 100 known biochemical modifications of RNA, such as methylation or pseudouridylation, explain differences in activation potential? Recently, it was shown that several of the modifications that occur primarily in mammalian, but not in bacterial, RNAs protected transfected cells from intracellular TLR stimulation (17). This situation is analogous to the protective methylation of DNA in mammalian cells. Finally, the high abundance of U1 RNA (106 copies per nucleus) and the tight binding between the nucleic acid and the core proteins may confer a relative insensitivity to nucleases and the persistence of the particles both outside and inside the cell.
If immunostimulatory DNA and RNA sequences or structures help to account for autoantibody specificity in SLE, and very likely in Sjogren's syndrome (18), a prediction is that the dominant nucleoprotein autoantigens in other systemic diseases, such as systemic sclerosis and polymyositis, will also stimulate TLRs.
Innate immunity to self-nucleoproteins
If self–nucleic acids are capable of activating TLRs, how does the immune system avoid autoimmune activation of the sort described by Lau et al. (1) and Vollmer et al. (2)? The answer likely lies in the intracellular and intraendosomal location of TLR3, 7, 8, and 9, and the presence of highly abundant extra- and intracellular nucleases. Serum contains high levels of DNase and RNase activity, which presumably help dissolve nucleic acids that leak out of dead and dying cells, particularly at sites of inflammation. A plethora of nucleases also exist within the cell—some that assist in the processing of nucleic acid intermediates and others that appear to degrade foreign or ectopic nucleic acids. Type II DNases function optimally in an acidic environment (such as in endosomes/lysosomes), the DNase1-like (L) family have been variously proposed to act in the nucleus, cytosol, and extracellular space, and several other DNases (such as caspase-activated DNase and endonuclease G) are required for cell-autonomous DNA degradation (19–21). The inability to degrade DNA in DNase II−/− mice results in high levels of IFN-β production, although interestingly the inefficient removal of engulfed endogenous DNA stimulates innate immunity through a TLR9-independent pathway (22).
Extracellular or secretory RNases of the RNase A superfamily have a wide range of activities as demonstrated by differential catalytic activities on substrates such as single or double stranded RNA and poly-C or poly-U (for review see reference 23). Intracellular RNases are extremely diverse and comprise not only proteins but also catalytic RNAs. SmRNP itself, as part of the spliceosome, excises intronic RNA from pre-mRNA by transesterification reactions. Many RNases function in processing events at the 5′ or 3′ region of the RNA, whereas others are selectively catalytic for RNA/DNA hybrids (such as the RNase H family) or double stranded RNA (such as the RNase III superfamily), which include the recently recognized Dicer—the enzyme required for the generation of small interfering RNAs. Two enzymes, RNase L and ISG20, help to degrade viral RNAs and are of particular interest, as they are induced by type I IFNs.
As mentioned previously, U-rich RNAs in SmRNP may be relatively resistant to nuclease attack. Endosomal location cannot be the only explanation for this resistance, as snRNPs are assembled in the cytoplasm before their reimport into the nucleus. Whether resistance is conferred by high-affinity binding of RNAs to proteins or by specific patterns of protein shielding, nucleoprotein stability may be an important requirement for TLR stimulation.
Are TLRs the B all and end all?
Two important consequences of TLR7/8 activation were demonstrated in these new studies. Lau et al. (1) reported that immune complexes containing RNA stimulated B cell proliferation, but they did not show that stimulation led to B cell maturation and autoantibody production. Although lymphocyte proliferation is often followed by maturation, this is not always the case. For example, CD8+ T cell proliferation in response to self-antigen is followed by death—one mechanism of peripheral tolerance (24). Studies on the fate of B cells activated by nucleoprotein complexes are eagerly awaited.
In the study by Vollmer et al. (2), the major consequence of TLR7/8 activation by U-rich RNAs was the production of IFN-α, a cytokine long known to be elevated in patients with SLE. Although the low IFN response in TLR7−/− mice suggests a dominant role for this receptor in response to U1 RNA, it is relevant to note that both viral and mammalian nucleic acids may stimulate IFNs through TLR-independent routes, as recently shown (25–27). Of particular interest, RNA helicases containing caspase-recruiting domains (CARDs), such as RIG-1 and Mda5, stimulate IFN production in response to intracellular, double stranded viral RNA in a TLR-independent manner (28, 29). It is thus likely that additional nucleic acids sensors and pathways will be discovered.
Clinical implications
Regardless of whether TLR engagement is required for the initiation and/or progression of autoantibody production, modulation of certain TLR pathways impacts autoantibody production, at least in murine models of SLE. Although studies performed to date have used mice with a mixed genetic background, they have consistently shown that both MyD88 and TLR9 deficiencies reduced the level of serum autoantibodies (1, 30), suggesting that these pathways are required for high titer autoantibody production. TLR3 deficiency, in contrast, had no effect. Although TLR9 deficiency reduced some anti-DNA antibodies, it did not protect mice from glomerulonephritis (30). This could occur either because the lack of TLR9 altered the isotypes or affinity of the anti-DNA antibodies (not tested in this study), or because nephritis was caused by a different subset of autoantibodies. Since oligonucleotide-based inhibitors of both TLR9 and 7 have been developed (31), their therapeutic application for treatment of SLE is an exciting prospect (32).
Concluding remarks
There is currently no unifying hypothesis to explain autoantigen selection in systemic autoimmune disorders. Here, we propose that a necessary property for the selection of nucleoprotein autoantigens is their ability to activate intracellular TLRs. According to the Toll hypothesis, the nucleic acid component of RNPs serves as an adjuvant, and the protein component is processed by the activated APC and presented to T cells (Fig. 2). However, this is not a sufficient explanation for autoimmunity. Other critical abnormalities that sensitize immune cells and provide a constant source of antigen, or that facilitate uptake of nucleoproteins (Fig. 1), must act as predisposing factors. Nevertheless, at least part of the answer to the 50-yr-old puzzle of autoantibody specificity now seems to be solved.
Acknowledgments
We thank Alan Weiner, Gabriele Varani, and Gary Zieve for helpful discussions.
Our work is supported by the National Institutes of Health.
References
- 1.Lau, C.M., C. Broughton, A.S. Tabor, S. Akira, R.A. Flavell, M.J. Mamula, S.R. Christensen, M.J. Shlomchik, G.A. Viglianti, I.R. Rifkin, and A. Marshak-Rothstein. 2005. RNA-associated autoantigens activate B cells by combined B cell receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202:1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollmer, J., S. Tluk, C. Schmitz, S. Hamm, M. Jurk, A. Forsbach, S. Akira, K.M. Kelly, W.H. Reeves, S. Bauer, and A.M. Krieg. 2006. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. [DOI] [PubMed] [Google Scholar]
- 4.Boule, M.W., C. Broughton, F. Mackay, S. Akira, A. Marshak-Rothstein, and I.R. Rifkin. 2004. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 199:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 6.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 8.Lovgren, T., M.L. Eloranta, U. Bave, G.V. Alm, and L. Ronnblom. 2004. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50:1861–1872. [DOI] [PubMed] [Google Scholar]
- 9.Viglianti, G.A., C.M. Lau, T.M. Hanley, B.A. Miko, M.J. Shlomchik, and A. Marshak-Rothstein. 2003. Activation of autoreactive B cells by CpG dsDNA. Immunity. 19:837–847. [DOI] [PubMed] [Google Scholar]
- 10.Fritzler, M.J., and K.B. Elkon. 2003. Autoantibodies in SLE. In Rheumatology. M.C. Hochberg, A.J. Silman, J.S. Smolen, M.E. Weinblatt, and M.H. Weisman, editors. Mosby Publishing, London. 1337–1346.
- 11.Beg, A.A. 2002. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23:509–512. [DOI] [PubMed] [Google Scholar]
- 12.Kim, S.J., D. Gershov, X. Ma, N. Brot, and K.B. Elkon. 2003. Opsonization of apoptotic cells and its effect on macrophage and T cell immune responses. Ann. NY Acad. Sci. 987:68–78. [DOI] [PubMed] [Google Scholar]
- 13.Yurasov, S., H. Wardemann, J. Hammersen, M. Tsuiji, E. Meffre, V. Pascual, and M.C. Nussenzweig. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Will, C.L., and R. Luhrmann. 2001. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 13:290–301. [DOI] [PubMed] [Google Scholar]
- 15.Xue, D., D.A. Rubinson, B.K. Pannone, C.J. Yoo, and S.L. Wolin. 2000. U snRNP assembly in yeast involves the La protein. EMBO J. 19:1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornung, V., M. Guenthner-Biller, C. Bourquin, A. Ablasser, M. Schlee, S. Uematsu, A. Noronha, M. Manoharan, S. Akira, A. de Fougerolles, et al. 2005. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11:263–270. [DOI] [PubMed] [Google Scholar]
- 17.Kariko, K., M. Buckstein, H. Ni, and D. Weissman. 2005. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 23:165–175. [DOI] [PubMed] [Google Scholar]
- 18.Bave, U., G. Nordmark, T. Lovgren, J. Ronnelid, S. Cajander, M.L. Eloranta, G.V. Alm, and L. Ronnblom. 2005. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 52:1185–1195. [DOI] [PubMed] [Google Scholar]
- 19.Evans, C.J., and R.J. Aguilera. 2003. DNase II: genes, enzymes and function. Gene. 322:1–15. [DOI] [PubMed] [Google Scholar]
- 20.Baron, W.F., C.Q. Pan, S.A. Spencer, A.M. Ryan, R.A. Lazarus, and K.P. Baker. 1998. Cloning and characterization of an actin-resistant DNase I-like endonuclease secreted by macrophages. Gene. 215:291–301. [DOI] [PubMed] [Google Scholar]
- 21.Nagata, S. 2005. DNA degradation in development and programmed cell death. Annu. Rev. Immunol. 23:853–875. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida, H., Y. Okabe, K. Kawane, H. Fukuyama, and S. Nagata. 2005. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 6:49–56. [DOI] [PubMed] [Google Scholar]
- 23.Sorrentino, S. 1998. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell. Mol. Life Sci. 54:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honda, K., Y. Ohba, H. Yanai, H. Negishi, T. Mizutani, A. Takaoka, C. Taya, and T. Taniguchi. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 434:1035–1040. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda, K., P. Yu, C.J. Kirschning, B. Schlatter, F. Schmitz, A. Heit, S. Bauer, H. Hochrein, and H. Wagner. 2005. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J. Immunol. 174:6129–6136. [DOI] [PubMed] [Google Scholar]
- 27.Martin, D.A., and K.B. Elkon. 2006. Intracellular mammalian DNA stimulates myeloid dendritic cells to produce Type I interferons independently of MyD88 or TLR9. Arthritis Rheum. In press. [DOI] [PubMed] [Google Scholar]
- 28.Andrejeva, J., K.S. Childs, D.F. Young, T.S. Carlos, N. Stock, S. Goodbourn, and R.E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA. 101:17264–17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737. [DOI] [PubMed] [Google Scholar]
- 30.Christensen, S.R., M. Kashgarian, L. Alexopoulou, R.A. Flavell, S. Akira, and M.J. Shlomchik. 2005. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 202:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrat, F.J., T. Meeker, J. Gregorio, J.H. Chan, S. Uematsu, S. Akira, B. Chang, O. Duramad, and R.L. Coffman. 2005. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong, L., S. Ito, K.J. Ishii, and D.M. Klinman. 2005. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB x NZW mice. Arthritis Rheum. 52:651–658. [DOI] [PubMed] [Google Scholar]