Abstract
The expression of the Adenovirus serotype 5 (Ad5) E1A oncogene sensitizes tumor cells to natural killer (NK) cell–mediated killing and tumor rejection in vivo. These effects are dependent on the ability of E1A to bind the transcriptional coadaptor protein p300. To test the hypothesis that E1A up-regulates ligands recognized by the NKG2D-activating receptor, we stably transfected the highly tumorigenic mouse fibrosarcoma cell line MCA-205 with Ad5-E1A or a mutant form of E1A that does not interact with p300 (E1A-Δp300). Ad5-E1A, but not E1A-Δp300, up-regulated the expression of the NKG2D ligand retinoic acid early inducible (RAE)-1, but not murine ULBP-like transcript 1, another NKG2D ligand, in four independently derived MCA-205 transfectants. The up-regulation of RAE-1 by E1A targeted MCA-205 tumor cells to lysis by NK cells, resulting in NKG2D-dependent tumor rejection in vivo. Moreover, the up-regulation of NKG2D ligands by E1A was not limited to mouse tumor cells, as E1A also increased the expression of NKG2D ligands on primary baby mouse kidney cells, human MB435S breast cancer cells, and human H4 fibrosarcoma cells.
Adenoviruses (Ads) are ubiquitous human pathogens that typically cause self-limited viral infections. In the United States, 95% of adults are seropositive to one or more of the group C Ad, such as Ad serotype 2 or serotype 5 (Ad2/5). Although not known to be oncogenic in humans, Ad can transform mammalian cells, including human cells. In Ad-transformed cells, there is viral integration into the host genome and the expression of two viral genes (E1A and E1B) is consistently found. E1A is the primary immortalizing gene of Ad, and E1B serves as a “helper” gene to increase the efficiency of E1A-induced transformation (1 and references therein).
The E1A oncoprotein from all Ad serotypes expresses two highly homologous conserved regions, CR1 and CR2, which are essential for E1A-induced immortalization (1). The conserved regions of E1A serve as high-affinity binding sites for cell growth regulatory proteins, including retinoblastoma (Rb) family members and the transcriptional coadaptor molecules p300/CBP (1). (In this paper, we do not distinguish between the closely related transcriptional coadaptor molecules p300 and CBP and refer to both as p300). Mutations within CR1 or CR2 that eliminate binding to p300 or pRb abrogate the capacity of E1A to immortalize primary cells.
The expression of the Ad2/5-E1A oncogene sensitizes cells to lysis by NK cells, an activity that is dependent on the capacity of E1A to bind p300 (2). This activity of E1A leads to the NK cell–mediated rejection in vivo of tumor cells that express E1A (2, 3). Although the ability of E1A to sensitize cells to NK cells was first described nearly two decades ago, the molecular mechanism remains to be elucidated.
The balance of inhibitory and activating signals delivered by the target cell to the NK cells determines the susceptibility of a target cell to NK cell–mediated lysis. E1A does not block the capacity of class I MHC molecules expressed on target cells to inhibit NK cell–mediated killing (4). Therefore, we hypothesized that the capacity of E1A to sensitize cells to NK cell lysis was by the E1A-induced up-regulation of ligands on target cells that interact with activating NK cell receptors. NKG2D ligands are an attractive candidate because they are up-regulated by a variety of cellular stresses, including viral infection (5, 6) or conditions promoting DNA damage (7). Furthermore, the forced expression of NKG2D ligands on tumors sensitizes these cells to NK cell–mediated lysis in vitro and NK cell–dependent tumor rejection in vivo (8–10). Here, we have investigated the effect of E1A on the expression of NKG2D ligands on mouse and human cell lines and the recognition of these cells by NK cells in vivo and in vitro.
Results
E1A up-regulates NKG2D ligand expression on primary mouse cells, mouse tumor cells, and human tumor cells
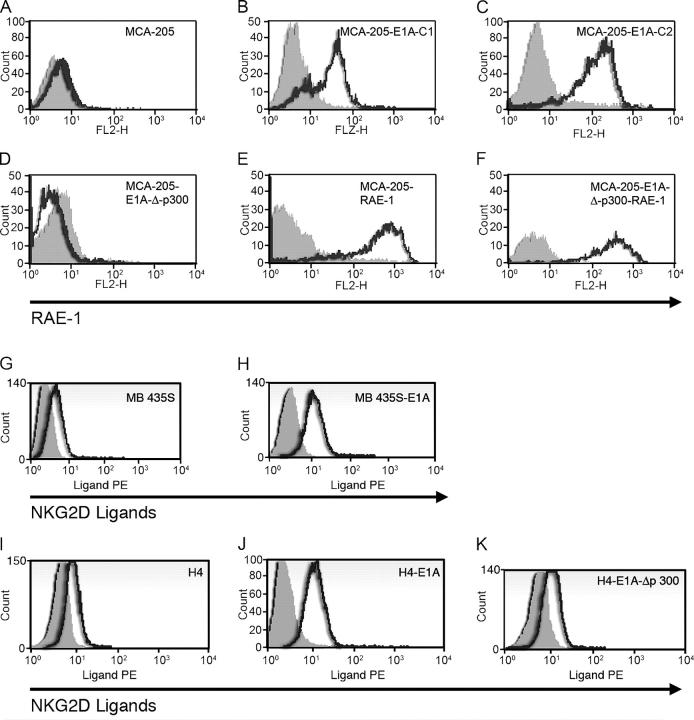
Mouse MCA-205 fibrosarcoma cells and MCA-205 cells stably transfected with E1A (see Materials and methods) were screened for expression of mouse NKG2D ligands by flow cytometry using a mouse NKG2D–Ig fusion protein. MCA-205 cells transfected with E1A expressed higher amounts of NKG2D ligands than parental MCA-205 cells as revealed by staining with mouse NKG2D–Ig fusion protein (not depicted). To determine which of the NKG2D ligands were up-regulated by E1A, MCA-205 and MCA-205-E1A cells were stained with mAbs against retinoic acid early inducible (RAE)-1 and murine ULBP-like transcript 1 (MULT1), another NKG2D ligand. MCA-205-E1A cells expressed substantially higher amounts of RAE-1 on the cell surface than parental MCA-205 cells (Fig. 1, A–C). The up-regulation of RAE-1 on MCA-205 cells by E1A was specific for RAE-1 because we detected no significant expression of MULT1 on the surface of the parental MCA-205 or MCA-205 E1A cells (not depicted).
Figure 1.
Expression of NKG2D ligands on the surface of parental and E1A-expressing murine (MCA-205) and human (MB435S and H4) tumor cells. Expression of RAE-1 on the surface of (A) MCA-205, (B and C) MCA-205-E1A, and (D) MCA-205-E1A-Δ-p300 tumor cells or (E) MCA-205 and (F) MCA-205-E1A-Δ-p300 cells infected with a retrovirus that expresses RAE-1ɛ. Expression of NKG2D ligands on the surface of (G) MB435S or (H) MB435S-E1A human breast cancer cells. Expression of NKG2D ligands on the surface of (I) H4, (J) H4-E1A, or (K) H4-E1A-Δp300 human fibrosarcoma cells.
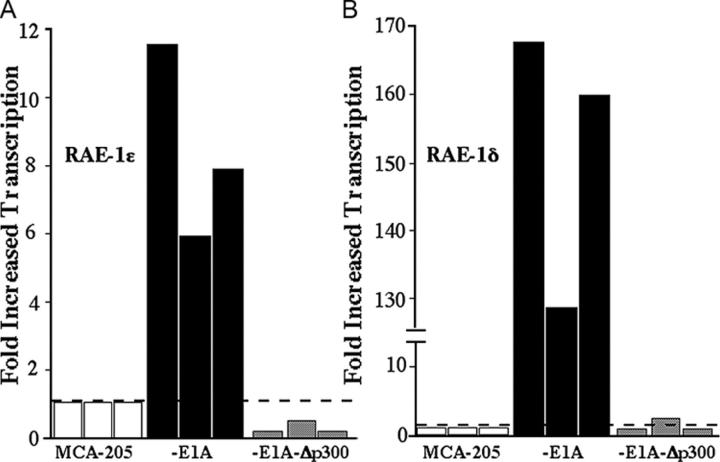
The best control to demonstrate that the ability of E1A to up-regulate NKG2D ligands is E1A-specific is to compare the effect of wild-type E1A with mutant forms of E1A that do not sensitize cells to NK cell lysis. Previous studies established that the expression of E1A, but not a mutant E1A unable to bind cellular p300 (E1A-Δp300), rendered tumor cells more sensitive to NK cell–mediated cytotoxicity (2, 11). Therefore, we determined if MCA-205 cells stably transfected with E1A-Δp300 would up-regulate RAE-1 on the cell surface (Fig. 1). Four independently derived MCA-205 cell lines transfected with E1A-Δp300 (designated MCA-205 E1A-Δp300-C1, -C2, -C3, and -C4) did not show enhanced NKG2D ligand expression (Fig. 1 and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20050240/DC1). In contrast, increased amounts of RAE-1 were expressed on four independently derived MCA-205-E1A transfectants (designated MCA-205-E1A-C1, -C2, -C3 and -C4; Fig.1 and Fig. S1). The different MCA-205 E1A or E1A-Δp300 transfectants were derived simultaneously and maintained in cell culture for an equivalent period of time. Therefore, the up-regulation of RAE-1 on MCA-205-E1A cells was due to the expression of E1A and not a result of a spontaneous up-regulation of RAE-1 due to prolonged cell culture of the cells. Real-time PCR demonstrated increased transcription of both RAE-1δ and RAE-1ɛ in MCA-205 tumor cells that expressed E1A, but not E1A-Δp300 (Fig. 2).
Figure 2.
The expression of mRNA for RAE-1δ and RAE-1ɛ in MCA-205, MCA-205-E1A, and MCA-205-Δp300 tumor cells as determined by real-time PCR. (A) mRNA for RAE-1δ and (B) RAE-1ɛ in MCA-205, MCA-205-E1A, and MCA-205-Δp300 tumor cells. mRNA levels for RAE-1δ and RAE-1ɛ were normalized to hypoxanthine-guanine phosphoribosyltransferase transcripts in each cell line, and the fold-induction of RAE-1 transcripts are reported compared with the parental MCA-205 cells (hatched line). The three bars indicated for each cell line represent the data obtained in three independent experiments.
The ability of E1A to up-regulate NKG2D ligands was not restricted to the MCA-205 fibrosarcoma cell line. The expression of NKG2D ligands was also up-regulated on primary baby mouse kidney (BMK) fibroblast cells derived from p53−/− mice that were transformed by E1A (Fig. S1; reference 12). Furthermore, human MB435S breast cancer cells and human H4 fibrosarcoma cells stably transfected with E1A demonstrated increased amounts of NKG2D ligands on the cell surface (Fig. 1, G–J). Consistent with our observations on MCA-205 cells, H4 cells expressing E1A-Δp300 did not up-regulate NKG2D ligand expression (Fig. 1 K). We observed increased amounts of both the MIC and ULBP proteins on the surface of these human tumor cell lines transduced with E1A (not depicted).
In our studies, we used two different mutant forms of E1A that do not bind p300. H4 cells were transfected with a mutant form of E1A that changed only one residue (arg to gly residue no. 2). MCA-205 cells were transfected with an E1A-Δp300 mutant that deletes residues 48–60 in conserved region 1 of E1A. Both of the mutant forms of E1A used do not interact with p300 and do not abolish the ability of E1A to bind Rb (13, 14). Consequently, the E2F-Rb–regulated G1/S checkpoint would be abrogated by both E1A and E1A-Δp300 (13, 14), yet only wild-type E1A affected expression of the NKG2D ligands.
Taken together, these studies showed that the expression of E1A increased the expression of NKG2D ligands on the surface of mouse (MCA-205) and human cancer cells (H4 and MB435S), as well as primary mouse cells (BMK) transformed by E1A. In contrast, the expression E1A-Δp300, an E1A mutant unable to bind p300 but able to bind Rb, failed to increase NKG2D ligands on either mouse or human cells. Additional studies will be necessary to define the effects of E1A on p300 that induce transcription of these different genes encoding distinct NKG2D ligands in human and mouse cells.
Up-regulation of NKG2D ligands by E1A sensitizes tumors to lysis by NK cells
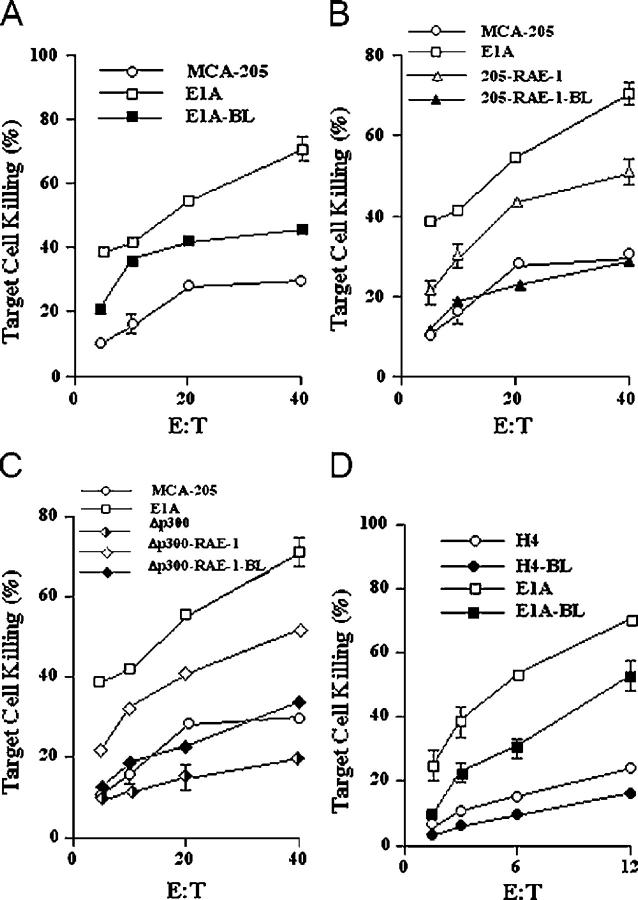
The relationship between the amounts of RAE-1 molecules expressed on the surface of the different MCA-205 cell lines and their sensitivity to NK cell lysis was examined in two ways. With the neutralizing anti–mouse NKG2D mAb CX5, we determined the effect on NK cell–mediated killing by blocking the interaction of NKG2D on NK cells and RAE-1 on the MCA-205-E1A cells. We also compared the NK cell–mediated lysis of MCA-205-E1A cells to MCA-205 and MCA-205-E1A-Δp300 cell lines that were transduced with a retrovirus encoding RAE-1ɛ (Fig. 3). The neutralizing anti–mouse NKG2D mAb substantially blocked the NK cell–mediated killing of the MCA-205-E1A cells. The inhibition of the NK cell killing was incomplete, as MCA-205-E1A cells were still more sensitive to NK cell lysis than parental MCA-205 cells (Fig. 3 A). MCA-205 or MCA-205-E1A-Δp300 cells that were transduced with a retrovirus encoding RAE-1ɛ and selected to express high amounts of RAE-1ɛ were more sensitive than their respective parental cells lines, but less sensitive to NK cell–mediated lysis than MCA-205-E1A cells (Fig. 3, A and B).
Figure 3.
The role of NKG2D ligands in the NK cell–mediated lysis of mouse and human tumor cells that express E1A. (A) MCA-205-E1A, (B) MCA-205-RAE-1ɛ (i.e., MCA-205 cells transduced with RAE-1ɛ), and (C) MCA-205-E1A-Δp300-RAE-1ɛ (MCA-205-E1A-Δp300 cells transduced with RAE-1ɛ) were incubated with IL-2–activated mouse NK cells at the indicated effector–target cell ratio with control rat IgG1 or the neutralizing anti–mouse NKG2D mAb CX5 in standard NK cytolysis assays. (B) Human NK cell–mediated cytolysis assays using human H4 or H4-E1A target cells with control mouse IgG1 or a neutralizing anti–human NKG2D antibody. BL refers to the addition of an antibody that blocks the interaction of NKG2D with its ligand. The graphs represent the mean of ± SEM of three (MCA-205) or four (H4) separate experiments. Data points in which the SEM was less than five are not shown on the graphs.
We also evaluated whether the up-regulation of NKG2D ligands by E1A sensitized human H4 tumor cells to lysis by human NK cells. A neutralizing anti–human NKG2D mAb significantly blocked the ability of human NK cells to lyse H4-E1A tumor cells. Again, blocking the interaction of NKG2D with its ligands did not completely inhibit the NK cell–mediated killing of H4-E1A cells. In both the mouse MCA-205 and human H4 tumor cell lines, the addition of an isotype-matched control antibody had no inhibitory effect on the NK cell–mediated killing of the target cells (not depicted). These data indicated that the capacity of E1A to sensitize cells to lysis by NK cells was dependent, in part, on the ability of E1A to up-regulate NKG2D ligands.
Up-regulation of RAE-1 by E1A on MCA-205 cells enhances NK cell–mediated tumor rejection
We examined whether the up-regulation of RAE-1 by E1A on MCA-205 cells would lead to a reduction in tumorigenicity by enhancing an NK cell–dependent antitumor response. To determine the tumorigenicity of the different MCA-205 tumor lines, quantitative tumor induction studies were performed and TPD50 values were calculated. Quantitative tumor induction studies were performed because they are superior to single-dose tumor induction studies and allow for quantitation of intermediate tumorigenic phenotypes (15).
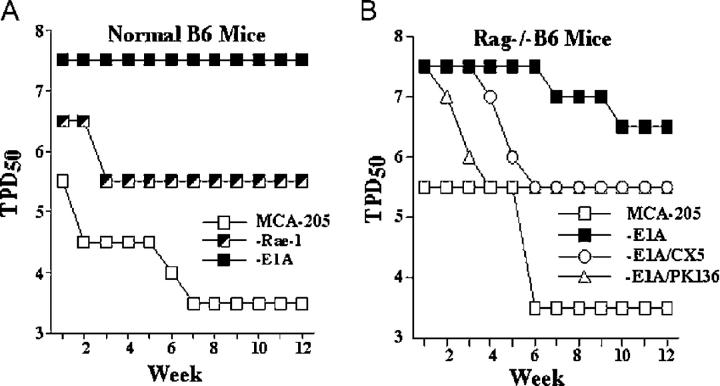
In wild-type C57BL/6 mice, the subcutaneous injection of MCA-205-E1A cells failed to cause progressive tumors, even with high doses (107 tumor cells/animal) of tumor cells. In contrast, progressive tumors were observed after injection of ∼3,000 MCA-205 or MCA-205-E1A-Δp300 cells (Fig. 4 A and not depicted). The stable expression of RAE-1 on MCA-205 tumor cells by transfection of RAE-1ɛ also resulted in a 100-fold reduction in tumorigenicity compared with MCA-205 cells (Fig. 4 A).
Figure 4.
Role of NKG2D in the NK cell–mediated rejection of MCA-205-E1A cells. (A) Wild-type B6 or (B) B6-Rag1 − / − mice were injected subcutaneously with serial log concentrations of cells and observed weekly for tumor development for 12 wk. Depletion of NK cells in vivo was performed by using the mAb PK136. The interaction between NKG2D and NKG2D ligands was blocked in vivo using the neutralizing anti-NKG2D mAb CX5. TPD50 values (log10 of the number of tumor cells required to produce tumors in 50% of the mice) were calculated on a weekly basis. Cells derived from progressive tumors were tested for E1A or RAE-1 expression by Western blot analysis and flow cytometry, respectively, to confirm expression of E1A and RAE-1 in tumors that emerged to exclude transgene loss as a reason for tumor growth.
To directly evaluate the role of NK cells and NKG2D in immunity against the MCA-205-E1A tumor, we performed tumor induction studies using B6-Rag1 − / − mice. Individual groups of mice were treated with control rat IgG1, the nondepleting neutralizing anti-NKG2D mAb, CX5 (16), or PK136, an NK cell–depleting mAb that recognizes the NK1.1 antigen. Mice were then injected with MCA-205-E1A tumor cells. Mice treated with control rat IgG1 and challenged with parental MCA-205 cells served as an additional control. Depletion of NK cells in the mice treated with anti-NK1.1 mAb and modulation of NKG2D on NK cells in mice treated with anti-NKG2D mAb was confirmed by flow cytometry (not depicted). Depletion of NK cells or blocking the NKG2D receptor substantially increased the tumorigenicity of MCA-205-E1A cells (Fig. 4 B). Approximately 3 × 106 MCA-205-E1A cells (TPD50 = 6.5) were required to form progressive tumors in 50% of control, rat IgG1-treated Rag1 − / − mice. In contrast, progressive tumors formed after the injection of roughly 300,000 MCA-205-E1A tumor cells in mice treated with PK136 or CX5. Thus, the NK cell–mediated, NKG2D-dependent antitumor response was capable of eliminating more than 2 × 106 MCA-205 tumor cells expressing E1A.
The reduction in tumorigenicity of MCA-205 cells by E1A was not entirely NKG2D-dependent. MCA-205 cells that were transfected with RAE-1ɛ were more tumorigenic than the MCA-205 cells expressing E1A. Moreover, MCA-205-E1A cells were less tumorigenic than parental MCA-205 cells in Rag1 − / − mice treated with PK136 or CX5 to deplete NK cells or block NKG2D function, respectively. E1A has other activities that likely explain the NKG2D-independent anti-tumorigenic activity of E1A. The expression E1A sensitizes cells to lysis by macrophages and immune effector molecules, such as perforin/granzyme, TNF-α, TRAIL, nitric oxide, and Fas-L (17 and references therein).
Discussion
Our results demonstrated that the expression of E1A up-regulated the expression of NKG2D ligands on primary mouse cells (BMK cells), mouse tumor cells (MCA-205 cells), and human tumor cells (H4 and MB435S cells). The molecular events that link the expression of E1A to the up-regulation of NKG2D ligands were not investigated in this study and remain unknown. E1A does not alter cellular transcription by binding DNA in a sequence-specific manner. Rather, E1A modulates cellular transcription by interacting with and altering the function of a diverse set of cellular proteins, such as transcription factors, transcriptional coadaptor molecules, and tumor suppressor gene products. For example, E1A binds the tumor suppressor protein pRb, resulting in the release of the cellular transcription factor E2F, thereby overriding the G1/S checkpoint and enhancing cellular proliferation. The mutant E1A-Δp300 binds Rb and does not affect the ability of E1A to override the G1/S checkpoint (14). Therefore, our data indicated that the ability of E1A to up-regulate NKG2D and sensitize cells to lysis by NK cells was independent of this activity of E1A.
Experiments reported herein are consistent with previous studies that indicate that E1A-p300 binding is required for the ability of E1A to sensitize cells to lysis by NK cells (2). Our data demonstrated that in contrast to wild-type E1A, the expression of E1A-Δp300 did not up-regulate the expression of NKG2D ligands on mouse MCA-205 or human H4 cells and did not sensitize MCA-205 or H4 cells to lysis by NK cells. p300 (and CBP) serve as coadaptor molecules for numerous cellular transcription factors and have the capacity to remodel chromatin, activities altered upon interacting with E1A. Several other viral oncoproteins also interact with and alter the function of p300. Therefore, it will be important to ascertain the molecular mechanism whereby the interaction of E1A with p300 up-regulates NKG2D ligands and determine if this mechanism is unique to E1A or a common pathway used by other viral oncoproteins.
Small DNA tumor viruses, such as Ads, human papillomaviruses (HPVs), and polyomaviruses (e.g., SV40), transform cells from diverse species by using similar molecular mechanisms. The primary immortalizing oncogenes of Ad (E1A), HPV (E7), and SV40 (large T antigen) contain homologous conserved regions (CR1 and CR2) that are essential for cellular immortalization and interchangeable in cellular transformation assays (18). The “helper” oncogenes of Ad (E1B), HPV (E6), and SV40 (large T antigen), which are necessary for complete cellular transformation, inhibit the function of the tumor suppressor gene product p53 (18). Despite substantial progress in defining the molecular mechanisms used by these viruses to transform mammalian cells, a fundamental question remains: Why do viruses that appear to transform cells through analogous molecular mechanisms differ so dramatically in their oncogenicity, even in species that serve as natural hosts for virus infection? For example, HPVs, but not Ads, are oncogenic in humans, their natural host.
Our observations may shed light on this question and help explain the lack of oncogenicity of Ads in humans. Ad-transformed human cells are tumorigenic in immunocompromised rodents (19), illustrating the potential oncogenicity of this common human pathogen. Studies using rodents established the importance of the cellular immune response in regulating the oncogenicity of Ad-transformed cells. Primary cells transformed by Ad2 or Ad5, which are not oncogenic in immunocompetent rodents, are tumorigenic in animals with defective NK cell or T cell responses (20, 21). The ectopic expression of NKG2D ligands on tumors has been shown to target these cells to lysis in vivo by both NK cells and T cells (9, 10, 22). Therefore, the up-regulation of NKG2D ligand by E1A during a transforming viral infection may also lead to the elimination of these transformed cells by both innate and adaptive immune responses.
Viral oncoproteins derived from different small DNA tumor viruses may vary in their ability to up-regulate ligands, such as NKG2D ligands, recognized by activating NK cell receptors on innate effector cells. Virally transformed cells that do not activate innate immune responses may also fail to elicit effective adaptive immune responses and evade host cellular antitumor immunity, thereby enhancing their oncogenicity. This hypothesis is consistent with the inability of E7 to sensitize cells to lysis by NK cells or macrophages and the high oncogenicity of HPV in humans (3). This hypothesis is also consistent with the well-described inverse relationship between the sensitivity of cells transformed by several small DNA tumor viruses to lysis by NK cells in vitro and the oncogenicity of these virus-transformed cells in vivo (23).
In summary, E1A, but not E1A-Δp300, up-regulated the expression of the NKG2D ligands on mouse MCA-205 tumor cells as well as human breast cancer and fibrosarcoma cells. To our knowledge, E1A is the first example of a viral oncoprotein that can up-regulate NKG2D ligands and thereby enhance NK cell–mediated tumor rejection.
Materials and Methods
Plasmids and retroviruses.
The pLSXN-E1A and pLSXN-E1A-Δp300 retroviruses express wild-type Ad5 E1A and Ad5 E1A-Δp300, respectively. The E1A-Δp300 (dl1104) mutant has a deletion in amino acids 48–60 in CR1, which abrogates binding to p300. Retroviruses containing a RAE-1ɛ cDNA were generated by using the pMx-puro vector (24).
Antibodies and NKG2D fusion proteins.
CX5 is a neutralizing, nondepleting rat IgG1 mAb directed against mouse NKG2D that blocks the binding of NKG2D to its ligands and modulates expression of NKG2D from the cell surface (25). mAb 186107 (rat IgG2b) binds to RAE-1α, RAE-1β, RAE-1γ, RAE-1δ, and RAE-1ɛ (5), mAb 237104 (rat IgG2a) recognizes MULT1 (26), and mAb 149810 (mouse IgG1) binds the human NKG2D receptor and blocks the interaction of the NKG2D receptor with NKG2D ligands on human cells (27). PK136 is an NK cell–depleting mouse IgG2a mAb that recognizes NK1.1 (28). Mouse NKG2D–Ig fusion protein is composed of the extracellular domain of mouse NKG2D and the Fc fragment of human IgG1 (8).
Cell lines.
The C57BL/6-derived MCA-205 fibrosarcoma cell line was provided by N. Restifo (National Cancer Institute, National Institutes of Health, Bethesda, MD). p53−/− BMK cells transformed by E1A (12) were provided by E. White (Rutgers University, Piscataway, NJ). Stable cell lines were generated by a single transfection of the MCA-205 cell line with pLSXN-E1A or pLSXN-E1A-Δp300 by using the Superfect transfection reagent (QIAGEN). After transfection, the cells were plated into six-well plates followed by selection in 1 mg/ml G418 (Sigma-Aldrich). Geneticin-resistant colonies from separate wells were expanded and screened for expression of E1A or E1A-Δp300 by Western blot analysis (3). MCA-205-E1A and MCA-205-E1A-Δp300 cell lines that expressed high levels of E1A or E1A-Δp300 proteins were subsequently analyzed for NKG2D ligand expression by using mouse NKG2D–Ig fusion protein. MCA-205 and MCA-205-E1A-Δp300 cell lines were infected with retroviruses containing RAE-1ɛ cDNA. After selection in medium containing 1 μg/ml puromycin, transduced cells stably expressing RAE-1ɛ were isolated by flow cytometry. H4, a human fibrosarcoma cell line, and H4-E1A, an Ad5 E1A-transfected H4 cell line (29 and references therein), were provided by S. Frisch (La Jolla Cancer Research Institute, La Jolla, CA). H4-E1A-Δp300 is an H4 cell line that expresses a mutant form of E1A unable to bind p300 (29). MB435S, a human breast cancer line, and MB435S-E1A, an Ad5 E1A-transfected MB435S cell line, were provided by J. Cook (University of Illinois, Chicago, IL). All cell lines were maintained in DMEM supplemented with 5% fetal calf serum, 15 mM glucose, and antibiotics.
NK cell cytolysis assays.
Purified populations of polyclonal mouse NK cells or human NK cells were used as effectors in a standard 4-h 51Cr-release cytotoxicity assay, as previously described (8). Human NK cells were isolated from PBMCs by negative selection using the RosetteSep enrichment cocktail (Stem Cell Technologies, Vancouver, BC) according to the manufacturer's instructions. Polyclonal mouse and human NK cell cultures contained >90% NK cells as determined by flow cytometry.
Tumor induction studies.
Quantitative tumor induction studies were performed as described previously (2). Mice (two [B6 Rag1 − / −] or three [B6 mice] animals per cell line at each dose) were injected subcutaneously with serial log concentrations of cells and observed weekly for tumor development for 12 wk. Animals were killed when tumors reached 20 mm mean diameter or at the end of the 12-wk observation period. Tumor induction experiments were repeated twice in B6 mice. Tumor cells from animals injected with MCA-102-E1A were tested for E1A or RAE-1 expression. TPD50 values, the log10 of the number of tumor cells required to produce tumors in 50% of the mice, were calculated as described previously (2). Experiments involving mice were conducted using protocols approved by the National Jewish Medical and Research Center Animal Care and Use Committee and the University of California, San Francisco, Committee on Animal Research.
Real-time PCR.
The expression of mRNA for RAE-1δ and RAE-1ɛ in MCA-205, MCA-205-E1A, and MCA-205-Δp300 was determined by real-time PCR. Real-time PCR, including probe sets for RAE-1δ, RAE-1ɛ, and hypoxanthine-guanine phosphoribosyltransferase, were performed using an ABI 7700 with Sequence Detector Software (Applied Biosystems), and the conditions were described previously (25, 30).
Online supplemental material.
Fig. S1 shows the amounts of RAE-1 expressed on two additional, independently derived MCA-205-E1A and MCA-205-E1A-Δp300 cell lines and the expression of NKG2D ligands on primary and E1A-transformed BMK cells derived from p53−/− mice. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20050240/DC1.
Acknowledgments
We thank Gabriele Cheatham for secretarial assistance.
L.L. Lanier is an American Cancer Society Research Professor. This work was supported by National Institutes of Health grants CA76491 to J.M. Routes and CA89189 to L.L. Lanier.
The authors have no conflicting financial interests.
Abbreviations used: Ad, adenovirus; BMK, baby mouse kidney; HPV, human papillomavirus; MULT1, murine ULBP-like transcript 1; RAE, retinoic acid early inducible; Rb, retinoblastoma.
A. Cerwenka's present address is Deutsches Krebsforschungszentrum, DKFZ, 69120 Heidelberg, Germany.
References
- 1.Sang, N., J. Caro, and A. Giordano. 2002. Adenoviral E1A: everlasting tool, versatile applications, continuous contributions and new hypotheses. Front. Biosci. 7:d407–d413. [DOI] [PubMed] [Google Scholar]
- 2.Cook, J.L., C.K. Krantz, and B.A. Routes. 1996. Role of p300-family proteins in E1A oncogene induction of cytolytic susceptibility and tumor cell rejection. Proc. Natl. Acad. Sci. USA. 93:13985–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Routes, J.M., S. Ryan, J. Steinke, and J.L. Cook. 2000. Dissimilar immunogenicities of human papillomavirus E7 and adenovirus E1A proteins influence primary tumor development. Virology. 277:48–57. [DOI] [PubMed] [Google Scholar]
- 4.Routes, J.M., J. Ryan, S. Ryan, and M. Nakamura. 2001. MHC class I molecules on adenovirus E1A-expressing tumor cells inhibit NK cell killing but not NK cell mediated tumor rejection. Int. Immunol. 13:1301–1307. [DOI] [PubMed] [Google Scholar]
- 5.Lodoen, M., K. Ogasawara, J.A. Hamerman, H. Arase, J.P. Houchins, E.S. Mocarski, and L.L. Lanier. 2003. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J. Exp. Med. 197:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groh, V., R. Rhinehart, J. Randolph-Habecker, M.S. Topp, S.R. Riddell, and T. Spies. 2001. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2:255–260. [DOI] [PubMed] [Google Scholar]
- 7.Gasser, S., S. Orsulic, E.J. Brown, and D.H. Raulet. 2005. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 436:1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerwenka, A., A.B. Bakker, T. McClanahan, J. Wagner, J. Wu, J.H. Phillips, and L.L. Lanier. 2000. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 12:721–727. [DOI] [PubMed] [Google Scholar]
- 9.Cerwenka, A., J.L. Baron, and L.L. Lanier. 2001. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA. 98:11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diefenbach, A., A.M. Jamieson, S.D. Liu, N. Shastri, and D.H. Raulet. 2000. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 1:119–126. [DOI] [PubMed] [Google Scholar]
- 11.Cook, J.L., B.A. Routes, T.A. Walker, K.L. Colvin, and J.M. Routes. 1999. E1A oncogene induction of cellular susceptibility to killing by cytolytic lymphocytes through target cell sensitization to apoptotic injury. Exp. Cell Res. 251:414–423. [DOI] [PubMed] [Google Scholar]
- 12.Degenhardt, K., G. Chen, T. Lindsten, and E. White. 2002. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell. 2:193–203. [DOI] [PubMed] [Google Scholar]
- 13.Wang, H.-G.H., Y. Rikitake, M.C. Carter, P. Yaciuk, S.E. Abraham, B. Zerler, and E. Moran. 1993. Identification of specific adenovirus E1A N-terminal residues critical to the binding of cellular proteins and to the control of cell growth. J. Virol. 67:476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe, J.A., and S.T. Bayley. 1992. Effects of Ad5 E1A mutant viruses on the cell cycle in relation to the binding of cellular proteins including the retinoblastoma protein and cyclin A. Virology. 186:15–24. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, A., Jr., S. Banks, S. Soddu, and J. Cook. 1995. The effects of end point overdispersions on the validity of single dose tumorigenicity assays. Cancer Lett. 93:179–186. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara, K., J.A. Hamerman, L.R. Ehrlich, H. Bour-Jordan, P. Santamaria, J.A. Bluestone, and L.L. Lanier. 2004. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 20:757–767. [DOI] [PubMed] [Google Scholar]
- 17.Cook, J.L., and J.M. Routes. 2005. Adenovirus E1A gene-induced tumor cell rejection through cellular sensitization to immune and nonimmune apoptotic injuries. Front. Biosci. 10:1396–1414. [DOI] [PubMed] [Google Scholar]
- 18.Munger, K., A. Baldwin, K.M. Edwards, H. Hayakawa, C.L. Nguyen, M. Owens, M. Grace, and K. Huh. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook, J.L., T.A. Miura, D.N. Ikle, A.M. Lewis Jr., and J.M. Routes. 2003. E1A oncogene-induced sensitization of human tumor cells to innate immune defenses and chemotherapy-induced apoptosis in vitro and in vivo. Cancer Res. 63:3435–3443. [PubMed] [Google Scholar]
- 20.Cook, J., A. Lewis Jr., and C. Kirkpatrick. 1979. Age-related and thymus-dependent rejection of adenovirus 2-transformed cell tumors in the Syrian hamster. Cancer Res. 39:3335–3340. [PubMed] [Google Scholar]
- 21.Kenyon, D.J., J. Dougherty, and K. Raska Jr. 1991. Tumorigenicity of adenovirus-transformed cells and their sensitivity to tumor necrosis factor α and NK/LAK cell cytolysis. Virology. 180:818–821. [DOI] [PubMed] [Google Scholar]
- 22.Bauer, S., V. Groh, J. Wu, A. Steinle, J.H. Phillips, L.L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, A.M., Jr., and J.L. Cook. 1985. A new role for DNA virus early proteins in viral carcinogenesis. Science. 227:15–20. [DOI] [PubMed] [Google Scholar]
- 24.Onishi, M., S. Kinoshita, Y. Morikawa, A. Shibuya, J. Phillips, L.L. Lanier, D.M. Gorman, G.P. Nolan, A. Miyajima, and T. Kitamura. 1996. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 24:324–329. [PubMed] [Google Scholar]
- 25.Ogasawara, K., J.A. Hamerman, H. Hsin, S. Chikuma, H. Bour-Jordan, T. Chen, T. Pertel, C. Carnaud, J.A. Bluestone, and L.L. Lanier. 2003. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 18:41–51. [DOI] [PubMed] [Google Scholar]
- 26.Lodoen, M.B., G. Abenes, S. Umamoto, J.P. Houchins, F. Liu, and L.L. Lanier. 2004. The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60–NKG2D interactions. J. Exp. Med. 200:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrlich, L.I., K. Ogasawara, J.A. Hamerman, R. Takaki, A. Zingoni, J.P. Allison, and L.L. Lanier. 2005. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J. Immunol. 174:1922–1931. [DOI] [PubMed] [Google Scholar]
- 28.Koo, G.C., F.J. Dumont, M. Tutt, J. Hackett Jr., and V. Kumar. 1986. The NK-1.1(−) mouse: a model to study differentiation of murine NK cells. J. Immunol. 137:3742–3747. [PubMed] [Google Scholar]
- 29.Miura, T.A., H. Li, K. Morris, S. Ryan, K. Hembre, J.L. Cook, and J.M. Routes. 2004. Expression of an E1A/E7 chimeric protein sensitizes tumor cells to killing by activated macrophages but not NK cells. J. Virol. 78:4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamerman, J.A., K. Ogasawara, and L.L. Lanier. 2004. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J. Immunol. 172:2001–2005. [DOI] [PubMed] [Google Scholar]