Abstract
The renin-angiotensin system (RAS) has been traditionally linked to blood pressure and volume regulation mediated through the angiotensin II (ANG II) type 1 (AT1) receptor. Here we report that ANG II via its ANG II type 2 (AT2) receptor promotes the axonal elongation of postnatal rat retinal explants (postnatal day 11) and dorsal root ganglia neurons in vitro, and, moreover, axonal regeneration of retinal ganglion cells after optic nerve crush in vivo. In retinal explants, ANG II (10−7–10−5 M) induced neurite elongation via its AT2 receptor, since the effects were mimicked by the AT2 receptor agonist CGP 42112 (10−5 M) and were entirely abolished by costimulation with the AT2 receptor antagonist PD 123177 (10−5 M), but not by the AT1 receptor antagonist losartan (10−5 M). To investigate whether ANG II is able to promote axonal regeneration in vivo, we performed optic nerve crush experiments in the adult rats. After ANG II treatment (0.6 nmol), an increased number of growth-associated protein (GAP)-43–positive fibers was detected and the regenerating fibers regularly crossed the lesion site (1.6 mm). Cotreatment with the AT2 receptor antagonist PD 123177 (6 nmol), but not with the AT1 receptor antagonist losartan (6 nmol), completely abolished the ANG II–induced axonal regeneration, providing for the first time direct evidence for receptor-specific neurotrophic action of ANG II in the central nervous system of adult mammals and revealing a hitherto unknown function of the RAS.
Keywords: axonal regeneration, angiotensin receptor, PD 123177, losartan, apoptosis
The renin–angiotensin system (RAS)1 is phylogenetically one of the oldest hormone systems. Renin, an acid protease generating the angiotensin peptides, was discovered one hundred years ago, but only recently two angiotensin receptor subtypes, designated AT1 (angiotensin II type 1) and AT2 (angiotensin II type 2), were cloned. The established actions of angiotensin II (ANG II) within blood pressure control and body fluid homeostasis as well as the growth-promoting effects of this peptide in several organs have been clearly assigned to the AT1 receptor subtype (for review see reference 1). With the help of highly selective receptor ligands (2) it is now possible to functionally characterize the second receptor subtype, AT2, and thus to unveil major aspects of the RAS that have escaped our recognition in the past, such as the role of AT2 receptor in growth control and cell differentiation (3–6). AT2 receptors are abundantly present during development in brain and peripheral tissues but recede after birth (7–9). In adult individuals, the AT1 receptor dominates in most organs with few exceptions, but AT2 receptors can be drastically upregulated upon tissue injury, for instance in the heart after myocardial infarction (10) and in nervous tissue after central nervous system (CNS) lesion (11) or axotomy in sciatic nerves and dorsal root ganglia (DRG) neurons (12). These observations, together with previous reports on AT2 receptor–induced antiproliferative actions (3–6, 13), neuronal differentiation in PC12W- (6, 14, 15) and NG108-15 cells (16), and cellular damage in PC12W- (17) and ovarian granulosa cells (18), suggest a role of this receptor in cellular programs of regeneration after neuronal axon injury.
Immature mammalian CNS possesses the ability to regenerate (19), whereas adult CNS neurons are in most cases unable to reinnervate their target regions after injury, probably due to the actions of inhibitory molecules from CNS myelin (20). However, it has been shown that CNS neurons are able to regenerate new processes over long distances and to reinnervate their target region (21, 22) if they are supplied with growth-promoting substrates, e.g., transplants of fetal CNS tissue (23) or peripheral nerves (24). These observations, in conjunction with the above-mentioned evidence for AT2 receptor–mediated neuronal differentiation, prompted us to investigate the potential role of ANG II, acting through the AT2 receptor, as a neurotrophic factor in vitro and in vivo.
In the retina, a local RAS with high ANG II levels has been described (25) and related to the modulation of calcium currents of retinal ganglion cells (RGCs) (26). Adult retinal explants have frequently been used for in vitro studies of axonal regeneration (e.g., 27, 28). The results presented here demonstrate that ANG II promotes both the neurite elongation of RGCs and DRG in vitro and the axonal regeneration of RGCs after optic nerve crush in vivo via its AT2 receptor. Thus, these results are the first to assign a new role for the AT2 receptor in neuronal regeneration and after lesion in the CNS of adult mammals.
Materials and Methods
Preparation of Retinal Explants.
Rat retinal explants (postnatal day 11) were prepared as previously described (30). In brief, the eyes were dissected and the retinae were prepared and collected in L-15 medium (Biochrom KG, Berlin, Germany). Retinal explants were punched out with a sharpened syringe needle (400-μm diameter) and collected before plating in L-15 medium. All preparations were carried out at 4°C.
Three-dimensional Culture System/Bioassay.
The retinal explants were cultured in a fibrin gel (fibrinogen concentration ∼3 mg/ ml) in serum-free medium, supplemented with 0.5 mg/ml amino-n-caproic acid (Sigma, München, Germany) as a plasmin inhibitor to prevent destruction of the fibrin gel as previously described (30). After precoating coverslips with 10 μl (3 NIH units) thrombin (Topostasin®; Hoffmann La Roche, Basel, Switzerland), a 20-μl drop of fibrinogen was given on the coverslip to start coagulation of the fibrin gel. The retinal explants were placed in the gel just before coagulation and were cultivated for 3 d in 24-well plates (Costar, Bodenheim, Germany), filled with 2 ml of serum-free culture medium at 37°C in humidified CO2/air (7.5:92.5%).
Evaluation of Neurite Outgrowth.
After 3 d in vitro, the explant cultures were fixed in 2.5% glutaraldehyde by microwave irradiation (10 s at 50°C), dehydrated in ethanol, stained with sudan black (1 min of microwave irradiation at 50°C), and embedded in a mixture of glycerol and gelatin. Neurite outgrowth of RGCs was assessed using a computerized image analysis system (Analysis; SIS, Münster, Germany). The axonal domain of the retinal explant was plotted by connecting the tips of the outgrown neurites, and the area of the solid retinal explant was subtracted. The axonal domain was expressed as a percentage of control cultures (control = 100%).
Preparation of DRG Neurons.
Newborn rat DRG were dissected after removal of the dorsal skin, muscles, and the vertebral arches. The spinal ganglia were picked carefully and washed thoroughly in PBS and dissociated in PBS with 0.1% trypsin and 0.1% collagenase at 37°C for 15 min, followed by a second enzymatic dissociation step with 0.5% trypsin solution (37°C for 10 min). After centrifugation (5 min, 500 g), the cell pellet was resuspended in DMEM with 10% FCS with the following supplements: 2 mM l-glutamine, 10 ng/ml nerve growth factor (Sigma), 50 IU/ml penicillin, and 50 μg/ml streptomycin. Cells were mechanically dissociated in this culture medium by gentle pipetting through siliconized Pasteur pipettes with decreasing inner diameter, filtered through a nylon mesh (35 μm pore size), and preplated in 100-mm culture dishes (Sarstedt, Inc., Newton, NC) for 2 h. This neuron-enriched cell suspension was plated on laminin-coated coverslips (5 μg/ml; Sigma) at a density of 2,000 cells/cm2. At appropriate times the cells were fixed in phosphate-buffered 2.5% glutaraldehyde.
Schwannoma Cell Cultures.
Schwannoma cells were cultivated in DMEM with 10% FCS. The confluent monolayer was rinsed in fresh medium, removed with a rubber policeman, and centrifuged (10 min, 40 g) for the preparation of cell pellets.
Addition of ANG II and Selective Receptor Agonists and Antagonists.
ANG II (Bachem, Bubendorf, Switzerland) was dissolved in DMEM to yield stock solutions of 10−3 M. Losartan (AT1 receptor antagonist, a gift from Dr. R. Smith, DuPont Merck Pharmaceutical Company, Wilmington, DE), PD123177 (AT2 receptor antagonist, a gift from Dr. D. Taylor, Parke Davis Pharmaceutical Research, Ann Arbor, MI), and CGP 42112 (AT2 receptor agonist, a gift from Dr. M. DeGasparo, Ciba-Geigy Pharmaceutical Division, Basel, Switzerland) were prepared from 10−3 M stock solutions in DMEM. All substances were freshly prepared and later diluted to the desired working concentrations.
Surgery.
Adult female Wistar rats (180–200 g) were used for the optic nerve crush experiments. After deep anesthesia by an intraperitoneal injection of chloral hydrate (400 mg/kg), we exposed the optic nerve through a supraorbital approach as previously described (23). The optic nerve was crushed ∼2 mm behind its bulbar exit using fine forceps for 10 s. After transection of the rectus superior muscle, a collagen foam (Lysostypt®) soaked with 0.6 nmol ANG II alone or in combination with the AT1 or AT2 receptor antagonists (6 nmol) was introduced into the vitreous body after a scleral incision. Schwannoma cell pellets were implanted in an identical manner. After wound closure, the animals were allowed to survive for 14 d. Five animals served as operated control and five received the collagen foam only to exclude unspecific effects. After each operation, the retinal blood supply was controlled by indirect ophthalmoscopy to verify that the central retinal artery was not damaged by the crush lesion and, thus, that the physiologic blood supply after optic nerve crush was unaffected.
Immunohistochemistry.
The rats were killed by transcardiac perfusion with 4% paraformaldehyde in PBS, and the left optic nerve was removed. Paraffin-embedded optic nerves were stained for (a) growth associated protein (GAP)-43 immunoreactivity to label regenerating axons, (b) neurofilament (clone RT 97) for the detection of other axons, and (c) glial fibrillary acidic protein (GFAP) to demonstrate the glial reaction at the lesion site. 7-μm sections were incubated in 0.75% BSA solution in PBS for 20 min to block unspecific binding, washed three times in PBS, and incubated for 1 h with a monoclonal rabbit GAP-43 antiserum (Sigma; 1:400), a neurofilament antibody (clone RT 97; Boehringer, Mannheim, Germany; 1:100), or GFAP antibody (Boehringer; 1:50). After washing in PBS (3 times for 10 min), the sections were incubated with peroxidase-conjugated rabbit anti– mouse secondary antibody (Sigma; 1:100) for 30 min, washed, and stained with goat anti–rabbit IgG (Sigma; 1:200, 30 min), before processing with diaminobenzidine and embedding in Aquatek (Merck, Inc., Hawthorne, NY). Sections were photographed with a microscope (Axioplan; Carl Zeiss, Inc., Thornwood, NY) on Kodak TMAX 100 (Eastman Kodak Co., Rochester, NY).
Reverse Transcriptase PCR Analysis.
Total RNA from retinae and optic nerves after crush was isolated and reverse transcribed according to the manufacturer's recommended protocols (GIBCO BRL, Eggenstein, Germany). For determination of AT2 receptor mRNA expression 3, 8, and 14 d after lesion with respect to the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase), the reverse transcriptase (RT)-PCR assay was thoroughly established as previously described (14) to confirm that the RT-PCR precisely reflects relative changes in the respective AT2 receptor levels. In brief, for experiments with respect to the housekeeping gene GAPDH, the reaction mixture was equally split into two tubes before specific primers and PCR reagents were added. To optimize the RT-PCR assay, the relationship of signal strength to the number of PCR cycles and the amount of input cDNA was assessed for AT2 receptors and GAPDH for optic nerves as well as for retinae. For AT2 receptor PCR, 25 amplification cycles were used for retinae and optic nerves, whereas 17 and 20 cycles, respectively, were used for GAPDH. Under the experimental conditions used, the signals increased linearly when increasing amounts of cDNA were subjected to PCR accurately reflecting relative differences in the respective cDNA levels.
Each reaction mixture was denatured at 95°C for 5 min and samples were amplified in a programmable thermal controller (PTC-100; MJ Research Inc., Watertown, MA) using specific AT1 and AT2 primers. For amplification of AT1, the sense primer d(TGTAAGATTGCTTCAGCCAGC) and the antisense primer d(GCCCTGTCCACAATATCTGC) were used. The sense primer for AT2 receptor PCR d(TTGCTGCCACCAGCAGAAAC) was used in combination with the antisense primer d(GTGTGGGCCTCCAAACCATTGCTA) generating a 1,179-bp product. Aliquots of each sample were subjected to electrophoresis on 1.2% agarose gels and were stained with ethidium bromide. The identity of PCR products was verified by Southern blot hybridization using 32P-labeled probes according to manufacturer's recommendations (Stratagene, Heidelberg, Germany). Hybridizations were carried out in a RapidHyb solution (Amersham, Braunschweig, Germany) and washed to stringencies of 0.1 × SSPE/0.1% SDS at 60°C. For hybridization, an AT1 and AT2 cDNA (3) was used as probe.
Statistics.
Statistical comparison of the data was performed using one-way analysis of variance (ANOVA) followed by an appropriate post-hoc test (Bonferroni). P ≤0.05 was considered significant. Further details of statistical analysis are given in the legends to the figures.
Results
In Vitro Effects of ANG II on the Neurite Elongation of Rat Retinal Explants.
To study the effects of ANG II on neurite elongation, we prepared retinae from postnatal day 11 rats (29, 30), which are comparable to adult animals in their regenerative response, and thus represent an appropriate model for the study of neuronal regeneration. After 3 d in vitro, there were many regenerating but short neurites visible in control explants, which grew up to 200 μm out of the whole circumference of the retinal explants (Fig. 1 a). Staining with antibodies against Thy-1.1 (31) confirmed that the regenerating neurites were processes of RGCs (data not shown). The responsiveness of RGCs to neurotrophic factors was verified by treatment with brain- derived neurotrophic factor (BDNF; 10 ng/ml), a known neurotrophic factor for RGCs (27) (Figs. 1, b, and 2). Compared with control cultures (100%), BDNF elongated the neurites up to 160% (± 3%, SEM, n = 36). Most of the RGCs extended their axons for >300 μm, some of them up to 500 μm.
Figure 1.
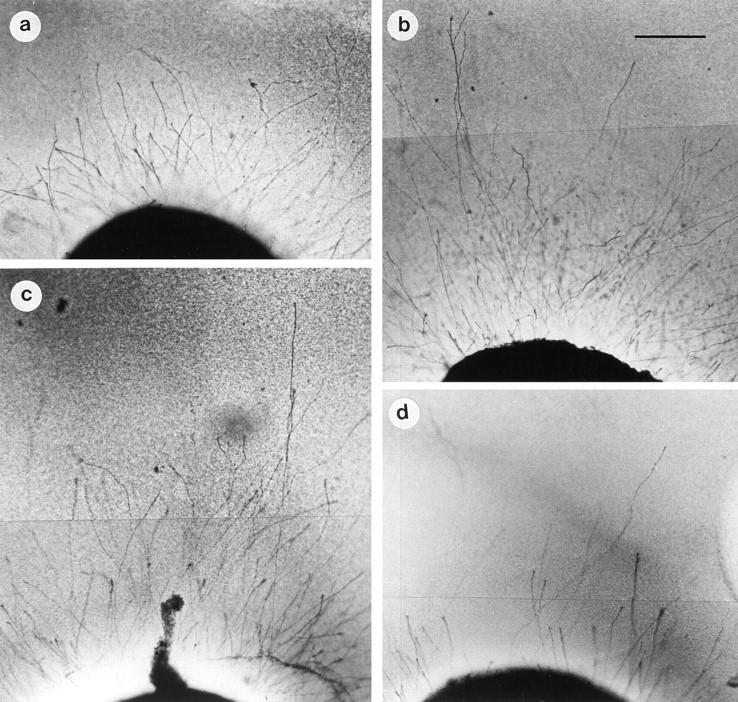
(a) Retinal explant (postnatal day 11) in control cultures after 3 d in vitro. Many regenerating, but short neurites were detected. (b) Addition of BDNF (10 ng/ml) enhanced axonal growth up to 160 ± 3%. (c) In the presence of ANG II (10−6 M), the number and length of neurites was increased to comparable amounts (162 ± 4%), whereas (d) the AT2 receptor antagonist, PD123177 (10−5 M), blocked axonal regrowth (102 ± 3%). On the other hand, the AT1 receptor-antagonist, losartan (10−5 M), did not influence the ANG II–induced axonal elongation (data not shown). Bar = 100 μm.
The addition of ANG II to the retinal explants dose- dependently (10−5–−7 10 M, data not shown) enhanced the length and number of regenerating neurites. In a concentration of 10−6 M, ANG II increased the neurite elongation up to 162% (control = 100%, Figs. 1, c, and 2). The length of the majority of axons was in the range of >350 μm, and single axons reached distances up to 600 μm. Similarly, the neurite-promoting capacity of ANG II (10−7 M) was also detectable in neuronal cultures of postnatal rat DRG (data not shown).
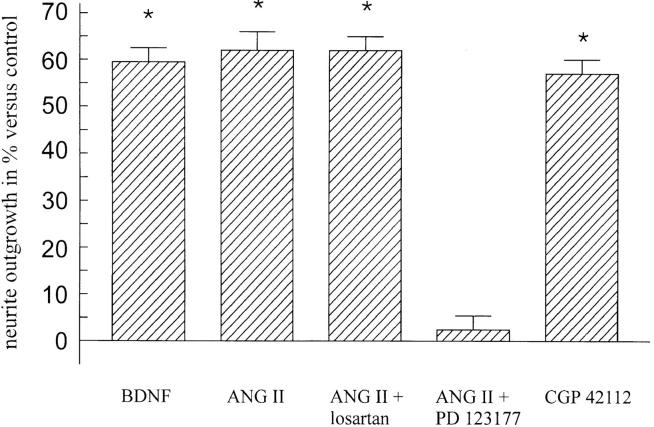
In the presence of drugs selectively interacting with the two angiotensin receptor subtypes, the outgrowth of neurites was markedly changed. The compound CGP 42112, an AT2 receptor agonist at higher concentrations (32), showed comparable effects on neurite regeneration at 10−5 M as obtained with ANG II (Fig. 2). Coincubation with ANG II did not further increase this response (data not shown). In the presence of the selective AT2 receptor antagonist, PD123177 (10−5 M), the regenerative response was reduced to control levels (Figs. 1, d, and 2) whereas the AT1 receptor antagonist, losartan (10−5 M), was not able to block the ANG II–induced neurite elongation (Fig. 2).
Figure 2.
Effect of ANG II on neurite outgrowth of retinal explants (postnatal day 11). Compared with control cultures, treatment with both, BDNF (10 ng/ml) and ANG II (10−6 M), resulted in increased axonal regeneration of RGCs. The AT1 receptor antagonist, losartan (10−5 M), had no effect on ANG II-induced neurite extension, whereas treatment of explants with ANG II and the AT2 receptor antagonist, PD 123177 (10−5 M), completely inhibited these effects. The peptide CGP 42112, an AT2 receptor agonist at 10−5 M, entirely mimicked ANG II–mediated effects (n = 36 for all experimental groups, mean ± SEM, *P <0.05 compared with controls).
In Vivo Effects of ANG II after Optic Nerve Crush.
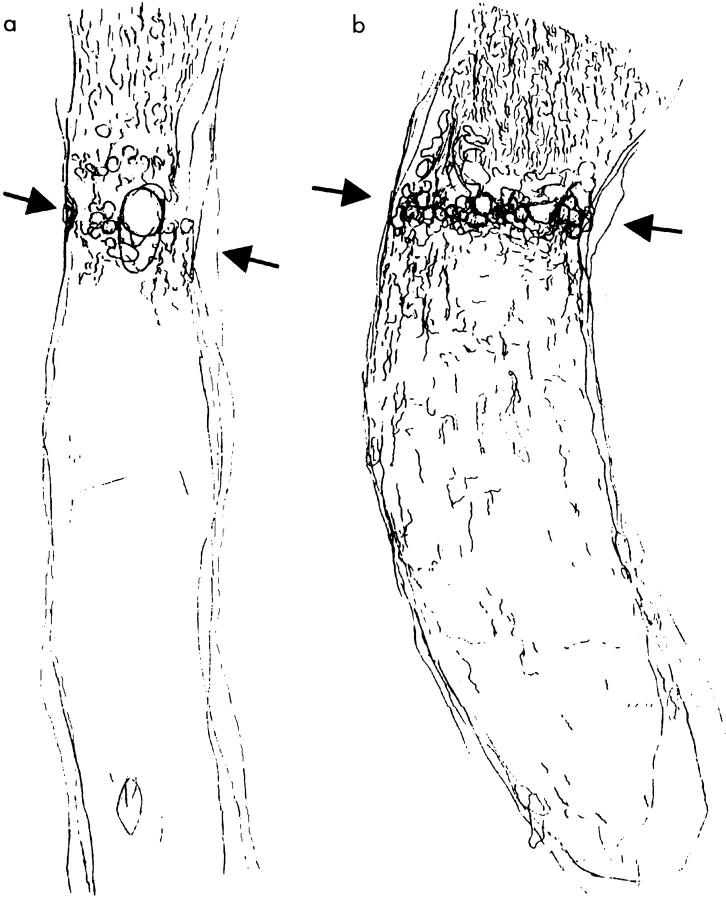
To investigate whether the in vitro results apply to CNS lesions in vivo, we performed an optic nerve crush in adult rats. Axonal regrowth was quantified by GAP-43 immunohistochemistry, which selectively recognizes an intracellular phosphoprotein in axonal growth cones and is mandatory for axon elongation. In control-operated animals, only a few regenerating fibers were detectable as marked by the GAP-43 staining and regrowth was limited up to the lesion site. No unspecific growth stimulation was detectable in animals receiving the collagen foam immediately after crush into the vitreous body. GFAP-positive astrocytes marked the lesion site, in which cystic changes occurred (Figs. 3 a and 4 a).
Figure 3.
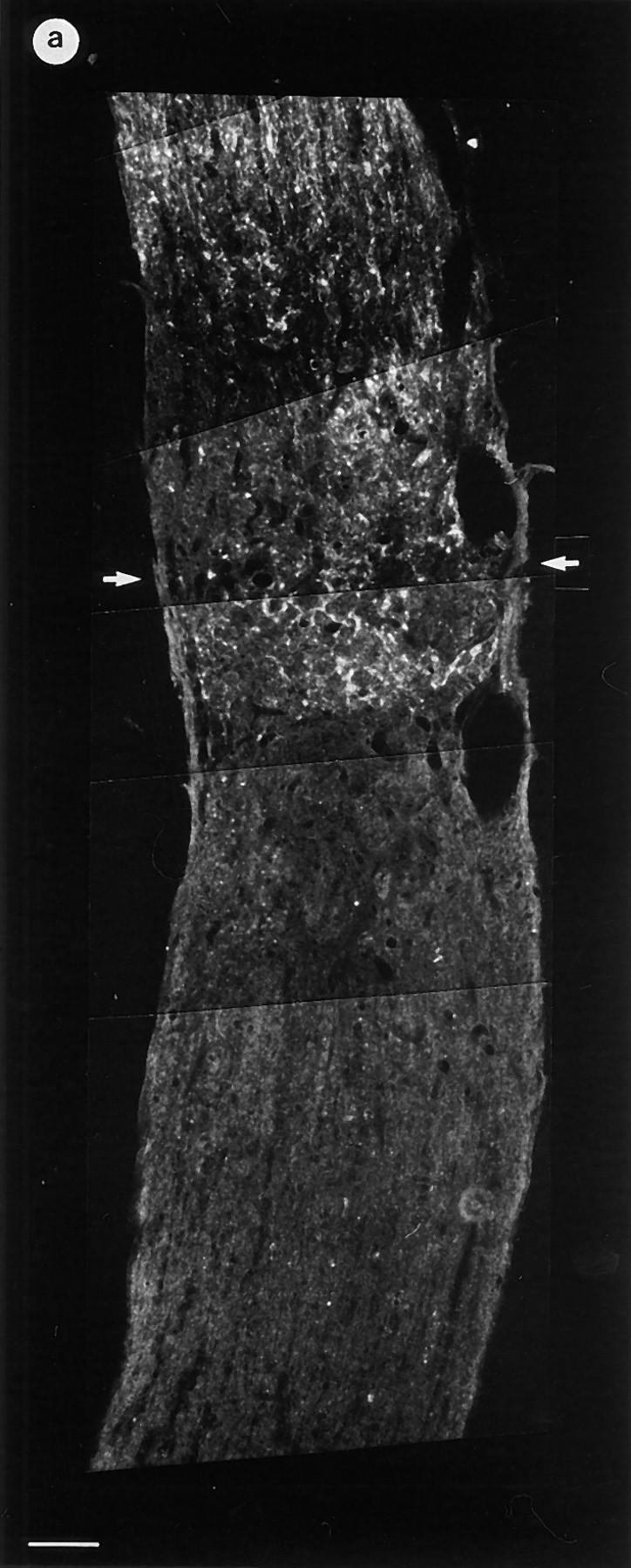
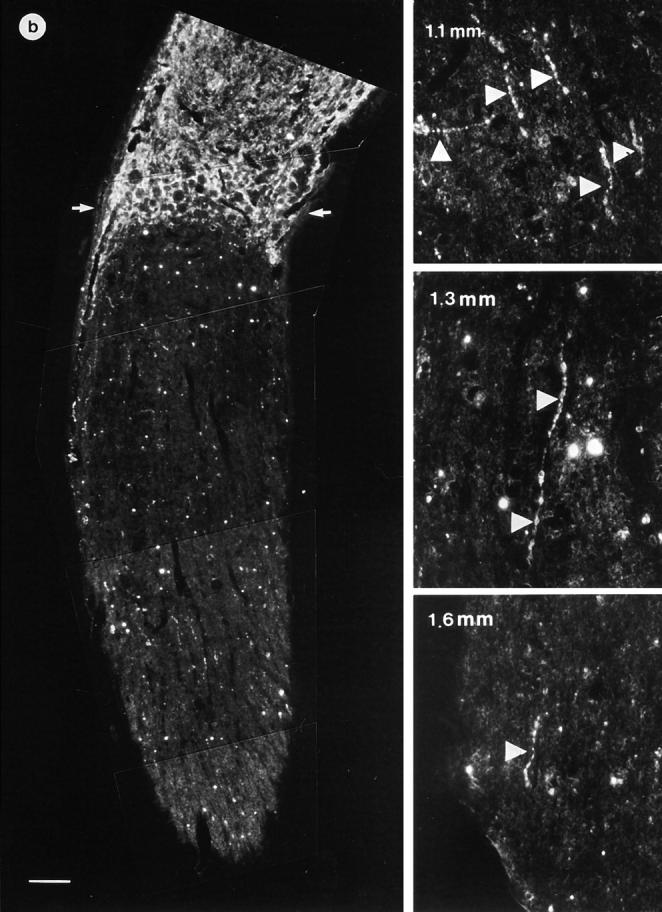
Paraffin-embedded sections of optic nerves of control and ANG II–treated animals (GAP-43 staining). (a) In control animals (original magnification: ×40), regenerating fibers reached the lesion site (demarcated by two arrows) but rarely grew further. (b) In contrast, animals receiving an ANG II–soaked gel foam (original magnification: ×40) showed regenerating fibers that regularly crossed the lesion site and grew over a distance of several millimeters. Higher magnification (right side in b, original magnification: ×640) shows GAP-43–positive fibers (arrowheads) reaching a distance up to 1.6 mm distal to the lesion site (bar = 100 μm).
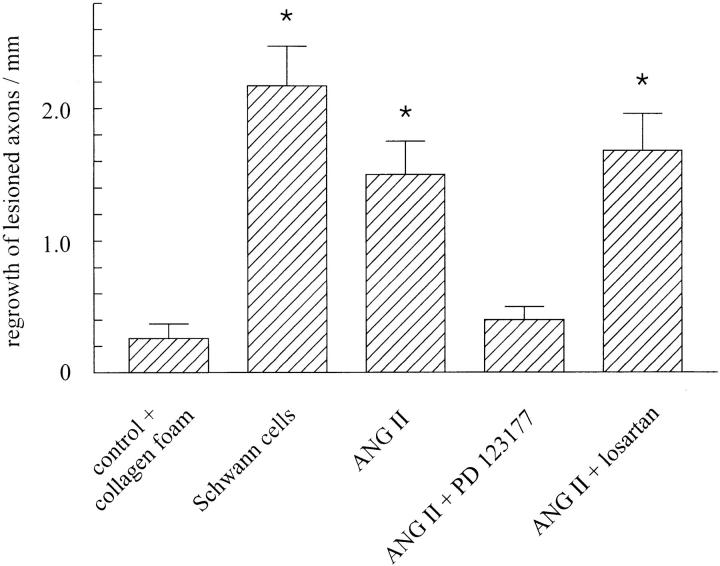
To evaluate the regenerative capacity of ANG II, animals received an ANG II–soaked collagen foam (0.6 nmol), resulting in the outgrowth of large axon bundles within the proximal optic nerve. Since the gel foam was placed into the vitreous body after scleral incision, indirect effects of ANG II on glial cells or inflammatory cells at the lesion site resulting in alterations of the nonpermissive nature of the optic nerve (33) could be excluded. The GAP-43–positive fibers reached the lesion site and many of them grew over the lesion site reaching regrowth distances of several millimeters (Figs. 3 b and 4 b). The effects of ANG II were completely abolished by coadministration of the AT2 receptor antagonist, PD 123177 (6 nmol), reducing the regenerative response to that of control-operated animals (Fig. 5). On the other hand, the AT1 receptor antagonist, losartan (6 nmol), did not influence the ANG II–mediated growth response (Fig. 5).
Figure 5.
Measurement of regrowth of lesioned axons in the optic nerve. Evaluations were made in a blinded fashion by number-coded GAP-43–positive serial sections. Only rats with regenerative fibers/ sprouts distal to the lesion were included in this analysis. The length of regenerated axons was measured by a video image processing program (Analysis; SIS) from the distal end of the lesion site. Compared with control-operated animals, the application of ANG II evoked an increase in the regenerative response that was completely suppressed by costimulation with the AT2 receptor antagonist, PD 123177, but not by the AT1 receptor antagonist, losartan (n = 5 for all experimental groups, mean ± SEM, *P <0.05 compared with controls).
Schwannoma cells, known to secrete several neurotrophic factors (e.g., BDNF), were implanted into the vitreous body as an internal control (34), evoking a strong regenerative response (Fig. 5). The regenerative capacity observed after ANG II treatment was as pronounced as the one detected after implantation of these cells, emphasizing the relevance of this finding.
RT-PCR Analysis.
The presence of AT1 (data not shown) and AT2 receptors (Fig. 6) in the retinal explants and optic nerves was verified by RT-PCR. In separately collected tissues from operated animals, both of the angiotensin receptor subtypes were detectable. To investigate whether optic nerve crush evokes alterations in the AT2 receptor mRNA expression, we determined the AT2 receptor levels in both retinae and optic nerves 3, 8 and 14 d after optic nerve crush with respect to the housekeeping gene GAPDH. In retinae as well as in optic nerves, we observed a moderate increase in AT2 receptor mRNA levels after 14 d by 35 and 22%, respectively (Fig. 6).
Figure 6.
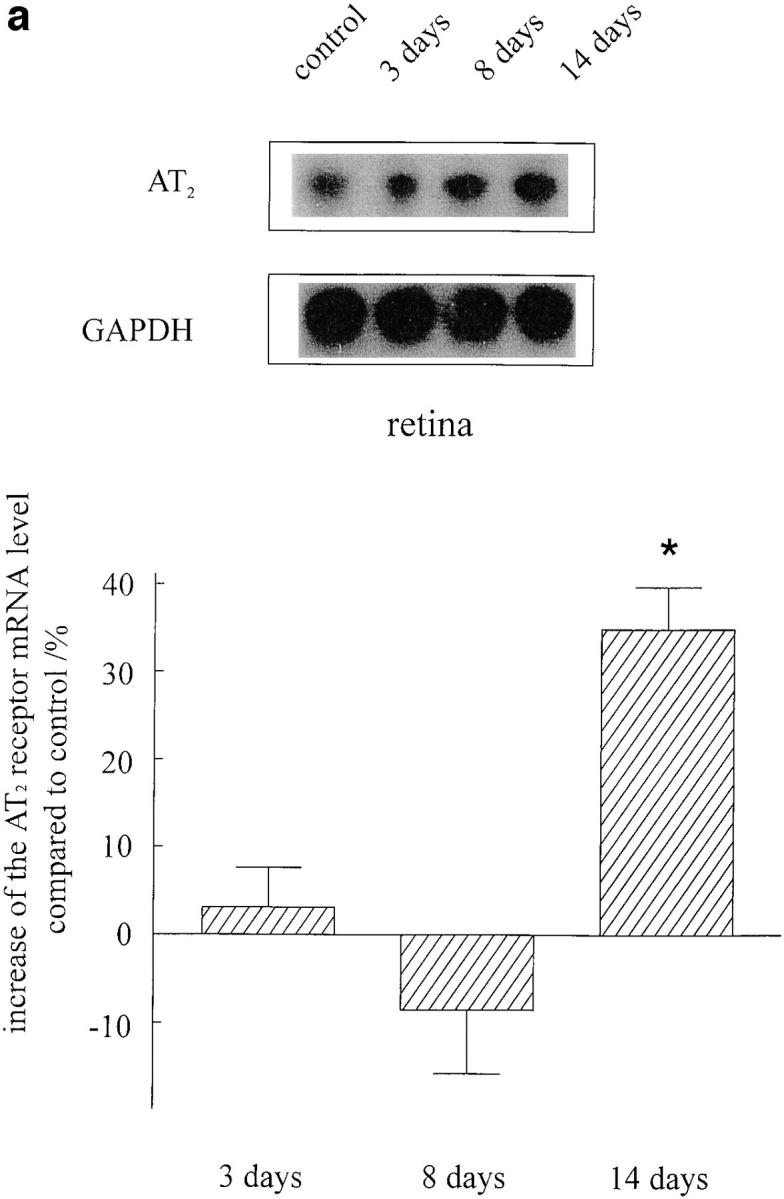
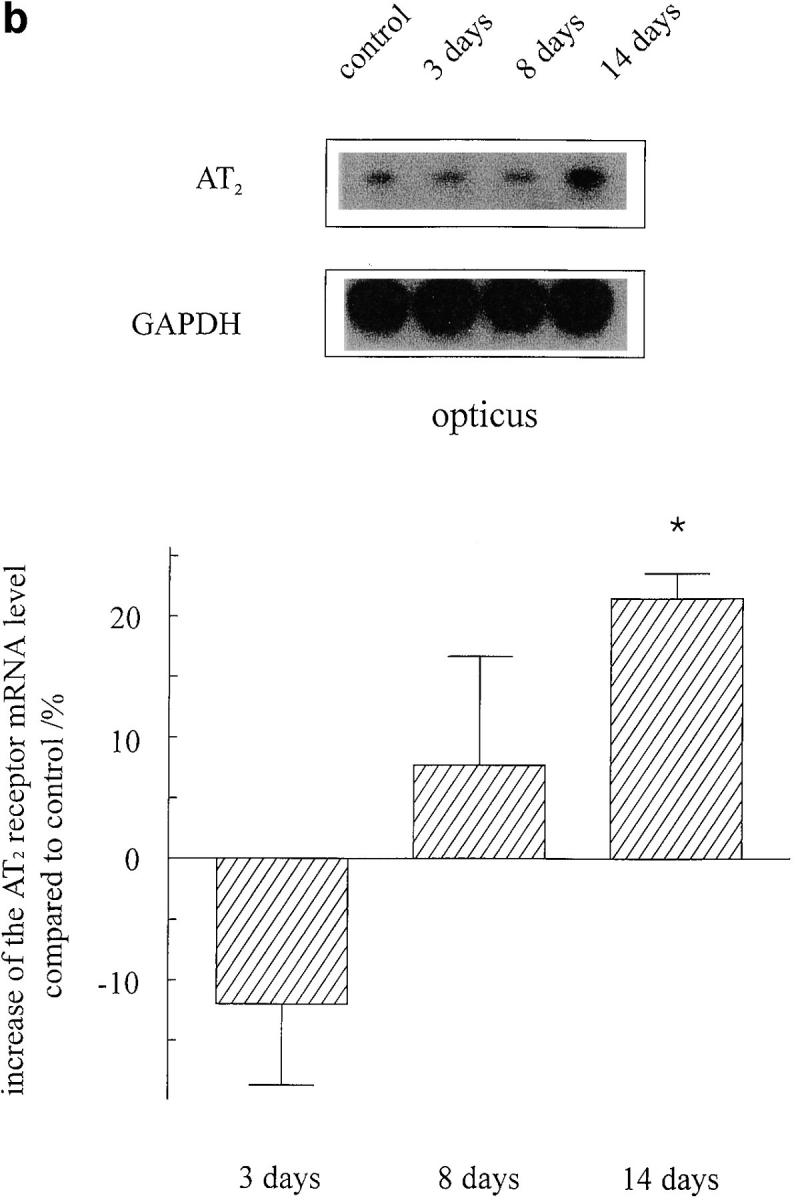
AT2 receptor expression in retinae (a) and optic nerves (b) of adult rats 3, 8, and 14 d after optic nerve crush (n = 4 for each condition). To investigate whether optic nerve injury results in changes of AT2 receptor expression, we performed an RT-PCR assay with respect to the housekeeping gene GAPDH. Although the expression of AT2 receptors remained unchanged after 3 and 8 d, a significant increase was observed in both tissues 14 d after lesion. The identity of PCR products was confirmed by Southern blot hybridizations (top) that were quantified by densitometric analysis (mean ± SEM, *P <0.05 compared with controls).
Discussion
The salient finding of this study is the observation that ANG II via its AT2 receptor is able to promote the axonal regeneration in postnatal retinal explants and DRG in vitro as well as after optic nerve crush in vivo. The results not only ascribe a hitherto unknown physiological function to ANG II as a neurotrophic factor through its AT2 receptor, but open up a new dimension with respect to our general understanding of the RAS, one of the most universal endocrine and paracrine systems.
In the three-dimensional in vitro culture system used (29, 30), activation of the AT2 receptor leads to regeneration of neurites from postnatal RGCs comparable to those from adult animals (35, 36) in their capacity to regenerate. The finite regenerative capacity of RGCs was reflected in our control experiments, where the explants were cultured in serum-free medium and only a limited outgrowth of neurites was observed. However, the regeneration of neurites can be influenced either by addition of BDNF, a known neurotrophic factor for RGCs (27), or by cultivating the explants in the presence of cocultivated astrocytes (30). Therefore, the RGCs are able to regenerate their axons if they are supplied with neurotrophic substances. A similar effect as after BDNF treatment (10 ng/ml) was observed by ANG II application, pointing to a role of this peptide as a neurotrophic factor.
In addition, ANG II treatment of DRG isolated from neonatal rats resulted in an AT2 receptor–mediated neurite extension indicating that the promotion of axonal regeneration in response to AT2 receptor stimulation is a general feature in neuronal cells.
To investigate the effects of ANG II on the axonal elongation of RGCs in vitro, we used, compared with other in vitro assays, slightly elevated ANG II concentrations (10−6 M), since the retinal explants were cultured in a fibrin clot. Thus, the ANG II was not freely accessible for the RGCs and the effective ANG II concentration at the explants differed from the one in the cell culture medium. Moreover, it has been shown by Kohler et al. (25) that the ANG II levels in the retina are 10-fold higher than in the plasma and that RGCs require higher ANG II concentrations to be activated (26).
The quantification of the regeneration process was performed by staining against GAP-43. The expression of this protein is increased after optic nerve lesion (37–39) and identifies those RGCs that are capable of regenerating their axons (40). An overexpression of GAP-43 enables neurons to sprout new terminals (41), whereas the absence of GAP-43 leads to a poor adherence and unstable lamellar extensions of neurites. These findings point to a role of this protein in the process of regulated neurite outgrowth during both development and regeneration (42). Moreover, it has been shown (43) that the GAP-43 induction is paralleled by an increased potential of injured CNS neurons to regenerate. Therefore, the AT2 receptor–mediated increased GAP-43 expression after optic nerve crush reflects an increased axonal regeneration of these neurons.
The octapeptide ANG II has been implicated in the process of angiogenesis. However, for the following reasons it is very unlikely that an enhanced blood supply induced by ANG II is responsible for the axonal regeneration described here. First, ANG II also induced the axonal elongation of RGCs and dorsal root ganglion neurons in vitro via its AT2 receptor (Figs. 1 and 2). In these assays, vascularization events can be excluded. Second, in a study published by Fernandez et al. (44), it was observed that ANG II induces vascularization in the cornea. However, even when this ANG II–induced angiogenesis was not attributed to any angiotensin receptor subtype, these effects of ANG II can be explained by stimulation of AT1 receptors since all known growth-promoting effects of ANG II are mediated by AT1 receptors. In contrast, all effects presented in this study in terms of neuronal regeneration are clearly mediated by AT2 receptors since they were completely abolished by PD 123177 and not affected by losartan. In a study in the rat cremaster muscle (4), it was demonstrated that the AT1 receptor mediated angiogenic actions in the microcirculation, whereas an AT2 receptor stimulation caused an inhibition of angiogenesis. Le Noble et al. (45) demonstrated that the angiogenic effects induced by ANG II were mediated by a “novel” angiotensin receptor since they could neither be inhibited by PD 123319 nor by losartan. Investigating the role of ACE (angiotensin-converting enzyme) inhibitors and AT1 receptor antagonists, it has been shown by Gohlke et al. (46) that a treatment with ACE inhibitors but not with losartan increased the capillary density in the heart. The failure of losartan to exert this effect was attributed (besides bradykinin) to the chronic overstimulation of AT2 receptors that are left unopposed by an AT1 receptor antagonism. Finally, if the effects presented in our manuscript were due to enhanced blood supply, this would neither result in an increased expression of GAP-43 nor in an enhanced axonal regrowth beyond the glial scar.
For these reasons, we feel that our statement is justified attributing the observed AT2 receptor–mediated axonal regeneration after optic nerve crush to direct effects and not to indirect vascularization.
In previous studies, we and others have shown that the AT2 receptor can induce neuronal differentiation in PC12W cells (6, 14, 15) and neurite outgrowth in the neuroblastoma–glioma hybrid cell line NG108-15 (16). Moreover, the AT2 receptor mRNA is upregulated after axotomy in sciatic nerves and DRG neurons (12). These findings are compatible with the hypothesis that ANG II, acting via the AT2 receptor, represents a factor capable of promoting neuronal regeneration. AT2 receptors have been implicated in antiproliferative and differentiation-promoting effects (3, 5, 6), in contrast to the growth-inducing actions of AT1 receptors, and play a role in tissue repair and wound healing (10, 37). The latter is of special interest in brain injury and, indeed, experiments by Viswanathan and Saavedra (11) have already shown increased tissue AT2 levels after CNS lesions. Besides this, AT2 receptors are involved in the modulation of the proteolytic activity of the extracellular microenvironment of neurons as suggested by the AT2 receptor–mediated regulation of serine protease inhibitors in cultured Schwann cells (48).
These findings gave rise to the hypothesis that AT2 receptors are also involved in axonal regeneration in the CNS. Our data show for the first time that AT2 receptor stimulation indeed leads to enhanced regeneration of axons from postnatal RGCs and DRG. These effects are totally abolished in the presence of PD 123177, a selective AT2 receptor antagonist, and are mimicked by agonistic concentrations of the selective AT2 receptor agonist, CGP 42112, whereas regeneration is not suppressed by the AT1 receptor antagonist, losartan.
The intracellular signal transduction cascade of AT2 receptors has not yet been clearly established (for review see reference 49). One recently reported effect is the inhibition of mitogen-activated protein (MAP) kinases by dephosphorylation (17). Interestingly, an antiapoptotic protein (Bcl-2; references 50–52) is phosphorylated by MAP kinase, a process which is reversed after AT2 receptor stimulation, resulting in the inactivation of Bcl-2 and the induction of apoptosis (53).
The question arises as to how a factor suspected to be involved in apoptotic processes can at the same time be contributing to tissue regeneration. This dual role is conceivable in view of the fact that the risk of apoptosis and the potency for axonal regeneration are closely associated. With an increasing ability to regenerate, neurons are in danger of entering programmed cell death (54). If, for example, mammalian CNS neurons are lesioned close to their cell bodies, a strong cell body response, a strong regenerative potency, and, simultaneously, a high risk for cell death are observed. On the other hand, a distal transection evokes a weak cell body response resulting in weak regenerative efforts, but the CNS neurons are also somehow protected from apoptosis. These observations suggest that neuronal injury initiates a series of molecular events that are initially identical for both apoptosis and regeneration.
Considering the above-mentioned studies, it is tempting to speculate that AT2 receptors play a role in either neuroregenerative or apoptotic events. Although AT2 receptors can induce apoptosis in different cell types (17, 18, 53), an increasing body of evidence demonstrates an AT2 receptor–mediated promotion of regenerative actions in both neuronal and nonneuronal cells (our data and references 10, 12, 47). It appears now that the well-known growth-promoting effects of the AT1 receptor, which can engender neuroplastic as well as pathological structural changes in several organs, are counteracted within the RAS itself by growth arrest, differentiation, and tissue repair, effected through the AT2 receptor, and that a (disturbed) balance between the opposing actions of these two receptors determines the net effects of the RAS in a given disease situation.
Our findings on the neurotrophic actions of the AT2 receptor may provide a basis for the design of new, receptor-directed therapeutic strategies in the failure of axonal regeneration in the mammalian CNS. This is of particular interest considering the current difficulties in applying neurotrophic factors after nerve fiber damage. Moreover, AT2-mediated tissue regeneration may not be confined to axonal regrowth but may constitute a general phenomenon to be exploited by therapeutic intervention. The clinical relevance of this approach becomes apparent with the increasing use of AT1 receptor antagonists as antihypertensive drugs in, as of this time, more than two million patients worldwide. Since AT2 receptors are unmasked and ANG II levels are increased by AT1 receptor antagonists, part of the organ-protective actions of these drugs might be ascribed to an agonistic action of ANG II at the AT2 receptor site.
Figure 4.
Camera-lucida projections from serial sections of paraffin-embedded and GAP-43–stained optic nerves. The lesion site is demarcated by two arrows. In control animals (a), only few fibers grew over the lesion, whereas in animals receiving the ANG II gel foam (b), numerous axons crossed the lesion site and regenerated over a distance of several millimeters. ANG II increased the number of GAP-43–positive axons in the proximal optic nerve stump compared with controls (b). The AT2 receptor–mediated regeneration was completely abolished by the selective AT2 receptor antagonist, PD 123177 (see Fig. 6), whereas the selective AT1 receptor antagonist, losartan, had no effect (see Fig. 6).
Acknowledgments
We thank Sibille Piontek for her expert technical assistance.
This work was supported by a grant from the German Research Foundation (Lu 617/1-1) and the Hensel-Foundation, University of Kiel (to R. Lucius), and a grant from the German Institute for High Blood Pressure Research, Heidelberg (to T. Unger and S. Gallinat).
Abbreviations used in this paper
- ANG
angiotensin
- AT1
ANG II type 1
- AT2
ANG II type 2
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- DRG
dorsal root ganglia
- GAP
growth-associated protein
- GAPDH
glyceralaldehyde-3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- RAS
renin–angiotensin system
- RGC
retinal ganglion cell
- RT-PCR
reverse transcriptase PCR
Footnotes
R. Lucius and S. Gallinat contributed equally to this work.
References
- 1.Unger T, Chung O, Csikos T, Culman J, Gallinat S, Gohlke P, Höhle S, Meffert S, Stoll M, Stroth U, Zhu Y-Z. Angiotensin receptors. J Hypertens. 1996;14:s95–103. [PubMed] [Google Scholar]
- 2.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 3.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension (Dallas) 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, Horiuchi M, Pratt RE, Dzau VJ. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T. The angiotensin AT2receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol. 1996;122:59–67. doi: 10.1016/0303-7207(96)03873-7. [DOI] [PubMed] [Google Scholar]
- 7.Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2angiotensin II receptor subtypes in the brain during development. Proc Natl Acad Sci USA. 1991;88:11440–11444. doi: 10.1073/pnas.88.24.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin receptor subtypes (AT1 and AT2) in the rat brain. Am J Physiol. 1991;216:209–216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 9.Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2receptors in the developing rat fetus. J Clin Invest. 1991;88:921–923. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J Clin Invest. 1995;95:46–54. doi: 10.1172/JCI117675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanathan M, Saavedra JM. Expression of angiotensin AT2receptors in the rat brain during wound healing. Peptides. 1992;13:783–786. doi: 10.1016/0196-9781(92)90187-8. [DOI] [PubMed] [Google Scholar]
- 12.Gallinat S, Yu MH, Zhu Y-Z, Herdegen T, Unger T. Up-regulation of angiotensin receptors after myocardial infarction and sciatic nerve transection. Hypertension (Dallas) 1997;30:999. . (Abstr.) [Google Scholar]
- 13.Tsuzuki S, Eguchi S, Inagami T. Inhibition of cell proliferation and activation of protein tyrosine phosphatase mediated by angiotensin II type 2 (AT2) receptor in R3T3 cells. Biochem Biophys Res Commun. 1996;228:825–830. doi: 10.1006/bbrc.1996.1739. [DOI] [PubMed] [Google Scholar]
- 14.Gallinat S, Csikos T, Meffert S, Herdegen T, Stoll M, Unger T. The angiotensin AT2receptor down-regulates neurofilament M in PC12W cells. Neurosci Lett. 1997;277:29–32. doi: 10.1016/s0304-3940(97)00291-7. [DOI] [PubMed] [Google Scholar]
- 15.Stroth U, Meffert S, Gallinat S, Unger T. Angiotensin II and NGF differentially influence microtubule proteins in PC12W cells: role of the AT2receptor. Mol Brain Res. 1998;53:187–195. doi: 10.1016/s0169-328x(97)00298-2. [DOI] [PubMed] [Google Scholar]
- 16.Laflamme L, de Gasparo M, Gallo J-M, Payet MD, Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2receptors in NG108-15 cells. J Biol Chem. 1996;271:22729–22735. doi: 10.1074/jbc.271.37.22729. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka M, Ohnishi J, Ozawa Y, Sugimoto M, Usuki S, Naruse M. Characterization of angiotensin II receptor type 2 during differentiation and apoptosis of rat ovarian cultured granulosa cells. Biochem Biophys Res Commun. 1995;207:593–598. doi: 10.1006/bbrc.1995.1229. [DOI] [PubMed] [Google Scholar]
- 19.Shewan D, Berry M, Cohen J. Extensive regeneration in vitro by early embryonic neurons on mature and adult CNS tissue. J Neurosci. 1995;15:2057–2062. doi: 10.1523/JNEUROSCI.15-03-02057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwab M, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 21.Björklund, A., and U. Stenevi, editors. 1985. Neural Grafting in the Mammalian CNS. Elsevier. Amsterdam. 700 pp.
- 22.Aguayo, A.J. 1985. Axonal regeneration from injured neurons in the adult mammalian nervous system. In Synaptic Plasticity. C.W. Cotman, editor. Guilford Press, New York. 457–484.
- 23.Hausmann B, Sievers J, Hermanns J, Berry M. Regeneration of axons from the adult rat optic nerve: influence of fetal brain grafts, laminin and artificial basement membrane. J Comp Neurol. 1989;281:447–466. doi: 10.1002/cne.902810309. [DOI] [PubMed] [Google Scholar]
- 24.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 25.Kohler K, Wheeler-Schilling T, Jurklies B, Guenther E, Zrenner E. Angiotensin II in the rabbit retina. Vis Neurosci. 1997;14:63–71. doi: 10.1017/s0952523800008762. [DOI] [PubMed] [Google Scholar]
- 26.Guenther E, Schmid S, Hewig B, Kohler K. Dual effects of angiotensin II on voltage-dependent calcium currents in rat retinal ganglion cells. Brain Res. 1996;718:112–116. doi: 10.1016/0006-8993(96)00077-7. [DOI] [PubMed] [Google Scholar]
- 27.Thanos S, Bähr M, Barde YA, Vanselow J. Survival and axonal elongation of adult retinal ganglion cells: in vitro effects of lesioned sciatic nerve and brain derived neurotrophic factor (BDNF) Eur J Neurosci. 1989;1:19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 28.Bähr M, Hopkins JM, Bunge RP. In vitro myelination of regenerating adult rat retinal ganglion cell axons by Schwann cells. Glia. 1991;4:529–533. doi: 10.1002/glia.440040512. [DOI] [PubMed] [Google Scholar]
- 29.Lucius R, Young HP, Tidow S, Sievers J. Growth stimulation and chemotropic attraction of rat retinal ganglion cell axons in vitro by co-cultured optic nerves, astrocytes and astrocyte conditioned medium. Int J Dev Neurosci. 1996;14:387–398. [PubMed] [Google Scholar]
- 30.Lucius R, Sievers J. Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996;743:56–62. doi: 10.1016/s0006-8993(96)01029-3. [DOI] [PubMed] [Google Scholar]
- 31.Barnstable CJ, Drager UC. Thy-1 antigen: a ganglion specific cell marker in rodent retina. Neuroscience. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- 32.Brechler V, Jones PW, Levens NR, de Gasparo M, Bottari SP. Agonistic and antagonistic properties of angiotensin analogs at the AT2 receptor in PC12W cells. Regul Pept. 1993;44:207–213. doi: 10.1016/0167-0115(93)90244-3. [DOI] [PubMed] [Google Scholar]
- 33.David S, Bonchard C, Tsatas O. Macrophages can modify the non-permissive nature of the adult mammalian central nervous system. Neuron. 1990;5:463–469. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- 34.Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- 35.Allcutt D, Berry M, Sievers J. A quantitative comparison of the reaction of retinal ganglion cells to optic nerve crush in neonatal and adult mice. Brain Res. 1984;16:219–230. doi: 10.1016/0165-3806(84)90027-0. [DOI] [PubMed] [Google Scholar]
- 36.Allcutt D, Berry M, Sievers J. A qualitative comparison of the reaction of retinal ganglion cells to optic nerve crush in neonatal and adult mice. Brain Res. 1984;16:231–240. doi: 10.1016/0165-3806(84)90028-2. [DOI] [PubMed] [Google Scholar]
- 37.Wodarczyk L, Merrill VK, Perry GW. Differential regulation of fast axonally transported proteins during the early response of rat retinal ganglion cells to axotomy. J Neurochem. 1997;68:1114–1123. doi: 10.1046/j.1471-4159.1997.68031114.x. [DOI] [PubMed] [Google Scholar]
- 38.Meyer RL, Miotke JA, Benowitz LI. Injury induced expression of growth-associated protein GAP-43 in adult mouse retinal ganglion cells in vitro. Neuroscience. 1994;63:591–602. doi: 10.1016/0306-4522(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 39.Campbell G, Anderson PN, Turmaine M, Lieberman AR. GAP-43 in the axons of mammalian CNS neurons regenerating into peripheral nerve grafts. Exp Brain Res. 1991;87:67–74. doi: 10.1007/BF00228507. [DOI] [PubMed] [Google Scholar]
- 40.Schaden H, Stuermer CA, Baehr M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J Neurobiol. 1994;25:1570–1578. doi: 10.1002/neu.480251209. [DOI] [PubMed] [Google Scholar]
- 41.Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 42.Aigner L, Caroni P. Absence of persistent sprouting, branching, and adhesion in GAP-43–depleted growth cones. J Cell Biol. 1995;128:647–660. doi: 10.1083/jcb.128.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doster SK, Lozano AM, Aguayo AJ, Willard MB. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991;6:635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez LA, Twickler J, Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985;105:141–145. [PubMed] [Google Scholar]
- 45.Le Noble FA, Schreurs NH, van Straaten HW, Slaaf DW, Smits JF, Rogg H, Struijker-Boudier HA. Evidence for a novel angiotensin II receptor involved in angiogenesis in chick embryo chorioallantoic membrane. Am J Physiol. 1993;264:R460–R465. doi: 10.1152/ajpregu.1993.264.2.R460. [DOI] [PubMed] [Google Scholar]
- 46.Gohlke P, Kuwer I, Schnell A, Amann K, Mall G, Unger T. Blockade of bradykinin B2 receptors prevents the increase in capillary density induced by chronic angiotensin-converting enzyme inhibitor treatment in stroke-prone spontaneously hypertensive rats. Hypertension (Dallas) 1997;29:478–482. doi: 10.1161/01.hyp.29.1.478. [DOI] [PubMed] [Google Scholar]
- 47.Kimura B, Sumners C, Phillips MI. Changes in skin angiotensin II receptors in rats during wound healing. Biochem Biophys Res Commun. 1992;187:1083–1090. doi: 10.1016/0006-291x(92)91308-d. [DOI] [PubMed] [Google Scholar]
- 48.Bleuel A, de Gasparo M, Whitebread S, Puttner I, Monard D. Regulation of protease nexin-1 expression in cultured Schwann cells is mediated by angiotensin II receptors. J Neurosci. 1995;15:750–761. doi: 10.1523/JNEUROSCI.15-01-00750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nahmias C, Strosberg AD. The angiotensin AT2 receptor: searching for signal-transduction pathways and physiological function. TIPS (Trends Pharmacol Sci) 1995;16:223–225. doi: 10.1016/s0165-6147(00)89030-6. [DOI] [PubMed] [Google Scholar]
- 50.Farlie PG, Dringen R, Rees SM, Kannourakis G, Bernard O. bcl-2transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci USA. 1995;92:4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence MS, Ho DY, Sun GH, Steinberg GK, Sapolsky RM. Overexpression of Bcl-2 with Herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci. 1996;16:486–496. doi: 10.1523/JNEUROSCI.16-02-00486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen D, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 53.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 54.Herdegen T, Skene P, Bähr M. The c-Jun transcription factor — bipotential mediator of neuronal death, survival and regeneration. TINS (Trends Neurosci) 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]