Abstract
The formation of the pre-B cell receptor (BCR) corresponds to an important checkpoint in B cell development that selects pro-B (pre-BI) cells expressing a functionally rearranged immunoglobulin μ (Igμ) heavy chain protein to undergo the transition to the pre-B (pre-BII) cell stage. The pre-BCR contains, in addition to Igμ, the surrogate light chains λ5 and VpreB and the signal transducing proteins Igα and Igβ. The absence of one of these pre-BCR components is known to arrest B cell development at the pre-BI cell stage. Disruption of the Pax5 gene, which codes for the B cell–specific activator protein (BSAP), also blocks adult B lymphopoiesis at the pre-BI cell stage. Moreover, expression of the mb-1 (Igα) gene and VH-to-DHJH recombination at the IgH locus are reduced in Pax5-deficient B lymphocytes ∼10- and ∼50-fold, respectively. Here we demonstrate that complementation of these deficiencies in pre-BCR components by expression of functionally rearranged Igμ and chimeric Igμ-Igβ transgenes fails to advance B cell development to the pre-BII cell stage in Pax5 (−/−) mice in contrast to RAG2 (−/−) mice. Furthermore, the pre-BCR is stably expressed on cultured pre-BI cells from Igμ transgenic, Pax5-deficient bone marrow, but is unable to elicit its normal signaling responses. In addition, the early developmental block is unlikely to be caused by the absence of a survival signal, as it could not be rescued by expression of a bcl2 transgene in Pax5-deficient pre-BI cells. Together, these data demonstrate that the absence of Pax5 arrests adult B lymphopoiesis at an early developmental stage that is unresponsive to pre-BCR signaling.
Keywords: B cell–specific activator protein, Pax5, pro-B cell development, pre-B cell receptor, Igμ transgene
An important checkpoint in B cell development controls the transition from the pro-B (pre-BI) to the pre-B (pre-BII) cell stage that is initiated upon completion of a productive rearrangement at the immunoglobulin heavy chain (IgH) locus. A consequence of expressing the membrane-bound Igμ protein is the transient formation of the pre-B cell receptor (BCR).1 Signaling initiated by this receptor promotes allelic exclusion at the IgH locus, stimulates proliferative cell expansion, and induces differentiation to small pre-BII cells undergoing Ig light chain gene rearrangements (for review see reference 1). In addition to the Igμ protein, the pre-BCR consists of the two nonpolymorphic, surrogate light chains, λ5 and VpreB, as well as the signal transducing proteins Igα and Igβ whose expression is initiated early in B lymphopoiesis (for review see reference 2). B cell development is arrested at the pro-B (pre-BI) cell stage in mice that lack one component of either the pre-BCR (mIgμ [reference 3], λ5 [reference 4], and Igβ [reference 5]) or of the V(D)J recombination machinery [RAG1; reference 6), RAG2 (7), DNA-dependent protein kinase (DNA-PK; reference 8)]. However, expression of a functionally rearranged Igμ transgene is able to complement the recombination defects of both severe combined immunodeficiency (scid) and RAG mutant mice, thus resulting in pre-BCR formation and subsequent progression to the small pre-BII cell stage (9–11). The early expression of a rearranged Igμ transgene significantly shortens the duration of pro-B cell development by directly inducing differentiation to small pre-BII cells (12). Likewise, expression of a functionally rearranged κ light chain gene is capable of activating the pre-B cell transition in λ5-deficient mice (13, 14).
The Igα and Igβ proteins form a disulfide-linked heterodimer that is associated through its transmembrane domain with the Ig molecule in the pre-BCR and BCR. This heterodimer is not only essential for surface transport of Igs, but also constitutes the signal transducing unit of these receptors (for review see references 2, 15). The Igα and Igβ proteins both initiate signaling via immunoreceptor tyrosine-based activation motifs (ITAMs), which become phosphorylated upon receptor engagement and recruit intracellular effectors such as protein-tyrosine kinases to the receptor (2, 15). Apart from these motifs, the cytoplasmic tails of Igα and Igβ differ considerably in sequence, but yet appear to fulfill redundant functions in B cell development. Chimeric receptors, consisting of the Igμ protein fused to the cytoplasmic domain of either the Igα or Igβ protein, are each independently sufficient to induce the pre-B cell transition (16, 17) and to signal B cell maturation (18) in transgenic mice.
Insight into the transcriptional control of early B cell development has recently been gained by gene targeting in the mouse. One of the critical transcription factors thus implicated in early B lymphopoiesis is the B cell–specific activator protein (BSAP), which is encoded by the Pax5 gene (for review see references 19, 20). Pax5 is expressed from the earliest B lineage–committed precursor cell up to the mature B cell stage (21–23), and, consistent with this expression pattern, is essential for B lineage commitment in the fetal liver (24). However, in adult bone marrow, Pax5 is required later for the progression of B cell development beyond the early pro-B (pre-BI) cell stage (24, 25). Interestingly, the VH-to-DHJH recombination at the IgH locus is ∼50-fold reduced in Pax5-deficient pre-BI cells (24). Moreover, the mb-1 (Igα) gene, which has been identified as one of five direct BSAP (Pax5) targets, is expressed at an ∼10-fold lower level in these pre-BI cells, whereas Pax5 is not involved in the control of λ5, VpreB, and B29 (Igβ) expression (24, 26). Hence, the synthesis of two pre-BCR components, Igμ and Igα, is affected in early B lymphocytes of Pax5 mutant mice.
Here we have tested the hypothesis that the inability to express a pre-BCR might be the cause for the B cell developmental block in the bone marrow of Pax5-deficient mice. For this purpose, we have introduced functionally rearranged Igμ and chimeric Igμ–Igβ transgenes into the Pax5 mutant background. These transgenes were able to neither advance B cell development to the small pre-BII cell stage nor to elicit normal signaling responses, although the pre-BCR was expressed on the Igμ transgenic, Pax5-deficient pre-BI cells. Moreover, expression of a bcl2 transgene was also incapable of rescuing the early developmental block which is thus unlikely to result from the absence of a survival signal in Pax5 mutant B lymphocytes. These data therefore demonstrate that Pax5 fulfills an essential function during pro-B cell development before the pre-BCR stage.
Materials and Methods
Mice.
The different mouse strains were maintained on the hybrid C56BL/6 × 129/Sv background. The genotype of Pax5 mutant mice (25) was determined by PCR analysis as previously described (24). RAG2 mutant mice (7) were genotyped by PCR amplification with the following oligonucleotides: 5′-GCAACATGTTATCCAGTAGCCGGT-3′ (primer 1), 5′-TTGGGAGGACACTCACTTGCCAGT-3′ (primer 2), and 5′-GTATGCAGCCGCCGCATTGCATCA-3′ (primer 3). A 605-bp PCR product was amplified from the wild-type RAG2 allele with primer pair 1 and 2 and a 1-kb DNA fragment from the mutant RAG2 allele with the pair 1 and 3. For simplicity, the mouse– human hybrid transgene mIgμ–Igβ (YS:VV; references 16, 27) is referred to as Igμ–Igβ in this manuscript and the functionally rearranged mouse Igμ transgene of the line M54 (28) as Igμ. The presence of the Igμ transgene expressing the membrane form of the μ heavy chain was detected by Southern blot analysis with radiolabeled pBR322 DNA as previously described (28). The Igμ–Igβ transgene was identified by PCR amplification with the primers 5′-GCCTTTGAGAACCTGTGGGC-3′ and 5′-CCTCATTCCTGGCCTGG-3′ (100-bp PCR product). The transgenic mouse strain Eμ-bcl-2-36 (29), which expresses a human bcl-2 cDNA under the control of the SV40 promoter and IgH Eμ enhancer in B and T lymphocytes (30), was genotyped by PCR using the primers 5′-GCAGACACTCTATGCCTGTGTGG-3′ and 5′-GGAACCTTACTTCTGTGGTGTGA-3′ (504-bp PCR product).
Pre-BI Cell Cultures.
Cell suspensions prepared from mouse bone marrow or fetal liver (at embryonic day 16.5 or 17.5) were plated at limiting dilutions on a semiconfluent layer of γ-irradiated stromal ST2 cells in the presence of IL-7–containing medium as previously described (24). After 1 wk of in vitro culture, individual pre-BI cell colonies were collected and further propagated as a cell pool. The long-term proliferation potential of these pre-BI cell pools was analyzed for at least 1 mo.
Antibodies and Flow Cytometry.
The following mAbs were purified from hybridoma supernatants on protein G–sepharose columns (Pharmacia Biotech AB, Uppsala, Sweden) and conjugated with sulfo-NHS-biotin (Pierce Chemical Co., Rockford, IL) as recommended by the suppliers: anti-c-kit mAb (ACK4; reference 31), anti-μ mAb (M41.42; reference 32), anti-λ5 mAb (LM34; reference 33), and anti-pre-BCR mAb (SL156; reference 33). The following reagents were purchased from PharMingen (San Diego, CA): biotinylated anti-CD25 mAb (7D4), biotinylated anti-CD43 mAb (S7), biotinylated anti-CD2 mAb (RM2-5), FITC- and PE-coupled anti-B220/CD45R mAb (RA3-6B2), FITC-conjugated anti-μ mAb (R6-60.2), APC-coupled anti– c-kit mAb (ACK45), purified anti–human Bcl-2 mAb (Bcl-2/ 100), and PE-conjugated streptavidin.
8–11-d-old mice were used for flow cytometric analysis, as older Pax5 mutant mice suffer from disease and generally die within 3 wk (25). Cultured pre-BI cells or single-cell suspensions prepared from the bone marrow of these mice were stained with different antibody combinations and subsequently analyzed on a FACScan® flow cytometer (Becton Dickinson, San Jose, CA) as previously described (25).
Intracellular Antibody Staining.
The cytoplasmic μ heavy chain protein was detected in bone marrow pre-BI cells as previously described (34). In brief, bone marrow cells were incubated with PE-coupled anti-B220 (RA3-6B2) and allophycocyanin (APC)- conjugated anti–c-kit (ACK45) antibodies at 4°C, washed twice with PBS, and then fixed with 2% paraformaldehyde (Fluka AG, Buchs, Switzerland) in PBS at room temperature for 20 min, followed by two washes with PBS. The fixed cells were subsequently permeabilized with 0.5% saponin (Sigma Chemical Co., St. Louis, MO) in 2% FCS/PBS and were simultaneously stained with FITC-conjugated anti-μ antibody (R6-60.2) for 40 min at 4°C, then washed twice in saponin buffer and once in 2% FCS/PBS before analysis on a FACSVantage® TSO flow cytometer (Becton Dickinson). Cultured bcl-2 transgenic, Pax5 (−/−) pre-BI cells were analyzed for expression of the human Bcl-2 protein by cytoplasmic staining with an anti–human Bcl-2 mAb (Bcl-2/100; detected with a PE-coupled goat anti–mouse IgG antibody) as described above.
Western Blot Analysis.
Whole cell extracts of in vitro cultured pre-BI cells were prepared by lysis in 0.25 M Tris, pH 7.5, and 0.1% Triton X-100, followed by removal of insoluble material by centrifugation. Total protein (10 μg) was separated by 10% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and then incubated with a rabbit polyclonal anti-Igβ antiserum (27) (diluted at 1:1,000). Anti-Igβ antibodies were detected by enhanced chemiluminescence using a horseradish peroxidase–conjugated donkey anti–rabbit secondary antibody (ECL; Amersham International, Arlington Heights, IL).
RNase Protection Analysis.
A mouse terminal deoxynucleotidyl transferase (TdT) riboprobe was generated by inserting a 244-bp cDNA fragment of the mouse TdT mRNA (35) into the HindIII and EcoRI sites of pSP64. This cDNA fragment was amplified from RNA of 70Z/3 cells by reverse transcriptase PCR using the following primers: 5′-GCGGAATTCAAGGTGGATGCTCTCGACCAT-3′ and 5′-GCGAAGCTTCGTGGTTGTCCAGCATCATCT-3′. Total RNA was prepared from cultured pre-BI cells and analyzed by RNase protection assay exactly as previously described (24).
Results
Pax5 (BSAP) Is Essential for Early B Cell Development before the Pre-BCR Stage.
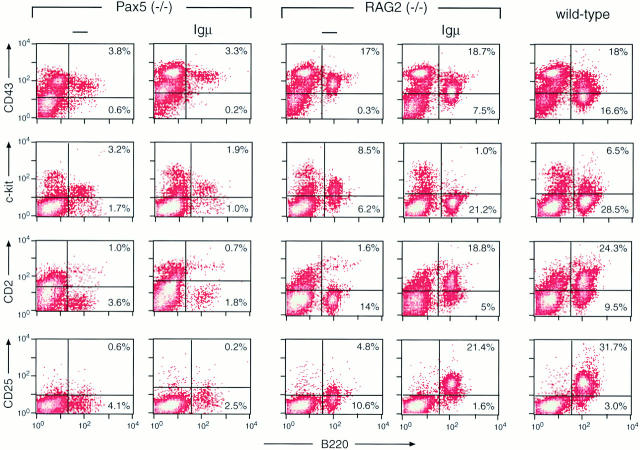
Based on the expression of cell surface markers, we have recently demonstrated that B cell development is arrested in the bone marrow of Pax5 mutant mice (24) at a similar pro-B (pre-BI) cell stage as in mice that are deficient in one of the components of the pre-BCR (μMT [reference 36], λ5 [reference 36], and Igβ [reference 5]) or the V(D)J rearrangement machinery (RAG1 [reference 9] and RAG2 [reference 7]). Moreover, Pax5-deficient pre-BI cells are essentially unable to synthesize the Igμ protein, an important constituent of the pre-BCR, due to an ∼50-fold reduction of the VH-to-DHJH recombination frequency at the IgH locus (24). The inability to form a functional pre-BCR could therefore explain the early B cell developmental block observed in Pax5 mutant mice. This hypothesis makes the clear prediction that expression of a functionally rearranged Igμ transgene in Pax5 mutant mice should result in the formation of the pre-BCR, thus traversing this important checkpoint and advancing B cell development to the pre-BII cell stage. To test this hypothesis, we have introduced a rearranged murine Igμ transgene, which directs expression of a membrane-bound Igμ protein under the control of a VH gene promoter (28) into Pax5 (−/−) mice. As the chosen Igμ transgene has not yet been used for similar experiments, we have also tested its ability to guide B cell development to the pre-BII cell stage in RAG2-deficient mice. The transition from the pre-BI to the pre-BII cell stage is known to be accompanied by the downregulation of the early markers CD43 and c-kit, by the initiation of CD2 and CD25 expression, and by an increase in the total B cell number (9, 10, 37). B lymphocytes from RAG2 (−/−) bone marrow lacking or containing the Igμ transgene were compared by flow cytometric analysis (Fig. 1), demonstrating that the synthesis of CD43 and c-kit was indeed downregulated, the expression of CD2 and CD25 was initiated, and the number of B220+ cells was increased by about twofold in the presence of the transgene. In marked contrast, the B lymphocyte number and cell surface phenotype did not change in the bone marrow of Pax5 (−/−) mice irrespective of the presence or absence of the Igμ transgene (Fig. 1). Hence, the Pax5 and RAG2 gene mutations clearly differ, as the presence of a rearranged Igμ transgene is unable to rescue the early B cell developmental block in Pax5-deficient mice in contrast to RAG2-deficient mice.
Figure 1.
Expression of a rearranged Igμ transgene fails to advance B cell development in Pax5 mutant mice. Bone marrow cells from 8–11-d-old mice of the indicated genotype were analyzed by flow cytometry using an FITC-conjugated anti-B220 antibody (RA3-6B2) in combination with biotinylated anti-CD25 (7D4), anti-CD43 (S7), anti-CD2 (RM2-5), or anti–c-kit (ACK4) antibodies. The biotin-conjugated antibodies were revealed by incubation with PE-coupled streptavidin. The percentage of B220+ cells is indicated in each quadrant. The number of B220+ cells was consistently lower in the bone marrow of Pax5 mutant mice compared with RAG2 mutant mice, which may reflect the poorer health of Pax5-deficient mice (25), the ultrasensitivity of Pax5 (−/−) pre-BI cells to apoptotic signals (Nutt, S.L., data not shown), or blockage at different stages of pro-B cell development in the two mutant mice (see Discussion).
Possible trivial explanations for the failure of the Igμ transgene to induce the pre-B cell transition could be that Pax5 itself is involved in the transcriptional control of the transgene or that B cell development is arrested before the initiation of transgene expression in Pax5 mutant mice. To investigate these possibilities, we have analyzed the presence of cytoplasmic Igμ protein in c-kit+ B220+ pre-BI cells of Pax5-deficient bone marrow (Fig. 2 A). No cytoplasmic Igμ protein could be detected by intracellular staining in Pax5 (−/−) pre-BI cells in agreement with the fact that the VH-to-DHJH recombination is drastically reduced in these cells (24). In contrast, the Igμ protein was expressed in the majority of Pax5 (−/−) pre-BI cells carrying the transgene. We therefore conclude that early expression of a rearranged Igμ transgene is not sufficient to trigger the pre-B cell transition in Pax5 mutant mice.
Figure 2.
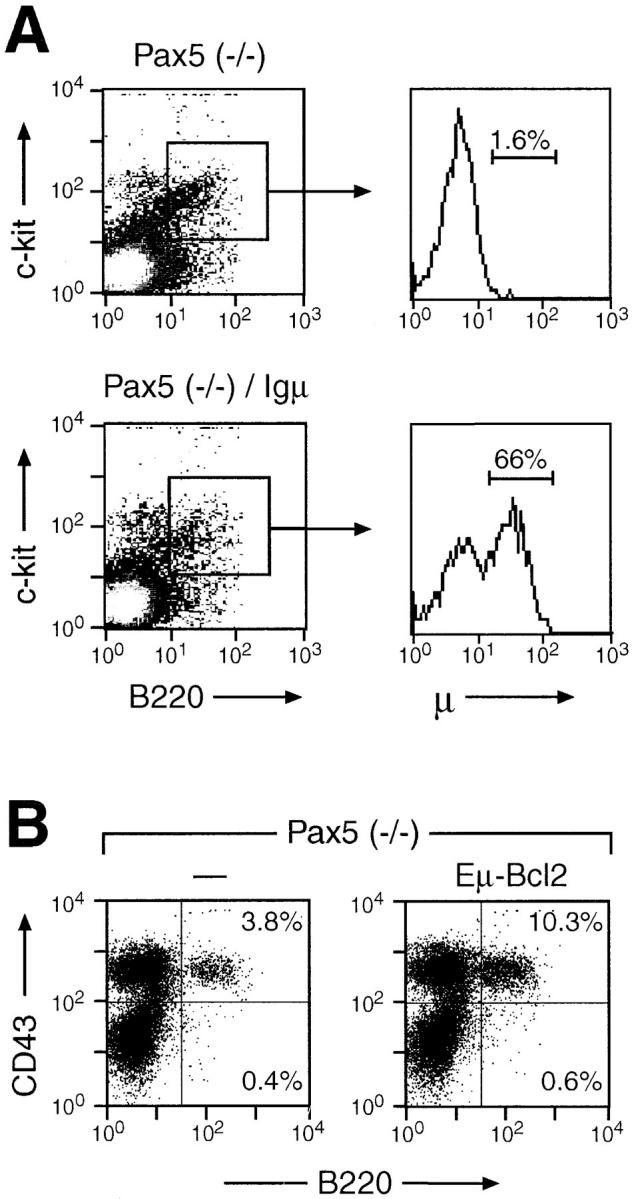
Igμ and bcl-2 transgene expression in pre-BI cells of Pax5- deficient bone marrow. (A) Intracellular Igμ staining. Bone marrow cells of Pax5 mutant mice containing or lacking the Igμ transgene were analyzed by intracellular staining for expression of the Igμ protein (see Materials and Methods). Pre-BI cell were identified by staining with APC-coupled anti–c-kit (ACK45) and PE-conjugated anti-B220 (RA3-6B2) antibodies. The presence of the intracellular Igμ protein within the c-kit+ B220+ cell population was detected with an FITC-conjugated anti-μ antibody (R6-60.2) and is displayed to the right. (B) Expression of a bcl-2 transgene fails to rescue the early B cell developmental block in Pax5-deficient mice. The transgene of the Eμ-bcl-2-36 strain (29, 30) was crossed into the Pax5 mutant background, and the bone marrow of 10–14-d-old Pax5 (−/−) mice was analyzed by flow cytometry using FITC-conjugated anti-B220 (RA3-6B2) and biotinylated anti-CD43 (S7) antibodies (revealed by PE-streptavidin). The percentage of B220+ cells is indicated in each quadrant. 10 mice of each genotype were analyzed, indicating that the CD43+ B220+ cell population corresponded on average to 5.4% [Pax5 (−/−)], 7.1% [Pax5 (−/−), bcl-2 transgene], 7.2% [Pax5 (+/+)], and 10.8% [Pax5 (+/+), bcl-2 transgene] of the total bone marrow cells (data not shown).
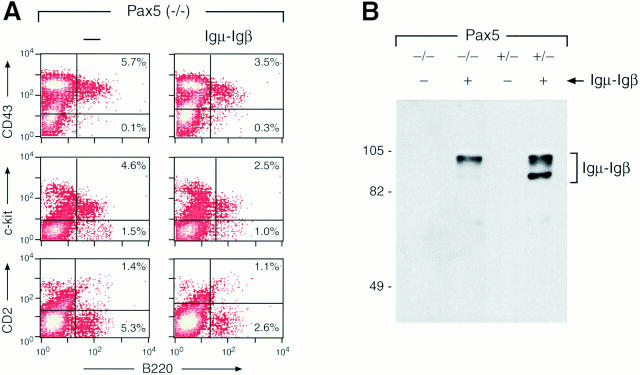
The mb-1 gene coding for Igα was recently shown to be a direct BSAP (Pax5) target whose expression is reduced ∼10-fold in Pax5-deficient pre-BI cells compared with wild-type cells (26). In addition to the Igμ protein, Igα is therefore a second component of the pre-BCR that is expressed under the control of Pax5. As the heterodimer consisting of the proteins Igα and Igβ constitutes the signal transducing unit of the pre-BCR (2), it is conceivable that the reduced Igα expression in Pax5-deficient pre-BI cells prevents the formation of a functional pre-BCR even in the presence of a rearranged Igμ transgene. To address this question, we have introduced a chimeric Igμ–Igβ transgene (16) into the Pax5 (−/−) background. The Igμ component of this transgene codes for a membrane-bound Ig with two transmembrane mutations (Y587V, S588V) which prevent its normal association with the Igα–Igβ dimer (27). The cytoplasmic domain of the fusion protein is encoded by Igβ and directly mediates signaling independent of the presence of endogenous Igα or Igβ proteins (27). Furthermore, the chimeric Igμ–Igβ receptor was shown to efficiently activate transition to the pre-BII cell stage and to induce allelic exclusion at the IgH locus in RAG-deficient mice (16). Hence, signaling of this chimeric receptor should be independent of the reduced expression levels of both Igα and Igμ proteins that are observed in Pax5-deficient mice. Nevertheless, the chimeric Igμ–Igβ gene was unable to advance B cell development in the bone marrow of Pax5 mutant mice, since its presence neither altered the expression of cell surface markers nor increased the number of B220+ cells (Fig. 3 A). However, the Igμ–Igβ fusion protein was expressed in pre-BI cells regardless of the Pax5 genotype (Fig. 3 B). Together, these in vivo data indicate that expression of the pre-BCR is not sufficient to rescue the early B cell developmental block in Pax5-deficient mice. Hence, the Pax5 mutation appears to arrest B lymphopoiesis at an early stage that is not responsive to pre-BCR signaling.
Figure 3.
Expression of the Igμ-Igβ transgene is not sufficient to rescue the early developmental arrest in Pax5 mutant mice. (A) Flow cytometric analysis. Bone marrow cells of Pax5 mutant mice containing or lacking the Igμ-Igβ transgene (16) were analyzed by flow cytometry as described in the legend to Fig. 1. (B) Expression of the Igμ–Igβ fusion protein. Bone marrow pre-BI cells from mice of the indicated genotypes were enriched by short-term culturing in IL-7 containing medium and then analyzed for Igμ–Igβ protein expression by immunoblotting (see Materials and Methods). The faster migrating polypeptide corresponds in size to the unglycosylated form of the Igμ-Igβ fusion protein. The size of marker proteins (given in kD) is indicated to the left.
The survival of B cell precursors is controlled by differential expression of the antiapoptotic genes bcl-2 and bcl-xL during B lymphopoiesis (38, 39). Interestingly, the bcl-xL but not the bcl-2 gene is consistently expressed at a 10-fold lower level in Pax5-deficient pre-BI cells compared with wild-type cells, although this downregulation was shown to be an indirect consequence of the absence of Pax5 (26). In agreement with this finding, the pre-BI cells of Pax5 mutant bone marrow proved to be ultrasensitive to growth factor withdrawal, as they rapidly undergo apoptosis ex vivo in the absence of survival signals emanating from the IL-7 receptor (data not shown). In this context it is interesting to note that the expression of a bcl-2 transgene was previously shown to promote B cell development in scid mice (40) that also exhibit a defect in V(D)J recombination of Ig genes (for review see reference 41). Hence, we investigated the possibility that sustained cell survival may also rescue the early developmental block in Pax5-deficient bone marrow. For this purpose, the same Eμ-bcl-2-36 transgenic mouse, carrying a human bcl-2 cDNA under the control of the IgH Eμ enhancer (29), was crossed with Pax5 mutant mice. Expression of the bcl-2 transgene in Pax5 (−/−) pre-BI cells was demonstrated by cytoplasmic staining with an anti–human Bcl-2 antibody as well as by its ability to completely block apoptosis upon IL-7 withdrawal (data not shown). Nevertheless, the bcl-2 transgene was unable to advance B cell development to the pre-BII cell stage, as no CD43− B220+ B lymphocytes were observed in the bone marrow of bcl-2 transgenic, Pax5 (−/−) mice (Fig. 2 B). Instead, deregulated bcl-2 expression led to a modest increase in CD43+ B220+ pre-BI cells similar to the situation observed in RAG2 mutant mice carrying a bcl-2 transgene (42). Therefore, these data demonstrate that blocking apoptosis is not sufficient to promote B cell development in Pax5 mutant mice.
Stable Expression of the Pre-BCR and Absence of its Normal Signaling Response in Igμ Transgenic, Pax5-deficient Pre-BI Cells.
Expression of a functionally rearranged Igμ chain has previously been shown to alter the IL-7 responsiveness of precursor B cells in wild-type and RAG2 mutant mice (10). The proliferative response to IL-7 was considerably decreased in bone marrow cells of Igμ transgenic mice, thus preventing the establishment of long-term pre-BI cell cultures (10). One possible reason for this phenomenon may be the downregulation of c-kit expression in response to pre-BCR activation (33, 37), which eliminates an essential costimulatory signal for IL-7–dependent proliferation of B lymphoid precursor cells (43). To further study the function of the pre-BCR, we have established pre-BI cell cultures from bone marrow of Pax5-deficient mice carrying an Igμ(-Igβ) transgene. These pre-BI cells were cultured in the presence of stromal ST2 cells and IL-7, and their long-term proliferation potential was assessed after 1 mo of in vitro culture. Surprisingly, Pax5-deficient pre-BI cells could be efficiently established and maintained even in the presence of transgenic Igμ or chimeric Igμ–Igβ proteins (Table 1). In contrast, no pre-BI cell cultures with long-term proliferation capacity were obtained from homozygous or heterozygous RAG2 mutant mice carrying an Igμ transgene, as previously described (10). Thus, these data indicate that expression of the Igμ protein does not interfere with the proliferation potential of Pax5-deficient pre-BI cells in contrast to control B lymphocytes.
Table 1.
Long-term Proliferation of Pax5-deficient Pre-BI Cells Despite the Expression of Igμ Transgenes
| Genotype | Transgene | Long-term pre-BI cell growth/mouse | ||
|---|---|---|---|---|
| Pax5 (−/−) | – | 4/4 | ||
| Pax5 (−/−) | Igμ | 6/6 | ||
| Pax5 (−/−) | Igμ-Igβ | 7/7 | ||
| RAG2 (−/−) | – | 9/9 | ||
| RAG2 (−/−) | Igμ | 0/7 | ||
| RAG2 (+/−) | – | 7/7 | ||
| RAG2 (+/−) | Igμ | 0/8 |
Bone marrow cells of Pax5 (−/−) mice or fetal liver cells of RAG2 mutant embryos were plated under limiting dilution conditions in the presence of IL-7 and stromal ST2 cells. Pre-BI cell colonies were subsequently pooled, and their long-term proliferation potential was assessed after 1 mo of in vitro culture. Bone marrow instead of fetal liver had to be used as a source of B lymphoid progenitors for culturing Pax5-deficient pre-BI cells, as Pax5 is required for B lineage commitment in the fetal liver (24).
Given the possibility to grow Igμ transgenic, Pax5 (−/−) precursor cells, we next investigated whether these cells could assemble the pre-BCR on their surface. As shown by flow cytometric analysis, Pax5-deficient pre-BI cells containing or lacking the Igμ transgene expressed a similar level of the surrogate light chain λ5 on their surface (Fig. 4 A). In contrast, the Igμ protein was only found on the transgenic pre-BI cells. Furthermore, staining with a monoclonal antibody (SL156), which recognizes a conformational epitope present on the surrogate light chain-Igμ complex of the pre-BCR (33), demonstrated that the Igμ protein was part of the pre-BCR (Fig. 4 A). Three conclusions can be drawn from these data. First, the pre-BCR is stably expressed on the surface of Igμ transgenic, Pax5 (−/−) pre-BI cells despite the fact that the pre-BCR is only transiently expressed and rapidly internalized on wild-type precursor B cells (33, 44, 45). Second, the surrogate light chains are expressed at normal levels on transgenic, Pax5 (−/−) pre-BI cells, although their expression is usually downregulated in response to pre-BCR signaling (9, 10, 37, 38, 44). Third, the Igα protein is known to be essential for cell surface transport of Igs (46, 47), and yet the 10-fold lower mb-1 expression in Pax5-deficient pre-BI cells (26) seems to provide sufficient Igα protein for pre-BCR formation.
Figure 4.
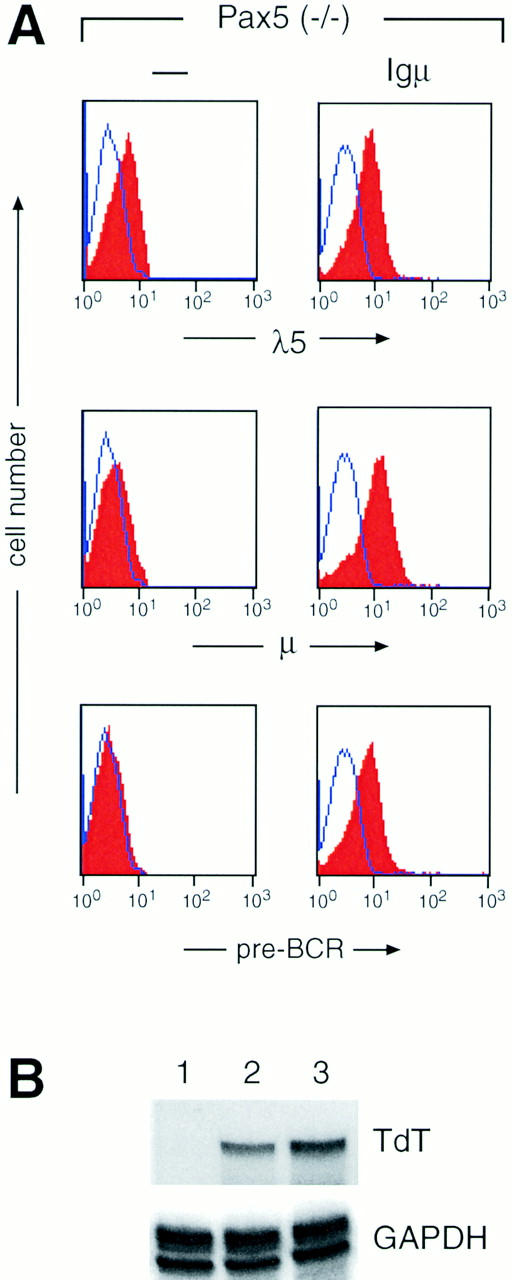
The Pax5 function is required for normal signaling responses of the pre-BCR. (A) Constitutive expression of the pre-BCR on Pax5-deficient pre-BI cells carrying the Igμ transgene. Pre-BI cells from Pax5 mutant mice were grown for 3 wk on stromal ST2 cells in the presence of IL-7 followed by flow cytometric analysis with biotinylated anti-λ5 (LM34), anti-μ (M41.42), and anti–pre-BCR (SL156) antibodies. Incubation with PE-coupled streptavidin was used to visualize the biotinylated antibodies. Unstained control cells are indicated by a line. Note that the cell surface expression of λ5 in the absence of Igμ is in agreement with the finding that the surrogate light chains λ5 and VpreB are expressed in association with an unidentified 130 kD glycoprotein on the surface of pre-BI cells before any productive V(D)J rearrangement (44). (B) Expression of the TdT gene in transgenic, Pax5-deficient pre-BI cells. Total RNA (10 μg) isolated from cultured pre-BI cells was simultaneously analyzed for TdT and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression by RNase protection assay. Only the relevant parts of the autoradiograph containing the RNase-protected fragments are shown. Pre-BI cells derived from Pax5 (−/−) bone marrow lacking (lane 2) or containing the Igμ transgene (lane 3) were compared with pre-BI cells established from the fetal liver of a control embryo (lane 1). Note that the TdT gene is not expressed in fetal B lymphopoiesis (38).
The expression of the TdT gene is rapidly downregulated during the pre-B cell transition in response to expression of a functionally rearranged Igμ protein (37, 38, 48). The TdT gene is therefore considered to be a downstream target in the signaling cascade initiated by the pre-BCR (48). As shown by RNase protection analysis, the level of TdT transcripts was similar in Pax5-deficient pre-BI cells regardless of the presence of the Igμ transgene (Fig. 4 B, lanes 2 and 3). In summary, the different results obtained with cultured pre-BI cells all demonstrate that the pre-BCR is unable to elicit its normal signaling response in the absence of Pax5 function.
Discussion
The transcription factor Pax5 (BSAP) is involved in the control of VH-to-DHJH recombination and in the transcriptional regulation of the mb-1 gene, which results in reduced expression of the two pre-BCR components, Igμ and Igα, in Pax5-deficient pre-BI cells (24, 26). Here we have demonstrated that complementation of these deficiencies by the expression of Igμ and Igμ–Igβ transgenes is not sufficient to initiate the pre-B cell transition in Pax5 mutant mice. Hence, the inability to form a pre-BCR cannot be the cause of the early B cell developmental block in mice lacking Pax5. Instead, the absence of Pax5 arrests B cell development by a different mechanism compared with mice which lack a component of the pre-BCR (mIgμ [reference 3], λ5 [reference 4], or Igβ [reference 5]) or of the V(D)J recombination machinery (RAG1 [reference 6], RAG2 [reference 7], or DNA-PK [reference 8]). Consistent with this conclusion, the lack of Pax5 or RAG2 function has opposite effects on the in vitro differentiation potential of B lymphocytes. Pre-BI cells of RAG2 mutant mice efficiently differentiate ex vivo to the mature B cell stage upon stimulation with IL-4 and anti-CD40 antibodies, which by-passes in vitro the requirement of Ig gene rearrangements for further development (49). In contrast, Pax5 mutant pre-BI cells entirely fail to differentiate under the same in vitro conditions, further demonstrating a strict dependency of early B lymphopoiesis on Pax5 (Nutt, S.L., unpublished data).
It has been notoriously difficult to demonstrate expression of the pre-BCR on the surface of precursor B cells (33, 44), which reflects both a slow, inefficient cell surface transport and rapid, tyrosine phosphorylation–dependent internalization of this receptor (2, 45). Quite in contrast, we have now observed stable expression of the pre-BCR on the surface of Igμ transgenic, Pax5-deficient pre-BI cells. Interestingly, the constitutive cell surface expression of the pre-BCR correlates with the absence of normal signaling responses. The transgenic, Pax5-deficient pre-BI cells neither lost their long-term proliferation potential in the presence of IL-7 and stromal cells nor did they downregulate expression of the TdT or surrogate light chain genes, which are normal responses to pre-BCR signaling in wild-type precursor B cells (9, 10, 37, 38, 44, 48). Therefore, it is conceivable that Pax5 may either regulate the expression of an essential component of the signal transduction cascade or act in the nucleus as the critical mediator of pre-BCR signaling. Stimulation of the BCR is known to result in the phosphorylation and association of the Igα– Igβ heterodimer with the protein-tyrosine kinases Lyn, Fyn, Blk, Btk, and Syk (50–53). Moreover, the Syk kinase has been shown to play an important role in pre-BCR signaling (54, 55). However, none of these tyrosine kinase genes is expressed under the control of Pax5, as shown by a comprehensive analysis of putative BSAP (Pax5) target genes (26). Hence, there is at present no evidence that Pax5 is involved in the expression of cytoplasmic signal transducers. Moreover, an exclusive role of Pax5 in mediating signal transduction of the pre-BCR seems unlikely for several reasons. First, Pax5 expression is already initiated at B lineage commitment long before the pre-BCR stage and thereafter is maintained at a rather constant level throughout B lymphopoiesis (21–23). Second, all our attempts have so far failed to demonstrate any alteration in the posttranslational modification pattern of BSAP (Pax5) in response to signal transduction (M. Busslinger, unpublished data). Third, the developmental arrest in Pax5 mutant mice is tight (25) rather than leaky as it would be expected, in analogy to the syk (−/−) mouse (54, 55), for a mutation in a downstream component of the signal transduction pathway. Last but not least, a role for Pax5 in the regulation of λ5, VpreB, or TdT has recently been excluded (26), although the expression of these genes is downregulated in response to pre-BCR signaling.
The mouse scid mutation affects the XRCC7 gene coding for the catalytic subunit of the DNA-PK, which is essential for V(D)J recombination and double-stranded DNA break repair (8, 41). The phenotype of the scid mouse is known to be leaky in contrast to the RAG mutations, as scid B lymphocytes are still able to generate DH-to-JH and VH-to-DHJH rearrangements at a very low frequency (41). Hence, the scid and Pax5 mutations appear to be comparable with regard to their low efficiency of Ig gene rearrangements and early B cell developmental block. However, expression of a bcl-2 transgene in scid mice results in the accumulation of almost normal numbers of B lymphocytes that express many markers of mature B cells (40, 56). Due to the increased life span, the early progenitor cells present in the bcl-2 transgenic scid bone marrow seem to have a higher probability to generate productive DH-to-JH rearrangements in reading frame 2 and thus to express the truncated Dμ protein that promotes maturation to later B cell stages (56). Interestingly, we have previously shown that DH-to-JH rearrangements occur relatively frequently in reading frame 2 in pre-BI cells of Pax5 mutant bone marrow (24). Nevertheless, expression of the same bcl-2 transgene fails to promote B cell development in Pax5-deficient mice, thus further demonstrating that the early arrest of B lymphopoiesis is neither caused by a rearrangement defect nor by the lack of a survival signal.
The inability of Igμ and bcl-2 transgenes to advance B cell development and the constitutive cell surface expression of the pre-BCR strongly argue that B lymphopoiesis is arrested in Pax5 mutant mice at an early stage that is not responsive to pre-BCR signaling in the absence of Pax5 function. Consistent with this notion, the cell surface marker BP-1, which is specifically expressed on late pro-B (pre-BI) cells (57), is absent on bone marrow cells of Pax5-deficient mice (24). Therefore, it appears that Pax5 controls a critical step between initial B lineage commitment and the pre-BCR stage of adult B lymphopoiesis. In this context it is interesting to note that the interruption of Ras signaling also arrests early B cell development well before the pre-BCR stage (58). Our analysis of Pax5-deficient pre-BI cells has recently demonstrated a pleiotropic role of the transcription factor BSAP (Pax5) in gene regulation during early B lymphopoiesis (26). Hence, it will be a challenge for the future to identify the critical, and thus far unknown, BSAP target gene(s) that mediates the Pax5-dependent control of early B cell development.
Acknowledgments
We thank F. Alt (Harvard Medical School, Boston, MA) for providing the RAG2-deficient mouse; T. Imanishi-Kari (Tufts University, Boston, MA) for the transgenic M54 mouse; S. Cory (Walter and Eliza Hall Institute, Melbourne, Australia) for the Eμ-bcl2-36 strain; M. Nussenzweig (The Rockefeller University, New York) for the mIgμ–Igβ (YS:VV) mouse and anti-Igβ antiserum; T. Rolink (Basel Institute for Immunology, Switzerland) for monoclonal antibodies; and P. Steinlein for help with flow cytometric analysis.
This work was supported by the Research Institute of Molecular Pathology, and in part by a grant from the Austrian Industrial Research Promotion Fund.
Abbreviations used in this paper
- APC
allophycocyanin
- BCR
B cell receptor
- BSAP
B cell–specific activator protein
- DNA-PK
DNA-dependent protein kinase
- IgH
immunoglobulin heavy chain
- RAG
recombination activating gene
- scid
severe combined immune deficiency
- TdT
terminal deoxynucleotidyl transferase
Footnotes
Claire Thévenin's present address is Department of Medical and Molecular Parasitology, New York University Medical Center, New York, NY 10016.
References
- 1.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Borst J, Jacobs H, Brouns G. Composition and function of T-cell receptor and B-cell receptor complexes on precursor lymphocytes. Curr Opin Immunol. 1996;8:181–190. doi: 10.1016/s0952-7915(96)80056-2. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell–deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura D, Kudo A, Schaal S, Müller W, Melchers F, Rajewsky K. A critical role of λ5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 5.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Igβ. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 6.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1–deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 7.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2–deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 8.Blunt T, Finnie NJ, Toccioli GE, Smith GCM, Demengeot J, Gottlieb TM, Mizuta R, Varghese AJ, Alt FW, Jeggo PA, Jackson SP. Defective DNA- dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scidmutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 9.Spanopoulou E, Roman CAJ, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, Nussenzweig MC, Shinton SA, Hardy RR, Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1–deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 10.Young F, Ardman B, Shinkai Y, Landford R, Blackwell TK, Mendelsohn M, Rolink A, Melchers F, Alt FW. Influence of immunoglobulin heavy- and light-chain expression on B-cell differentiation. Genes Dev. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y, Bosma GC, Bosma MJ. Development of B cells in scid mice with immunoglobulin transgenes: implications for the control of V(D)J recombination. Immunity. 1995;2:607–616. doi: 10.1016/1074-7613(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 12.Arakawa H, Takeda S. Early expression of Ig μ chain from a transgene significantly reduces the duration of the pro-B stage but does not affect the small pre-B stage. Int Immunol. 1996;8:1319–1328. doi: 10.1093/intimm/8.8.1319. [DOI] [PubMed] [Google Scholar]
- 13.Pelanda R, Schaal S, Torres RM, Rajewsky K. A prematurely expressed Igκ transgene, but not a VκJκ gene segment targeted into the Igκ locus, can rescue B cell development in λ5-deficient mice. Immunity. 1996;5:229–239. doi: 10.1016/s1074-7613(00)80318-0. [DOI] [PubMed] [Google Scholar]
- 14.Papavasiliou F, Jankovic M, Nussenzweig MC. Surrogate or conventional light chains are required for membrane immunoglobulin Mu to activate the precursor B cell transition. J Exp Med. 1996;184:2025–2030. doi: 10.1084/jem.184.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFranco AL. Transmembrane signaling by antigen receptors of B and T lymphocytes. Curr Opin Cell Biol. 1995;7:163–175. doi: 10.1016/0955-0674(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 16.Papavasiliou F, Misulovin Z, Suh H, Nussenzweig MC. The role of Igβ in precursor B cell transition and allelic exclusion. Science. 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- 17.Papavasiliou F, Jankovic M, Suh H, Nussenzweig MC. The cytoplasmic domains of immunoglobulin (Ig)α and Igβ can independently induce the precursor B cell transition and allelic exclusion. J Exp Med. 1995;182:1389–1394. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teh Y-M, Neuberger MS. The immunoglobulin (Ig)α and Igβ cytoplasmic domains are independently sufficient to signal B cell maturation and activation in transgenic mice. J Exp Med. 1997;185:1753–1758. doi: 10.1084/jem.185.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison AM, Nutt SL, Thévenin C, Rolink A, Busslinger M. Loss- and gain-of-function mutations reveal an important role of BSAP (Pax-5) at the start and end of B cell differentiation. Semin Immunol. 1998;10:133–142. doi: 10.1006/smim.1998.0115. [DOI] [PubMed] [Google Scholar]
- 20.Busslinger, M., and S.L. Nutt. 1998. Role of the transcription factor BSAP (Pax-5) in B-cell development. In Molecular Biology of B-Cell and T-Cell Development. J.G. Monroe and E.V. Rothenberg, editors. Humana Press Inc., Totowa, NJ. 83–110.
- 21.Barberis A, Widenhorn K, Vitelli L, Busslinger M. A novel B-cell lineage–specific transcription factor present at early but not late stages of differentiation. Genes Dev. 1990;4:849–859. doi: 10.1101/gad.4.5.849. [DOI] [PubMed] [Google Scholar]
- 22.Adams B, Dörfler P, Aguzzi A, Kozmik Z, Urbánek P, Maurer-Fogy I, Busslinger M. Pax-5encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992;6:1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- 23.Li Y-S, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 24.Nutt SL, Urbánek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgHlocus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- 25.Urbánek P, Wang Z-Q, Fetka I, Wagner EF, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 26.Nutt SL, Morrison AM, Dörfler P, Rolink A, Busslinger M. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO (Eur Mol Biol Organ) J. 1998;17:2319–2333. doi: 10.1093/emboj/17.8.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez M, Misulovin Z, Burkhardt AL, Mahajan S, Costa T, Franke R, Bolen JB, Nussenzweig M. Signal transduction by immunoglobulin is mediated through Igα and Igβ. J Exp Med. 1993;178:1049–1055. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosschedl R, Weaver D, Baltimore D, Costantini F. Introduction of a μ immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibodies. Cell. 1984;38:647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- 29.Strasser A, Harris AW, Vaux DL, Webb E, Bath ML, Adams JM, Cory S. Abnormalities of the immune system induced by dysregulated bcl-2expression in transgenic mice. Curr Topics Microbiol Immunol. 1990;166:175–181. doi: 10.1007/978-3-642-75889-8_22. [DOI] [PubMed] [Google Scholar]
- 30.Strasser A, Harris AW, Cory S. bcl-2transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S-I, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S-I. Expression and function of c-kitin hematopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leptin M, Potash MJ, Grützmann R, Heusser C, Shulman M, Köhler G, Melchers F. Monoclonal antibodies specific for murine IgM. I. Characterization of antigenic determinants on the four constant domains of the μ heavy chain. Eur J Immunol. 1984;14:534–542. doi: 10.1002/eji.1830140610. [DOI] [PubMed] [Google Scholar]
- 33.Winkler TH, Rolink A, Melchers F, Karasuyama H. Precursor B cells of mouse bone marrow express two different complexes with the surrogate light chain on the surface. Eur J Immunol. 1995;25:446–450. doi: 10.1002/eji.1830250221. [DOI] [PubMed] [Google Scholar]
- 34.ten Boekel E, Melchers F, Rolink AG. Changes in the VHgene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 35.Koiwai O, Yokota T, Kageyama T, Hirose T, Yoshida S, Arai K. Isolation and characterization of bovine and mouse terminal deoxynucleotidyltransferase cDNAs expressible in mammalian cells. Nucleic Acids Res. 1986;14:5777–5792. doi: 10.1093/nar/14.14.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlich A, Schaal S, Gu H, Kitamura D, Müller W, Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993;72:695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 37.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor α chain (CD25,TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 38.Li Y-S, Hayakawa K, Hardy RR. The regulated expression of B lineage–associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang W, Mueller DL, Pennell CA, Rivard JJ, Li Y-S, Hardy RR, Schlissel MS, Behrens TW. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xLtransgene. Immunity. 1996;4:291–299. doi: 10.1016/s1074-7613(00)80437-9. [DOI] [PubMed] [Google Scholar]
- 40.Strasser A, Harris AW, Corcoran LM, Cory S. Bcl-2 expression promotes B- but not T-lymphoid development in scidmice. Nature. 1994;368:457–460. doi: 10.1038/368457a0. [DOI] [PubMed] [Google Scholar]
- 41.Bosma MJ, Carroll AM. The scid mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 42.Young F, Mizoguchi E, Bhan AK, Alt FW. Constitutive bcl-2 expression during immunoglobulin heavy chain-promoted B cell differentiation expands novel precursor B cells. Immunity. 1997;6:23–33. doi: 10.1016/s1074-7613(00)80239-3. [DOI] [PubMed] [Google Scholar]
- 43.Rolink A, Streb M, Nishikawa S-I, Melchers F. The c-kit–encoded tyrosine kinase regulates the proliferation of early pre-B cells. Eur J Immunol. 1991;21:2609–2612. doi: 10.1002/eji.1830211044. [DOI] [PubMed] [Google Scholar]
- 44.Karasuyama H, Rolink A, Shinkai Y, Young F, Alt FW, Melchers F. The expression of Vpre-B/λ5 surrogate light chain in early bone marrow precursor B cells of normal and B cell-deficient mutant mice. Cell. 1994;77:133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 45.Salamero J, Fougereau M, Seckinger P. Internalization of B cell and pre-B cell receptors is regulated by tyrosine kinase and phosphatase activities. Eur J Immunol. 1995;25:2757–2764. doi: 10.1002/eji.1830251007. [DOI] [PubMed] [Google Scholar]
- 46.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990;343:760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 47.Venkataraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352:777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 48.Wasserman R, Li Y-S, Hardy RR. Down-regulation of terminal deoxynucleotidyl transferase by Ig heavy chain in B lineage cells. J Immunol. 1997;158:1133–1138. [PubMed] [Google Scholar]
- 49.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2gene product is required for Sμ-Sε heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 50.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci USA. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell M-A, Sefton BM. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992;12:2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman C, Cambier JC. The B cell antigen receptor complex: association of Ig-α and Ig-β with distinct cytoplasmic effectors. Science. 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 53.Saouaf SJ, Mahajan S, Rowley RB, Kut SA, Fargnoli J, Burkhardt AL, Tsukada S, Witte ON, Bolen JB. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz WLJ. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 55.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 56.Tarlinton DM, Corcoran LM, Strasser A. Continued differentiation during B lymphopoiesis requires signals in addition to cell survival. Int Immunol. 1997;9:1481–1494. doi: 10.1093/intimm/9.10.1481. [DOI] [PubMed] [Google Scholar]
- 57.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Kayakawa K. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO (Eur Mol Biol Organ) J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]