Abstract
The germinal center (GC) is an anatomic compartment found in peripheral lymphoid organs, wherein B cells undergo clonal expansion, somatic mutation, switch recombination, and reactivate immunoglobulin gene V(D)J recombination. As a result of somatic mutation, some GC B cells develop higher affinity antibodies, whereas others suffer mutations that decrease affinity, and still others may become self-reactive. It has been proposed that secondary V(D)J rearrangements in GCs might rescue B cells whose receptors are damaged by somatic mutations. Here we present evidence that mature human tonsil B cells coexpress conventional light chains and recombination associated genes, and that they extinguish recombination activating gene and terminal deoxynucleotidyl transferase expression when their receptors are cross-linked. Thus, the response of the recombinase to receptor engagement in peripheral B cells is the opposite of the response in developing B cells to the same stimulus. These observations suggest that receptor revision is a mechanism for receptor diversification that is turned off when antigen receptors are cross-linked by the cognate antigen.
Keywords: secondary V(D)J recombination, germinal center, recombination activating gene, surrogate light chain, terminal deoxynucleotidyl transferase
Blymphocytes develop clonally restricted antigen specific receptors by randomly joining Ig variable (V), diversity (D), and joining (J) gene segments by V(D)J recombination (1). V(D)J recombination is a highly regulated process that requires coordinate expression of a series of lymphoid-specific and non-lymphoid-specific genes (2). Among the lymphoid-restricted components, only recombination activating genes RAG1 1 and RAG2 are essential for V(D)J recombination (3–6). Together, these two proteins form a complex that initiates Ig genes rearrangements by recognizing the recombination signal sequences (RSSs) and cleaving DNA (7–13). There are three distinct waves of RAG expression in the bone marrow (14, 15). These waves of RAG expression correspond to heavy and light chain gene rearrangements and receptor editing (16–18).
In addition to RAG1 and RAG2, B cell progenitors also express a series of other proteins that are developmentally restricted, B cell specific, and required for efficient antibody gene assembly (19, 20). These include the terminal deoxynucleotidyl transferase (TdT) and murine λ5 or human λ-like, and V-preB proteins. TdT increases antibody diversity by adding nontemplated nucleotides to V(D)J junctions (21, 22) whereas λ5 and V-preB associate with each other to form the surrogate light chain (ΨL) (23–26). The ΨL is believed to enhance the efficiency of B cell development by pairing with heavy chains before a conventional light chain is assembled. In the absence of λ5, B cell development is severely impaired at the pro-B cell to pre-B cell transition because pro-B cells that express a heavy chain in the absence of a light chain are unable to develop into pre-B cells (23–26). Expression of all of these genes is thought to be terminated before mature B cells exit the bone marrow, ensuring that each B cell produces a single fixed antigen receptor that can be clonally selected and expanded in the periphery in response to specific antigen (19, 20).
Clonal expansion of specific antigen receptor–bearing B cells occurs in the germinal center (GC) (27–29). During the GC reaction, B cells with low affinity antigen receptors can improve the affinity of their receptors by somatic hypermutation (30–32). In addition, mouse GC B cells have been shown to be able to reactivate V(D)J recombination (33–37). The finding that V(D)J recombination occurs in mature B cells was unexpected because V gene rearrangements alter antibody specificity, and clonal selection implies that cells expanded in the GC retain their specificity. However, if appropriately regulated, new recombination might function to rescue B cells that make deleterious somatic mutations, or serve as an alternative mechanism to somatic mutation for producing cells with high affinity antibodies.
Here, we report experiments that show V(D)J rearrangement in mature peripheral B cells in humans and that suggest that recombination in these cells is regulated by antigen receptor cross-linking.
Materials and Methods
Human Cell Isolation and Fractionation.
Tonsil B cells and B cell subsets were prepared as previously described (38). In brief, after depletion of non-B cells, tonsil mononuclear cells were stained with biotin-labeled goat anti–human IgD (Amersham Pharmacia Biotech, Piscataway, NJ), which was visualized with streptavidin-FITC (Immunotech, Westbrook, ME), and PE-labeled mouse anti–human CD38 (Becton Dickinson, San Jose, CA). Cells were then sorted into the following fractions: (a) IgD+CD38− follicular mantle (FM) B cells; (b) IgD−CD38+ GC B cells; and (c) IgD−CD38− memory B cells. Alternatively, tonsil mononuclear cells were stained with PE-labeled mouse anti–human CD38 (Becton Dickinson) and rat anti–human CD77 (Immunotech) that was visualized with FITC-labeled sheep anti–rat IgM (Serotec, Ltd., Kidlington, UK) and sorted into three fractions: (a) CD38+CD77+ centroblasts; (b) CD38+CD77− centrocytes; and (c) a CD38−CD77− fraction that contained both naive FM B cells and memory B cells. To analyze for V-preB expression, tonsil cells were stained with tricolor anti-CD38 (Caltag Labs., San Francisco, CA), PE-labeled IgG1 monoclonal anti–V-preB antibody (4G7) (Schiff, C., manuscript in preparation), and FITC labeled anti-IgM (DAKO Corp., Carpinteria, CA), or anti-κ (DAKO Corp.) and/or anti-λ (Ortho Diagnostic Systems, Raritan, NJ) mAb.
Cell Culture.
Human FM and GC B cells were maintained in tissue culture as previously reported (38). In brief, 5 × 105 cells were cocultured during 3 d with CD40L-transfected L cells and stimulated with: IL-2 (10 U/ml), or IL-4 (50 U/ml), or IL-10 (100 ng/ml) or anti-κ, or anti-λ, or both, or Fab′2 anti-κ + anti-λ, or irrelevant control IgG, at the indicated concentrations in micrograms per milliliter (Kallestad Lab., Inc., Austin, TX).
Linker-ligation PCR.
DNA was extracted from 2 × 106 cells in agarose plugs (35) and ligated to the BW linker at 20 pM as previously described (39). PCR was performed using AmpliTaq Gold Taq polymerase (PE Applied Biosystems, Foster City, CA). In the first round of PCR we used linker primer BW-1 or BW-1H (39) and either JH6-1 or Jκ5-1 (JH6-1, 5′GCTGGTCTGGGGTGACCTCTCTCCGCTTC3′; Jκ5-1, 5′CAAACGTAAGTGCACTTTCCTAATGC3′). Samples were amplified for 15 cycles of 40 s at 94°C, 40 s at 66°C, and 1 min at 72°C, followed by a final 10-min extension step at 72°C. 1 μl was then used for an additional 40 cycles of PCR containing a second nested locus-specific primer (JH6-2, 5′GTGGTGGGACTCTGTCCGCTCCAAGGC3′; Jκ5-2, 5′GTTTGAGATATTAGCTCAGGTCAATTC3′) and either BW-1 or the closely related BW-1H linker primers (39). The expected size for the specific JH6 or Jκ5 RSS signal break products are 322 and 249 bp, respectively. Control PCR assays used the Jκ5-R primer specific for the human Jκ5 coding exon (Jκ5-R, 5′GTTTAATCTCCAGTCGTGTCCCTTG3′) combined with Jκ5-2 to amplify a germline 260-bp internal Ig κ locus fragment from the same linker-ligated DNA samples. PCR products were analyzed on 2% agarose gels and hybridized with either JH6 or Jκ5 internal probes (JH6, 5′GCTTGCGGTTGGACTTCCCAGCCGACAGTGGTGGTCTGGCTTCTGA3′; Jκ5, 5′GAACAGCCAAGCGCTAGCCAGTTAAGTGAGGCATCTCAATTGCAAG3′).
Cloning and Sequencing.
PCR products were gel purified with the Qiaquick kit (QIAGEN Inc., Chatsworth, CA) and cloned using the TA cloning system (Invitrogen Corp., Carlsbad, CA). Double-stranded DNA sequences were obtained using T7 and M13 primers with a Dye Terminator Cycle Sequencing kit (PE Applied Biosystems). The sequencing reactions were run and analyzed on a genetic analyzer (model 310; PE Applied Biosystems).
RNA Preparation and Reverse Transcriptase PCR.
Total RNA was extracted from 2 × 105 cells using TRIzol Reagent (GIBCO BRL, Gaithersburg, MD) and reverse transcribed in 20 μl with Superscript II (GIBCO BRL). For reverse transcriptase (RT)-PCR reactions, 2 μl of cDNA were amplified for 26, 28, or 30 (Igβ, λ-like, and V-preB) or 36, 38, or 40 (RAG1, RAG2, and TdT) cycles of 30 s at 94°C, 30 s at 60°C and 30 s at 72°C with a final 10-min extension at 72°C using Platinum Taq DNA polymerase (GIBCO BRL) and the following primers: RAG1 sense 5′TGCAGACATCTCAACACTTTGG3′, antisense 5′ACATCTGCCTTCACATCGATCC3′; RAG2 sense exon 1A 5′AGCAGCCCCTCTGGCCTTCAG3′, exon 1B 5′CCTTCAGACTGCGGTCTCCAG3′, antisense 5′TCCCTCGGCTGTACACCACATTAATG3′; TdT sense 5′GACACCACCAGATGGGCCAGCCAGAG3′, antisense 5′TGTTCTCTGCTACAATGTGGGTGAC3′; Igβ sense 5′ATGGCCAGGCTGGCGTTGTCTC3′, antisense 5′GAGGCGCTGTTCATGTAGCAGTG3′. Since human RAG2 transcripts derive from two different alternative first exons, RT-PCR was done with two 5′ primers specific for exon 1A or exon 1B. Amplification of either of the two possible mature mRNAs results in the same size product. λ-like– and V-preB–specific primers have been previously described (40). RT-PCR products were analyzed on 2% agarose gels and hybridized with cognate cDNAs amplified from the human pro-B cell line JEA-2, which expresses all these transcripts (40). PhosphorImager analysis was performed on samples amplified in the linear range.
Analysis of Mouse Cells.
4–6-wk-old λ5 targeted mice (41) and C57Bl/6 controls were bred and maintained under specific pathogen-free conditions. Mice were immunized with 50 μg of NP-CGG in alum 10 d before analysis. Spleen cells from immunized mice were stained with allophycocyanin-labeled anti-B220, PE-labeled anti-κ, PE-labeled anti–λ-1 and -2, FITC-labeled anti-GL7 (PharMingen, San Diego, CA), and biotin-labeled LM34 or SL156 anti-λ5 antibodies (42) visualized with streptavidin-PerCP (Becton Dickinson). Four-color analysis was performed on a FACScalibur® using Cellquest 3.1 (Becton Dickinson).
Immunohistochemistry.
Acetone-fixed cryostat tonsil sections (5 μm) were incubated with primary mouse mAbs against V-preB (4G7) (Schiff, C., manuscript in preparation), followed by Alexa488-conjugated goat anti–mouse Ig (Molecular Probes, Eugene, OR), and/or anti-CD21 mAb (DAKO Corp.) followed by biotinylated horse anti–mouse IgG (Vector Labs., Burlingame, CA) and Texas red–conjugated streptavidin (Molecular Probes). Confocal laser scanning microscopy was performed along the x and y axes with a confocal laser scanning microscope (Leica Inc., Deerfield, IL) equipped with an ×20 oil objective.
Results and Discussion
To study the regulation of V(D)J recombination in peripheral B cells, we turned to human tonsils as a source of large numbers of B cells that are readily fractionated into naive FM B cells and GC B cells (38). To determine whether human GC B cells actively undergo V(D)J recombination we developed an assay for detecting blunt 5′ phosphorylated human Igμ and Igκ RSS signal ends which are specific intermediate products of the V(D)J recombination reaction (references 39, 43; Fig. 1 a). JH6 and Jκ5 were selected because both are at the 3′ ends of their respective J regions and are least likely to be deleted during V(D)J recombination in the bone marrow. Using this assay PCR products corresponding to the expected 5′ phosphorylated human JH6 and Jκ5 signal ends were readily detected in human bone marrow, and were verified as specific by cloning and sequencing (Fig. 1 b, and data not shown).
Figure 1.
Ligation-mediated (LM)-PCR assay for detecting signal breaks in human GC B cells. (a) Scheme for LM-PCR strategy to detect RSS DNA breaks within the human Ig κ light chain locus. A primary VκJκ1 rearrangement is represented at the κ locus, and after V(D)J recombinase activation, downstream Jκ segments may be chosen for a secondary rearrangement; Jκ5 is depicted. Blunt signal ends at the nonamer–heptamer sequences (triangles) are ligated to BW linkers. Linker-ligated DNA molecules are then amplified by PCR using two sets of specific primers (see Materials and Methods). (b) Jκ signal breaks in human GC B cells. LM-PCR for JH6 (top) and Jκ5 (middle) signal breaks in human mononuclear bone marrow (BM) cells, naive CD38−IgD+ FM tonsil B cells, and CD38+IgD− GC B cells (left) and in centroblasts CD38+CD77+ and centrocytes CD38+CD77− (right). PCR products were visualized with locus-specific probes. PCR from a region overlapping the germline Jκ5 segment was used as a control for DNA loading (bottom).
Having established the validity of the assay we examined B cells from tonsils fractionated into IgD+CD38− naive FM B cells, and IgD−CD38+ GC cells. We found no RSS signal breaks in the naive FM B cells (Fig. 1 b). In contrast, GC B cells displayed Igκ but no Igμ RSS signal breaks. The number of Igκ RSS breaks in GC B cells could not be determined precisely because the RSS break assay involves a less than stoichiometric ligation reaction and two rounds of amplification. Nevertheless, the amount DNA breaking detected in GC samples was always 5–10-fold lower than in bone marrow as determined by dilution. We conclude that human GC B cells are actively undergoing V(D)J recombination at the Igκ locus.
The absence of Igμ RSS breaks in GC B cells may be due to the 12/23 rule. DH segments are 12/23 compatible with both VH and JH gene segments, but all DH segments are deleted by any V to DJH joining event. VH to JH joining is prohibited in the absence of DH segments by 12/23 incompatible RSSs (1). All mature B cells carry at least one productive VDJH on one allele and in addition many B cells have a nonproductive VDJH on the second allele (44). Thus, there is a relative dearth of potential 12/23 compatible targets for recombination at the heavy chain locus. Consistent with this idea, heavy chain RSS breaks can be found in activated B cells when DH segments are artificially preserved by allelic exclusion in mice that carry targeted VH genes (35). VH gene recombination using internal cryptic heptamers as signals for recombination has been documented but would not be detected by our assay (45, 46).
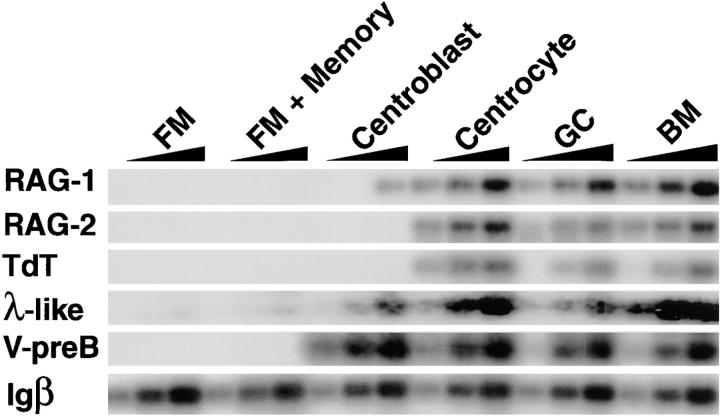
Immunohistochemistry revealed that mouse RAG1 protein expression is most prominent in the GC light zone, suggesting that recombination is activated in centrocytes (33). To determine whether expression of other recombinase components is restricted to a specific subset of mature B cells, we separated human tonsil B lymphocytes into five fractions by cell sorting: (a) FM cells, IgD+CD38−; (b) mixed FM cells and memory B cells, CD38−CD77−; (c) total GC B cells, IgD−CD38+; (d) GC centroblasts CD38+CD77+; and (e) GC centrocytes, CD38+CD77− (38). Centroblasts are rapidly dividing early GC cells that initiate somatic mutation, whereas switch recombination occurs primarily in the centrocyte fraction that is derived from the centroblasts (38, 47). RAG1, RAG2, TdT, λ-like, and V-preB were not expressed in resting FM B cells or in mixtures of FM B cells and post-GC memory B cells (Fig. 2). In contrast, all of these mRNAs were found in the GC fraction and RAG1, RAG2, and TdT were specifically enriched in centrocytes (Fig. 2). Although the PCR assay we used is only semiquantitative, it is in the linear range and shows that the levels of RAG1, RAG2, TdT, λ-like, and V-preB mRNAs in centrocytes are comparable to the levels of these mRNAs found in unfractionated adult bone marrow samples as measured by phosphorimaging. Low levels of RAG1 were also found in centroblasts (20% of the levels in centrocytes by phosphorimaging), but RAG2 was not detected in these cells (Fig. 2). In accordance with the RAG mRNA expression data, Igκ RSS breaks were found in centrocytes and not in centroblasts (Fig. 1 b). Thus, receptor revision by secondary V(D)J recombination is found in B cells that have already passed through the centroblast stage where somatic mutation is initiated (38).
Figure 2.
RAG, TdT, λ-like, and V-preB gene expression in human tonsil B cell subsets. RNA from FACS®-sorted CD38−IgD+ FM B lymphocytes, CD38−CD77− FM and memory B cells, CD38+CD77+ GC centroblasts, CD38+CD77− GC centrocytes, CD38+IgD− total GC B cells, and mononuclear bone marrow (BM) cells was analyzed by semiquantitative RT-PCR and visualized with labeled oligonucleotide probes. Igβ RT-PCR was used as a B cell–specific mRNA loading control. PCR assays were designed to distinguish between genomic DNA and mRNA using primers on different exons. PhosphorImager analysis showed that there is a fivefold difference in the amount of RAG1 mRNA amplified between centroblasts and centrocytes.
In developing B cells in the bone marrow, λ-like and V-preB proteins associate with each other as ΨLs that combine with nascent heavy chains to form the pre-B cell receptor (42, 48). The number of human bone marrow cells that express surface ΨL is very small (1–3% of B cells; references 40, 48, 49). Nevertheless, in both mouse and human, B cell development is inefficient in the absence of the ΨL (41, 50), presumably due to loss of B cells that express a heavy chain but no light chain. ΨL may play a similar role in the GC B cell. According to this hypothesis, ΨL would combine with heavy chains to rescue GC B cells that have lost conventional light chain expression (33, 34, 37). To characterize the GC B cells that reexpress ΨL, we stained human tonsil lymphocytes with anti–V-preB antibodies in conjunction with anti-CD38, anti-Igκ, and anti-Igλ antibodies (Fig. 3 a). Surface V-preB expression was found on 0.85–11% of CD38+ GC B cells in six independent samples (both ends of the spectrum are shown in Fig. 3 a), whereas CD38− cells did not express V-preB (Fig. 3 a). In all cases, GC cells that expressed V-preB co-expressed either Igκ or Igλ (Fig. 3 a and data not shown). The low number of ΨL-positive cells in most of the human GC samples is reminiscent of the low levels of ΨL on the surface of human bone marrow cells (40, 48, 49). In contrast to the human, ΨL are readily detected on the surface of mouse B cells in the bone marrow (42). To confirm our findings in human cells, we examined mouse spleen B cells for λ5 expression using two different anti-λ5 antibodies (reference 42; Fig. 3 b). In immunized mice, 15–20% of B220+GL7+ B cells expressed cell surface λ5 (five consecutive experiments), and, as in humans, these cells coexpressed either κ or λ light chains (Fig. 3 b). The relative absence of variability in ΨL expression on mouse peripheral B cells as compared with human may be due to controlled immunizations and the use of inbred mouse strains. Control B220+GL7− non-GC B cells did not express surface ΨLs and neither did B220+GL7+ B cells from λ5 targeted mice (Fig. 3 b). Furthermore, ΨLs are coexpressed with RAGs in B220+GL7+ B cells as determined by cell sorting and RT-PCR analysis (data not shown). We conclude that ΨLs are coexpressed with conventional light chains in GL7+ mature spleen B cells in mice and human, and that loss of conventional light chains is not a prerequisite for activation of ΨL expression in these cells.
Figure 3.
ΨL cell surface expression on GC B cells. (a) Flow cytometric analysis were performed on human tonsil B cells using anti-CD38, anti–V-preB, anti-Igκ, or anti-Igλ. Two different patient samples are shown. (b) Flow cytometric analysis of immunized wild-type and λ5-targeted (λ5−/−) mouse spleen cells using anti-B220, anti-GL7, anti-κ, and anti-λ, and either LM34 or SL156 anti-λ5 antibodies (42).
GC B cells may not be the only peripheral B cells that are CD38+IgD− (38). To directly confirm that ΨLs are expressed in GC B cells, we stained human tonsil tissue sections with anti-CD21 and anti–V-preB antibodies. Consistent with the cell fractionation experiments, ΨL expression was found in GCs (Fig. 4). In addition, occasional ΨL-positive B cells were found in the mantle and T cell zones. These occasional ΨL-positive cells represent CD38+IgD− non-GC B cells because isolated CD38−IgD+ FM B cells are always ΨL negative as measured by immunofluorescence (data not shown). ΨL expressing non-GC CD38+IgD− B cells may be recent bone marrow immigrants that are CD38+IgM+IgD−. We conclude that ΨLs are expressed in human GC B cells but are also expressed in other tonsillar B cells.
Figure 4.
ΨL expression in human tonsil B cells. Immunohistochemical staining of human tonsil sections was performed using anti–V-preB (left), anti-CD21 (middle), or both (right). GCs, FM, and the T cell zones (T zone) are indicated.
To define the requirements for RAG induction in peripheral B cells, we cocultured purified IgD+CD38− FM and IgD−CD38+ GC B cells with fibroblasts that express CD40L and ILs (51). After 3 d of culture with CD40L, resting FM B cells were induced to express high levels of TdT mRNA, but did not express RAG1, RAG2, λ-like, or V-preB (Fig. 5 a). IgD−CD38+ B cells differ from FM B cells in that they express RAG1, RAG2, λ-like, and V-preB, and all of these mRNAs are maintained in cocultures of GC B cells and CD40L-expressing fibroblasts (Figs. 2 and 5, a and b). Consistent with the results obtained with FM cells, only TdT mRNA expression is upregulated in the GC B cells in culture (Fig. 5 a). Addition of IL-2, IL-4, or IL-10 was not sufficient for RAG induction in the resting FM B cells, and did not alter the levels of RAGs or TdT expressed by GC B cells (Fig. 5 a). These results are in contrast to experiments with mouse spleen B cells that appeared to be induced to express RAGs in response to CD40L plus IL-4 or LPS plus IL-4 (35, 36). We conclude that reinduction of TdT in mature human B cells is mediated through CD40, and that distinct pathways are required to activate RAG expression.
Figure 5.
Regulated expression of RAG, TdT, λ-like, and V-preB genes. (a) Human tonsil CD38−IgD+ FM or CD38+IgD− GC B cells were sorted (d0) and cultured on CD40L-transfected fibroblasts with or without anti-κ+λ (α Ig) at 10 μg/ml, or IL-2, IL-4, or IL-10 for 3 d (d3). Gene expression was analyzed by RT-PCR as described above. (b) CD38+IgD− GC B cells were cultured with CD40L alone or with CD40L and an irrelevant IgG control (Control Ig), or with intact anti-κ (Ig α–κ) or anti-λ (Ig α–λ) or both (Ig α–κ + α–λ) or with Fab′2 anti-κ or anti-λ (Fab′2 α–κ + α–λ). Antibody concentrations are indicated in micrograms per milliliter. (c) GC cells reanalyzed by flow cytometry using anti-CD38 and anti–V-preB mAbs after 3 d of culture with CD40L and the indicated antibodies. GC B cells cultured with Ig α–κ + α–λ or Fab′2 α–κ + α–λ antibodies contained 1.5–2-fold more B cells than those with cultured with control antibody, and the percentage of dead cells was 40–50% in all of cultures as determined by trypan blue exclusion.
Immature autoreactive IgM+IgD- bone marrow B cells can be induced to reexpress RAGs when their receptors are cross-linked by self-antigens (15, 52). Reexpression of RAGs in these self-reactive B cells has been shown to delete the auto-reactive receptors, and this process is referred to as receptor editing (16–18). To determine whether RAG expression in peripheral B cells could also be modulated by receptor cross-linking, we added anti-κ and anti-λ (anti-κ+λ) antibodies to cultured IgD+CD38− and IgD−CD38+ B cells (Fig. 5, a and b). In contrast to the activation of RAG expression in bone marrow B cells, addition of anti-B cell receptor (BCR) antibodies to cultured peripheral B cells inhibited the expression of RAG1, RAG2, and TdT (Fig. 5, a and b). Anti-κ+λ antibodies also downregulated CD40L-induced TdT expression in FM cells (Fig. 5 a). To further characterize the suppressive effect of anti-BCR antibodies on RAG expression, we performed dose–response experiments with both intact anti-κ+λ antibodies and Fab′2 fragments as well as control nonspecific antibody preparations (Fig. 5 b). mRNA levels were measured by semiquantitative RT-PCR and phosphorimaging. RAG1 and RAG2 downregulation was observed with 10 μg/ml of intact anti-κ+λ and as little as 2 μg/ml of Fab′2 fragments of anti-κ+λ antibody but was difficult to detect with 10 μg/ml of intact anti-κ or anti-λ alone (RAG1 expression was decreased by a factor of 6–8 after treatment with 10 μg/ml of intact anti-κ+λ antibody, and up to 20× after treatment with 10 μg/ml of Fab′2 fragments of anti-κ+λ; Fig. 5 b). Direct measurements of cell viability showed that addition of either intact or Fab′2 fragments of anti-κ+λ antibodies to the cultures did not result in increased cell death (see legend to Fig. 5). More importantly, FACS® analysis showed that the number cells undergoing “neoteny” (33) did not change after anti-BCR cross-linking as determined by anti–V-preB staining and confirmed by RT-PCR analysis of V-preB mRNA levels (Fig. 5, a–c). Thus, the effects of anti-BCR antibodies on RAG expression cannot be explained by preferential killing of the specific subset of GC B cells that are undergoing receptor revision (36). We conclude that the effect of receptor cross-linking on recombination-associated gene expression in peripheral B cells is the opposite of the effect of cross-linking the same receptor on immature IgM+IgD− bone marrow B cells (15).
What is the function of receptor revision in the periphery? Our experiments indicate that new antigen receptor assembly does not require light chain inactivation and is not likely to be a mechanism for removing autoreactive receptors (Figs. 3 and 5). On the contrary, activation of recombination in cells that fail to bind antigen suggests that secondary recombination may participate in repertoire diversification by improving very low affinity receptors (35, 36). Expression of TdT lends further support to the notion that new receptor diversification by gene recombination occurs in mature B cells in the periphery since the only known function for TdT is to increase the diversity in the repertoire by adding N nucleotides to V(D)J junctions (21, 22).
The idea that specific antibodies might be induced during an immune response is difficult to accept because it is directly contradictory to current interpretations of the clonal selection theory (53, 54). Nevertheless, receptor assembly in GC B cells is already a well established mechanism for antibody diversification in avian immune systems (55). In the chicken, Ig gene conversion is a prominent feature of the GC reaction, resulting in groups of interrelated clones of B cells (55). In mice and humans, Ig recombination in GC B cells may be limited to the light chain genes. Since much of the antibody-binding pocket is shaped by the heavy chain, changing just the light chain could result in a group of related B cells with antibodies that have a spectrum of antigen binding affinities. Our findings are consistent with the idea that secondary recombination is terminated when antigen receptors are cross-linked and suggest a mechanism for positive selection of B cells that produce high affinity receptors by receptor revision in the periphery.
Acknowledgments
We thank members of the Nussenzweig lab for comments on the manuscript and helpful discussions, and Dr. Jim Young for help obtaining bone marrow samples.
Abbreviations used in this paper
- GC
germinal center
- FM
follicular mantle
- ΨL
surrogate light chain
- LM
ligation-mediated
- RAG
recombination activating gene
- RSS
recombination signal sequences
- RT
reverse transcriptase
- TdT
terminal deoxynucleotidyl transferase
Footnotes
E. Meffre was supported by a grant from the Philippe Foundation, and P. Cohen by National Institutes of Health (NIH) Medical Scientist Training Program grant GM-77039. This work was supported by grants from the NIH to M.C. Nussenzweig. M.C. Nussenzweig is an associate investigator of the Howard Hughes Medical Institute.
References
- 1.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Willerford DM, Swat W, Alt FW. Developmental regulation of V(D)J recombination and lymphocyte differentiation. Curr Opin Genet Dev. 1996;6:603–609. doi: 10.1016/s0959-437x(96)80090-6. [DOI] [PubMed] [Google Scholar]
- 3.Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- 4.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 5.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 6.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1 deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 7.Hiom K, Gellert M. A stable RAG-1-RAG-2– DNA complex that is active in V(D)J cleavage. Cell. 1996;88:65–72. doi: 10.1016/s0092-8674(00)81859-0. [DOI] [PubMed] [Google Scholar]
- 8.McBlane JF, van Gent DC, Ramsden DA, Romeo C, Cuomo CA, Gellert M, Oettinger MA. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 9.van Gent DC, McBlane JF, Ramsdem DA, Sadofsky MJ, Hesse JA, Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995;81:925–934. doi: 10.1016/0092-8674(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 10.Cuomo CA, Mundy CL, Oettinger MA. DNA sequence and structure requirements for cleavage of recombination signal sequences. Mol Cell Biol. 1996;16:5683–5690. doi: 10.1128/mcb.16.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Difilippantonio MJ, McMahan CJ, Eastman QM, Spanopoulou E, Schatz D. RAG1 mediates signal sequence recognition and recruitment of RAG2 in V(D)J recombination. Cell. 1996;87:253–262. doi: 10.1016/s0092-8674(00)81343-4. [DOI] [PubMed] [Google Scholar]
- 12.Spanopoulou E, Zaitseta F, Wang F-H, Santagata S, Baltimore D, Panayotou G. The homeodomain region of Rag-1 reveals the parallel mechanisms of bacterial and V(D)J recombination. Cell. 1996;87:263–276. doi: 10.1016/s0092-8674(00)81344-6. [DOI] [PubMed] [Google Scholar]
- 13.Ramsden DA, McBlane JF, van Gent DC, Gellert M. Distinct DNA sequence and structure requirement for the two steps of V(D)J recombination signal cleavage. EMBO (Eur Mol Biol Organ) J. 1996;15:3197–3206. [PMC free article] [PubMed] [Google Scholar]
- 14.Grawunder U, Leu TMJ, Schatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 15.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD−bone marrow B cells in vitro. Immunity. 1997;6:429–436. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 16.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radic MZ, Erickson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 20.Rolink A, Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991;66:1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- 21.Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- 22.Komori T, Okada A, Stewart V, Alt FW. Lack of N regions of antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S, Baltimore D. Formation of disulfide-linked μ2ω2 tetramers in pre-B cells by the 18k ω-immunoglobulin light chain. Nature. 1987;329:172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- 24.Karasuyama H, Kudo A, Melchers F. The proteins encoded by the VpreB and λ5pre-B cell–specific genes can associate with each other and with μ heavy chain. J Exp Med. 1990;172:969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsubata T, Reth M. The products of pre-B cell– specific genes (λ5 and VpreB) and the immunoglobulin μ chain form a complex that is transported onto the cell surface. J Exp Med. 1990;172:973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimoto N, Kubagawa H, Ohno T, Gartland GL, Stankovic AK, Cooper MD. Normal pre-B cells express a receptor complex of μ heavy chains and surrogate light-chain proteins. Proc Natl Acad Sci USA. 1991;88:6284–6288. doi: 10.1073/pnas.88.14.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 28.Nossal GJ. Differentiation of the secondary B-lymphocyte repertoire: the germinal center reaction. Immunol Rev. 1994;137:173–183. doi: 10.1111/j.1600-065x.1994.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- 30.Weigert MG, Cesari IM, Yonkovich SJ, Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- 31.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 32.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 33.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 34.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 35.Papavasiliou F, Casellas R, Qin XF, Besmer E, Suh H, Pelanda R, Nemazee D, Rajewsky K, Nussenzweig MC. V(D)J recombination in mature B cells: a new mechanism for diversification of antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 36.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 37.Hikida M, Mori M, Kawabata T, Takai T, Ohmori H. Characterization of B cells expressing recombination activating genes in germinal centers of immunized mouse lymph nodes. J Immunol. 1997;158:2509–2512. [PubMed] [Google Scholar]
- 38.Pascual V, Liu Y-J, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlissel M, Constantinescu A, Morrow T, Baxter M, Peng A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5′-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 1993;7:2520–2532. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- 40.Meffre E, Fougereau M, Argenson J-N, Aubaniac J-M, Schiff C. Cell surface expression of surrogate light chain (ΨL) in the absence of μ on human pro-B cell lines and normal pro-B cells. Eur J Immunol. 1996;26:2172–2180. doi: 10.1002/eji.1830260932. [DOI] [PubMed] [Google Scholar]
- 41.Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of λ5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 42.Karasuyama H, Rolink A, Shinkai Y, Young F, Alt FW, Melchers F. The expression of VpreB/λ5 surrogate light chain in early bone marrow precursor B cells of normal and B cell deficient mutant mice. Cell. 1994;77:133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 43.Roth DB, Zhu C, Gellert M. Characterization of broken DNA molecules associated with V(D)J recombination. Proc Natl Acad Sci USA. 1993;90:10788–10792. doi: 10.1073/pnas.90.22.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 45.Reth M, Gehrmann P, Petrac E, Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. Nature. 1986;322:840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- 46.Kleinfield R, Hardy RR, Tarlinton D, Dangl J, Herzenberg LA, Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and germline variable gene segment in a Ly 1+B-cell lymphoma. Nature. 1986;322:843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- 47.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, Max EE, Martinez-Valdez H. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–250. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 48.Lassoued K, Nunez CA, Billips L, Kubagawa H, Monteiro RC, LeBien TW, Cooper MD. Expression of surrogate light chain receptors is restricted to a late stage in pre-B cell differentiation. Cell. 1993;73:73–86. doi: 10.1016/0092-8674(93)90161-i. [DOI] [PubMed] [Google Scholar]
- 49.Ghia P, ten Boekel E, Sanz E, de la Hera A, Rolink A, Melchers F. Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain loci. J Exp Med. 1996;184:2217–2229. doi: 10.1084/jem.184.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minegishi Y, Coustan-Smith E, Wang Y-H, Cooper MD, Campana D, Conley ME. Mutations in the human λ5/14.1gene result in B cell deficiency and agammaglobulinemia. J Exp Med. 1998;187:71–77. doi: 10.1084/jem.187.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu Y-J. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 52.Melamed D, Benschop RJ, Cambier JC, Nemazee D. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173–182. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 53.Burnet, F.M. 1959. The Clonal Selection Theory Of Acquired Immunity. The University Press, Cambridge, UK.
- 54.Talmage DW. Clonal selection theory. Science. 1959;129:1643–1648. doi: 10.1126/science.129.3364.1643. [DOI] [PubMed] [Google Scholar]
- 55.Arakawa H, Furusawa S, Ekino S, Yamagishi H. Immunoglobulin gene hyperconversion ongoing in chicken splenic germinal centers. EMBO (Eur Mol Biol Organ) J. 1996;15:2540–2546. [PMC free article] [PubMed] [Google Scholar]